Introduction
With the rapid development of technology for cardiac
surgery, cardiopulmonary bypass (CPB) and anesthesia, the number of
cardiac surgical procedures, particularly CPB, in China has
significantly increased. Many patients suffer postoperative central
nervous system complications (1).
CPB open-heart surgery is complicated by postoperative cognitive
dysfunction (POCD) manifesting in neurological and mental
disorders, including dysmnesia, disorientation and visual-spatial
ability (2,3). POCD has become a leading cause of
mortality and disability in patients following CPB open-heart
surgery. The need to prevent and treat POCD initiated by CPB
cardiac surgery has hindered the development of cardiac
surgery.
Reactive oxygen species (ROS) are chemically
reactive chemical species containing oxygen. ROS are implicated in
mediating apoptosis, or programmed cell death, and ischemic injury.
During CPB, ischemia and perfusion injury can activate neutrophils
and lead to excessive ROS. ROS levels can increase markedly,
causing damage in numerous cellular molecules, including lipids,
proteins and DNA (4). Drugs with
antioxidant properties may reduce ROS bursts and oxidative stress,
reducing POCD during and following CPB (5).
Kappa opioid receptors (KORs) are important in
regulating ischemic brain damage. Studies have confirmed that KORs
alleviate brain damage and improve functional recovery in animal
models with general and regional cerebral ischemia (6,7).
KOR agonists can significantly improve the hippocampal nerve damage
caused by ischemia, thereby alleviating cognitive dysfunction
(6). Another report shows that
KORs are beneficial for the activation of hippocampal cholinergic
neurons (8). KOR agonists can
block the transport of acetylcholine through the KOR-mediated
opioid nervous system to improve learning and memory dysfunction
(9). However, the specific
regulatory mechanisms by which KOR agonists improve cognitive
dysfunction remain unclear.
The Janus kinase 2/signal transducer and activator
of transcription 3 (JAK2/STAT3) signaling pathway is involved in
the anti-inflammatory response following activation of the
acetyl-choline receptor (10,11). Numerous studies have confirmed
that JAK2/STAT3 activation is involved in the anti-apoptotic
process following transient focal cerebral ischemia (12). However, the potential effects of
KOR agonists on POCD in CPB rats through the JAK2/STAT3 signaling
pathway remain to be fully established.
In the present study, a model of POCD was
established in CPB rats. Using this model, the effects of KOR
agonists on neurological damage, brain damage, inflammation,
oxida-tive stress and neuronal apoptosis were investigated, and the
effect on JAK2/STAT3 signaling pathway-related proteins was further
analyzed. The present study aimed to identify a novel therapy for
POCD in CPB rats, which may provide theoretical and experimental
evidence for the treatment of patients with POCD.
Materials and methods
Experimental animals and groupings
A total of 50 male Sprague-Dawley rats (SPF grade),
6 months old, weighing 350-450 g, were provided by the Animal
Experimental Department of the General Hospital of Northern Theater
Command [Shenyang, China; license for rodent use: SYXK (Military)
20120007; production license for rodents: SCXK (Military) 20120006]
in accordance with The Guide to the Care and Use of Laboratory
Animals published by the Canadian Council on Animal Care. All rats
were cultivated in individual ventilated cages at 24±2°C) and
40-70% humidity in a 12-h light/dark cycle. Standard pelleted chow
and drinking water were available ad libitum. All animal
protocols were approved by the Experimental Animal Ethics Committee
of the General Hospital of Northern Theater Command (no.
GHNTC2018018).
The rats were randomly divided into five groups
(n=10): Sham operation (Sham group), CPB surgery (CPB group), KOR
agonist (U50488H) + CPB (K group), KOR agonist (U50488H) +
norbinaltorphimine (nor-BNI) + CPB (NK group), KOR agonist
(U50488H) + JAK2-STAT3 pathway inhibitor (AG490) + CPB (AG group).
In the K group, the rats were administered with an intravenous
injection of U50488H (1.5 mg/kg, cat. no. 0495/25, Tocris
Bioscience, Bristol, UK) 30 min before the CPB assay; in the NK
group, an intravenous injection of U50488H (1.5 mg/kg) was
administered when rats were catheterized, and nor-BNI (2 mg/kg,
Sigma-Aldrich; Merck KGaA) was administered intravenously 30 min
later; in the AG Group, U50488H (1.5 mg/kg) was administered
intravenously when the rats were anesthetized and catheterized, and
AG490 (5 mg/kg) was injected intravenously 30 min later. When the
water maze test was completed, 7 days after CPB bypass, the rats
were anesthetized and catheterized, 5 ml of blood was drawn through
the right internal vein, and serum was separated by centrifugation
at 500 x g for 5 min. The samples were stored at −80°C until
examination. The bilateral hippocampus was immediately removed,
with one side stored at −80°C and the other side fixed in 10%
formalin at room temperature for 48 h.
Preparation of the CPB model
CPB surgery was performed as previously reported
(7), with minor modifications.
Briefly, the rats were injected intraperitoneally (i.p.) with 4%
chloral hydrate (300 mg/kg; Shanghai Ziyuan Pharmaceutical Co.,
Ltd., Shanghai, China) to induce anesthesia (13). During surgery, anesthesia was
maintained with isoflurane (MAC=1.5%, Hangzhou Minsheng
Pharmaceutical Co., Ltd., Hangzhou, China). Photopic oral
intubation was performed using a 16-G intravenous catheter, and
animals were mechanically ventilated with a small animal ventilator
(frequency, 60 beats/min; tidal volume, 3 ml/kg; inspiratory to
expiratory ratio, 1:1.5) connected to a monitor to observe the
heart rate, oxygen saturation and rectal temperature.
The puncture site was sterilized with iodophor
(Shandong Lierkang Disinfection Technology Co., Ltd., Dezhou,
China), followed by exposure and puncture of the vein. Right
femoral vein catheterization (24-G) was performed to open the fluid
path, which was transfused with 6% hydroxyethyl starch (Guangdong
Jiabao Pharmaceutical Co., Ltd., Qingyuan, China) and connected to
a microinfusion pump. The left femoral artery was catheterized
(22-G) and used to monitor blood pressure. Coccygeal artery
catheterization (22-G) and right internal jugular vein
catheterization (18-G) were performed to drain blood for CPB. The
drainage tube, a homemade blood storage device, a constant
peristaltic pump (Baoding Longer Precision Pump Co., Ltd., Baoding,
China), silicone tubing (internal diameter, 4 mm) and a rat
membrane oxygenator (Guangdong Kewei Medical Instrument Co. Ltd.,
Dongguan, China) were installed between the two puncture sites to
establish the CPB circuit. Heparin sodium (300 IU/kg; Shenyang
Haitong Pharmaceutical Co., Ltd., Shenyang, China) was injected
into the left femoral vein once the activated clotting time reached
480 sec.
CPB was performed with the membrane oxygenator to
supply oxygen. The low-flow CPB velocity was 35 ml/kg/min, which
was later increased to 100-120 ml/kg/min at full-flow bypass. To
prevent air embolism, 1-2 ml of blood was retained in the blood
storage device. The mean arterial pressure was maintained at >60
mmHg, partial CO2 pressure at 35-45 mmHg, base excess at
−3-3 mmol/l mmHg, pH at 7.35-7.45 and hematocrit at >0.25. The
rats were treated with 2-20 μg/100 g epinephrine
hydrochloride (Wuhan Grand Pharmaceutical Group Co., Ltd., Wuhan,
China) and fluids during surgery to maintain a stable
circulation.
Water maze assessment
After 24 h of CPB, the water maze test was performed
for 7 days consecutively, which included hidden platform tests and
space exploration tests. For the hidden platform test, the rats
were placed into water from any quadrant facing the pool wall and
made to swim for 90 sec to locate the hidden platform. The
incubation period of escape was recorded as the time required to
locate the hidden platform in the pool. If the platform was not
found after 90 sec, the rat was directed to the platform and the
score was counted as 90 sec. The rats were assessed for 5 days,
with the first 4 days used for training. Any rats with a score of
90 sec were eliminated, and the test scores on day 5 were recorded
as the spatial learning and memory scores of the animals.
