Introduction
Lumbar laminectomy is considered an effective
treatment approach for diseases of the lumbar vertebrae, such as
slipped disc, spinal stenosis and intraspinal tumor, which are
often accompanied by neuropathic pain and other clinical symptoms
(1-3). However, epidural fibrosis, which
occurs in >20% of patients undergoing lumbar laminectomy
worldwide, remains an intractable clinical complication, that
results in failed back surgery syndrome (FBSS), which is
characterized by chronic lower back and leg pain, as well as
disability (4-6). It has been reported that the
excessive proliferation and migration of fibroblasts in the lumbar
laminectomy area serve a significant role in the development of
epidural fibrosis. The aggregation of fibroblasts can promote the
deposition of abundant extracellular matrix (ECM) components,
eventually leading to the formation of local fibrosis and
lumbosacral adhesive arachnoiditis (7-9).
Previous studies have suggested that E8002, a biological
antiadhesive membrane, as well as immunosuppressants and cytotoxic
drugs could attenuate FBSS by regulating fibroblast proliferation
and migration (10-12). However, their controversial
clinical safety has limited the further advancement of this
treatment strategy. Therefore, further research is required in this
field.
Laminins are significant biofunctional glycoproteins
of the ECM, which are composed of three polypeptide chains, namely
α, β and γ, linked with disulfide bonds. Accumulating evidence has
suggested that there are five α chains (α1-α5), four β chains
(β1-β4) and three γ chains (γ1-γ3), which are assembled into >16
different laminin molecules (13-16). In terms of function, laminins are
associated with several cell surface receptors, and are involved in
the transmission of biochemical signals in the microenvironment
inside and outside the cell, in order to regulate cellular
biological behaviors, such as proliferation, migration, adhesion
and differentiation (17-20). Furthermore, the biological
activity of laminins is principally governed by five different α
chains (laminin α1, laminin α2, laminin α3, laminin α4 and laminin
α5), which exhibit a greater effect compared with β and γ chains
(21-23). However, the underlying biological
mechanism of laminin α1 in regulating the biological behaviors of
fibroblasts remains unknown and requires further investigation. To
the best of our knowledge, the present study was the first to
investigate the role of laminin α1 in the proliferation, apoptosis
and migration of fibroblasts in epidural fibrosis. The present
study also investigated the association between the expression of
laminin α1 and activation of the classical AKT/mechanistic target
of rapamycin (mTOR) signaling pathway. Overall, the results of the
current study may provide a novel target for the prevention and
treatment of epidural fibrosis.
Materials and methods
Animal preparation and establishment of a
laminectomy model
A total of 48 Sprague-Dawley (SD) male rats (weight,
250 g; age, 8 weeks) were purchased from the Hubei Provincial
Center for Disease Control and Prevention. SD rats were acclimated
for 1 week to the laboratory environment of 25±2°C and 30-50%
humidity, under a 12-h light/dark cycle, and were provided with
ad libitum access to food and water. A total of 36 SD rats
were numbered and randomly divided into the 2-, 3- and 4-weeks
groups using the random-number-table function in Excel version 2016
(Microsoft Corporation); the number of weeks indicates when rats
were sacrificed post operation. The remaining 12 SD rats were set
as the control group, which did not undergo laminectomy, and were
directly sacrificed to provide a control epidural area. During the
preparation process, all rats were appropriately treated according
to the Regulations of Laboratory Animals of Hubei province
(24).
To simulate laminectomy in clinical patients, a
laminectomy model was established in SD rats by carefully excising
the vertebral plate, as previously described (3). Briefly, SD rats were anesthetized
with 1% pentobarbital sodium (50 mg/kg) through intraperitoneal
injection, and their backs were shaved above the first and second
lumbar vertebrae to clearly expose the surgical area. The operative
skin was sterilized three times with iodine. Following skin
incision, the local soft tissue was separated to expose the
vertebral plate. Subsequently, the vertebral plates of the first
and second lumbar were carefully excised using a rongeur to expose
the spinal cord. After appropriate hemostasis, the wound was
sutured using Vicryl Plus antibacterial sutures (Johnson &
Johnson). During establishment of the laminectomy model, all
procedures were performed in sterile conditions by professional
orthopedists. After the surgery, the following signs were
considered humane endpoints and if these endpoints were met, the
rats were sacrificed by cervical dislocation under anesthesia [1%
pentobarbital sodium (50 mg/kg), intraperitoneal injection]: i)
Persistent tiredness and failure to clean the fur (rough and dull);
ii) weakness, dehydration, decreased food and water intake, urine
and stool volume; iii) abnormal physical responses to human touch
(including excessive withdrawal, limping, unusual aggression,
screaming and abdominal clamping); iv) weight loss of >20% (the
maximum percentage of body wight loss was 20% in the present
study); v) paralysis, unable to walk normally; vi) abnormal body
temperature, pulse and breathing (rates were too high or too low);
vii) persistent self-harming behavior, self-harm of the incision
site and post-operative area; viii) severe inflammation and
infection at the surgical site; ix) abnormal central nervous system
reactions (convulsions, shaking, paralysis, head tilting).
Histopathological analysis
At 2, 3 and 4 weeks following the lumbar
laminectomy, the SD rats from the three groups were sacrificed. The
SD rats in the control group were directly sacrificed after the
1-week acclimation. To evaluate the degree of fibrosis, the spinal
tissue from the surgical area was collected and a histopathological
analysis was then carried out. Briefly, all of the SD rats in each
group were anesthetized with 1% pentobarbital sodium (50 mg/kg)
through intraperitoneal injection and cardiac perfusion was
performed using 4% paraformaldehyde. When decreased corneal
reflexes, abnormal pulse and respiration were observed, cervical
dislocation was performed to ensure the rats were euthanized.
