Introduction
Parastomal hernia (PH) is a common complication
following an ileostomy or colostomy (1). PH rarely appears in the early stages
after surgery (0-3%); however, it normally occurs in the first 2
years post-surgery (30-50%), and the risk may persist for >2
decades (2). In addition, PHs may
have an adverse effect on the body and quality of life of patients
(3). The treatment of PH is
difficult as it has a high recurrence rate (4); to the best of our knowledge, the
best method for overcoming this is to control initial PH
development (5).
PH development may be attributed to multiple risk
factors, including age, sex, aperture size, body mass index (BMI)
and hypertension (6,7). However, the exact pathogenesis of PH
formation remains unclear. It has been demonstrated that a
decreased ratio of collagen I/collagen III is involved in PH
formation (8). Collagen, as a
primary component of the extracellular matrix (ECM), is relevant to
the tensile strength in abdominal wall fascial layers. Fibroblasts
are a common type of cell involved in the synthesis and metabolism
of collagen (9). Therefore, we
hypothesized that the synthesis of collagen was associated with the
development of parastomal hernia and that the potential regulation
mechanism involved fibroblast activity.
To the best of our knowledge, alterations to the ECM
are regulated by the matrix metalloproteinases (MMPs), which may
lead to collagen degradation (10). The MMPs, which belong to a
zinc-dependent endopeptidases family, are essential for ECM
remodeling and modification of almost all ECM components, including
collagens, fibronectins and proteoglycans (11) that are also involved in normal
physiological and pathological processes including angiogenesis and
neoplasm metastasis (12,13). MMPs also serve a role in wound
healing, in addition to growth factors, cytokines and adhesion
molecules (14). In addition,
MMPs have been revealed to be associated with each phase of skin
wound healing (15,16). Chronic wounds have been
demonstrated to be associated with aberrant ECM, and elevated MMPs
levels including MMP-2 and MMP-9 (14,17,18). Tissue inhibitor of
metalloproteinases (TIMPs) and intrinsic inhibitors of MMPs may
regulate the ECM. Metalloproteinase inhibitor 1 (TIMP-1) has also
been suggested to be able to affect fibrosis. For example, TIMP-1
was demonstrated to be involved in organ fibrosis, including the
liver and the heart (19,20). A recent study also indicated that
TIMP-1 regulated pulmonary fibrosis by activating fibroblasts and
promoting proliferation (21). In
addition, TIMP-1 contributes to types I and III collagen
degradation (22).
However, the association between TIMP-1 and collagen
in parastomal hernia remains unclear. Therefore, the aim of the
present study was to investigate the potential effect of collagen
synthesis in PH.
Materials and methods
Clinical data and specimens
Data from 157 patients with rectal cancer who
received permanent colostomy between March 2008 and September 2013
at Beijing Chao-Yang Hospital (Beijing, China) were reviewed
retrospectively. All patients underwent abdominal perineal
resections. Abdominal CT scans were performed every 6 to 12 months
to evaluate the development of PH during the follow-up stage. PH
was identified in a total of 55 cases. Information concerning age,
sex, BMI (kg/m2), diabetes, hypertension, radiation
history, length of hospital stay (days), aperture size and
expression of types I and III procollagen mRNA from each patient
were collected during outpatient visits in the hospital. Informed
consent was provided by each patient and all experiments were
authorized by the Ethics Committee of Beijing Chao-Yang Hospital.
The dermal tissues and matched normal tissues were obtained from 55
patients with PH for the reverse transcription quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis.
Skin fibroblast culture
Briefly, fibroblasts were taken from tissues 1-mm
distance away from the center of the skin samples. The tissues were
placed on the upper wall of the culture bottle, to which was added
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS
(both Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 mg/ml streptomycin in a 90% humidified incubator with 5%
CO2 at 37°C. After 4 h, the culture bottle was turned
over carefully and the tissues were immersed into the medium.
Primary fibroblast cells were formed and subjected to cell
identification following tissue culture for 3-14 days. The cells
were amplified, passaged weekly at 80-90% confluence and used at
early (between 2nd and 4th) passages (P) to avoid in vitro
replicative-induced ageing. At P2 or P3, the cells were seeded at
5,000 cells/cm2 for all the experiments, unless
otherwise stated.
Immunofluorescence microscopy
The fibroblasts were fixed in 4% paraformaldehyde
for 20 min at room temperature and extracted in 0.5% Triton X-100
for 10 min. Following washing in PBS, the samples were incubated
with rabbit anti-human vimentin monoclonal antibody (cat. no.
AX10005; 1:200; Abgent, Inc.) for 1 h at room temperature and then
with fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG
(cat. no. R37119; 1:100; Molecular Probes; Thermo Fisher
Scientific, Inc.). Nuclei were stained with DAPI (5 μg/ml;
Abgent, Inc.) for 15 min in the dark and images were observed with
a fluorescence DM5000 B microscope (magnification, ×200; Leica
Microsystems, Inc.).
RNA extraction, cDNA synthesis and
RT-qPCR
Total RNA from PH tissues or tissues without PH
(WPH) and skin fibroblasts was extracted by TRIzol®
(Thermo Fisher Scientific, Inc.). TRIzol® reagent and
chloroform were added to the samples and mixed for 5 min. The
samples were then centrifuged at 2,000 x g for 10 min at room
temperature to recover the supernatant. Next, the supernatant was
incubated with an equal volume of isopropyl alcohol at 0°C for 5
min, followed by centrifugation at 12,000 x g at 4°C for 10 min.