For the space exploration test, the platform was
removed 24 h after the hidden platform test had ended. The rats
were placed in the water at the same place as previously, and their
swimming paths were recorded for 60 sec. The duration of rats in
the original station quadrant and the number of times the original
station location was crossed were recorded. The trajectories of the
rats were recorded, and information processing was performed using
the Morris water maze video analysis system (WMT-100S,
Taimeng).
Neurological function scores
After 1, 3, and 7 days of CPB model preparation, the
Garcia score scale was used for detecting the neurological
functions of the experimental animals (Table I) (14,15).
 | Table IGarcia score scale. |
Table I
Garcia score scale.
| Test item | 0 point | 1 point | 2 points | 3 points |
|---|
| Free in cage for 5
min | No movement | Rats almost unable
to move | The rats can move,
with the range within three sides in cage | Range of movement
reaches at least three sides in cage |
| Movement symmetry
of arms of legs | No movement of left
lateral limb | Left lateral limb
can move slightly | Left lateral limb
can move slowly | Left lateral limb
moves symmetrically |
| Movement symmetry
of forelimb | Left lateral limb
cannot move | Left lateral limb
can stretch gently only | Movement and
stretch of right lateral limb are better than that of left | Both forelimbs can
stretch symmetrically |
| Climbing in metal
cage | No | Rats cannot
climb | Left limb slightly
weak | Rats can climb
normally |
| Response on
touching both sides of body | No | No response on left
side | Weak response on
left side | Symmetric
response |
| Whisker
response | No | No response on left
side | Weak response on
left side | Symmetric
response |
Hematoxylin and eosin (H&E)
staining
The formalin-fixed tissue samples were placed in 70,
80, 90, 95 and 100% alcohol. Xylene was used to clear the sample.
The samples were embedded into paraffin blocks, cut into
4-μm sections and then dewaxed. Hematoxylin staining was
performed for 5 min at room temperature, following which the slides
were washed in PBS, immersed in 1% hydrochloric acid, stained with
eosin for 30 sec and then dehydrated in gradient alcohol. Neutral
gum was used for sealing. Pathological changes in each group of
tissues were observed under a light microscope.
TUNEL assay
The in situ cell death detection kit (Roche
Diagnostics GmbH, Mannheim, Germany) was used according to the
manufacturer's instructions: The 5-μm sections of
paraffin-embedded hippocampal tissue were deparaffinized by
dimethylbenzene for twice, 10 min per time, permeabilized by
gradient elution of alcohol (100, 95, 90, 80 and 70%). The sections
were treated with 50 μl TUNEL reaction solution for 60 min
in a humid dark box at 37°C. Subsequently, 50 μl of
streptavidin-HRP working solution was added to the sections in the
dark box for 30 min. The nuclei were fluorescently stained with
DAPI, followed by conventional dehydration, decolorization and
fixation. The apoptotic rates were examined and images were
captured using a light microscope (Olympus Corporation, Tokyo,
Japan) at a magnification of ×400, and densitometric scanning was
analyzed using the MetaMorph BX41 image analysis system (Olympus
Corporation). A total of five images were captured randomly for
each section at ×400 magnification and integral optical density was
calculated using the microscopic image analyzer (MetaMorph BX41
image analysis system). The total nuclei and TUNEL-positive nuclei
were counted, and the proportions of TUNEL-positive cells above the
number in the untreated controls were calculated as follows: %
apoptosis=(number of TUNEL-positive cells/total cells) ×100.
ELISA assessment
ELISA kits were used to detect inflammatory factors
IL-1β (cat. no. CSB-E08055r, CUSABIO, Wuhan, China), IL-6 (cat. no.
SEA079Ra, USCN, Wuhan, China), TNF-α (cat. no. SEA133Si, USCN) and
IL-10 (cat. no. SEA056Ra, USCN) in rat serum, stress indicators
superoxide dismutase (SOD; cat. no. SES134Hu, USCN),
malondialdehyde (MDA; cat. no. CEA597Ge, USCN) and nitric oxide
(NO; cat. no. IS100, USCN), and brain damage markers S-100β (cat.
no. SEA567Ra, USCN) and neuron-specific enolase (NSE; cat. no.
SEA537Ra, USCN). The kit was equilibrated to room temperature and
the required reaction plate was removed. Subsequently, 100
μl of the standard product and 100 μl of the diluted
sample were successively added to the well of the corresponding
reaction plate, the plate was mixed by gently shaking for 30 sec
and then incubated for 20 min at room temperature. The reaction
plate was washed with a washing machine, following which 100
μl serum was added to each well and incubated at 37°C for 2
h. The plate was washed, and l00 μl HRP-labeled secondary
antibody provided in the kit was added per well and incubated at
37°C for 30 min. The plate was washed and 50 μl each of
color developing solutions A and B were added for 15 min in the
dark, following which 50 μl of stop solution was added. The
optical density (OD) value at 450 nm was read on a micro-plate
reader (EXL808 BioTek Instruments, Inc., Winooski, VT, USA).
Using the OD value as the vertical coordinate and
the standard concentration as the horizontal coordinate allowed for
a standard curve to be drawn and the curve equation and r value to
be calculated to determine the corresponding concentration values
of each sample.
Immunofluorescence
The paraffin-embedded hippocampal tissues were
dewaxed and placed into water and then immersed in 3% hydrogen
peroxide solution for 15 min and washed with PBS. Subsequent
antigen recovery was performed with 0.1 M sodium citrate solution.
The tissues were blocked with goat serum (cat. no. SL038, Beijing
Solarbio Science & Technology) at 37°C for 30 min and the serum
was then decanted without washing. phosphorylated (p-)JAK2 (1:100,
cat. no. ab32101, Abcam) and p-STAT3 (1:100, cat. no. ab76315,
Abcam) antibodies were added for incubation overnight at 4°C. The
following day, the sections were washed in PBS and then incubated
with Goat Anti-Rabbit IgG H&L (Cy3®, 1:500, cat. no.
ab6939) antibody at 37°C for 30 min and then washed again with PBS.
DAPI dye at 300 μM was added for 10 min at room temperature,
following which the tissues were washed with PBS, sealed with
neutral gum, and observed under a fluorescence microscope.
Western blotting
Following homogenization of the hippo-campus,
pre-cooled RIPA (Thermo Fisher Scientific, Inc., Waltham, MA, USA,
cat. no. 89900) lysate was added and was lysed on ice for 30 min.
Following collection of the supernatant, the concentration of the
collected protein solution was determined using a BCA (Thermo
Fisher Scientific, Inc., cat. no. 23225) protein quantification
kit. The proteins (30 μg/well) were then separated by 12%
SDS-PAGE electrophoresis and transferred onto a PVDF membrane. The
membranes were blocked with 5% skim milk at room temperature for
1.5 h. JAK2 (1:2,000, cat. no. ab108596, Abcam), p-JAK2 (1:2,000,
cat. no. ab32101, Abcam), STAT3 (1:2,000, cat. no. ab119352,
Abcam), p-STAT3 (1:2,000, cat. no. ab76315, Abcam), Bc1-2 (1:1,000,
cat. no. ab59348, Abcam), Bax (1:2,000, cat. no. ab32503, Abcam),
pro-caspase-3 (1:1,000, cat. no. ab32150, Abcam) and cleaved
caspase 3 (1:500, cat. no. ab49822, Abcam) primary antibodies were
added for incubation overnight at 4°C. Following washing with PBS,
Goat Anti-Rabbit IgG H&L (HRP, 1:10,000, cat. no. ab6721)
antibody was added and incubated for 2 h at room temperature before
developing ECL luminescence. A gel imaging system (Gel Doc™ XR;
Bio-Rad Laboratories, Inc) was used for capturing images.