Subsequently, the postoperative lumbar column with external soft
tissues was excised and fixed in 4% paraformaldehyde at room
temperature for 1 week. After fixation, the column was immersed in
EDTA for 40 days for decalcification. Finally, for
histopathological analysis, the column was embedded in paraffin and
was then sliced into 4-µm transverse sections. For
hematoxylin and eosin (H&E) staining, the sections were first
stained with hematoxylin for 5 min and then with eosin for an
additional 5 min. For Masson's trichrome staining, the sections
were immersed overnight in 50% potassium dichromate, stained with
hematoxylin for 3 min and incubated in Ponceau S dye for 5 min. The
sections were then washed and incubated with 1% phosphomolybdic
acid for 2 min prior staining with aniline blue for 5 min. All of
the procedures were performed at room temperature. The tissue
sections were finally observed under an optical photographic light
microscopy (magnifications, ×40 and ×200) to ascertain the local
fibroblast proliferative level, epidural fibrotic level and the
content of collagen in epidural tissues. The results were collected
and processed using Image Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Immunohistochemical staining
Immunohistochemical staining was performed using the
Ready-to-use HP IHC detection kit (cat. no. abs957; Absin
Bioscience, Inc.). Briefly, the tissue sections were deparaffinized
in xylene at room temperature and rehydrated with a descending
alcohol series (100, 85 and 75%). For antigen retrieval, the tissue
sections were immersed in sodium citrate at 100°C for 20 min
followed by blocking in 3% hydrogen peroxide (from kit) and 100%
FBS (Gibco; Thermo Fisher Scientific, Inc.) for 10 and 15 min,
respectively, at room temperature. Subsequently, the sections were
first incubated with primary antibodies against laminin α1 (1:200;
cat. no. bs-4973R; BIOSS) and α smooth muscle actin (α-SMA; 1:200;
cat. no. NBP2-33006; Novus Biologicals, Ltd.) at 4°C overnight and
then with the corresponding secondary antibody provided by the HP
IHC detection kit at room temperature for 2 h. The tissue sections
were then stained with DAB reagent and hematoxylin for 2 min each
at room temperature. Finally, the sections were observed under an
optical photographic light microscope at ×200 magnification and the
expression levels of laminin α1 and α-SMA were analyzed using Image
Pro Plus 6.0 software.
Cell culture
Primary human fibroblasts were purchased from
ScienCell Research Laboratories, Inc. (cat. no. #2320), and were
cultured in DMEM supplemented with 15% FBS and 1% penicillin and
streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.) at
37°C in an incubator containing 5% CO2. Fibroblasts at
passages 3-8 were collected and divided into three groups: The
control group, negative control group and small interfering (si)RNA
group for the subsequent experiments.
Transfection with siRNA
The siRNA against laminin α1 (5′-TGCC ATA GAT GGC
ACC AAT AAC T-3′) and the corresponding negative control siRNA
(5′-GGC TCT AGA AAA GCC TAT GC-3′) were purchased from Guangzhou
RiboBio, Co., Ltd. Human fibroblasts (60% confluence) were
transfected with 50 nM siRNA (siRNA against laminin α1 for siRNA
group, and negative control siRNA for negative control group) for
48 h at 37°C with Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.)
and Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) to knock down the expression of laminin
α1. The cells in the control group were not transfected. All
procedures were performed according to the protocol of Guangzhou
RiboBio, Co., Ltd. The transfection efficiency was assessed by
reverse transcription-quantitative PCR (RT-qPCR) and
immunofluorescence staining. The transfected cells were collected
for the subsequent experiments within 48 h to guarantee
transfection efficiency.
RNA preparation and RT-qPCR
Total RNA was extracted from the transfected cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. RNA
was then reverse transcribed into cDNA using the FastKing DNA
Dispelling RT SuperMix (Tiangen Biotech Co., Ltd.), according to
the manufacturer's instructions. Subsequently, qPCR was performed
using the SYBR®-Green Master Mix kit (Vazyme Biotech
Co., Ltd.). The primer sequences used are shown in Table I. The thermocycling conditions
were as follows: Initial denaturation at 95°C for 5 min; followed
by 40 cycles at 95°C for 10 sec and 60°C for 30 sec; then, 95°C for
15 sec, 60°C for 60 sec and 95°C for 15 sec. The relative mRNA
expression levels were calculated using the 2−ΔΔCq
method (25). GAPDH served as the
internal reference.
 | Table IPrimers for reverse
transcription-quantitative PCR. |
Table I
Primers for reverse
transcription-quantitative PCR.
| Gene | Primer sequence,
5′-3′ |
|---|
| Laminin α1 | F:
AGAGGGCTACAAAGTCCAGT |
| R:
GCTCTAGTCCAATGGCATCC |
| GAPDH | F:
GAAGCTTGTCATCAATGGAAAT |
| R:
TGATGACCCTTTTGGCTCCC |
Immunofluorescence staining
The fibroblasts were seeded in 24-well plates
containing glass slides until they reached 60% confluence
(generally within 24 h). After fixing in 4% paraformaldehyde at
room temperature for 15 min, the fibroblasts were immersed in 0.1%
Triton X-100 for permeabilization and blocked in 3% BSA (Gibco;
Thermo Fisher Scientific, Inc.) for 30 min at room temperature.
Subsequently, the cells were incubated with anti-laminin α1 (1:100)
at 4°C overnight and then with Alexa Fluor®
647-conjugated anti-rabbit IgG antibody (1:200; cat. no. ab150075;
Abcam) for 1 h at room temperature. The nuclei were stained with
Hoechst for 5 min at room temperature and the cells were observed
under a Zeiss inverted fluorescence microscope (magnification,
×200; Zeiss AG). The fluorescence density of laminin α1 in each
group was analyzed using Image Pro Plus 6.0 in five randomly
selected fields with the same size.
Western blot analysis
Total protein was extracted from the fibroblasts
using RIPA lysis buffer (Beyotime Institute of Biotechnology) and
protein concentration was determined using the BCA kit (Thermo
Fisher Scientific, Inc.). Subsequently, 30 µg proteins/lane
were separated by SDS-PAGE on 10% gels and transferred onto a PVDF
membrane. After blocking in 5% skim milk in TBS with 0.05% Tween-20
for 2 h at room temperature, the membranes were incubated with the
primary antibodies at 4°C overnight and then with the corresponding
secondary antibodies (anti-rabbit; 1:2,000; cat. no. #7074; Cell
Signaling Technology, Inc.) at room temperature for 2 h. The
following primary antibodies were used: Anti-proliferating cell
nuclear antigen (PCNA; 1:1,000; cat. no. #13110), anti-cyclin D1
(1:1,000; cat. no. #55506), anti-matrix metalloproteinase (MMP)-2
(1:1,000; cat. no. #40994), anti-MMP-9 (1:1,000; cat. no. #13667),
anti-AKT (1:1,000; cat. no. #4685), anti-phosphorylated (p)-AKT
(1:1,000; cat. no. #4060), anti-mTOR (1:1,000; cat. no. #2983),
anti-p-mTOR (1:1,000; cat. no. #5536), anti-Bax (1:1,000; cat. no.
#5023), anti-Bcl-2 (1:1,000; cat. no. #4223) and anti-GAPDH
(1:1,000; cat. no. #5174) (all from Cell Signaling Technology,
Inc.). GAPDH served as an internal loading protein. The protein
expression level were then detected using BeyoECL Moon (cat. no.
P0018FS; Beyotime institute of Biotechnology) and the ChemiDoc XRS+
system (Bio-Rad Laboratories, Inc.) according to the manufacturer's
protocol. ImageJ version 1.46r software (National Institute of
Health) was used to perform densitometric analysis of all protein
bands.
