Introduction
Lung cancer is considered to be one of the leading
causes of cancer-associated death worldwide (1,2).
Non-small-cell lung cancer (NSCLC) accounts for ~85% of all lung
cancer cases and exhibits a dismal prognosis, with a 5 year
survival rate of 17.1% (3).
Despite the comprehensive treatment strategies, including surgery,
radiotherapy, immunotherapy and targeted therapies, the clinical
outcomes of patients with NSCLC are slightly improved. However, due
to recurrence and metastasis, NSCLC is still considered to be a
global challenge (4,5). Similar to other cancer cell types,
NSCLC cells are character-ized by their sustained proliferation
(6). Therefore, there is an
urgent requirement for the identification of the molecular
mechanisms underlying the development and progression of NSCLC, and
for the investigation into potential therapeutic targets and
agents, which may improve clinical survival rates.
Interleukin (IL)-21, which is a member of the IL-2
family, is associated with the immune responses of B cells, T cells
and natural killer (NK) cells (7). An accumulation of evidence has
suggested that IL-21 exerts an antitumor effect in a variety of
cancers, including gastric, colon and epithelial ovarian cancer
(8-10). A previous study has demonstrated
the association between IL-21 polymorphisms and NSCLC risk in a
Chinese Han population, indicating the potential role of IL-21 in
lung cancer detection and treatment (11). Notably, a recent study has
suggested that IL-21 levels in the serum of patients with NSCLC
were significantly decreased (12). It has also been documented that
IL-21 conducts signal transduction through binding to its receptor
IL-21R, and can subsequently promote antitumor function (13). However, the role of IL-21/IL-21R
in NSCLC, and the underlying mechanisms, remain poorly
elucidated.
Previous studies have reported that the inactivation
of Wnt/β-catenin signaling protects against NSCLC (14,15). Additionally, active Wnt/β-catenin
signaling can lead to T-cell exclusion and resistance to
anti-programmed death 1 ligand 1 (PD-L1)/anti-cytotoxic
T-lymphocyte antigen 4 (CTLA-4) monoclonal antibody therapy in
melanoma (16). Emerging evidence
has demonstrated that IL-21 suppresses tumor growth and metastasis
through the inhibition of Wnt/β-catenin signaling in epithelial
ovarian cancer (10). The present
study aimed to investigate the role of IL-21/IL-21R in NSCLC. IL-21
and IL-21R expression was measured in NSCLC peripheral blood,
tissues and a human NSCLC cell line. It was hypothesized that
IL-21/IL-21R signaling may inhibit cell proliferation, invasion and
migration in NSCLC via inhibiting Wnt/β-catenin signaling and PD-L1
expression. These results may provide a novel molecular target for
use in NSCLC therapy.
Materials and methods
Patient samples
A total of 30 pairs of NSCLC tissue samples and
matched adjacent normal tissues were collected from patients (15
males and 15 females; age range, 20-45) who underwent surgery at
Fujian Medical University Union Hospital from 2017 to 2018. All
fresh specimens were placed immediately into liquid nitrogen
following surgery. Additionally, peripheral blood samples from 30
NSCLC patients (15 males and 15 females; age range, 20-45) and 30
healthy controls were obtained (15 males and 15 females; age range,
20-45), at the same time interval as the NSCLC tissues and at the
same hospital. All cases were diagnosed using postoperative
pathological sections, and other major organic diseases were
excluded. The current study was approved by the Ethics Committee of
Fujian Medical University Union Hospital and all patients provided
written informed consent. All of the procedures were in compliance
with the Declaration of Helsinki and relevant policies in
China.
Cell culture
The human NSCLC cell line A549 was obtained from the
Type Culture Collection of the Chinese Academy of Sciences. The
cells were maintained in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS (Biological
Industries, Ltd.) at 37°C in a 5% CO2 incubator.
