Introduction
Apoptosis is the active process of endogenous
programmed cell death, which plays an important role in
developmental processes, maintenance of homeostasis, and
elimination of damaged cells. Apoptosis is a tightly regulated
process characterized by a series of distinct morphological and
biochemical alterations to cells, including plasma membrane
blebbing, cell shrinkage, cell surface expression of
phosphatidylserine, depolarization of mitochondria, chromatin
condensation, and DNA fragmentation. Many gene products have been
demonstrated as critical in regulation of apoptosis. In general,
apoptosis can be initiated through two well-recognized pathways in
cells; the death receptor-mediated (extrinsic) pathway and the
mitochondria-dependent (intrinsic) pathway (1,2). In
the former case, plasma membrane death receptors are involved and
the apoptosis signal is provided by ligation between ligands and
cell surface death receptors, and activation of caspase-8, which
activates downstream effector caspases (−3, −6, and/or −7). The
mitochondrion-mediated intrinsic pathway begins with disruption of
mitochondrial membrane potential (MMP,
ΔΨm) and release of apoptogenic proteins,
such as cytochrome c, into the cytosol. Once in the cytosol,
cytochrome c can activate caspase-9, which in turn cleaves
and activates caspase-3. Thus, caspases, a group of cysteine
proteases, play key roles in both apoptotic pathways. Caspases are
synthesized as proenzymes, which are activated by cleavage of the
prodomain at a specific aspartic acid cleaving site. Caspase
activation is often regulated by various cellular factors,
including members of the Bcl-2 family and/or inhibitor of apoptosis
(IAP) family proteins (3,4). Although these pathways act
independently to initiate apoptosis, a delicate balance and
cross-talk between the extrinsic and intrinsic pathways occurs in
many cell types. However, most cancer cells block apoptosis, which
allows for survival of malignant cells despite genetic and
morphologic transformations. Thus, induction of apoptosis in tumor
cells has been shown to be the most common anti-cancer mechanism
targeted by many cancer therapies (5,6).
Therefore, there is a need to identify potential therapeutic
anti-tumor agents with potent and cancer cell selective apoptotic
effects.
Metastasis is a sequential multi-step process, which
ultimately leads to outgrowth of the cancer in a different organ
from which it had originated. Metastasis is a major barrier to
treatment of cancer and a single event that results in the death of
most patients with cancer. This process involves the following
steps: invasion of adjacent tissues, intravasation, transport of
cancer cells through the circulatory system, arrest at a secondary
site, and extravasation and growth in a secondary organ (7–9).
Therefore, inhibition of tumor cell migration and invasion are
important mechanisms in the anti-metastatic properties of
anti-cancer drugs. Recently, substantial data have indicated that
inverted expression of matrix metalloproteinases (MMPs) and tissue
inhibitors of metalloproteinases (TIMPs) suggest that they function
as key regulators in cancer progression, invasion, and metastasis.
MMPs, a family of zinc-dependent endopeptidases, are known to
process a broad spectrum of cell surface molecules and to function
in several important biological processes. They are collectively
capable of cleavage of virtually all extracellular matrix (ECM)
substrates, and degradation of matrix is a key event in
progression, invasion, and metastasis of potentially malignant and
malignant lesions (10,11). Among various MMPs, MMP-2 and MMP-9
appear to play an important role in tumor invasion and metastasis
and are highly expressed in epithelial cancer cells, including lung
carcinoma cells (12–14). On the other hand, TIMPs are
naturally occurring inhibitors of MMPs, which inhibit catalytic
activity of MMPs through binding to activated MMPs and control of
breakdown of ECM (14,15). TIMPs can also inhibit
proliferation, invasion, and metastasis of malignant cells.
Disturbance in balance of MMPs and TIMPs is found in various
pathologic conditions, including cancer (16). Therefore, balance between MMPs and
TIMPs plays a vital role in maintaining the integrity of healthy
tissues, and MMP inhibitors, as well as TIMP activators, are
expected to be useful chemo-therapeutic agents for treatment of
malignant cancer.
Amphibian skin extract has been used as a
traditional Chinese medicine for centuries for alleviation of human
suffering (17). Toads,
particularly members of the genus Bufo, have been identified as a
particularly useful and readily available source of granular gland
secretions, which commonly contain biogenic amines, bufodienolides,
alkaloids and steroids, and peptides and proteins (18,19).
Chan Su, also called toad venom, is dried white venom prepared from
skin secretions of giant toads, such as Bufo bufo
gargarizans Cantor and B. melsanostictus Schneider. It
is widely used in clinics for treatment of various cancers and
heart failure (20–22). Chan Su has been applied in numerous
clinical situations; however, the bioactive compounds in Chan Su
are not fully known. Until recently, only a few studies have
reported on its pharmacological effects, and bufadienolides,
including bufalin, are generally agreed upon to be the major
bioactive components (19,23). Several studies have demonstrated
the potency of Chan Su and its major components in treatment of
various cultured human cancer cells by induction of cell cycle
arrest and apoptosis (23–28). The significance of Chan Su in
induction of apoptosis against human bladder carcinoma cells, which
was mediated by modulation of Bcl-2 family proteins and proteolytic
activation of caspases, has recently been reported (29). The apoptotic effects of Chan Su
were also associated with specific inhibition of cyclooxygenase-2
expression and prostaglandin E2 production (29). The effects of extract of Chan Su
induced apoptosis in non-small cell lung cancer (NSCLC) A549 cells
accompanied by modulation of the death receptor system, Bcl-2
family members, mitochondrial dysfunction, and activation of
caspases have been demonstrated (30). However, the precise anti-cancer
effects of Chan Su in human malignant cells are largely
unknown.