Cell viability assay
Cell viability was assessed using the Cell Counting
Kit-8 (CCK-8) assay (cat. no. CK04; Dojindo Molecular Technologies,
Inc.). Briefly, the prepared fibroblasts from each group were
cultured in 96-well plates and then treated with 10 µl CCK-8
reagent for 2 h at 37°C. Subsequently, optical density (OD) values
were measured at a wavelength of 450 nm using a microplate
absorbance reader (Elx800; Biotek Instruments, Inc.). Finally, the
cell survival rate was calculated according to the formula in the
manufacturer's protocol: [(As-Ab)/(Ac-Ab)] ×100. As indicates the
absorbance of the samples (negative control group or siRNA group),
Ac indicates the absorbance of the control group, and Ab indicates
the absorbance of wells without cells.
EdU incorporation assay
The EdU incorporation assay was performed to
evaluate cellular DNA synthesis using the Click-iT EdU Alexa Fluor
488 Imaging kit (Nanjing KeyGen Biotech Co., Ltd.), according to
the manufacturer's protocol. Briefly, fibroblasts from each group
were cultured in 24-well plates until they reached 60% confluence
(generally within 24 h). Subsequently, cells were incubated in 10
µmol/l EdU working solution at 37°C for 2 h followed by
fixing in 4% paraformaldehyde at room temperature for 30 min and
treatment with 0.5% Triton X-100 for 20 min in the dark at room
temperature. Cells were then immerged in the click-iT mixture
system and cell nuclei were stained with Hoechst at room
temperature for 5 min. Finally, the fibroblasts were observed under
a Zeiss inverted fluorescence microscope (magnification, ×200) to
analyze the EdU-positive fluorescent signal. The EdU-positive rate
was calculated using ImageJ.
Flow cytometric analysis
Flow cytometry was performed to determine the
apoptotic rate (early + late apoptosis) of fibroblasts according to
the protocol of the Annexin V-FITC Apoptosis Detection kit (cat.
no. C1062L; Beyotime institute of Biotechnology). Briefly, the
fibroblasts from each group were cultured in 6-well plates at a
density of 106 cells/well. When the cells reached 80%
confluence, the supernatant was removed. The prepared cells were
then collected and stained with Annexin V (5
µl/105 cells) and PI (10 µl/105
cells) at room temperature for 20 min. After staining, the cells
were washed twice with PBS, resuspended in PBS and detected by flow
cytometry (BD Accuri C6; BD Biosciences). The data were analyzed
using FlowJo 10.8.1 software (FlowJo, LLC).
Scratch wound assay
The fibroblasts from each group were cultured into
6-well plates until they covered the entire bottom of the well (80%
confluence) (26,27). Subsequently, a 10-µl
pipette tip was used to scratch the bottom of the well. The scraped
cells were removed after washing with PBS. Subsequently, the
complete culture medium was replaced with DMEM supplemented with
0.1% FBS. The fibroblasts were observed under an optical
photographic light microscopy (magnification, ×40). The migratory
ability of fibroblasts from each group was assessed at 0, 12 and 24
h after scratching. The results were collected and processed using
ImageJ software. The wound closure rate was calculated as follows:
Wound closure rate=the area of migrated cells in the total
scratched area at 0, 12 or 24 h/the total scratched area.
Statistical analysis
All of the data in the present study are presented
as the mean ± standard deviation and the statistical analysis was
performed by SPSS 19.0 statistical software (IBM Corp.). Each
experiment was performed in triplicate. The differences among
multiple groups were assessed using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Laminin α1 is positively and
time-dependently associated with epidural fibrosis
As shown in Fig.
1, the results of H&E staining and Masson's trichrome
staining showed a low level of epidural fibrosis, few fibroblasts
(spindle cells indicated with red arrows) and low collagen content
in the SD rats of the control group, which illustrated the normal
epidural site without laminectomy. By contrast, following the
laminectomy, the level of epidural fibrosis, the number of
fibroblasts and the collagen content were increased in the 4-weeks
group. These changes were also apparent but were more moderate in
the 3-weeks group and were even more moderate in the 2-weeks group
(Fig. 1A-F). These findings
indicated that the level of postoperative epidural fibrosis in the
laminectomy area was increased in a time-dependent manner. In
addition, some lymphocytes were observed in the epidural area after
laminectomy (round cells indicated with green arrows).
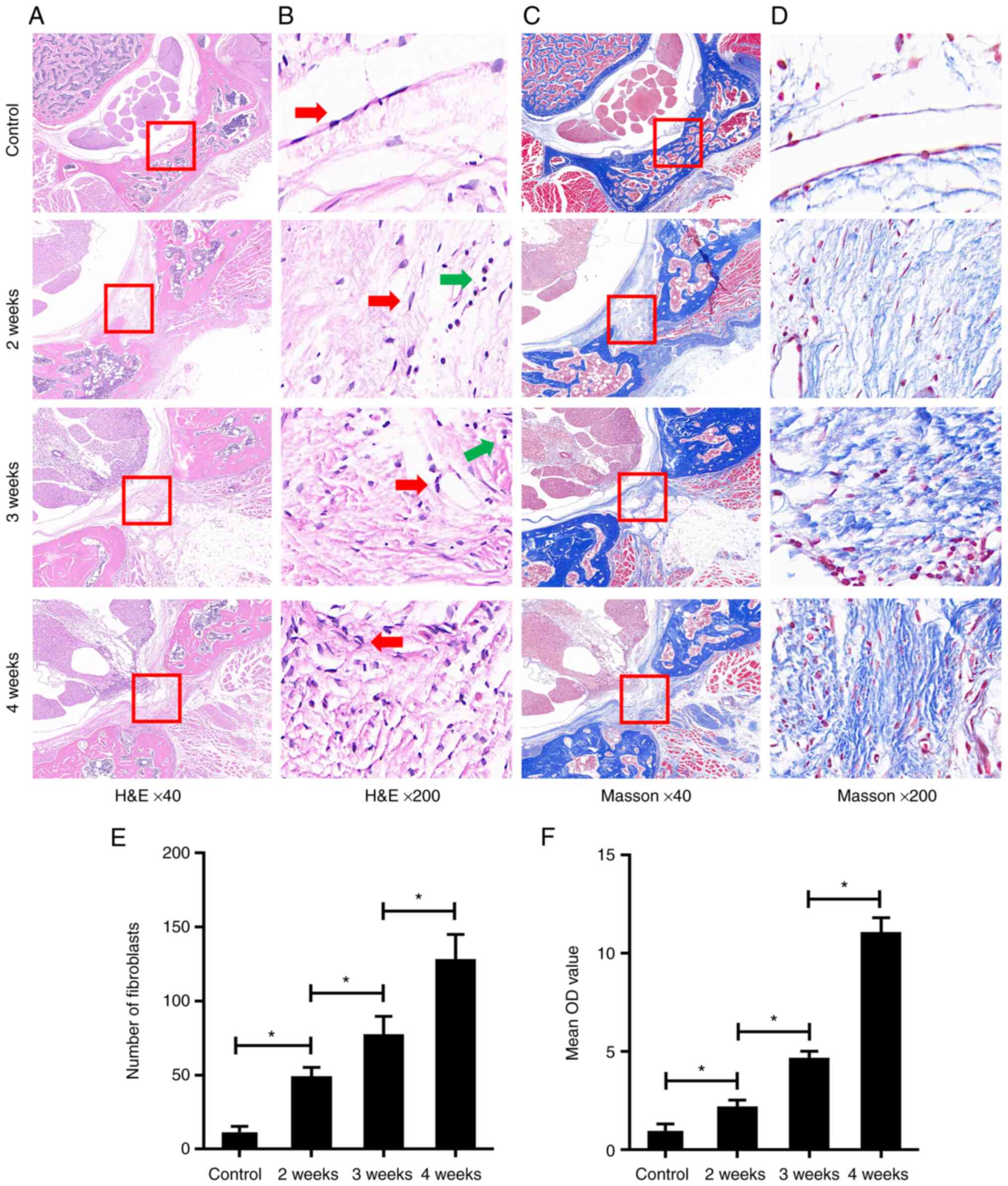 | Figure 1Postoperative epidural fibrosis was
increased in the laminectomy area in a time-dependent manner. (A)
As determined by H&E staining, compared with the control group,
excessive epidural fibrosis (red rectangle) with thick adherence to
the spinal dura was observed in the three operated groups; this
increased in a time-dependent manner. Magnification, ×40. (B) As
determined by H&E staining, compared with the control group,
the epidural fibroblasts (red arrows) in the three operated groups
were observed increasing in a time-dependent manner. Lymphocytes
are shown with green arrows. Magnification, ×200. (C and D) As
determined by Masson's trichrome staining, compared with the