Cell treatments
Cells were seeded into 6-well plates at a density of
1×106 cells/well. When 40-60% confluence was achieved,
different doses of IL-21 (0, 10, 20, 50 and 100 ng/ml) were used to
treat A549 cells for 12 h. For IL-21R silencing, cells were
transfected with 50 nM IL-21R-targeting small interfering RNA
(siRNA)1 (sense, 5′-CCUGCCACAUGGAUGUAUUTT-3′ and antisense,
5′-AAUACAUCCAUGUGGCAGGTT-3′), siRNA2 (sense,
5′-CCGCAAAGACUCGAGCUAUTT-3′ and antisense,
5′-AUAGCUCGAGUCUUUGCGGTT-3′) and control siRNA (sense,
5′-UUCUCCGAACGUGUCAGGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′), in Opti-MEM with Lipofectamine
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.). Following
incubation for 24 h, successful transfections were determined using
reverse transcription-quantitative PCR (RT-qPCR) and western blot
analysis. Additionally, in order to investigate the role of
Wnt/β-catenin signaling in NSCLC, the Wnt inhibitor LiCl was
applied to cells for 12 h, followed by treatment with 50 ng/ml
IL-21 for 24 h.
Cell Counting Kit-8 (CCK-8) assay
A549 cells were seeded into 96-well plates and
incubated at 37°C in a 5% CO2 humidified incubator. Cell
viability was determined using the CCK-8 reagent (Dojindo Molecular
Technologies, Inc.), according to the manufacturer's protocol.
After transfection for 24, 48 and 72 h, 10 μl CCK-8 solution
(Dojindo Molecular Technologies, Inc.) was added to each well for 4
h, then the optical density was measured at 450 nm using a
microplate reader.
Colony formation assay
To evaluate the long-term effects of IL-21, colony
formation experiments were performed using A549 cells. Cells were
seeded at 500 cells/well into 6-well plates and cultured for 7-12
days. Following this, the medium was removed, and the colonies were
fixed with 4% paraformaldehyde for 20 min and stained with 0.2%
crystal violet. Following being washed and air dried at room
temperature, the colonies were visualized under a light microscope
(Nikon Corporation; magnification, ×100).
Cell invasion assay
A Transwell assay was used to investigate the
invasive properties of A549 cells. Cells in serum-free DMEM were
seeded into the upper chamber which consisted of 8 μm-pore
inserts coated with Matrigel (BD Biosciences) in culture plates.
DMEM supplemented with 10% FBS was added to the lower chamber.
Following a 48 h incubation, the Matrigel and the cells remaining
in the upper chamber were removed with a cotton-tipped swab.
Subsequently, the cells were fixed in 4% polyformaldehyde for 10
min at room temperature and stained with 0.1% crystal violet for 15
min. The number of invasive cells in five random fields
(magnification, ×200) was counted using a light microscope (Olympus
Corporation).
Scratch wound healing assay
Cells were seeded into 12-well plates at a density
of 1×105 cells/well for adherent culture. When cells
reached 80% confluence, monolayer cells were scraped off using a 10
μl sterile pipette tip. A phase-contrast microscope (IX711;
Olympus Corporation) was used to monitor cells at the borders of
the scratches. The degree of scratch healing was observed and
images were captured in each group at 0 and 24 h.
ELISA
For measurements of patient sera, all peripheral
blood samples were centrifuged at 1,000 × g for 20 min, then the
supernatant was used for ELISA. For measurements of cells in
vitro, A549 cells were seeded at 1×106 cells/well in
6-well plates, allowed to adhere for 18 h, then treated as
indicated, and finally culture supernatant was used for ELISA. The
concentrations of IL-1β (cat. no. ab2105), tumor necrosis factor
(TNF)-α (cat. no. ab6671), IL-21 (cat. no. ab53655), IL-21R (cat.
no. ab13268) and soluble PD-L1 (cat. no. ab237726) were measured
using ELISA kits purchased from Abcam, according to the
manufacturer's protocol. The experimental detection wavelength was
450 nm.