Following removal of the supernatant, the 75% ethanol was added to
wash the precipitPate and the RNA was eluted with nuclease-free
water. The purity and content for the reverse transcription were
determined using NanoDrop 2000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). The cDNA was
obtained by RNA with mixture in PrimeScript™ 1st Strand cDNA
Synthesis kit (Takara Biotechnology, Co., Ltd.). The reactions were
conducted using the following primers: α1 (I) procollagen forward,
5′-GTTCGTCCTTCTCAG GGTAG-3′; α1 (I) procollagen reverse,
5′-TTGTCGTAGCAGGGTTCTTT-3′; α1 (III) procollagen forward,
5′-CGAGGTAACAGAGGTGAAAGA-3′; α1 (III) procollagen reverse,
5′-AACCCAGTATTCTCCACTCTT-3′; β-actin forward,
5′-GGTTACCTCCCATCAGCT-3′; and β-actin reverse:
5′-CAGTGTCCGGAAATCTCC-3′, using a LightCycler system (Roche
Diagnostics) using the under the following thermocycler conditions:
94°C for 4 min, then 40 cycles at 94°C for 45 sec, 56°C for 45 sec
and 72°C for 2 min. The results were calculated using the
2−ΔΔCq method (23).
Cell proliferation assay
Cells (~5×103) were maintained on 96-well
plates for the measurement of proliferation using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). Following
culture for 12, 24 and 48 h, the cells were incubated with CCK-8
solution (10 μl) at 37°C for 2 h. Absorbance was read at 450
nm using an iMark plate reader (Bio-Rad Laboratories, Inc.).
Cell transfection
A total of 2 μg small interfering RNA (siRNA;
5′-UCAACCAGACCACCUUAU AdT dT-3′; Shanghai GenePharma Co., Ltd.) was
used to silence TIMP-1 expression. A negative control (NC; cat. no.
A06001; Shanghai GenePharma Co., Ltd.) was also included. The cells
(4×105) were seeded in 6-well plates. After culture for
24 h, the medium was replaced by Opti-MEM (Invitrogen; Thermo
Fisher Scientific, Inc.). The fibroblasts were transfected with
TIMP-1 siRNA using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). According to the manufacturer's
protocol. The transfection efficiency was assessed using western
blot analysis. The subsequent experiments were performed 24 h after
transfection.
Wound healing assay
Cell migratory ability was measured using specific
wound assay chambers (ibidi GmbH). Fibroblasts (1×106)
were seeded onto 24-well plates. Following attachment of the cells,
a wound between the fibroblasts was created using a sterile pipette
tip (10 μl). Then, the fibroblasts were washed and incubated
for 24 h. The cell migration from 0 to 24 h was observed under a
phase-contrast inverted microscope (magnification, ×200; Olympus
Corporation) with a TUCSEN camera. The cell migration was measured
with Wimasis Image Analysis (Onimagin Technologies SCA) at 0 and 24
h.
Transwell assay
The fibroblast invasion capabilities were determined
using a Matrigel-coated Transwell assay (Corning Incorporated).
Following serum starvation overnight, the fibroblasts were added
into the upper chambers in DMEM containing 1% FBS. Concomitantly,
the lower chambers were filled with 500 ml DMEM containing 20% FBS.
The fibroblasts were incubated in an incubator for 24 h at 37°C.
The non-invading fibroblasts blocked by the Matrigel were removed
from the upper surface using a wet cotton swab. Following rinsing
with PBS, the bottom surface of the filter was fixed with 95%
ethanol at room temperature for 10 min and stained with 0.1%
crystal violet at room temperature for 5 min. Invasion was detected
by counting the stained cells under a light microscope
(magnification, ×200; Olympus Corporation).
Western blot analysis
The fibroblasts and PH tissues or tissues WPH and
were lysed in lysis buffer (RIPA; Beyotime Institute of
Biotechnology) and centrifuged at 2,000 × g for 10 min at room
temperature for supernatant recovery. The protein concentrations
were determined using a BCA kit (Beyotime Institute of
Biotechnology). Following separation on 10% SDS-PAGE, the proteins
(20 μg/lane) were transferred onto nitrocellulose membranes
(EMD Millipore) and blocked with 5% non-fat milk for 1 h at room
temperature. Then the membranes were incubated at 4°C overnight
with anti-MMP-2 (cat. no. 40994; 1:1,000; Cell Signaling
Technology, Inc.), anti-MMP-9 (cat. no. sc-21733; 1:500; Santa Cruz
Biotechnology, Inc.), anti-TIMP-1 (cat. no. sc-365905; 1:1,000;
Santa Cruz Biotechnology, Inc.), anti-collagen I (cat. no.
sc-293182; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-collagen
III (cat. no. sc-514601; 1:1,000; Santa Cruz Biotechnology, Inc.)
and anti-β-actin (cat. no. 4970; 1:1,000; Cell Signaling
Technology, Inc.) followed by incubation with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat. no.