The present study attempts to elucidate the
anti-cancer potential of the whole skin of Venenum bufonis
(SVB) in the NSCLC A549 cell line and the underlying intracellular
signal transduction pathways involved in regulation of apoptosis
and metastasis. Results of this study demonstrated that SVB induces
apoptosis of A549 cells through a signaling cascade of death
receptor-mediated extrinsic and mitochondria-mediated intrinsic
caspase pathways. Our data also indicated that SVB inhibits cell
motility and invasion of A549 cells through inhibition of the
activities of MMPs, while concurrently inducing TIMP
expression.
Materials and methods
Cell culture and SVB preparation
The NSCLC A549 cell line was obtained from the
American Type Culture Collection (Rockville, MD, USA) and cultured
in RPMI-1640 medium (Gibco BRL, Gaithersburg, MD, USA) supplemented
with 10% heat-inactivated fetal bovine serum (FBS, Gibco BRL), 2 mM
glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in a
humidified environment with 5% CO2 at 37°C. The whole
skin of V. bufonis (SVB) was obtained from Dunsan Oriental
Hospital (Daejeon, South Korea). For preparation of extracts of
SVB, SVB was pan fried at 90°C for 1 min; 45.4 g of SVB was then
washed with distilled water, and boiled in 1 l water at 80°C for 6
h. Solid particles and aggregates were removed by centrifugation at
3,000 × g for 30 min, followed by lyophilisis of the supernatants.
Finally, 19.8 g lyophilized SVB were obtained and used in this
experiment. The lyophilized extract was stored at −20°C until used
or dissolved to a 100 mg/ml concentration with medium, and the
stock solution was then diluted with medium to the desired
concentration prior to use.
Cell proliferation and viability assay,
and morphological study
For the cell proliferation study, cells were
cultured in the absence and presence of variable concentrations of
SVB for 24 h. Cells were trypsinized, washed with
phosphate-buffered saline (PBS), and viable cells were scored using
a hemo-cytometer through exclusion of trypan blue. Measurement of
cell viability was determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT,
Sigma Chemical Co., St. Louis, MO, USA) assay, which is based on
the conversion of MTT to MTT-formazan by mitochondrial enzymes. For
morphological study, the cells were treated with SVB for 24 h and
directly photographed with an inverted microscope (Carl Zeiss,
Germany).
Nuclear staining with DAPI
For evidence of apoptosis, morphological changes of
nuclei were visualized following DNA staining using the fluorescent
dye 4,6-diamidino-2-phenylindole (DAPI, Sigma). After treatment of
A549 cells with SVB, the cells were harvested, washed in ice-cold
PBS, and fixed with 3.7% paraformaldehyde (Sigma) in PBS for 10 min
at room temperature. Fixed cells were collected using cytospin,
washed with PBS, and stained with DAPI solution for 10 min at room
temperature. The cells were washed two more times with PBS and the
nuclear morphology of the cells was examined using a fluorescence
microscope (Carl Zeiss, Germany).
Flow cytometry analysis for measurement
of sub-G1 phase
After treatment with various concentrations of SVB
for 24 h, the cells were harvested and washed twice with ice-cold
PBS, fixed in ice-cold 70% ethanol, and stored at 4°C. Prior to
analysis, the cells were washed once again with PBS, suspended in 1
ml of a cold propidium iodide (PI, Sigma) solution containing 100
μg/ml RNase A, 50 μg/ml PI, 0.1% (w/v) sodium citrate, and 0.1%
(v/v) NP-40, and further incubated on ice for 30 min in the dark.
DNA content at sub-G1 phase was then determined by flow cytometer
(FACSCaliber, Becton-Dikinson, San Jose, CA, USA) and CellQuest
software was used for determination of the relative DNA content
based on the presence of a red fluorescence (31).
Mitochondrial membrane potential (MMP,
ΔΨm) assay
MMP (ΔΨm) values were
measured using a flow cytometer with a lipophilic cationic probe
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra-ethylbenzimidazolylcarbocyanine
lodide (JC-1, Calbiochem, San Diego, CA, USA). JC-1 is a
ratiometric, dual-emission fluorescent dye that is internalized and
concentrated by respiring mitochondria and can reflect changes in
MMP (ΔΨm) in living cells. There are two
excitation wavelengths, 527 nm (green) for the monomer form and 590
nm (red) for the J-aggregate form. With normal mitochondrial
function, MMP (ΔΨm) is high and the red
fluorescence is predominant. However, when there is mitochondrial
injury, MMP (ΔΨm) is reduced, leading to
an increase in green fluorescence. Quantitation of red and green
fluorescent signals reflects whether mitochondria are damaged. For
this study, cells treated with SVB were trypsinized and the cell
pellets were re-suspended in 500 μl of PBS and incubated with 10 μM
JC-1 for 20 min at 37°C. The cells were subsequently washed once
with cold PBS, suspended in a total volume of 500 μl, and analyzed
using a flow cytometer.