Absorbance values were analyzed using ImageJ (v1.8.0; National
Institutes of Health).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA) software. Multiple comparisons were
analyzed with one-way analysis of variance, followed by an
appropriate multiple comparison test (Tukey's procedure). P<0.05
was considered to indicate a statistically significant
difference.
Results
Successful preparation of the CPB rat
model
Compared with rats in the Sham group, there were no
significant changes in rectal temperature, pH, partial pressure of
carbon dioxide (PaCO2) or partial pressure of oxygen
(PaO2) in rats in the CPB group, K group, NK group and
AG group (P<0.05), as shown in Fig. 1. Compared with rats in the Sham
group, the mean arterial pressure (MAP), heart rate (HR), left
ventricular diastolic pressure (LVDP), highest rate of change in
pressure development (+dP/dtmin) and hemoglobin (Hb) were decreased
in the CPB group, and this effect was significantly reversed under
KOR agonist treatment in the K group (P<0.05) (Fig. 1).
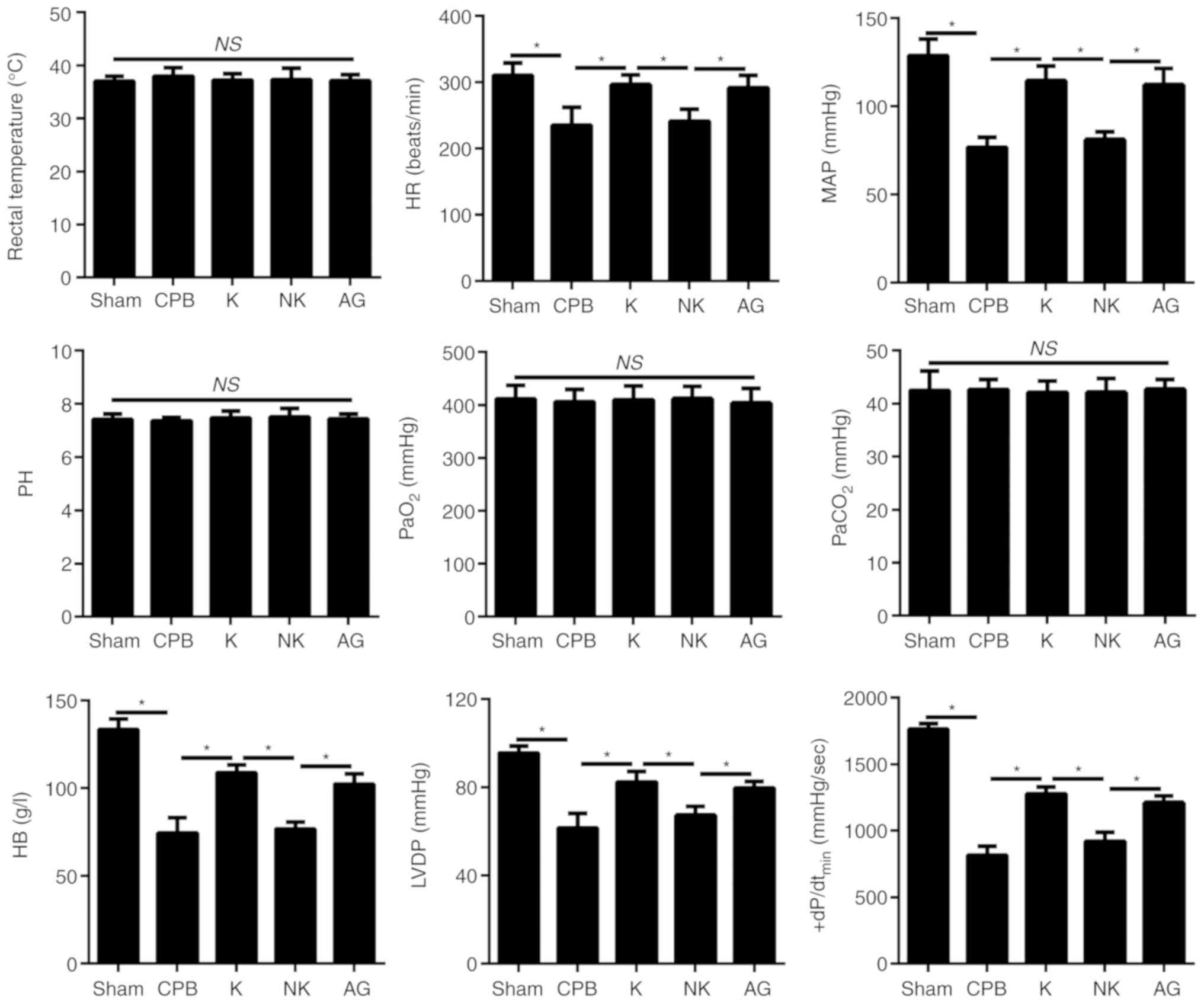 | Figure 1Changes in the hemodynamics of rats.
Changes in the rectal temperature, HR, MAP, pH, PaO2,
PaCO2, HB, LVDP and +dP/dtmax of rats in each group.
*P<0.05. CPB, cardiopulmonary bypass; KOR, kappa
opioid receptor; K, KOR agonist + CPB; NK, KOR agonist +
norbinaltorphimine + CPB; AG, KOR agonist + JAK2-STAT3 specific
pathway inhibitor + CPB; KOR, HR, heart rate; MAP, mean arterial
pressure; PaO2, partial pressure of oxygen; partial
pressure of CO2; HB, hemoglobin; LVDP, left ventricular
diastolic pressure; ns, not significant. |
KOR agonists alleviate neurological
dysfunction in CPB rats
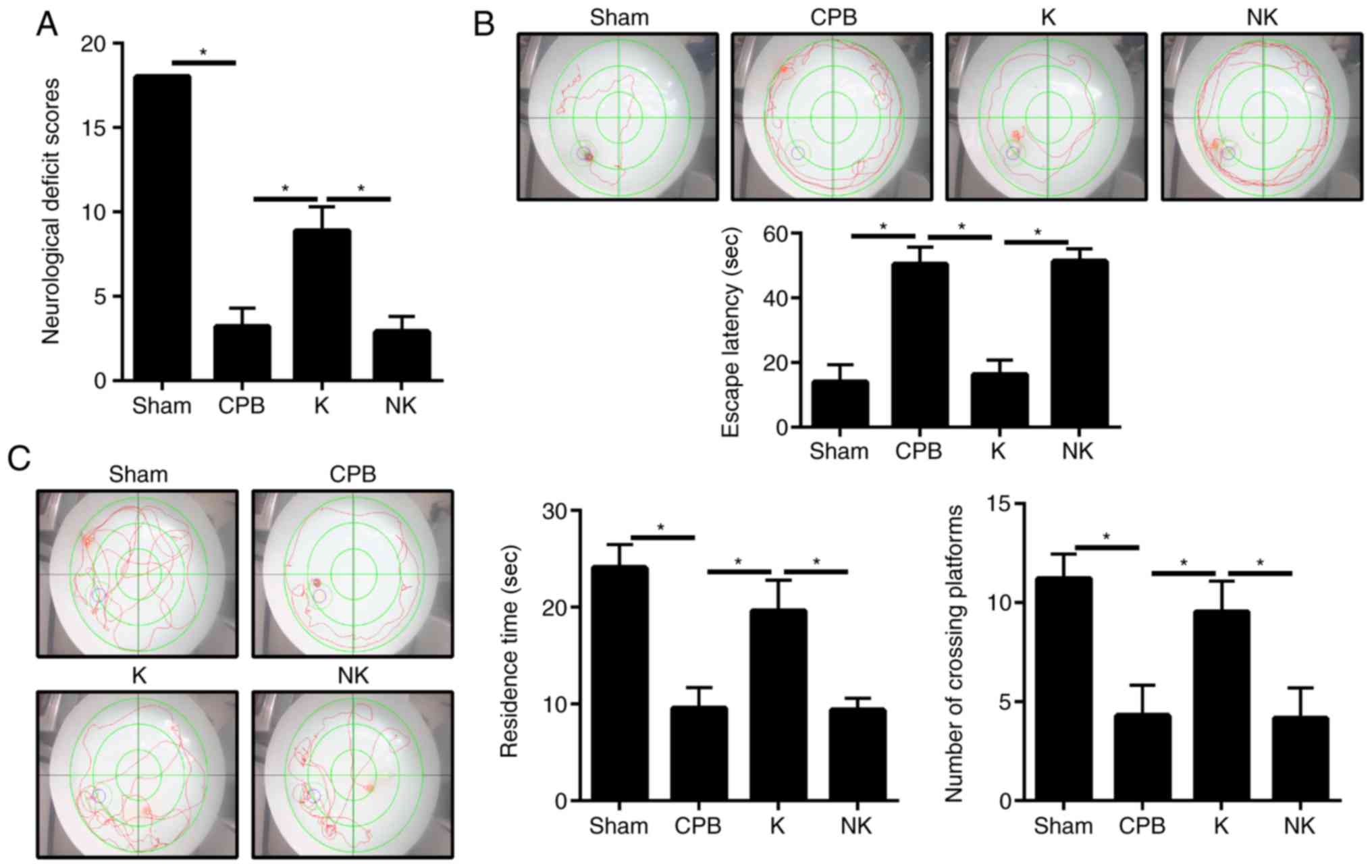
The Garcia neurological function score of the CPB
group was 3.2±1.1, which was significantly lower than that of the
Sham group (P<0.05; Fig. 2A).