10285-1-AP; 1:2,000, ProteinTech Group, Inc.) and HRP-goat
anti-mouse IgG H&L (cat. no. ab205719; 1:2,000; Abcam)
secondary antibodies at room temperature for 1 h. The proteins were
visualized using an ECL kit (Amersham; GE Healthcare). The density
of the blots was measured using the Quantity One software version
2.4 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Differences between groups for categorical data were
analyzed by the χ2 test and Fisher's exact test and
continuous data were analyzed using a Student's t-test. The
cumulative incidence rate of PH was calculated by the Kaplan-Meier
estimate analysis with log-rank test. Correlations among the risk
factors were assessed using logistic regression analysis with a 95%
confidence interval (CI) for the inclusion of additional prediction
into the model. The clinical features were used as independent
variables, while the presence or absence of PH was treated as a
dependent variable. The differences between multiple groups were
assessed with one-way analysis of variance and Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. SPSS 16.0 software (SPSS Inc.) was used for
the analysis and the results were represented as mean ± standard
deviation.
Results
Clinicopathological parameters
A total of 157 patients, including 55 patients with
PH and 102 patients without PH, were reviewed. Among them, several
parameters (age, history of diabetes, hypertension, radiation
history, length of hospital stay and α1 (I) procollagen expression)
exhibited no marked association with PH occurrence. However, the
sex, BMI, aperture size and α1 (III) procollagen expression levels
in the patients with PH were closely associated with the PH
(Table I). The hernias occurred
at between 7-57 months post-surgery, with an average of 23
months.
 | Table IPatients characteristics. |
Table I
Patients characteristics.
| Variable | Patients with PH
(n=55) (%) | Patients without PH
(n=102) (%) | P-value |
|---|
| Age, years | 66.9±10.4 | 66.3±9.1 | 0.708a |
| Sex | | | <0.001b |
| Male | 19 (22.6) | 65 (77.4) | |
| Female | 36 (49.3) | 37 (50.7) | |
| Body mass index
(kg/m2) | 24.7±3.3 | 23.0±3.4 | 0.003a |
| Diabetes | | | 0.671b |
| Yes | 6 (40.0) | 9 (60.0) | |
| No | 49 (34.5) | 93 (65.5) | |
| Hypertension | | | 0.306b |
| Yes | 20 (40.8) | 29 (59.2) | |
| No | 35 (32.4) | 73 (67.6) | |
| Radiation
history | | | 0.884b |
| Yes | 5 (35.7) | 9 (64.3) | |
| No | 43 (37.7) | 71 (62.3) | |
| Length of hospital,
days | 15.8±8.2 | 15.5±7.6 | 0.819a |
| Aperture size,
cm | 3.3±0.8 | 2.9±0.7 | 0.001a |
| α1 (I) procollagen
mRNA (ΔCq) | 1.91±0.58 | 1.99±0.62 | 0.431a |
| α1 (III)
procollagen mRNA (ΔCq) | 2.10±0.54 | 2.69±0.55 | <0.001a |
Among the 55 pairs of tissues, no marked difference
in the α1 (I) procollagen expression was observed between the
dermal tissues of patients with PH and that in tissues from
patients without PH (Fig. 1A). By
contrast, the α1 (III) procollagen level in dermal tissues of