Immunohistochemistry assay
Tissues were fixed in 10% formaldehyde for 24 h at
room temperature and then embedded in paraffin. Paraffin-embedded
specimens were cut into 4 μm thick sections, deparaffinized
and rehydrated with a graded ethanol and xylene series. They were
then blocked for 30 min using Endogenous Biotin Blocking kit
(Beyotime Institute of Biotechnology). Next, slides were incubated
with primary antibodies targeting IL-21 (1:200; cat. no. ab53655;
Abcam), IL-21R (1:200; cat. no. ab13268; Abcam) and PD-L1 (1:200;
cat. no. ab237726; Abcam) overnight at 4°C. Slides were then
incubated with horseradish peroxidase-secondary antibody (1:1,000;
cat. no. ab181658; Abcam) for 30 min at 37°C, stained with
diaminobenzidine (Beyotime Institute of Biotechnology), and
counterstained with hematoxylin. Three representative sections from
each patient sample were used to calculate the average staining
degree for image analysis using an optical microscope. Brown
nuclear staining was considered to indicate positive protein
expression.
Immunofluorescence assay
A549 cells were washed with PBS, fixed with 4%
paraformaldehyde for 30 min at a room temperature, permeabilized
with 0.2% Triton X-100 for 5 min, and blocked with 5% BSA for 1 h
at a room temperature. Cells were then incubated with primary
antibodies targeting IL-21R (1:100; cat. no. ab13268; Abcam) and
soluble PD-L1 (1:100; cat. no. ab237726; Abcam) overnight at
4°C. After washing with PBS, cells were incubated with
fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit
secondary antibody (1:2,000; cat. no. BM2004; Boster Biological
Technology) for 1 h at room temperature in the dark. Images were
acquired using a fluorescence microscope (Nikon Corporation) after
staining the cell nuclei with DAPI (Boster Biological
Technology).
RT-qPCR
Cells were seeded into 6-well plates at the density
of 1×106 cells/well. Total RNA was extracted from A549
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA was then reverse transcribed into cDNA using
a PrimeScript™ reverse transcription reagent kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
qPCR was performed using iTaq™ Universal SYBR®-Green
Supermix (Bio-Rad Laboratories, Inc.) on an ABI 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
amplification conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. The primers
used were: IL-21R, forward, 5′-ACCAGTCTGGCAACTACTCC-3′ and reverse,
5′-GGCAGGGTCTTCGTAATCTGAG-3′; GAPDH, forward, 5′-TAT
GATGATATCAAGAGGGTAGT-3′ and reverse, 5′-TGTATCCAAACTCATTGTCATAC-3′.
GAPDH was used as an internal control. Relative expression was
analyzed using the 2-∆∆Cq method (17).
Western blot analysis
A549 cells were seeded at 1×106
cells/well in 6-well plates. Proteins were extracted from tumor
tissues or A549 cells using a protein lysis buffer (RIPA; Beyotime
Institute of Biotechnology). The concentration of protein was
determined using a bicinchoninic acid assay protein assay kit
(Beyotime Institute of Biotechnology). Proteins (25 μg/lane)
were resolved using 10% SDS-PAGE and transferred to PVDF membranes
(EMD Millipore). The membranes were then incubated with primary
antibodies (all at 1:1,000), followed by goat anti-rabbit
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat. no. ab181658; Abcam) at room temperature for 1 h. Proteins
were visualized using Image Quant™ LAS 4000 (GE Healthcare Life
Sciences) and quantified using Image J (version 1.46; National
Institutes of Health). Anti-IL-21 (cat. no. ab5978), anti-IL-21R
(cat. no. ab5980), anti-PD-L1 (cat. no. ab205921) and anti-Wnt
(cat. no. ab28472) were purchased from Abcam. Anti-β-catenin (cat.
no. 8480T), anti-cyclinD1 (cat. no. 3300T) and anti-GAPDH (cat. no.
5174S) antibodies were obtained from Cell Signaling Technology,
Inc.
Statistical analysis
All experiments were performed for at least three
independent repeats and all the data are presented as mean ±
standard deviation. Statistical analysis was performed using SPSS
software 16.0 (SPSS, Inc.). Statistical comparisons were made using
a one-way ANOVA followed by a post hoc Dunnett's test. P<0.05
was considered to indicate a statistically significant result.