When the KOR agonist was administered, the neurological score of
rats in the K group (8.9±1.4; P<0.05) was significantly higher
than that of rats in the CPB group. The neurological function score
of rats in the NK group was significantly lower than that of rats
in the K group (2.9±0.9; P<0.05). The water maze test was used
to judge the cognitive function of the rats (Fig. 2B and C). In the hidden platform
training test, the latency in finding the platform in the CPB, K
and NK groups were all prolonged compared with that in the Sham
group (P<0.05). The latency of finding the platform in the K
group was significantly shorter compared with that in the CPB group
(P<0.05), whereas the latency of finding the platform in the NK
group was significantly prolonged compared with that in the K group
(P<0.05). In the space exploration experiment, the duration the
animal stayed in the original station quadrant and the number of
times the original station in the target quadrant was crossed were
significantly reduced in the CPB, K and NK groups compared with
those in the Sham group (P<0.05). The duration the rat remained
in the original station quadrant and the number of times the
original station in the target quadrant was crossed were
significantly increased in the K group compared with those in the
CPB group (P<0.05). In the NK group, there were no significant
changes in swimming distance or the duration the rat remained in
the target quadrant compared with the CPB group (P>0.05),
although the time spent in the original station quadrant and the
number of times the original station was crossed were lower in the
NK group compared with those in the K group (P<0.05). This
suggested that KOR agonists can alleviate neurological dysfunction
in CPB rats.
KOR agonists improve brain dysfunction in
CPB rats
As shown in Fig.
3A, H&E staining revealed that hippocampal neurons in the
Sham group were arranged in a regular and tight manner with clear
cell boundaries and intact cell bands. The cells were arranged
neatly with a normal cell structure. Only a small number of
inflammatory cells were present. The hippocampus in the CPB group
exhibited severe damage, with disordered cells, widened
intercellular spaces and increased astrocyte and vascular
proliferation. In the Sham group, the nerve cells in the
hippocampal region were normal, arranged regularly, exhibited
staining of the cytoplasm, nuclei were round or oval and there were
no obvious lesions. In the CPB group, the nerve cells were
disordered with nuclei dissolution. Neuronal cell and cone cell
death were observed and cell numbers were significantly decreased
in the hippocampus. In the K group, the arrangement of cells in the
hippocampus was more regular than that in the CPB group, and the
number of degenerative/necrotic nerve cells was significantly
lower.
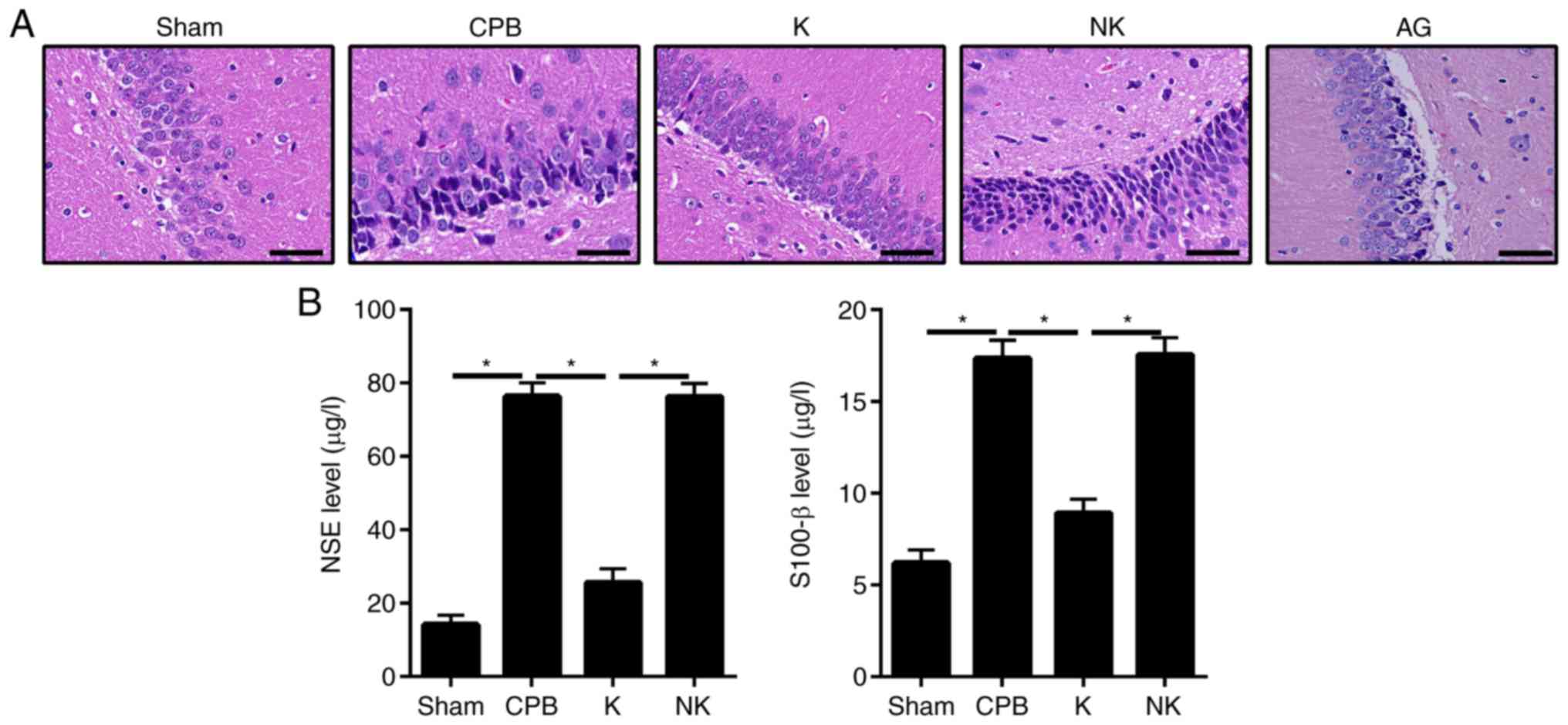 | Figure 3KOR agonists can improve brain
dysfunction in CPB rats. H&E staining was used to observe
hippocampal neurons. ELISA was used to detect the levels of brain
injury markers. (A) H&E staining (scale bar=50 μm). (B)
Brain damage markers detected by ELISA; *P<0.05. CPB,
cardiopulmonary bypass; KOR, kappa opioid receptor; NSE,
neuron-specific enolase; K, KOR agonist + CPB; NK, KOR agonist +
norbinaltorphimine + CPB; AG, KOR agonist + JAK2-STAT3 specific
pathway inhibitor + CPB; AG, KOR agonist + JAK2-STAT3 specific
pathway inhibitor + CPB; H&E, hematoxylin and eosin; JAK2,
Janus kinase 2; STAT3, signal transducer and activator of
transcription 3. |
Staining in the K group revealed less damage than
that in the CPB group. The cells were arranged neatly and the cell
band was incomplete. The NK group exhibited the most damage. The
cells were sparsely and unevenly distributed, and the number of