patients with PH was markedly increased compared with those in the
tissues from patients without PH (P<0.05; Fig. 1B). The protein levels of collagen
I were irregular while the collagen III levels were notably
increased in the dermal tissues of patients with PH (Fig. 1C). Furthermore, the ratio of
collagen I/III was clearly decreased in the dermal tissues of
patients with PH (Fig. 1D).
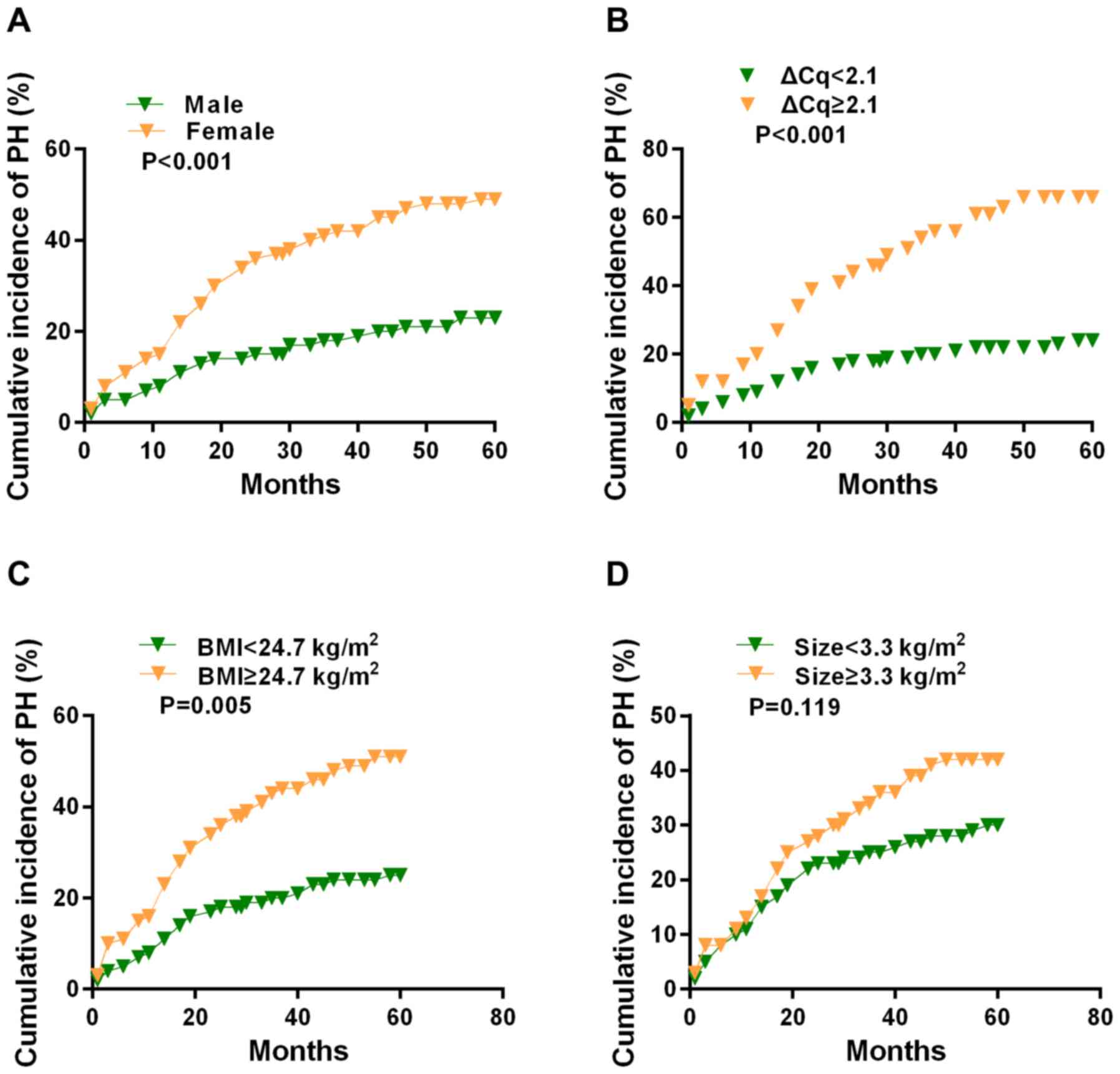
According to the Kaplan-Meier analysis, the 5-year
cumulative incidence of PH was calculated based on the clinical
parameters. The incidence rate in female patients (50.5%) was
significantly increased compared with that of male patients (25.4%;
P<0.001; Fig. 2A). Patients
with increased α1 (III) procollagen expression (68.9%) exhibited a
higher incidence rate compared with those with decreased α1 (III)
procollagen levels (26.7%) (P<0.001; Fig. 2B). The 5-year cumulative incidence
rate of patients with a BMI >24.7 kg/m2 was 53.1%,
which was remarkably increased compared with that of patients with
a BMI <24.7 kg/m2 (25.4%) (P=0.005; Fig. 2C). In addition, PH incidence rate
of patients with an aperture size >3.3 cm (41.3%) had no
significant difference compared with that of patients with an
aperture size <3.3 cm (31.5%) (P=0.119; Fig. 2D).
In the logistic analysis, female sex [odds ratio,
2.59; 95% confidence interval (CI), 1.107-6.059; P=0.028], aperture
size (Odds ratio, 2.247; 95% CI, 1.280-3.946; P=0.005) and α1 (III)
procollagen (Odds ratio, 0.109; 95% CI, 0.045-0.265; P<0.001)
were independent risk factors for PH formation, however, BMI was
not associated with PH incidence (Table II).
 | Table IIFactors associated with the
occurrence of parastomal hernia. |
Table II
Factors associated with the
occurrence of parastomal hernia.
| Variable | Odds ratio | 95% confidence
interval | P-value |
|---|
| Sex | | 1.107-6.059 | 0.028 |
| Male | 1 | | |
| Female | 2.590 | | |
| Body mass index
(kg/m2) | 1.116 | 0.983-1.266 | 0.089 |
| Aperture size | 2.247 | 1.280-3.946 | 0.005 |
| α1 (III)
procollagen mRNA (ΔCq) | 0.109 | 0.045-0.265 | <0.001 |
Effect of fibroblasts on PH
Positive vimentin expression was observed in the
fibroblasts derived from tissues from patients with PH and without
PH. The cells exhibited long shuttle or flat star shapes (Fig. 3A and B).
The wound closure of the fibroblasts from tissues
from patients without PH (control) reached ~80% by 24 h, while only
~50% of the area was denuded by the fibroblasts from PH dermal
tissues (Fig. 4A). The invasion
rate in the normal fibroblast group was evidently enhanced, while
it was notably inhibited in the hernia fibroblast group (Fig. 4B). The data demonstrated that the
migration and invasion rates of fibroblasts were significantly
suppressed in patients with PH (P<0.05; Fig. 4C and D). In addition, the
fibroblast viability was also decreased in patients with PH,
compared with patients without PH (Fig. 4E).