control group, epidural collagen (red rectangle) in the three
operated groups was observed synthesizing in a time-dependent
manner. Magnification, (C) ×40 and (D) ×200. (E)
Semi-quantification of H&E staining in (B); the number of
fibroblasts in each group are presented. (F) Semi-quantification of
Masson's trichrome staining in (D); the contents of epidural
collagen in each group are presented as the mean OD value. Data are
shown as the mean ± standard deviation. *P<0.05.
H&E, hematoxylin and eosin; OD, optical density. |
To determine whether laminin α1 was involved in the
progression of epidural fibrosis, the expression levels of laminin
α1 in the three groups were analyzed by immunohistochemical
staining. As shown in Fig. 2A,
laminin α1 was significantly increased in the 4-weeks group and was
moderately increased in the 3-weeks group compared with in the
2-weeks group. To exclude the influence of neovascularization,
which commonly occurs during fibrosis formation in local tissues,
the expression levels of α-SMA were also detected. The results
showed that there was no significant difference in the expression
of α-SMA among the three groups (Fig.
2B), thus suggesting that the expression of laminin α1 changed
with the progression of epidural fibrosis. Overall, these data
suggested that the expression of laminin α1 was positively and
time-dependently associated with epidural fibrosis and it could be
involved in postoperative FBSS.
siRNA transfection interferes with the
expression of laminin α1 in fibroblasts
The results of immunofluorescence staining and
RT-qPCR analysis confirmed transfection efficiency (Fig. 3A and B). After transfection, the
expression levels of laminin α1 were significantly reduced in the
siRNA group compared with in the control group, and a negligible
difference in expression was detected between the control and
negative control groups.
Laminin α1 regulates the proliferation,
apoptosis and migration of fibroblasts
The biological role of laminin α1 in fibroblast
proliferation, apoptosis and migration was subsequently assessed.
Compared with in the control group, the EdU-positive rate of the
siRNA group was decreased following laminin α1 knockdown (Fig. 4A), and the expression levels of
the proliferation-related proteins PCNA and cyclin D1 were also
significantly reduced; however, there was no difference between the
control group and negative control group (Fig. 4B). These results suggested that
laminin α1 could regulate the proliferation of fibroblasts.
 | Figure 4Laminin α1 regulates the
proliferation, apoptosis and migration of fibroblasts, and affects
their viability. (A) Fibroblast proliferation was detected using
the EdU incorporation assay. The proliferative rate was decreased
after laminin α1 was knocked down. Magnification, ×200. (B) Protein
expression levels of PCNA, cyclin D1, Bax, Bcl-2, MMP-2 and MMP-9
were detected by western blotting in each group. (C) Fibroblast
apoptosis was detected by Annexin V-FITC/PI double labeling and the
apoptotic rates are presented. (D) Fibroblast viability was
detected using the Cell Counting Kit-8 assay. (E) Fibroblast
migration was detected using the scratch wound assay
(magnification, ×40). Data are presented as the mean ± standard
deviation. *P<0.05. MMP, matrix metalloproteinase;
NS, not significant; PCNA, proliferating cell nuclear antigen;
siRNA, small interfering RNA. |
The results of flow cytometric analysis demonstrated
that compared with in the control group, the apoptotic rate of
fibroblasts in the siRNA group was significantly increased
following laminin α1 knockdown (Fig.
4C). Consistently, western blot analysis of the
apoptosis-related proteins Bax and Bcl-2 also indicated a similar
tendency; after laminin α1 was knocked down, the expression levels
of Bax were increased, whereas those of Bcl-2 were decreased
(Fig. 4B). Furthermore, the
results of the CCK-8 assay demonstrated that cell viability was
decreased in the siRNA group compared with in the control group
(Fig. 4D). These findings
indicated that laminin α1 could regulate the proliferation,
apoptosis and viability of fibroblasts.
The effect of laminin α1 on the migration of
fibroblasts was also assessed. It is widely accepted that several
members of the MMP family are involved in the degradation of
ECM-related proteins to promote cell migration (28). In addition, previous studies have
indicated that MMP-2 and MMP-9 (two classical MMPs) could promote
the proliferation, activation and migration of fibroblasts in an
inflammatory site, thus resulting in local fibrosis (29-31). As shown in Fig. 4B, the results of western blotting
revealed that after laminin α1 was knocked down, the expression
levels of MMP-2 and MMP-9 in the siRNA group were downregulated
compared with in the control group. These changes suggested that
migration may be inhibited in response to laminin α1 knockdown.
Moreover, the results of a scratch wound assay demonstrated that
after laminin α1 knockdown the migration of fibroblasts was
significantly reduced compared with in the control group, whereas
there was no obvious difference between the control group and
negative control group (Fig. 4E).
These results indicated that laminin α1 could regulate cellular
migration.