uneven cytoplasmic vacuoles was increased. This suggests that CPB
can severely damage the hippocampus of rats, whereas KOR agonists
can improve this damage. Compared with the K group, rats in AG
group exhibited notable damage as that in NK group. The cells were
scarcely and irregularly distributed and the number of uneven
cytoplasmic vacuoles was increased (Fig. 3A). To further examine the brain
damage, changes in the expression of brain damage markers were
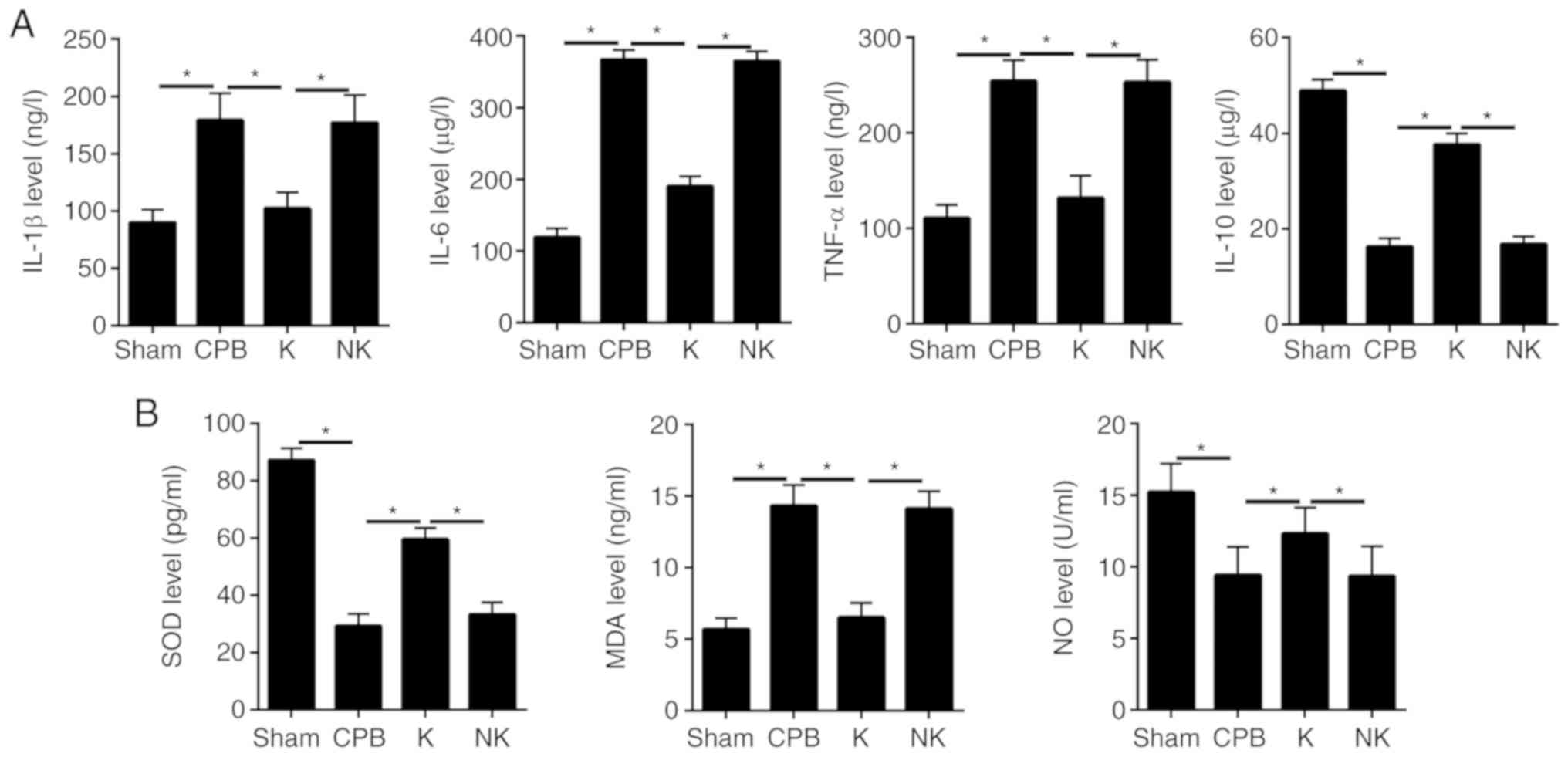
detected by ELISA (Fig. 3B).
Compared with those in the Sham group, serum concentrations of NSE
and S-100β were increased in the CPB, K and NK groups (P<0.05).
The serum concentrations of NSE and S-100β were significantly lower
in the K group than in the CPB group (P<0.05), whereas serum
concentrations of NSE and S-100β were significantly higher in the
NK and AG group than in the K group (P<0.05), suggesting that
KOR agonists can alleviate brain damage in CPB rats.
KOR agonists inhibit inflammation and
oxidative stress in CPB rats
Inflammatory factors (Fig. 4A) and oxidative stress factors
(Fig. 4B) in rat serum were
tracked by ELISA. The concentrations of IL-1β, IL-6 and TNF-α were
increased and that of IL-10 was significantly decreased in the CPB
group compared with that in the Sham group. (P<0.05). Serum
concentrations of IL-1β, IL-6 and TNF-α were significantly
decreased and that of IL-10 was significantly increased in the K
compared with the that in the CPB group (P<0.05). The analysis
of oxidative stress factors showed that serum concentrations of SOD
and NO were decreased and that of MDA was significantly increased
in the CPB group compared with that in the Sham group (P<0.05).
The serum concentrations of SOD and NO were significantly
increased, and the concentration of MDA was significantly reduced
in the K group compared with that in the CPB group (P<0.05).
This suggests that CPB triggered severe inflammation and oxidative
stress, both of which were reversed by KOR agonists.
KOR agonists improve neuronal apoptosis
in CPB rats
Neuronal apoptosis in the brain tissue was observed
by TUNEL staining (Fig. 5A). The
number of positive cells in the CPB group was significantly
increased compared with that in the Sham group (P<0.05). The
number of positive cells in the hippocampal brain tissue was
significantly lower in the K group than in the CPB group
(P<0.05), whereas the number of positive cells in the NK group
was significantly higher than in the K group (P<0.05). To detect
neuronal apoptosis, the expression of apoptosis-related factors
Bcl-2, Bax, pro-caspase-3 and cleaved caspase 3 were detected by
western blotting (Fig. 5B). Bcl-2
was significantly decreased and Bax was significantly increased in
the CPB group compared with the Sham group (P<0.05). The
expression of Bcl-2 was significantly increased in the K group
compared with that in the CPB group, whereas the expression of Bax
was significantly decreased (P<0.05). The expression of Bcl-2
was significantly decreased in the NK group compared with that in
the K group, whereas the expression of Bax was significantly
increased (P<0.05). In addition, the expression of pro-caspase-3
was significantly decreased and that of caspase 3 was significantly
increased in the CPB group compared with that in the Sham group
(P<0.05). The expression of pro-caspase-3 was significantly
increased and that of cleaved caspase 3 was significantly decreased
in the K group compared with that in the CPB group (P<0.05). The
expression of pro-caspase-3 was significantly decreased and that of
cleaved caspase 3 was significantly increased in the NK group
compared with that in the K group (P<0.05). These results
suggest that KOR agonists can inhibit neuronal apoptosis and
prevent neuronal degeneration in CPB rats.