To explore the potential effects of collagen
synthesis and the ECM on the PH, the protein levels of collagen I,
collagen III, MMP-2, MMP-9 and TIMP-1 were detected (Fig. 5A). The collagen I expression
levels in the fibroblasts from tissues from patients without PH
were not significantly different from those in the fibroblasts from
PH dermal tissues. However, among the fibroblasts from PH dermal
tissues, the levels of collagen III and TIMP-1 were markedly
increased while those of MMP-2 and MMP-9 were evidently inhibited
in comparison with the non-PH fibroblasts (P<0.05; Fig. 5B).
Silencing TIMP-1 improves the fibroblast
activity
For the fibroblasts from non-PH tissues, the
cell-free area in the control-NC group at 24 h was larger compared
with that in control-siTIMP-1 group. Similarly, among the
fibroblasts from PH dermal tissues, the cell-free area in the
hernia-NC group at 24 h was larger compared with that in the
hernia-siTIMP-1 group (Fig. 6A).
Silencing TIMP-1 notably increased the mean number of normal skin
fibroblasts migrating into the bottom chamber. In addition, the
number of fibroblasts from PH dermal tissues was significantly
increased by silencing TIMP-1, compared with that in hernia-NC
group (Fig. 6B). It was also
identified that silencing TIMP-1 markedly enhanced the migratory
and invasive abilities of fibroblasts (P<0.05; Fig. 6C and D).
Furthermore, the protein levels of the
ECM-associated molecules were detected (Fig. 7A). The TIMP-1 expression was
significantly suppressed in the groups transfected with siTIMP-1,
indicating a successful transfection. It was identified that TIMP-1
silencing markedly inhibited the collagen III expression levels. In
addition, the results also suggested that the levels of MMP-2 and
MMP-9 were evidently increased by silencing TIMP-1, which
indirectly indicated the negative correlation between MMP-2 or
MMP-9 and collagen III (P<0.05; Fig. 7B).
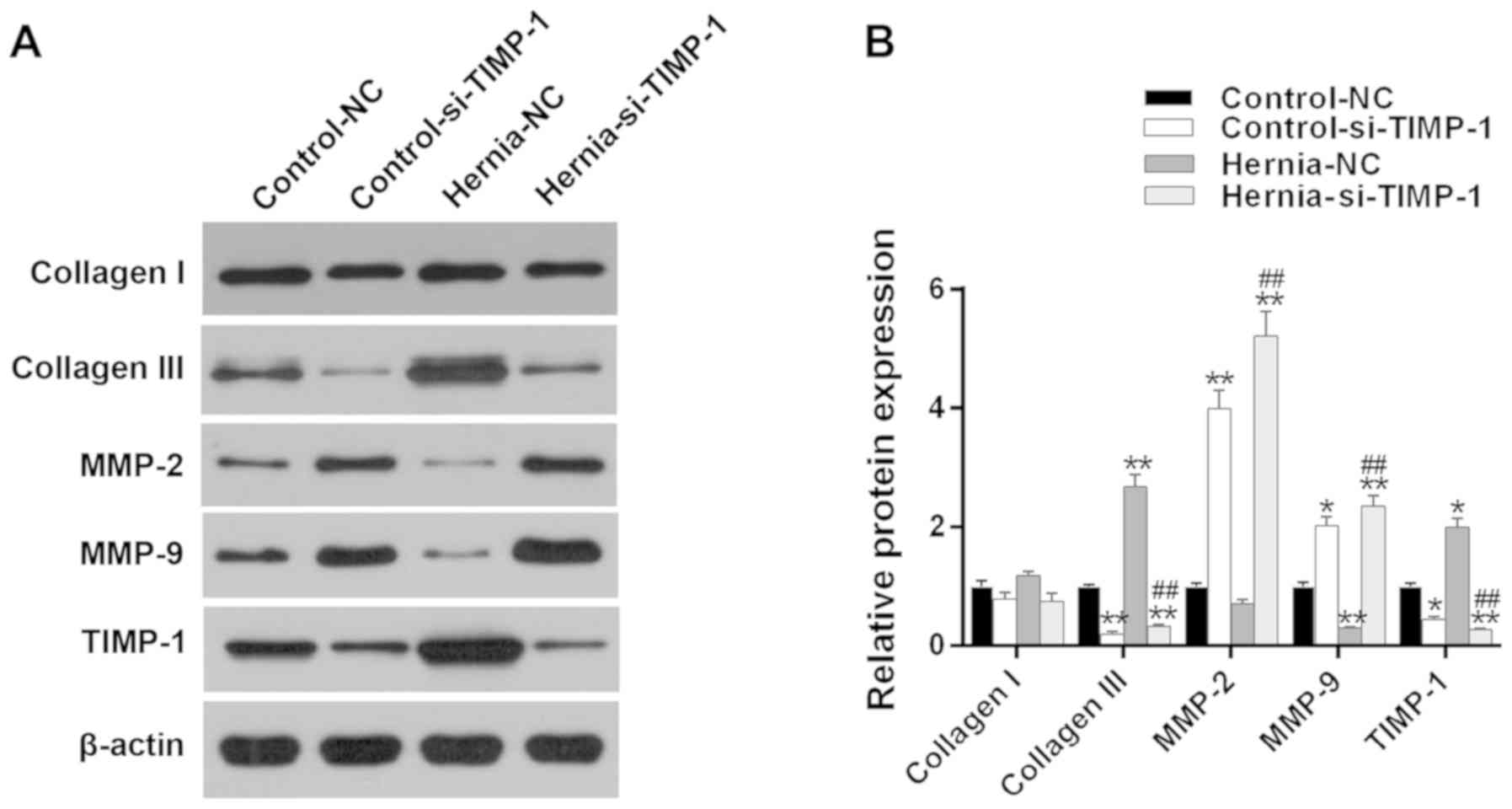 | Figure 7Effects of TIMP-1 silencing on the
expression of collagen I, collagen III, MMP-2 and MMP-9. (A) The
protein expressions of collagen I, collagen III, MMP-2, MMP-9 and
TIMP-1 were determined by western blot analysis in the fibroblasts
derived from patients with PH and those without PH (control). (B)
Quantitative data of protein expression were analyzed.