Results
Expression of IL-21, IL-21R and PD-L1 in
the serum and lung tissues of patients with NSCLC
The expression levels of IL-21, IL-21R and PD-L1 in
the serum and lung tissues of patients with NSCLC and healthy
controls (normal) were measured in the current study. As presented
in Fig. 1A-C, the protein levels
of IL-21 and IL-21R were decreased, whereas PD-L1 was increased, in
the serum of patients with NSCLC, compared with healthy controls.
The expression levels of these proteins in the lung tissues of
patients with NSCLC, as detected by western blotting (Fig. 2A-C), were in accordance with the
serum ELISA results. Finally, the levels of these proteins in the
lung tissues were also detected by immunohistochemistry assay, with
similar results (Fig. 2D and E).
Next, Pearson's correlation coefficients were used to analyze the
potential correlation between the expression levels of IL-21 and
PD-L1, and IL-21R and PD-L1. The results demonstrated that IL-21
(F=-0.2091; P<0.05) and IL-21R (F=-0.2337; P<0.05) were
negatively correlated with PD-L1 (Fig. 3A and B). These data indicated that
decreased expression of IL-21 and IL-21R, and increased expression
of PD-L1, may be correlated with NSCLC progression.
IL-21 treatment attenuates the
proliferation, invasion and migration of A549 cells
To investigate the effect of IL-21 on the NSCLC cell
line A549, different doses of IL-21 (0, 10, 20, 50 and 100 ng/ml)
were used to treat the cells. From the results of Fig. 4A and B, it was revealed that IL-21
treatment attenuated the proliferation of A549 cells in a
dose-dependent manner. Additionally, IL-21 treatment resulted in a
significant reduction of the invasive and migratory capacities of
the cells (Fig. 4C-F). These
results indicated that IL-21 was able to attenuate the
proliferation, invasion and migration of A549 cells.
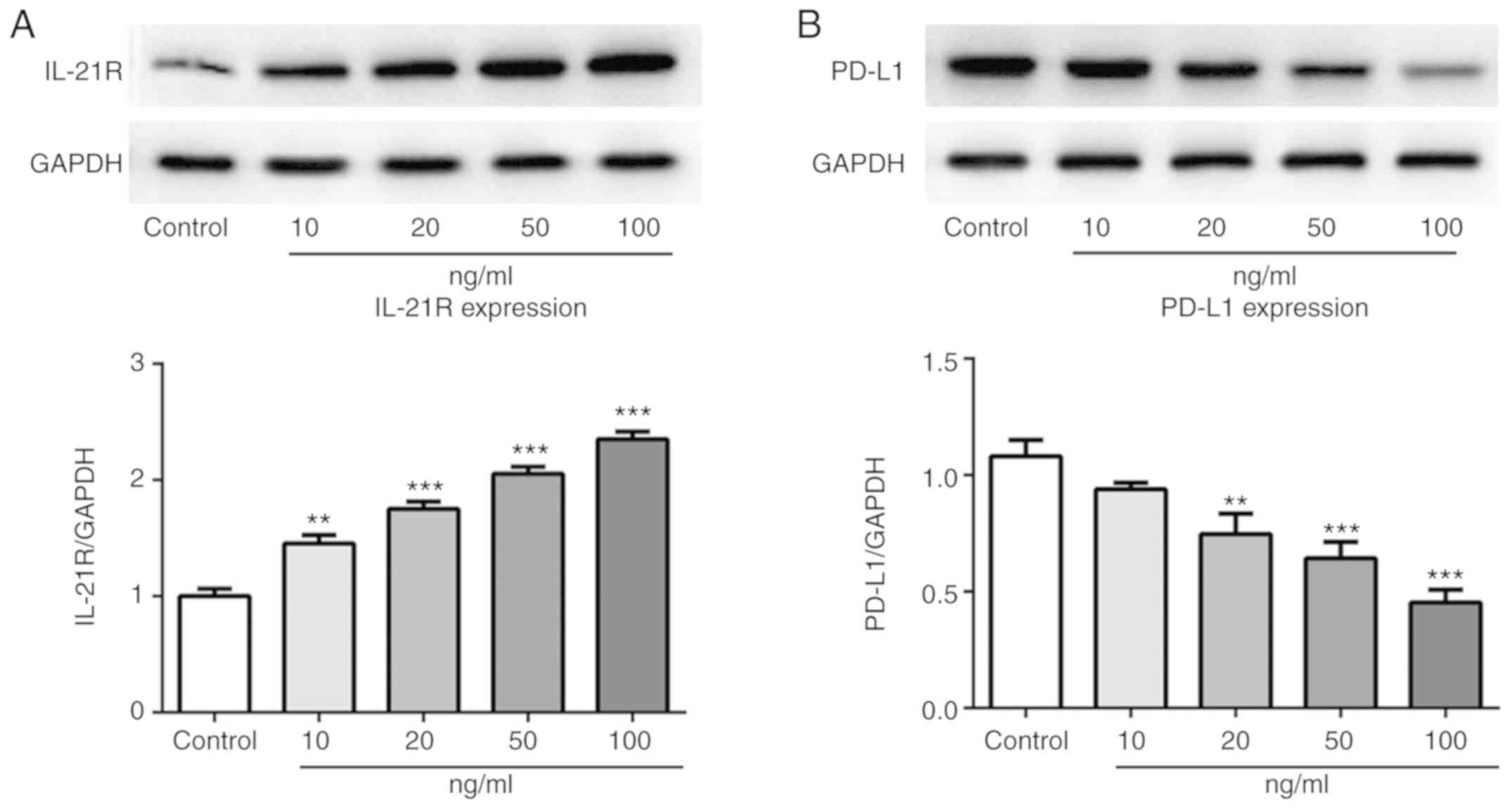
IL-21 treatment increases IL-21R and
decreases PD-L1 expression in A549 cells
Following treatment with IL-21, the expression
levels of IL-21R and PD-L1 in A549 cells were measured by western
blotting in the current study. As presented in Fig. 5A and B, the protein expression
levels of IL-21R were increased, while the PD-L1 expression levels