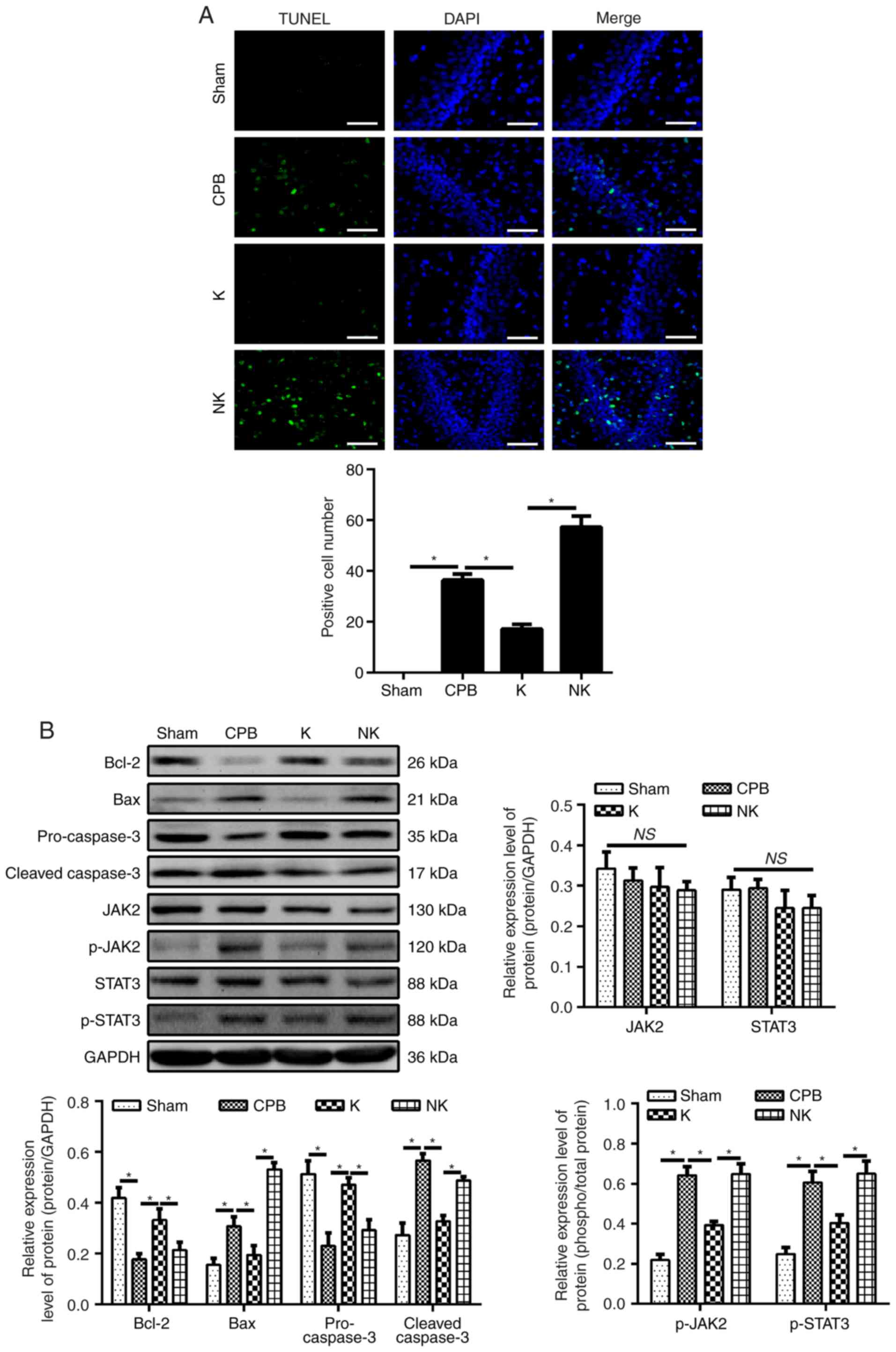 | Figure 5KOR agonists can improve neuronal
apoptosis and the effect of KOR agonists via the JAK2/STAT3
signaling pathway in CPB rats. TUNEL staining and western blotting
were used to detect apoptotic factors and neuronal apoptosis.
Western blotting was used to detect JAK2/STAT3 pathway-related
proteins. Immunofluorescence was used to detect p-JAK2 and p-STAT3.
(A) TUNEL staining (scale bar=50 μm). (B) Expression levels
of apoptosis-related proteins and JAK2/STAT3 signaling
pathway-related proteins were detected by western blotting. (C)
JAK2, p-JAK2, STAT3 and p-STAT3 in the hippocampus were detected by
immunofluorescence (scale bar=50 μm). (D) Levels of JAK2,
p-JAK2, STAT3 and p-STAT3 in the hippocampus were detected by
western blotting. *P<0.05. CPB, cardiopulmonary
bypass; KOR, kappa opioid receptor; NSE, neuron-specific enolase;
K, KOR agonist + CPB; NK, KOR agonist + norbinaltorphimine + CPB;
AG, KOR agonist + JAK2-STAT3 specific pathway inhibitor + CPB; AG,
KOR agonist + JAK2-STAT3 specific pathway inhibitor + CPB; JAK2,
Janus kinase 2; STAT3, signal transducer and activators of
transcription 3; p-, phosphorylated; ns, not significant. |
Effect of KOR agonist on the expression
of JAK2/STAT3 signaling pathway-related proteins in CPB rats
Numerous studies have reported that the JAK2/STAT3
signaling pathway serves an anti-inflammatory role (16) and has anti-apoptotic effects in
cerebral ischemic neurons (17,18). Upon treatment with KOR agonists
for POCD in CPB rats, western blotting (Fig. 5B) revealed significantly increased
levels of JAK2, p-JAK2, STAT3 and p-STAT3 in the hippocampus of the
CPB group compared with levels in the Sham group (P<0.05). The
levels of p-JAK2, and p-STAT3 in the hippocampus of the K group
were significantly decreased compared with those in the CPB group,
and the levels were significantly higher in the NK group compared
with those in the K group (P<0.05). Immunofluorescence further
validated this result (Fig. 5C).
The levels of JAK2 and STAT2 were not significantly altered among
that in different groups. Therefore, KOR agonists may attenuate
POCD in CPB rats through the JAK2/STAT3 signaling pathway.
KOR agonists improve POCD in CPB rats
through the p-JAK2/p-STAT3 rather than JAK2/STAT3
JAK2/STAT3 signaling pathway inhibitors were
administered as the AG group, then we identified that and it was
found that the levels of hippocampal p-JAK2, p-STAT3, but not the
levels of JAK2 and STAT3, were significantly decreased in the K
group compared with those in the CPB group (P<0.05). Compared
with that in the K group, the hippocampus of rats in the AG group
was still damaged, with some disordered cells, which exhibited
increasing astrocyte and vascular proliferation (Fig. 3A). The levels of p-JAK2 and
p-STAT3 in the hippocampus of the AG group were significantly
decreased compared with those in the CPB group (P<0.05). On
comparing the K group and AG group, the difference was not
statistically significant (P>0.05). There were no significant
differences in the levels of JAK2 and STAT3 among the CPB, K and AG
groups. Therefore, KOR agonists may improve POCD in CPB rats
through the JAK2/STAT3 signaling pathway (Fig. 5C and D).