*P<0.05 and **P<0.01 vs. control-NC
group. ##P<0.01 vs. hernia-NC group. TIMP-1,
metalloproteinase inhibitor 1; si, small interfering; MMP, matrix
metalloproteinase; PH, parastomal hernia; NC, negative control. |
Discussion
PHs are commonly presented as asymptomatic, however,
they may result in morbidities due to leakage, dermatitis,
perforation, intermittent obstruction and strangulation (24). The exact incidence rate of PH has
been very difficult to assess due to lack of a consistent
definitions (6). Several risk
factors have been identified to be crucial, in spite of a paucity
of reliable data. Age, sex, BMI, laparoscopic surgical approach,
trans-peritoneal route of stoma creation and aperture size have
been suggested as independent risk elements for PH development
(7,25). In the present study, it was
identified that sex, aperture size and collagen III expression were
independent risk factors for PH incidence. In addition, it was also
demonstrated that the decreased viability, migration and invasion
of the skin-derived fibroblasts from PH patients were enhanced by
silencing TIMP-1 and that TIMP-1 may regulate the genes associated
with collagen regulation and metabolism.
The occurrence of PH may be attributed to a delayed
or weakened healing process, but all the potential risk factors
have not been comprehensively identified. In addition, multiple
risk factors may be divided into three subcategories, including:
Disease (ulcerative colitis, constipation, obesity and cancer);
patient (age, sex, malnutrition, etc.); and surgery (emergency
operation, postoperative infection) (26,27). The follow-up time following stoma
has been demonstrated to be a vital factor in estimating an exact
incidence rate of PH; the PH incidence rate was increased with
longer follow-up time (28). In
the present study, it was demonstrated that risk factors, including
diabetes, hypertension, radiation history and length of hospital
stay were not markedly different between patients with PH and those
without PH. The average BMI of patients with PH was evidently
increased compared with those without PH. However, in the logistic
regression analysis, BMI was not identified as a significant
independent factor for PH development; this observation was
consistent with data from a previous study (29). Aperture size was revealed to be a
potential independent factor of herniation; however, whether
limiting the size of the aperture truly attenuates PH formation
remains unknown (7,30). In addition, age was a potential
risk factor for PH development. A retrospective review over a
>10-year follow-up period of 782 patients demonstrated that PH
incidence was increased in the elderly population (31). The present study identified that
the PH incidence was increased in female patients; this may be
explained by the fact that females usually have thinner abdominal
muscle and thicker fat layers (32).
Furthermore, the present study also demonstrated
that the levels of procollagen III expression were associated with
the PH development and that collagen III expression was increased
in patients with PH, indicating that collagen may be involved in PH
incidence. Disordered collagen levels and decreased type I collagen
and type III collagen expression levels have been observed
previously in tissue biopsies from patients with hernias (33). The collagen I/III ratio of
patients was revealed to be associated with hiatal hernia (34). Types I and III are the dominant
components of collagen in the derma. Type I collagen is mature,
mechanically stable and is demonstrated to be associated with
tissue strength (35). The
present study revealed that the collagen III expression levels were
elevated in patients with PH and that the ratio of collagen I/III
was markedly decreased, suggesting that collagen III might serve a
crucial role in PH occurrence.
Hernias have been suggested to be associated with a
poor quality ECM, which systemically alters collagen turnover
(36). ECM stability is directly
associated with the levels of collagen synthesis and degradation
(37). Due to the constituents of
the ECM, collagen degradation may exert a crucial effect on the
morphogenesis, development, tissue remodeling and repair processes
(38). Furthermore, ECM
degradation may be induced by MMPs (10). TIMPs have been demonstrated to
exert an effect on cell proliferation, differentiation, migration,
apoptosis and anti-angiogenesis processes (39). TIMP-1 may also regulate the levels
of MMP-2 and MMP-9. In the present study, it was observed that the
levels of MMP-2 expression were evidently decreased, while the
TIMP-1 levels were notably enhanced in the hernia fibroblasts,
suggesting that MMP-2 may be suppressed by TIMP-1. MMP-9 levels
were slightly decreased in the hernia fibroblasts, and this may be
due to an association between enhanced MMP-9 expression and poor
healing (40). To additionally
explore the effects of MMP-2 and MMP-9 on the levels of collagen
III, TIMP-1 expression was silenced. The results indicated that
silencing TIMP-1 reversed the decrease in viability, migration and
invasion of fibroblasts from skin tissues of patients with PH. It
has been indicated that MMP-2 is able to degrade interstitial
collagen I, II and III, while MMP-9 cleaves collagen I and III
(41,42). The results from the present study
are consistent with previous studies that silencing TIMP-1
increased the expression levels of MMP-2 and MMP-9 and inhibited
the expression of collagen III in the hernia fibroblasts. The
inhibited expression of collagen III through TIMP-1 silencing was
similar to the results from the clinicopathological analysis.