were decreased, following IL-21 treatment. Results from
immunofluorescence analysis for IL-21R (Fig. 6) and PD-L1 (Fig. 7) further confirmed the effects of
IL-21 treatment on their expression. These findings suggested that
IL-21 treatment upregulated the expression of IL-21R and
downregulated the expression of PD-L1 in A549 cells.
IL-21 treatment attenuates the
proliferation, invasion and migration of A549 cells by binding to
IL-21R
To investigate whether IL-21 was acting via binding
to IL-21R, IL-21R silencing was employed. The successful silencing
of IL-21R was confirmed using RT-qPCR and western blot analysis
(Fig. 8A and B). Cells were
treated with 50 ng/ml IL-21. As presented in Fig. 8C and D, following IL-21R
silencing, the effect of IL-21 on cell proliferation of A549 cells
was reversed, which indicated that IL-21 decreased proliferation by
binding to IL-21R. Similar results were demonstrated for cell
invasion (Fig. 8E and F) and
migration (Fig. 8G and H). These
results revealed that IL-21 attenuated the proliferation, invasion
and migration of A549 cells by binding to IL-21R.
IL-21 inhibits the Wnt/β-catenin
signaling pathway and PD-L1 expression
Following IL-21R silencing, IL-21 treatment
increased the protein expression levels of PD-L1 (Fig. 9A). IL-1β and TNF-α expression
levels were also increased (Fig.
9B and C). To investigate the regulatory mechanisms underlying
this effect, the expression levels of proteins associated with the
Wnt/β-catenin signaling pathway were measured. As presented in
Fig. 10A, IL-21 treatment
decreased the levels of Wnt, β-catenin and cyclin D1 compared with
the control. Subsequently, the Wnt/β-catenin signaling agonist LiCl
was used in the current study. It was demonstrated that the
expression of PD-L1 was upregulated following treatment with IL-21
and LiCl compared with the IL-21 alone control (Fig. 10B). Furthermore, the levels of
IL-1β and TNF-α were increased compared with the IL-21 alone
control (Fig. 10C and D). These
results revealed that IL-21 downregulated PD-L1 expression by
inhibiting the Wnt/β-catenin signaling pathway. These results
indicated that IL-21 attenuated NSCLC growth by inhibiting the
Wnt/β-catenin signaling pathway and PD-L1 expression.