Laminin α1 is involved in activation of
the AKT/mTOR signaling pathway
The AKT/mTOR signaling pathway serves an important
role in the regulation of diverse cellular functions, including
proliferation, differentiation, apoptosis and migration (32,33). In previous studies, it was
demonstrated that AKT/mTOR signaling could regulate fibroblast
behaviors, such as proliferation, apoptosis and autophagy (9,34).
To explore the potential interaction between laminin α1 and
AKT/mTOR signaling, the expression levels of the AKT/mTOR
signaling-related proteins (p-AKT, AKT, p-mTOR and mTOR) were
detected. The results of western blotting showed that following
laminin α1 knockdown, the expression ratios of p-AKT/AKT and
p-mTOR/mTOR were significantly reduced compared with in the control
group (Fig. 5). These findings
suggested that activation of the AKT/mTOR signaling pathway was
prevented after laminin α1 was knocked down.
Discussion
Lumbar laminectomy is often accompanied by an
increased incidence of FBSS and unavoidable symptoms, including
chronic lower back and leg pain (5). Although the clinical complications
are apparent to orthopedists, the underlying mechanisms of epidural
fibrosis have not been thoroughly explored. Therefore, it is
important to clearly study the cellular and molecular mechanisms,
which could be beneficial for the improved understanding of the
appearance and development of epidural fibrosis. An in-depth
understanding of the physiological mechanism is essential for the
development of effective methods to prevent and treat fibrosis. The
current dominant view is that fibroblasts have a critical role in
the progression of epidural fibrosis (3,12).
After lumbar laminectomy, local resident fibroblasts are recruited
to the operative region and start to deposit abundant ECM proteins,
including collagens and laminins (35). The ECM serves an important role in
cell construction, and regulates the cell biological behavior by
interacting with specific plasma membrane receptors in the
extracellular microenvironment. The ECM also affects the
physiological structure and characteristics of the cell basement
membrane (36,37). The abnormal parameters and
functions of ECM can lead to a variety of diseases, including
abnormal blood filtration, adipogenesis and fibrosis, muscle,
vessel and skin dyspoiesis, as well as tumorigenesis (38,39). As a type of important
biofunctional protein in the ECM, laminins can determine the
properties of basement membranes and serve a crucial role in
several cellular behaviors (20).
Across the five types of laminin α chains, laminin α1 has been
demonstrated to have a wide expression in human tissues, including
skin, retina, neural stem cells and testes, and may have an
important role in the biological behaviors of cells (40-43). Furthermore, increasing studies
have indicated that laminin α1 is upregulated in fibrotic diseases,
such as pulmonary fibrosis, liver fibrosis and keloid fibrosis
(44-46).
Based on the aforementioned evidence, the present
study first hypothesized that laminin α1 may be involved in the
formation of epidural fibrosis. According to the results of
histopathological analysis in the present study, after laminectomy,
the level of epidural fibrosis increased with the passage of
postoperative time compared with in the control group. Combined
with the results of immunohistochemical staining, the present study
demonstrated that the expression of laminin α1 was positively and
time-dependently associated with epidural fibrosis following lumbar
laminectomy. Furthermore, the expression levels of α-SMA were
detected to exclude the influence of neovascularization, which
ultimately confirmed the original hypothesis. Moreover, the results
of histopathological analysis indicated that several lymphocytes
were present in epidural fibrosis. Lymphocytes are involved in the
early stage of inflammation, and can produce inflammatory factors
and profibrotic molecules to promote inflammatory chemotaxis and
fibrosis (47,48). The presence of lymphocytes
indicated the development of inflammation at the postoperative
site. This finding was consistent with previous studies, which
demonstrated that following lumbar laminectomy, inflammation and
fibrosis occurred in the local area and finally led to clinical
FBSS (7-9). Therefore, it was further
hypothesized that laminin α1 may be related to fibroblast
proliferation, apoptosis and migration, which are considered the
predominant pathophysiological hallmarks of epidural fibrosis. A
stable siRNA-transfected system was thus established. Following
laminin α1 knockdown, the viability, proliferation, apoptosis and
migration of fibroblasts were detected using CCK-8 assay, scratch
wound assay, western blot analysis, EdU incorporation assay and
flow cytometry. Notably, the results revealed that the levels of
cellular viability and proliferation were significantly decreased
in response to laminin α1 knockdown. Furthermore, the expression
levels of proliferation-related proteins, such as PCNA and cyclin
D1, were also downregulated. The expression levels of
apoptosis-associated proteins, Bax and Bcl-2, and the results of
flow cytometry all demonstrated that apoptosis was promoted
following laminin α1 knockdown. These data indicated that the
laminin α1 could interfere with and regulate the proliferation and
apoptosis of fibroblasts.
MMPs are closely related to fibrosis and can
regulate a range of biological processes in the immune system and
tissue repair. A previous study demonstrated that several MMPs
could turn over ECM components, and influence cellular
proliferation, survival, gene expression and inflammation to impact
migration and fibrosis (49).
Another study revealed that the two classical MMPs, MMP-2
(gelatinase A) and MMP-9 (gelatinase B), could degrade type IV
collagen and gelatin substrates, which were highly associated with
cellular migration and invasion (50). Hence, the decreased expression of
MMP-2 and MMP-9 detected in response to laminin α1 knockdown in the
present study suggested that migration may be inhibited. The reason
for this could be attributed to the limited degradation of collagen
and gelatin. Combined with the results of the scratch wound assay,
it was suggested that the migratory ability of fibroblasts could be
suppressed after laminin α1 knockdown. It has been reported that
laminin α1 serves a critical role in the proliferation, migration
and invasion of esophageal squamous cells, which indicated a
potential prognostic and therapeutic significance for laminin α1 in
esophageal squamous cell carcinoma (51). Previous studies have also
indicated that laminin α1 is closely related to the melanoma
(52), pulmonary fibrosis
(44), colorectal carcinoma
(53), cerebellar hypoplasia
(54) and vitreoretinal disease
(55). The present findings also
indicated that laminin α1 could interfere with and regulate the
proliferation, apoptosis and migration of fibroblasts. As the
functioning cells of epidural fibrosis, the decreased migration and
proliferation, and enhanced apoptosis of fibroblasts may further
relieve epidural fibrosis during the postoperative period of lumbar
laminectomy, and laminin α1 could be an effective therapeutic
target in the treatment of epidural fibrosis.