This study had a number of limitations; for example,
the direct correlation between collagen III and MMP-2, the effect
of over-expressed TIMP-1 on MMP-2 and MMP-9 were not analyzed. A
more comprehensive and direct method of validating the results of
the present study will be conducted in the future.
In conclusion, the present study demonstrated that
collagen III levels were markedly and independently associated with
the occurrence of PH. In addition, the potential regulatory
mechanisms of collagen III were closely associated with TIMP-1,
MMP-2 and MMP-9 expression. These results may provide novel
therapeutic targets for PH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
FZ and FC made substantial contributions to the
conception and design of the study. XY, YL and JC were responsible
for data acquisition, analysis and interpretation. XY and YL were
involved in drafting the article and critically revising it for
important intellectual content. All authors provided final approval
of the version to be published. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experiments were authorized by the Ethics
Committee of Beijing Chao-Yang Hospital. All procedures involving
human participants were performed in accordance with the ethical
standards of the institutional and/or national research committee,
and with the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. Informed consent was provided by each
patient.
Patient consent for publication
Informed consent was provided by each patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carne PW, Frye JN, Robertson GM and
Frizelle FA: Parastomal hernia following minimally invasive stoma
formation. ANZ J Surg. 73:843–845. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Celik S, Firat Kocaay A and Akyol C:
Parastomal Hernia. pp. 185–199. 2017, http://dx.doi.org/10.5772/intechopen.68876Accessed
August 30, 2017.
|
|
3
|
van Dijk SM, Timmermans L, Deerenberg EB,
Lamme B, Kleinrensink GJ, Jeekel J and Lange JF: Parastomal hernia:
Impact on quality of life? . World J Surg. 39:2595–2601. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwiatt M and Kawata M: Avoidance and
management of stomal complications. Clin Colon Rectal Surg.
26:112–121. 2013. View Article : Google Scholar :
|
|
5
|
Shah NR, Craft RO and Harold KL:
Parastomal hernia repair. Surg Clin North Am. 93:1185–1198. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sohn YJ, Moon SM, Shin US and Jee SH:
Incidence and risk factors of parastomal hernia. J Korean Soc
Coloproctol. 28:241–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong SY, Oh SY, Lee JH, Kim DY and Suh KW:
Risk factors for parastomal hernia: Based on radiological
definition. J Korean Surg Soc. 84:43–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skibiński R, Pasternak A, Szura M, Solecki
R, Matyja M and Matyja A: Parastomal hernia-contemporary methods of
treatment. Pol Przegl Chir. 87:531–537. 2015. View Article : Google Scholar
|
|
9
|
Ma X, Liu Y, Wang Q, Chen Y, Liu M, Li X,
Xiang R, Wei Y, Duan Y and Han J: Tamoxifen induces the development
of hernia in mice by activating MMP-2 and MMP-13 expression.
Biochim Biophys Acta. 1852.1038–1048. 2015.
|
|
10
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han KY, Dugas-Ford J, Seiki M, Chang JH
and Azar DT: Evidence for the involvement of MMP14 in MMP2
processing and recruitment in exosomes of corneal fibroblasts.
Invest Ophthalmol Vis Sci. 56:5323–5329. 2015.
|
|
12
|
Siefert SA and Sarkar R: Matrix
metalloproteinases in vascular physiology and disease. Vascular.
20:210–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein T and Bischoff R: Active
metalloproteases of the A disintegrin and metalloprotease (ADAM)
family: Biological function and structure. J Proteome Res.
10:17–33. 2011. View Article : Google Scholar
|
|
14
|
Ishida Y, Kuninaka Y, Nosaka M, Kimura A,
Kawaguchi T, Hama M, Sakamoto S, Shinozaki K, Eisenmenger W and
Kondo T: Immunohistochemical analysis on MMP-2 and MMP-9 for wound
age determination. Int J Legal Med. 129:1043–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanriverdi-Akhisaroglu S, Menderes A and
Oktay G: Matrix metalloproteinase-2 and -9 activities in human
keloids, hyper-trophic and atrophic scars: A pilot study. Cell
Biochem Funct. 27:81–87. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gill SE and Parks WC: Metalloproteinases
and their inhibitors: Regulators of wound healing. Int J Biochem
Cell Biol. 40:1334–1347. 2008. View Article : Google Scholar
|
|
17
|
Schultz GS and Wysocki A: Interactions
between extracellular matrix and growth factors in wound healing.