Discussion
The present study demonstrated that IL-21 and IL-21R
were negatively correlated with PD-L1 in the lung tissues of
patients with NSCLC. IL-21 was revealed to exert a protective
effect on the growth and migration of NSCLC cells by binding to
IL-21R. Additionally, the results indicated that IL-21 inhibited
the Wnt/β-catenin signaling pathway and decreased PD-L1 expression.
Following treatment with LiCl, the decreasing effect of IL-21 on
PD-L1 was reversed. Therefore, the present results indicated that
IL-21/IL-21R served an antitumor role in NSCLC via repression of
Wnt/β-catenin signaling and PD-L1 expression.
IL-21, which is a member of the common γ-chain
family, is produced by activated CD4+ T cells, NK T
cells and follicular T-helper cells (18,19). Increasing evidence has indicated
that IL-21 exhibits an antitumor function in a variety of tumor
models (8,20). Additionally, the antitumor
activity of IL-21 can be potentiated when used in combination with
other immuno-stimulants, chemotherapy or with monoclonal antibodies
that recognize tumor antigens (19,21,22). A previous study reported that
IL-21, in combination with 5-fluorouracil, potentiated its
antitumor effect in human gastric cancer. The current study
demonstrated that IL-21 and IL-21R were upregulated in the
peripheral blood and lung tissues of patients with NSCLC, which was
in accordance with previous research (12). IL-21 treatment inhibited the
growth, invasion and migration of NSCLC cells in a dose-independent
manner, which revealed the antitumor effect of IL-21 on NSCLC.
PD-L1 is the major ligand of PD-1 and is expressed
in a variety of tumors, including in NSCLC (23,24). Overexpression of PD-L1 is
implicated in tumor immunity and inhibition of PD-L1 enhances
antitumor immunity by preventing tumor cells from escaping host
immune responses (25). A growing
number of studies have demonstrated that PD-L1 is closely
associated with tumorigenesis and invasiveness (26). In the present study, the
expression of PD-L1 in lung tissues of patients with NSCLC
increased, and this was negatively correlated with IL-21 and
IL-21R. Upon treatment of NSCLC cells with IL-21 in vitro,
the expression levels of PD-L1 were downregulated in a
dose-dependent manner, which indicated that IL-21 was able to
inhibit the expression of PD-L1.
It has been previously reported that the
Wnt/β-catenin signaling pathway is of great importance in
regulating tumor cell growth and metastasis (27,28), and inactivation of Wnt/β-catenin
signaling suppresses the growth and progression of numerous
cancers, including NSCLC (29).
Increasing evidence has revealed that active Wnt/β-catenin
signaling can lead to T-cell exclusion and resistance to
anti-PD-L1/anti-CTLA-4 monoclonal antibody therapy in melanoma
(16). Additionally, IL-21 can
suppress tumor growth and metastasis through the inhibition of
Wnt/β-catenin signaling in epithelial ovarian cancer (10). The current study demonstrated that
IL-21 treatment inhibited the expression of Wnt, β-catenin and
cyclin D1, while promoting PD-L1 expression. Following intervention
with LiCl, the suppressing effect of IL-21 on PD-L1 expression was
reversed. Therefore, the present results provided evidence that
IL-21/IL-21R protected against NSCLC via repression of the
Wnt/β-catenin signaling pathway and PD-L1 expression.