During the regulation of cellular behavior, laminins
can interact with cell surface receptors and lead to a series of
changes in intracellular signaling pathways (17). In previous studies, the AKT/mTOR
signaling pathway has been demonstrated to have an important role
in the regulation of cellular behaviors, such as proliferation,
differentiation, apoptosis and migration (32,33). To further confirm the underlying
molecular mechanism of laminin α1 knockdown, the activation status
of the AKT/mTOR signaling pathway was detected. In response to
laminin α1 knockdown, the AKT/mTOR signaling pathway was
significantly suppressed. These results suggested that laminin α1
could affect activation of the AKT/mTOR signaling pathway. A
previous study reported that the AKT/mTOR signaling pathway
promoted the cellular activities of fibroblasts; notably, after
intervening with LY294002 (an inhibitor of the AKT/mTOR signaling
pathway), cellular viability and proliferation were reduced
(3). Previous studies have also
confirmed the inhibitory effect of AKT/mTOR signaling inactivation
on fibroblast migration; notably, applying Morin (an anti-arthritis
compound) has been shown to reduce the migration of fibroblasts in
rheumatoid arthritis (56) and
Danlou tablet (a Chinese medicine used to treat cardiovascular
diseases) has been reported to restrain the migration of vascular
adventitial fibroblasts in atherosclerosis (57). Furthermore, Lu et al
revealed that an antibody against programmed cell death ligand 1
could inhibit fibroblast proliferation, migration and ECM
deposition in pulmonary fibrosis through regulating the AKT/mTOR
signaling pathway (58). In
combination with the changes in the AKT/mTOR signaling pathway
detected following laminin α1 knockdown, it may be hypothesized
that laminin α1 could regulate the proliferation, apoptosis and
migration of fibroblasts, partially via regulating the AKT/mTOR
signaling pathway.
The findings of the present study provided novel
insights into the treatment of epidural fibrosis and FBSS; however,
due to limitations associated with technology, time and finance,
there are some deficiencies that should be overcome to further
strengthen the conclusion of the present study. For example, the
following experiments may provide a better understanding of the
relationships and mechanisms among laminin α1, the AKT/mTOR
signaling pathway, epidural fibrosis and fibroblast behaviors: The
establishment of a laminin α1 knockdown animal model; more cellular
staining experiments; a Transwell migration assay; and a possible
reversal experiment (activating AKT/mTOR in fibroblasts in which
laminin α1 was knocked down and reconfirming the cellular
behavior). Furthermore, previous studies have used fibroblasts
derived from the epidural postoperative site to study the
mechanisms of epidural fibrosis (59,60); however, due to the difficulty of
extracting primary fibroblasts, a high cell contamination rate and
their low cell viability, there are limits to their application.
Scars and fibrosis after injury are caused by tissue repair induced
by the proliferation of surrounding fibroblasts, and the
physiological mechanisms and cellular functions of fibroblasts
around the dura may be similar to those around the derma.
Therefore, in the present study, commercially available human
dermal fibroblasts [classic fibroblasts usually applied in the
study of fibrosis (61)] were
purchased as an alternative, as they have better cell viability and
more stable cell passages. The results of the present study could
provide a novel idea in the clinical treatment of postoperative
epidural fibrosis. In addition, previous studies have selected L929
(mouse epithelial fibroblasts) and NIH-3T3 (mouse embryo
fibroblasts) cells to study epidural fibrosis (62,63). However, validation of the results
demonstrated in the present study in other fibroblast cell lines or
the fibroblasts extracted from the epidural site would still be
valuable. In the future, these could drive the directions for
further research.
In conclusion, the present study first illustrated
the positive association between laminin α1and epidural fibrosis,
and confirmed the regulatory effect of laminin α1 on the
proliferation, apoptosis and migration of fibroblasts. Furthermore,
a possible mechanism was revealed; the regulatory effect of laminin
α1 on proliferation, apoptosis and migration of fibroblasts may be
realized via the AKT/mTOR signaling pathway.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL, DZ and GH designed the study and performed most
of the experiments together. MX, YF, LL and JZ established the
animal models, helped to perform the experiments, and contributed
to the reagents, materials, data analysis and figure preparation.
MX and ZY guided and modified the whole study, interpretated the
data, and reviewed and amended the manuscript. PL and MX confirmed
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The purchase and application of human primary
fibroblasts in the present study were reviewed and approved by the
Medical Ethics Committee of Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology [(2021) IEC (approval
no. 554)], and the animal experiment was reviewed and approved by
the Institutional Animal Care and Use Committee of Huazhong
University of Science and Technology [(2021) IACUC (approval no.
2955)].
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was funded by the National Natural Science Fund of
China (grant nos. 81974355 and 82172524) and the Scientific
Research Project of Wuhan Municipal Health Commission (grant no.
WX20Q16).
References
|
1
|
Kong L, Shang XF, Zhang WZ, Duan LQ, Yu Y,
Ni WJ and Huang Y: Percutaneous endoscopic lumbar discectomy and
microsurgical laminotomy: A prospective, randomized controlled
trial of patients with lumbar disc herniation and lateral recess
stenosis. Orthopade. 48:157–164. 2019. View Article : Google Scholar
|
|
2
|
Sun F, Liang Q, Yan M, Wang H, Liu Z, Li
F, Dong J and Liu T: Unilateral laminectomy by endoscopy in central
lumbar canal spinal stenosis: Technical note and early outcomes.
Spine (Phila Pa 1976). 45:E871–E877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu P, Chen H, Yan L and Sun Y: Laminin α5
modulates fibroblast proliferation in epidural fibrosis through the
PI3K/AKT/mTOR signaling pathway. Mol Med Rep. 21:1491–1500.
2020.PubMed/NCBI
|
|
4
|
Guyer RD, Patterson M and Ohnmeiss DD:
Failed back surgery syndrome: Diagnostic evaluation. J Am Acad
Orthop Surg. 14:534–543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avellanal M, Diaz-Reganon G, Orts A,
Gonzalez-Montero L and Riquelme I: Transforaminal epiduroscopy in
patients with failed back surgery syndrome. Pain Physician.
22:89–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogerson A, Aidlen J and Jenis LG:
Persistent radiculopathy after surgical treatment for lumbar disc
herniation: Causes and treatment options. Int Orthop. 43:969–973.
2019. View Article : Google Scholar
|
|
7
|
Sun Y, Zhao S, Li X, Yan L, Wang J, Wang
D, Chen H, Dai J and He J: Local application of rapamycin reduces
epidural fibrosis after laminectomy via inhibiting fibroblast
proliferation and prompting apoptosis. J Orthop Surg Res.
11:582016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai J, Li X, Yan L, Chen H, He J, Wang S,
Wang J and Sun Y: The effect of suramin on inhibiting fibroblast
proliferation and preventing epidural fibrosis after laminectomy in
rats. J Orthop Surg Res. 11:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan Q, Chen H, Xiong G, Jiao R, Liu Y, Li
X, Sun Y, Wang J and Yan L: Artesunate protects against
surgery-induced knee arthrofibrosis by activating beclin-1-mediated
autophagy via inhibition of mTOR signaling. Eur J Pharmacol.