Discussion
In the present study, it was found that KOR agonists
significantly improved cognitive dysfunction in CPB rats. S-100β
and NSE detection showed that the KOR agonists alleviated brain
damage in the CPB rats, and this result was reversed by KOR
antagonists. KOR agonists significantly the reduced the
inflammatory response and oxidative stress on ELISA detection. The
KOR agonists were also detected to attenuate hippocampal neuronal
apoptosis, as shown by TUNEL staining and western blotting, and
downregulated the levels of p-JAK2, STAT3 and p-STAT3 compared with
those in the CPB group. These results indicate that KOR agonists
can improve cognitive dysfunction in POCD of CPB rats by inhibiting
the JAK2/STAT3 signaling pathway. CPB technology provides an
irreplaceable tool for doctors performing surgery (19). With the advancement of clinical
medicine, CPB has been increasingly expanded into an important
technology in clinical medicine (20,21). However, with the increasing
application of this technology, increasing complications are also
being exposed (22). The
perioperative period of CPB open-heart surgery, in-hospital
mortality rates and postoperative complications have all decreased
significantly. Only the incidence of neurological impairment has
not declined, and neurological impairment can significantly
increase in the perioperative period, with various complications
and postoperative mortality, in addition to prolonged hospital
admissions and an increase in the economic burden of patients.
Permanent nerve injury not only reduces patient quality of life,
but also requires re-admission to hospital and may even lead to
death (23).
In the present study, the neurological function
score and water maze test performance were assessed in in a POCD
model in CPB rats. H&E and TUNEL staining were used to observe
the hippocampus. Oxidative stress factors, brain injury markers,
inflammatory factors, apoptosis and JAK2/STAT3 signaling
pathway-associated proteins were examined to investigate the role
of KOR agonists in the development and progress of POCD in CPB
rats. A novel treatment for POCD was provided and its mechanism was
examined.
The results of the study showed that KOS may be a
suitable drug target in POCD therapy. In addition to central
nervous tissues, KOR is expressed in the hippocampal dentate gyrus,
hypothalamus, certain thalamic nuclei, cerebral cortex, caudate
nucleus, olfactory bulb, nucleus accumbens and spinal cords in rats
(24-26). U50488H, a specific KOR agonist,
blocks the transport of acetylcholine through the KOR-mediated
opioid nervous system, which inhibits the reduction of
acetylcholine release caused by mecamylamine (an
N-cholinoceptor-blocking drug), thus reversing the learning and
memory damage caused by mecamylamine (27,28). It has been shown that κ agonists
can improve memory damage caused by μ agonists; their effect is not
only opposite to the effect of μ receptor agonists, but they also
regulate components of the μ system, such as anti-nociceptive
effects (29). Studies on KOR
intervention (U50488H) in ischemia-induced hippocampal nerve injury
have shown that the agonist can significantly reduce cognitive
dysfunction (30). The present
study confirmed that KOR agonists in CPB rats can inhibit the
inflammatory response, reduce oxidative stress, inhibit neuronal
apoptosis and improve brain damage, thus reducing the occurrence
and development of POCD in CPB rats.
The JAK2/STAT3 signaling pathway is an important
pathway for the cholinergic anti-inflammatory pathway (CAP)
(31). With expanding research on
CAP, its protection of the brain has attracted attention. Studies
have shown that the anti-inflammatory effects of JAK2/STAT3 are
activated when the core α7 nicotinic acetylcholine receptor
(α7nAchR) of the CAP is activated (32,33). In addition to its involvement in
the inflammatory response, the JAK2/STAT3 signaling pathway is
involved in the anti-inflammatory response following a7nAchR
activation (34,35). Studies have confirmed that the
JAK2/STAT3 signaling pathway is involved in the anti-apoptotic
process of cerebral ischemic neurons (36,37).
In the present study, it was shown that KOR agonists
can improve POCD of CPB rats via the phosphorylation JAK2 and
STAT3, rather than affecting their expression. The phos-phorylation
or overexpression of JAK2 may be a mechanism of brain damage
(16). Reducing JAK2/STAT3
phosphorylation can decrease neuronal death, narrow infarct size
and prevent post-ischemic damage of nerve cells. Wang et al
reported a significant neuroprotective effect by reducing the
phosphorylation of STAT3 following cerebral ischemia through RNA
interference (38). Others have
found that electroacupuncture stimulation of focal cerebral
ischemia at the Baihui acupoint and Dazhui acupoint in rats
relieved nerve function deficit by reducing the expression of JAK2,
preventing abnormal JAK2 activation and downregulating the
phosphorylation of STAT3 (37).
In conclusion, the findings of the present study
suggest that KOR agonists provide neuroprotective effects against
POCD brain damage in CPB rats, which is partially mediated by
inhibition of the JAK2/STAT3 pathway. The findings regarding the
KOR agonist-mediated molecular mechanisms and signaling pathways
provide novel insight into, and a novel therapeutic target for,
POCD brain damage. Studies in the future should focus on other
possible relationships between JAK2/STAT3 and PI3K/AKT/mTOR in the
action of KOR agonists in POCD brain damage.
Acknowledgements
Not applicable.
Funding
This study was supported by the Liaoning Natural
Science Foundation (grant. no. 201602790) and the National Natural
Science Foundation of China (grant. no. 81471121).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, YS and YD conceived and designed the study and
drafted the manuscript. XL, YS, QJ and DS performed experiments and
interpreted the results. QJ and DS analyzed the data. YS and YD
contributed to acquisition of funding support. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the
Experimental Animal Ethics Committee of the General Hospital of
Northern Theater Command (no. GHNTC2018018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arrowsmith JE, Grocott HP, Reves JG and
Newman MF: Central nervous system complications of cardiac surgery.
Br J Anaesth. 84:378–393. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evered L, Scott DA, Silbert B and Maruff
P: Postoperative cognitive dysfunction is independent of type of
surgery and anesthetic. Anesth Analg. 112:1179–1185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinmetz J and Rasmussen LS:
Peri-operative cognitive dysfunction and protection. Anaesthesia.
71(Suppl 1): pp. S58–S63. 2016, View Article : Google Scholar
|
|
4
|
Noctor G, Lelarge-Trouverie C and Mhamdi
A: The metabolomics of oxidative stress. Phytochemistry. 112:33–53.
2015. View Article : Google Scholar
|
|
5
|
Zakkar M, Guida G, Suleiman MS and
Angelini GD: Cardiopulmonary bypass and oxidative stress. Oxid Med
Cell Longev. 2015.189863:2015.
|
|
6
|
Yang L, Shah K, Wang H, Karamyan VT and
Abbruscato TJ: Characterization of neuroprotective effects of
biphalin, an opioid receptor agonist, in a model of focal brain
ischemia. J Pharmacol Exp Ther. 339:499–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen J, Sun LN, Wu LP and Xia Q: Mito
K(ATP) and kappa-opioid receptor mediate the neuroprotective effect
of limb ischemic post-conditioning on rat brain
ischemia/reperfusion injury. Zhongguo Ying Yong Sheng Li Xue Za
Zhi. 25:368–372. 2009.In Chinese. PubMed/NCBI
|
|
8
|
Hiramatsu M, Murai M and Kameyama T:
Different modulation of cholinergic neuronal systems by dynorphin A
(1-13) in carbon monoxide-exposed mice. Biochem Pharmacol.
57:1321–1329. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olianas MC, Dedoni S, Ambu R and Onali P:
Agonist activity of N-desmethylclozapine at delta-opioid receptors
of human frontal cortex. Eur J Pharmacol. 607:96–101. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fei R, Zhang Y, Wang S, Xiang T and Chen
W: α7 nicotinic acetylcholine receptor in tumor-associated
macrophages inhibits colorectal cancer metastasis through the
JAK2/STAT3 signaling pathway. Oncol Rep. 38:2619–2628. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi S, Liang D, Bao M, Xie Y, Xu W, Wang
L, Wang Z and Qiao Z: Gx-50 inhibits neuroinflammation via α7 nAChR
activation of the JAK2/STAT3 and PI3K/AKT pathways. J Alzheimers
Dis. 50:859–871. 2016. View Article : Google Scholar
|
|
12
|
Hu GQ, Du X, Li YJ, Gao XQ, Chen BQ and Yu
L: Inhibition of cerebral ischemia/reperfusion injury-induced
apoptosis: Nicotiflorin and JAK2/STAT3 pathway. Neural Regen Res.