In conclusion, the present study demonstrated that
IL-21 and IL-21R expression levels were negatively correlated with
PD-L1 expression levels in the lung tissues of patients with NSCLC,
and that IL-21 exerted a protective effect on the growth and
invasion of NSCLC cells by binding to IL-21R. It was also revealed
that IL-21/IL-21R inactivated the Wnt/β-catenin signaling pathway
and decreased PD-L1 expression in NSCLC cells. These findings
indicated a tumor suppressive role of IL-21 in the development of
NSCLC, which may be useful for the development of novel therapies
for this disease.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National and
Fujian Province's Key Clinical Specialty Discipline Construction
Program, the Natural Science Foundation of Fujian Province,
China (grant no. 2017J01295), and the Joint Funds
for the Innovation of Science and Technology, Fujian province,
China (grant no. 2017Y9031).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DX and PY wrote the manuscript, interpreted the data
and performed experiments. QW and XL collected the data. LL and TL
searched the literature, designed the study and revised the
manuscript. All authors read and approval the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Fujian Medical University Union Hospital, and all
patients provided written informed consent. All of the procedures
were in compliance with The Declaration of Helsinki and relevant
policies in China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hao H, Zhou Z, Li S, Maquilan G, Folkert
MR, Iyengar P, Westover KD, Albuquerque K, Liu F, Choy H, et al:
Shell feature: A new radiomics descriptor for predicting distant
failure after radiotherapy in non-small cell lung cancer and cervix
cancer. Phys Med Biol. 63:0950072018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shaw AT and Engelman JA: Ceritinib in
ALK-rearranged non-small-cell lung cancer. N Engl J Med 3.
70:2537–2539. 2014.
|
|
3
|
Shroff GS, Viswanathan C, Carter BW,
Benveniste MF, Truong MT and Sabloff BS: Staging lung cancer:
Metastasis. Radiol Clin North Am. 56:411–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Legras A, Pécuchet N, Imbeaud S, Pallier
K, Didelot A, Roussel H, Gibault L, Fabre E, Le Pimpec-Barthes F,
Laurent-Puig P and Blons H: Epithelial-to-mesenchymal transition
and microRNAs in lung cancer. Cancers (Basel). 9. pp. E1012017,
View Article : Google Scholar
|
|
5
|
Pawlak K, Gabryel P, Kujawska A, Kasprzyk
M, Piwkowski C, Kuffel B and Dyszkiewicz W: Long-term results of
surgical treatment of non-small cell lung cancer in patients over
75 years of age. Kardiochir Torakochirurgia Pol. 15:65–71.
2018.PubMed/NCBI
|
|
6
|
Xu X, Chen D, Ye B, Zhong F and Chen G:
Curcumin induces the apoptosis of non-small cell lung cancer cells
through a calcium signaling pathway. Int J Mol Med. 35:1610–1616.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Monteleone I, Pallone F and Monteleone G:
Interleukin-23 and Th17 cells in the control of gut inflammation.
Mediators Inflamm. 2009.297645:2009.
|
|
8
|
Fu ZQ, Zhou Q, Zhu S and Liu W: Anti-tumor
mechanism of IL-21 used alone and in combination with
5-fluorouracil in vitro on human gastric cancer cells. J Biol Regul
Homeost Agents. 32:619–625. 2018.PubMed/NCBI
|
|
9
|
Chen C, Liu X and Ren Y: Interleukin 21
treatment in a murine model as a novel potential cytokine
immunotherapy for colon cancer. Adv Clin Exp Med. 27:583–589. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Wang J, Wu D, Li M, Zhao F, Ren
M, Cai Y and Dou J: IL-21-secreting hUCMSCs combined with miR-200c
inhibit tumor growth and metastasis via repression of Wnt/β-catenin
signaling and epithelial-mesenchymal transition in epithelial
ovarian cancer. Onco Targets Ther. 11:2037–2050. 2018. View Article : Google Scholar :
|
|
11
|
Liu L, Shi F, Li S, Liu X, Wei L, Zhang J,
Ju X and Yu J: IL-21 polymorphisms rs907715 and rs2221903 are
associated with decreased non-small cell lung cancer
susceptibility. Int J Clin Exp Med. 8:19460–19465. 2015.