854:149–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kikuchi K, Setoyama K, Terashi T, Sumizono
M, Tancharoen S, Otsuka S, Takada S, Nakanishi K, Ueda K, Sakakima
H, et al: Application of a novel anti-adhesive membrane, E8002, in
a rat laminectomy model. Int J Mol Sci. 19:15132018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Yan L, Wang J, Sun Y, Li X, Zhao
S, Wang D, Zhu G and Liang Y: Methotrexate prevents epidural
fibrosis through endoplasmic reticulum stress signalling pathway.
Eur J Pharmacol. 796:131–138. 2017. View Article : Google Scholar
|
|
12
|
Zeng L, Sun Y, Li X, Wang J and Yan L:
10-Hydroxycamptothecin induces apoptosis in human fibroblasts by
regulating miRNA-23b-3p expression. Mol Med Rep. 19:2680–2686.
2019.PubMed/NCBI
|
|
13
|
Aumailley M, Bruckner-Tuderman L, Carter
WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester
E, Jones JC, et al: A simplified laminin nomenclature. Matrix Biol.
24:326–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Durbeej M: Laminins. Cell Tissue Res.
339:259–268. 2010. View Article : Google Scholar
|
|
15
|
Atsuta I, Yamaza T, Yoshinari M, Goto T,
Kido MA, Kagiya T, Mino S, Shimono M and Tanaka T: Ultrastructural
localization of laminin-5 (gamma2 chain) in the rat peri-implant
oral mucosa around a titanium-dental implant by immuno-electron
microscopy. Biomaterials. 26:6280–6287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Wang TL, Toh WS and Pei M: The role
of laminins in cartilaginous tissues: From development to
regeneration. Eur Cell Mater. 34:40–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yurchenco PD: Basement membranes: Cell
scaffoldings and signaling platforms. Cold Spring Harb Perspect
Biol. 3:a0049112011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petz M, Them NC, Huber H and Mikulits W:
PDGF enhances IRES-mediated translation of Laminin B1 by
cytoplasmic accumulation of La during epithelial to mesenchymal
transition. Nucleic Acids Res. 40:9738–9749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petz M, Them NC, Huber H, Beug H and
Mikulits W: La enhances IRES-mediated translation of laminin B1
during malignant epithelial to mesenchymal transition. Nucleic
Acids Res. 40:290–302. 2012. View Article : Google Scholar :
|
|
20
|
Domogatskaya A, Rodin S and Tryggvason K:
Functional diversity of laminins. Annu Rev Cell Dev Biol.
28:523–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishiuchi R, Takagi J, Hayashi M, Ido H,
Yagi Y, Sanzen N, Tsuji T, Yamada M and Sekiguchi K: Ligand-binding
specificities of laminin-binding integrins: A comprehensive survey
of laminin-integrin interactions using recombinant alpha3beta1,
alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol.
25:189–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savino W, Mendes-da-Cruz DA, Golbert DC,
Riederer I and Cotta-de-Almeida V: Laminin-mediated interactions in
thymocyte migration and development. Front Immunol. 6:5792015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laperle A, Hsiao C, Lampe M, Mortier J,
Saha K, Palecek SP and Masters KS: α-5 Laminin synthesized by human
pluripotent stem cells promotes self-renewal. Stem Cell Reports.
5:195–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Department of Science and Technology of
Hubei Province: Regulations of Laboratory Animals of Hubei
Province. pp. 1–9. 2005, https://kjt.hubei.gov.cn/kjdt/ztzl/fzxczl/pfxcc/kjlflfg/202008/t20200826_2837541.shtml.
Accessed January 1, 2021.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Han G, Nguyen LN, Macherla C, Chi Y,
Friedman JM, Nosanchuk JD and Martinez LR: Nitric oxide-releasing
nanoparticles accelerate wound healing by promoting fibroblast
migration and collagen deposition. Am J Pathol. 180:1465–1473.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang M, Li J, Zhang S, You Y, Zhu X, Xiang
H, Yan L, Zhao F and Li Y: Effects of titanium dioxide
nanoparticles on cell growth and migration of A549 cells under
simulated microgravity. Nanomaterials (Basel). 12:18792022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar
|
|
29
|
Cabral-Pacheco GA, Garza-Veloz I,
Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA,
Guerrero-Rodriguez JF, Martinez-Avila N and Martinez-Fierro ML: The
roles of matrix metalloproteinases and their inhibitors in human
diseases. Int J Mol Sci. 21:97392020. View Article : Google Scholar :
|
|
30
|
Atkinson JJ and Senior RM: Matrix
metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol.
28:12–24. 2003. View Article : Google Scholar
|
|
31
|
Felsen CN, Savariar EN, Whitney M and
Tsien RY: Detection and monitoring of localized matrix
metalloproteinase upregulation in a murine model of asthma. Am J
Physiol Lung Cell Mol Physiol. 306:L764–L774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lam L, Hu X, Aktary Z, Andrews DW and
Pasdar M: Tamoxifen and ICI 182,780 increase Bcl-2 levels and
inhibit growth of breast carcinoma cells by modulating PI3K/AKT,
ERK and IGF-1R pathways independent of ERalpha. Breast Cancer Res
Treat. 118:605–621. 2009. View Article : Google Scholar
|
|
34
|
Wang S, Li X, Yan L, Nie Q, Dai J, Chen H,
Wang J and Sun Y: Tamoxifen inhibits fibroblast proliferation and
prevents epidural fibrosis by regulating the AKT pathway in rats.
Biochem Biophys Res Commun. 497:937–942. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng W, Qian Y, Chen S, Ruan H and Fan C:
Rapamycin protects against peritendinous fibrosis through
activation of autophagy. Front Pharmacol. 9:4022018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aumailley M: The laminin family. Cell Adh
Migr. 7:48–55. 2013. View Article : Google Scholar :
|
|
37
|
Simon-Assmann P: The laminin family:
Founding members of the basement membrane. Cell Adh Migr. 7:44–47.
2013. View Article : Google Scholar :
|
|
38
|
Pozzi A, Yurchenco PD and Iozzo RV: The
nature and biology of basement membranes. Matrix Biol. 57-58:1–11.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hynes RO: The evolution of metazoan
extracellular matrix. J Cell Biol. 196:671–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kurek M, Åkesson E, Yoshihara M, Oliver E,
Cui Y, Becker M, Alves-Lopes JP, Bjarnason R, Romerius P, Sundin M,
et al: Spermatogonia loss correlates with LAMA 1 expression in
human prepubertal testes stored for fertility preservation. Cells.
10:2412021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayashi H, Horinokita I, Yamada Y, Hamada
K, Takagi N and Nomizu M: Effects of laminin-111 peptide coatings
on rat neural stem/progenitor cell culture. Exp Cell Res.