Wound Repair Regen. 17:153–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seah CC, Phillips TJ, Howard CE, Panova
IP, Hayes CM, Asandra AS and Park HY: Chronic wound fluid
suppresses proliferation of dermal fibroblasts through a
Ras-mediated signaling pathway. J Invest Dermatol. 124:466–474.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu X, Wang H, Jiang YM, Zhang YY, Bao YF,
Zhang X, Zhang JP, Guo H, Yang F, Luan YC and Dong YS: Ameliorative
effects of tannic acid on carbon tetrachloride-induced liver
fibrosis in vivo and in vitro. J Pharmacol Sci. 130:15–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takawale A, Zhang P, Patel VB, Wang X,
Oudit G and Kassiri Z: Tissue inhibitor of matrix
metalloproteinase-1 promotes myocardial fibrosis by mediating
CD63-integrin β1 interaction. Hypertension. 69:1092–1103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong J and Ma Q: TIMP1 promotes
multi-walled carbon nanotube-induced lung fibrosis by stimulating
fibroblast activation and proliferation. Nanotoxicology. 11:41–51.
2017. View Article : Google Scholar
|
|
22
|
Chen G, Wang R, Chen H, Wu L, Ge RS and
Wang Y: Gossypol ameliorates liver fibrosis in diabetic rats
induced by high-fat diet and streptozocin. Life Sci. 149:58–64.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Chapman SJ, Wood B, Drake TM, Young N and
Jayne DG: Systematic review and meta-analysis of prophylactic mesh
during primary stoma formation to prevent parastomal hernia. Dis
Colon Rectum. 60:107–115. 2017. View Article : Google Scholar
|
|
25
|
Funahashi K, Suzuki T, Nagashima Y,
Matsuda S, Koike J, Shiokawa H, Ushigome M, Arai K, Kaneko T,
Kurihara A and Kaneko H: Risk factors for parastomal hernia in
Japanese patients with permanent colostomy. Surg Today.
44:1465–1469. 2014. View Article : Google Scholar :
|
|
26
|
Pilgrim CH, Mcintyre R and Bailey M:
Prospective audit of parastomal hernia: Prevalence and associated
comorbidities. Dis Colon Rectum. 53:71–76. 2010. View Article : Google Scholar
|
|
27
|
Murray BW, Cipher DJ, Pham T and Anthony
T: The impact of surgical site infection on the development of
incisional hernia and small bowel obstruction in colorectal
surgery. Am J Surg. 202:558–560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Höer J, Lawong G, Klinge U and Schumpelick
V: Factors influencing the development of incisional hernia. A
retrospective study of 2,983 laparotomy patients over a period of
10 years. Chirurg. 73:474–480. 2002. View Article : Google Scholar
|
|
29
|
Gurmu A, Matthiessen P, Nilsson S, Påhlman
L, Rutegård J and Gunnarsson U: The inter-observer reliability is
very low at clinical examination of parastomal hernia. Int J
Colorectal Dis. 26:89–95. 2011. View Article : Google Scholar
|
|
30
|
Hotouras A, Murphy J, Power N, Williams NS
and Chan CL: Radiological incidence of parastomal herniation in
cancer patients with permanent colostomy: What is the ideal size of
the surgical aperture? Int J Surg. 11:425–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jänes A, Cengiz Y and Israelsson LA:
Preventing parastomal hernia with a prosthetic mesh. Arch Surg.
139:1356–1358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanehisa H, Miyatani M, Azuma K, Kuno S
and Fukunaga T: Influences of age and sex on abdominal muscle and
subcutaneous fat thickness. Eur J Appl Physiol. 91:534–537. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henriksen NA, Yadete DH, Sorensen LT,
Agren MS and Jorgensen LN: Connective tissue alteration in
abdominal wall hernia. Br J Surg. 98:210–219. 2011. View Article : Google Scholar
|
|
34
|
Brown SR, Melman L, Jenkins E, Deeken C,
Frisella MM, Brunt LM, Eagon JC and Matthews BD: Collagen type
I:III ratio of the gastroesophageal junction in patients with
paraesophageal hernias. Surg Endosc. 25:1390–1394. 2011. View Article : Google Scholar
|
|
35
|
Shoulders MD and Raines RT: Collagen
structure and stability. Annu Rev Biochem. 78:929–958. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henriksen NA, Mortensen JH, Lorentzen L,
Ågren MS, Bay-Jensen AC, Jorgensen LN and Karsdal MA: Abdominal
wall hernias-A local manifestation of systemically impaired quality
of the extracellular matrix. Surgery. 160:220–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Basson MD: Invited research review:
Cell-matrix interactions in the gut epithelium. Surgery.
133:263–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31(Suppl 1):
pp. S177–S183. 2016, View Article : Google Scholar
|
|
39
|
Su Y, Wan D and Song W: Dryofragin
inhibits the migration and invasion of human osteosarcoma U2OS
cells by suppressing MMP-2/9 and elevating TIMP-1/2 through
PI3K/AKT and p38 MAPK signaling pathways. Anticancer Drugs.
27:660–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agren MS, Jorgensen LN, Andersen M,
Viljanto J and Gottrup F: Matrix metalloproteinase 9 level predicts
optimal collagen deposition during early wound repair in humans. Br
J Surg. 85:68–71. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bigg HF, Rowan AD, Barker MD and Cawston
TE: Activity of matrix metalloproteinase-9 against native collagen
types I and III. FEBS J. 274:1246–1255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Van Doren SR: Matrix metalloproteinase
interactions with collagen and elastin. Matrix Biol. 44-46:224–231.
2015. View Article : Google Scholar : PubMed/NCBI
|