|
|
12
|
Qiu L, Yu Q, Zhou Y, Zheng S, Tao J, Jiang
Q and Yuan G: Functionally impaired follicular helper T cells
induce regulatory B cells and CD14+ human leukocyte
antigen-DR- cell differentiation in non-small cell lung
cancer. Cancer Sci. 109:3751–3761. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaltenmeier C, Gawanbacht A, Beyer T,
Lindner S, Trzaska T, van der Merwe JA, Härter G, Grüner B,
Fabricius D, Lotfi R, et al: CD4+ T cell-derived IL-21
and deprivation of CD40 signaling favor the in vivo development of
granzyme B-expressing regulatory B cells in HIV patients. J
Immunol. 194:3768–3777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JY, Wang X, Wang XJ, Zheng BZ, Wang
Y, Wang X and Liang B: Curcumin inhibits the growth via
Wnt/β-catenin pathway in non-small-cell lung cancer cells. Eur Rev
Med Pharmacol Sci. 22:7492–7499. 2018.PubMed/NCBI
|
|
15
|
Wang XH, Cui YX, Wang ZM and Liu J:
Down-regulation of FOXR2 inhibits non-small cell lung cancer cell
proliferation and invasion through the Wnt/β-catenin signaling
pathway. Biochem Biophys Res Commun. 500:229–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:pp. 402–408. 2001,
View Article : Google Scholar
|
|
18
|
Santegoets SJ, Turksma AW, Powell DJ Jr,
Hooijberg E and de Gruijl TD: IL-21 in cancer immunotherapy: At the
right place at the right time = Oncoimmunology. 2:pp. e245222013,
PubMed/NCBI
|
|
19
|
Croce M, Rigo V and Ferrini S: IL-21: A
pleiotropic cytokine with potential applications in oncology. J
Immunol Res. 2015.696578:2015.
|
|
20
|
Moroz A, Eppolito C, Li Q, Tao J, Clegg CH
and Shrikant PA: IL-21 enhances and sustains CD8+ T cell
responses to achieve durable tumor immunity: Comparative evaluation
of IL-2, IL-15, and IL-21. J Immunol. 173:900–909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spolski R and Leonard WJ: Interleukin-21:
A double-edged sword with therapeutic potential. Nat Rev Drug
Discov. 13:379–395. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao J, Xu Q, Su S, Meng F, Zou Z, Chen F,
Du J, Qian X and Liu B: Engineered cells for costimulatory
enhancement combined with IL-21 enhance the generation of
PD-1-disrupted CTLs for adoptive immunotherapy. Cell Immunol.
320:38–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu QX, Xie F, Huang Q and Zhang XG:
Membranous and cytoplasmic expression of PD-L1 in ovarian cancer
cells. Cell Physiol Biochem. 43:1893–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Y, Fang W, Zhang Y, Hong S, Kang S,
Yan Y, Chen N, Zhan J, He X, Qin T, et al: The association between
PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced
non-small cell lung cancer patients treated with EGFR-TKIs.
Oncotarget. 6:14209–14219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mu CY, Huang JA, Chen Y, Chen C and Zhang
XG: High expression of PD-L1 in lung cancer may contribute to poor
prognosis and tumor cells immune escape through suppressing tumor
infiltrating dendritic cells maturation. Med Oncol. 28:682–688.
2011. View Article : Google Scholar
|
|
26
|
Pang L, Han S, Jiao Y, Jiang S, He X and
Li P: Bu Fei Decoction attenuates the tumor associated macrophage
stimulated proliferation, migration, invasion and immunosuppression
of non-small cell lung cancer, partially via IL-10 and PD-L1
regulation. Int J Oncol. 51:25–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li T, Ren J, Ma J, Wu J, Zhang R, Yuan H
and Han X: LINC00702/miR-4652-3p/ZEB1 axis promotes the progression
of malignant meningioma through activating Wnt/β-catenin pathway.
Biomed Pharmacother. 113:1087182019. View Article : Google Scholar
|
|
28
|
Jing JC, Feng Z, Chen ZH, Ji BN, Hong J,
Tang N, Yu JL and Wang SY: KDM4B promotes gastric cancer metastasis
by regulating miR-125b-mediated activation of Wnt signaling. J Cell
Biochem. 2018.
|
|
29
|
Yang Y, Liu L, Cai J, Wu J, Guan H, Zhu X,
Yuan J and Li M: DEPDC1B enhances migration and invasion of
non-small cell lung cancer cells via activating Wnt/β-catenin
signaling. Biochem Biophys Res Commun. 450:899–905. 2014.
View Article : Google Scholar : PubMed/NCBI
|