400:1124402021. View Article : Google Scholar
|
|
42
|
Truong AT, Hamada K, Yamada Y, Guo H,
Kikkawa Y, Okamoto CT, MacKay JA and Nomizu M: Evaluation of
extracellular matrix mimetic laminin bioactive peptide and
elastin-like polypeptide. FASEB J. 34:6729–6740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carney KR, Bryan CD, Gordon HB and Kwan
KM: LongAxis: A MATLAB-based program for 3D quantitative analysis
of epithelial cell shape and orientation. Dev Biol. 458:1–11. 2020.
View Article : Google Scholar :
|
|
44
|
Lee CM, Cho SJ, Cho WK, Park JW, Lee JH,
Choi AM, Rosas IO, Zheng M, Peltz G, Lee CG and Elias JA: Laminin
α1 is a genetic modifier of TGF-β1-stimulated pulmonary fibrosis.
JCI Insight. 3:e995742018. View Article : Google Scholar
|
|
45
|
Limandjaja GC, van den Broek LJ, Breetveld
M, Waaijman T, Monstrey S, de Boer EM, Scheper RJ, Niessen FB and
Gibbs S: Characterization of in vitro reconstructed human
normotrophic, hypertrophic, and keloid scar models. Tissue Eng Part
C Methods. 24:242–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nieto N and Cederbaum AI: Increased
Sp1-dependent transactivation of the LAMgamma 1 promoter in hepatic
stellate cells co-cultured with HepG2 cells overexpressing
cytochrome P450 2E1. J Biol Chem. 278:15360–15372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Della TE, Rigamonti E, Perugino C,
Baghai-Sain S, Sun N, Kaneko N, Maehara T, Rovati L, Ponzoni M,
Milani R, et al: B lymphocytes directly contribute to tissue
fibrosis in patients with IgG4-related disease. J Allergy Clin
Immunol. 145:968–981 e14. 2020. View Article : Google Scholar
|
|
48
|
Adusei KM, Ngo TB and Sadtler K: T
lymphocytes as critical mediators in tissue regeneration, fibrosis,
and the foreign body response. Acta Biomater. 133:17–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Giannandrea M and Parks WC: Diverse
functions of matrix metalloproteinases during fibrosis. Dis Model
Mech. 7:193–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Webb AH, Gao BT, Goldsmith ZK, Irvine AS,
Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC,
et al: Inhibition of MMP-2 and MMP-9 decreases cellular migration,
and angiogenesis in in vitro models of retinoblastoma. BMC Cancer.
17:4342017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou PL, Wu Z, Zhang W, Xu M, Ren J, Zhang
Q, Sun Z and Han X: Circular RNA hsa_circ_0000277 sequesters
miR-4766-5p to upregulate LAMA1 and promote esophageal carcinoma
progression. Cell Death Dis. 12:6762021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen J, Wu F, Shi Y, Yang D, Xu M, Lai Y
and Liu Y: Identification of key candidate genes involved in
melanoma metastasis. Mol Med Rep. 20:903–914. 2019.PubMed/NCBI
|
|
53
|
Mammadova-Bach E, Rupp T, Spenlé C, Jivkov
I, Shankaranarayanan P, Klein A, Pisarsky L, Méchine-Neuville A,
Cremel G, Kedinger M, et al: Laminin α1 orchestrates VEGFA
functions in the ecosystem of colorectal carcinoma. Biol Cell.
110:178–195. 2018. View Article : Google Scholar
|
|
54
|
Heng C, Lefebvre O, Klein A, Edwards MM,
Simon-Assmann P, Orend G and Bagnard D: Functional role of laminin
α1 chain during cerebellum development. Cell Adh Migr. 5:480–489.
2011. View Article : Google Scholar
|
|
55
|
Edwards MM, McLeod DS, Grebe R, Heng C,
Lefebvre O and Lutty GA: Lama1 mutations lead to vitreoretinal
blood vessel formation, persistence of fetal vasculature, and
epiretinal membrane formation in mice. BMC Dev Biol. 11:602011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang L, Cao N, Miao Y, Dai Y and Wei Z:
Morin acts as a USP7 inhibitor to hold back the migration of
rheumatoid arthritis fibroblast-like synoviocytes in a
'prickle1-mTORC2' dependent manner. Mol Nutr Food Res.
65:e21003672021. View Article : Google Scholar
|
|
57
|
Wang L, Wu T, Si C, Wang H, Yue K, Shang
S, Li X, Chen Y and Guan H: Danlou tablet activates autophagy of
vascular adventitial fibroblasts through PI3K/Akt/mTOR to protect
cells from damage caused by atherosclerosis. Front Pharmacol.
12:7305252021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lu Y, Zhong W, Liu Y, Chen W, Zhang J,
Zeng Z, Huang H, Qiao Y, Wan X, Meng X, et al: Anti-PD-L1 antibody
alleviates pulmonary fibrosis by inducing autophagy via inhibition
of the PI3K/Akt/mTOR pathway. Int Immunopharmacol. 104:1085042022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang S, Li X, Yan L, Chen H, Wang J and
Sun Y: Upregulation of P27Kip1 by mitomycin C induces
fibroblast apoptosis and reduces epidural fibrosis. Int J Clin Exp
Pathol. 10:11779–11788. 2017.
|
|
60
|
Dai J, Sun Y, Yan L, Wang J, Li X and He
J: Upregulation of NOXA by 10-Hydroxycamptothecin plays a key role
in inducing fibroblasts apoptosis and reducing epidural fibrosis.
PeerJ. 5:e28582017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tang J, Xu XY, Luo BL, Yang L, Zhang XL,
Sun YD, Hou ZQ and Yao G: Potential role of lnc-PTGS2 in fibrosis
progression after laminectomy via targeting EGR1. J Biol Regul
Homeost Agents. 34:2237–2244. 2020.PubMed/NCBI
|
|
62
|
Shi R, Huang Y, Zhang J, Wu C, Gong M,
Tian W and Zhang L: Effective delivery of mitomycin-C and meloxicam
by double-layer electrospun membranes for the prevention of
epidural adhesions. J Biomed Mater Res B Appl Biomater.
108:353–366. 2020. View Article : Google Scholar
|
|
63
|
Song Z, Wu T, Sun J, Wang H, Hua F,
Nicolas YSM, Kc R, Chen K, Jin Z, Liu J and Zhang M: Metformin
attenuates post-epidural fibrosis by inhibiting the TGF-β1/Smad3
and HMGB1/TLR4 signaling pathways. J Cell Mol Med. 25:3272–3283.
2021. View Article : Google Scholar : PubMed/NCBI
|



