12:96–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Silverman J and Muir WW III: A review of
laboratory animal anesthesia with chloral hydrate and chloralose.
Lab Anim Sci. 43:210–216. 1993.PubMed/NCBI
|
|
14
|
Garcia JH, Wagner S, Liu KF and Hu XJ:
Neurological deficit and extent of neuronal necrosis attributable
to middle cerebral artery occlusion in rats. Statistical validation
Stroke. 26:627–634; discussion 635. 1995.
|
|
15
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Satriotomo I, Bowen KK and Vemuganti R:
JAK2 and STAT3 activation contributes to neuronal damage following
transient focal cerebral ischemia. J Neurochem. 98:1353–1368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu Y, Zhou J, Xu C, Lin H, Xiao J, Wang Z
and Yang B: JAK/STAT and PI3K/AKT pathways form a mutual
transactivation loop and afford resistance to oxidative
stress-induced apoptosis in cardio-myocytes. Cell Physiol Biochem.
21:305–314. 2008. View Article : Google Scholar
|
|
18
|
Xie HF, Xu RX, Wei JP, Jiang XD and Liu
ZH: P-JAK2 and P-STAT3 protein expression and cell apoptosis
following focal cerebral ischemia-reperfusion injury in rats. Nan
Fang Yi Ke Da Xue Xue Bao. 27:208–211. 2182007.In Chinese.
|
|
19
|
Katz MG, Fargnoli AS, Yarnall C, Perez A,
Isidro A, Hajjar RJ and Bridges CR: Technique of complete heart
isolation with continuous cardiac perfusion during cardiopulmonary
bypass: New opportunities for gene therapy. J Extra Corpor Technol.
50:193–198. 2018.PubMed/NCBI
|
|
20
|
Dimarakis I: Miniaturized cardiopulmonary
bypass in adult cardiac surgery: A clinical update. Expert Rev
Cardiovasc Ther. 14:1245–1250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Melchior RW, Sutton SW, Harris W and
Dalton HJ: Evolution of membrane oxygenator technology for
utilization during pediatric cardiopulmonary bypass. Pediatric
Health Med Ther. 7:45–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sukumaran V, Tsuchimochi H, Fujii Y,
Hosoda H, Kangawa K, Akiyama T, Shirai M, Tatsumi E and Pearson JT:
Ghrelin Pre-treatment attenuates local oxidative stress and end
organ damage during cardiopulmonary bypass in anesthetized rats.
Front Physiol. 9:1962018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wimmer-Greinecker G, Matheis G, Brieden M,
Dietrich M, Oremek G, Westphal K, Winkelmann BR and Moritz A:
Neuropsychological changes after cardiopulmonary bypass for
coronary artery bypass grafting. Thorac Cardiovasc Surg.
46:207–212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ardianto C, Yonemochi N, Yamamoto S, Yang
L, Takenoya F, Shioda S, Nagase H, Ikeda H and Kamei J: Opioid
systems in the lateral hypothalamus regulate feeding behavior
through orexin and GABA neurons. Neuroscience. 320:183–193. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minowa S, Ishihara S, Tsuchiya S, Horie S,
Watanabe K and Murayama T: Involvement of glutamate and
gamma-amino-butyric acid receptor systems on gastric acid secretion
induced by activation of kappa-opioid receptors in the central
nervous system in rats. Br J Pharmacol. 138:1049–1058. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terman GW, Drake CT, Simmons ML, Milner TA
and Chavkin C: Opioid modulation of recurrent excitation in the
hippocampal dentate gyrus. J Neurosci. 20:4379–4388. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Q, Sun Y, Li J, Xing W, Zhang S, Gu
X, Feng N, Zhao L, Fan R, Wang Y, et al: Quaternary ammonium salt
of U50488H, a new K-opioid receptor agonist, protects rat heart
against ischemia/reperfusion injury. Eur J Pharmacol. 737:177–184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong G, Zhang B, Zhou X, Zhao J, Sun Z,
Tao Y, Pei J and Zhang W: Kappa-opioid agonist U50,488H-mediated
protection against heart failure following myocardial
ischemia/reperfu-sion: Dual roles of heme oxygenase-1. Cell Physiol
Biochem. 39:2158–2172. 2016. View Article : Google Scholar
|
|
29
|
Cheng MF, Ou LC, Chen SC, Chang WT, Law
PY, Loh HH, Chao YS, Shih C, Yeh SH and Ueng SH: Discovery,
structure-activity relationship studies, and anti-nociceptive
effects of
1-phenyl-3,6,6-trimethyl-1,5,6,7-tetrahydro-4H-indazol-4-one as
novel opioid receptor agonists. Bioorg Med Chem. 22:4694–4703.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi K, Nakagawasai O, Sugawara M,
Sato A, Nemoto W, Tadano T and Tan-No K: Kappa opioid receptor
agonist administration in olfactory bulbectomized mice restores
cognitive impairment through cholinergic neuron activation. Biol
Pharm Bull. 41:957–960. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatterjee PK, Al-Abed Y, Sherry B and
Metz CN: Cholinergic agonists regulate JAK2/STAT3 signaling to
suppress endothelial cell activation. Am J Physiol Cell Physiol.
297:C1294–C1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Wu S, Zhang H, Wang Y, Luo H, Zuo X
and Xiao X: Activation of nicotinic receptors inhibits
TNF-α-induced production of pro-inflammatory mediators through the
JAK2/STAT3 signaling pathway in fibroblast-like synoviocytes.
Inflammation. 38:1424–1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang YH, Li DL, Bi XY, Sun L, Yu XJ, Fang
HL, Miao Y, Zhao M, He X, Liu JJ and Zang WJ: Acetylcholine
inhibits LPS-induced MMP-9 production and cell migration via the α7
nAChR-JAK2/STAT3 pathway in RAW264.7 cells. Cell Physiol Biochem.
36:2025–2038. 2015. View Article : Google Scholar
|
|
34
|
Maldifassi MC, Atienza G, Arnalich F,
López-Collazo E, Cedillo JL, Martín-Sánchez C, Bordas A, Renart J
and Montiel C: A new IRAK-M-mediated mechanism implicated in the
anti-inflammatory effect of nicotine via α7 nicotinic receptors in
human macrophages. PLoS One. 9:pp. e1083972014, View Article : Google Scholar
|
|
35
|
Zhang W, Sun Q, Gao X, Jiang Y, Li R and
Ye J: Anti-inflammation of spirocyclopiperazinium salt compound
LXM-10 targeting α7 nAChR and M4 mAChR and inhibiting JAK2/STAT3
pathway in rats. PLoS One. 8:pp. e668952013, View Article : Google Scholar
|
|
36
|
Chen B, Yang L, Chen J, Chen Y, Zhang L,
Wang L, Li X, Li Y and Yu H: Inhibition of connexin43 hemichannels
with Gap19 protects cerebral ischemia/reperfusion injury via the
JAK2/STAT3 pathway in mice. Brain Res Bull. 146:124–135. 2019.
View Article : Google Scholar
|
|
37
|
Xu H, Zhang YM, Sun H, Chen SH and Si YK:
Electroacupuncture at GV20 and ST36 exerts neuroprotective effects
via the EPO-mediated JAK2/STAT3 pathway in cerebral ischemic rats.
Evid Based Complement Alternat Med. 2017.6027421:2017.
|
|
38
|
Wang F, Li M, Li X, Kinden R, Zhou H, Guo
F, Wang Q and Xiong L: 2-Arachidonylglycerol protects primary
astrocytes exposed to oxygen-glucose deprivation through a blockade
of NDRG2 signaling and STAT3 phosphorylation. Rejuvenation Res.
19:215–222. 2016. View Article : Google Scholar
|