Protein extraction, gel electrophoresis,
and Western blot analysis
Cells were treated with SVB for 24 h and harvested
with ice-cold PBS. Total cell lysates were lysed in an extraction
buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM
ethylenediaminetetra acetic acid, 1% Nonidet P-40, 0.1 mM sodium
orthovanadate, 2 μg/ml leupeptin, and 100 μg/ml
phenylmethylsulfonyl fluoride]. A Bio-Rad protein assay kit
(Bio-Rad, Hercules, CA, USA) was used for determination of protein
concentration. For Western blot analysis, proteins (~30–50 μg) were
separated by ~8–10% sodium dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis and then electrotransferred to a nitrocellulose
membrane (Schleicher & Schuell, Keene, NH, USA). Membranes were
blocked with 5% skim milk for 1 h and then subjected to immunoblot
analysis with the appropriate antibodies. Proteins were then
visualized by the enhanced chemiluminescence (ECL) method according
to the recommended procedure (Amersham Co., Arlington Heights, IL,
USA). Primary antibodies were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA) and Calbiochem.
Peroxidase-labeled donkey anti-rabbit immunoglobulin and
peroxidase-labeled sheep anti-mouse immunoglobulin were purchased
from Amersham (32).
Assay of caspase-3, -8 and -9
activity
Enzymatic activity of caspases induced by SVB was
assayed using a colorimetric assay kit according to the
manufacturer’s protocol (R&D Systems, Minneapolis, MN, USA).
Briefly, the cells were lysed in a lysis buffer for 30 min on an
ice bath. The lysed cells were centrifuged at 12,000 g for 10 min,
and 100 μg of the protein was incubated with 50 μl of a reaction
buffer and 5 μl of the colorimetric tetrapeptides, Asp-Glu-Val-Asp
(DEVD)-p-nitroaniline (pNA) for caspase-3, Ile-Glu-Thr-Asp
(IETD)-pNA for caspase-8 and Leu-Glu-His-Asp (LEHD)-pNA for
caspase-9, respectively, at 37°C for 2 h. Optical density of the
reaction mixture was quantified spectrophotometrically at a
wavelength of 405 nm (33).
Wound healing migration assay
Wound healing experiments were conducted in order to
assess the effect of SVB on A549 cell motility. In brief, cells
were grown to confluence on 30-mm cell culture dishes coated with
rat tail collagen (20 μg/ml, BD Biosciences, Bedford, MA, USA), and
then treated for 6 h with vehicle or 2 μg/ml of SVB, which induced
no cytotoxic effects, as shown by the results of the MTT assay. A
scratch was made in the cell layer using a pipette tip. After
washing with PBS, serum-free media (to prevent cell proliferation)
containing either vehicle or SVB was added. In order to monitor
cell movement into the wounded area, photographs of the wounded
area were taken immediately after the scratch was made and 12 and
24 h later.
Matrigel invasion assay
In order to determine the effects of SVB on A549
cell invasiveness, the cells were exposed for 6 h to 2 μg/ml of
SVB, and were evaluated by the Boyden chamber (BD Biosciences)
invasion assay. Briefly, treated cells (50,000) were plated onto
the apical side of Matrigel-coated filters in serum-free medium
containing either vehicle or SVB. Medium containing 20% FBS was
placed in a basolateral chamber as a chemoattractant. After 24 h,
cells on the apical side were wiped off with a Q-tip. Cells on the
bottom of the filter were stained with hematoxylin (Sigma) and
counted (three fields of each triplicate filter) using an inverted
microscope.
RNA extraction and reverse
transcription-PCR
Total RNA was prepared using an RNeasy kit (Qiagen,
La Jolla, CA, USA) and primed with random hexamers for synthesis of
complementary DNA using AMV reverse transcriptase (Amersham Corp.)
according to the manufacturer’s instructions. Polymerase chain
reaction (PCR) was performed in a Mastercycler (Eppendorf, Hamburg,
Germany) with the primers indicated in Table I. Conditions for PCR reactions were
1× (94°C for 3 min), 35× (94°C for 45 sec; 58°C for 45 sec; and
72°C for 1 min) and 1× (72°C for 10 min). Amplification products
obtained by PCR were electrophoretically separated on 1% agarose
gel and visualized by ethidium bromide (EtBr) staining.
 | Table ISequences of the primer pairs
employed in the RT-PCR reactions. |
Table I
Sequences of the primer pairs
employed in the RT-PCR reactions.
| Name | | Sequence of
primers |
|---|
| TIMP-1 | Sense |
5′-TGG-GGA-CAC-CAG-AAG-TCA-AC-3′ |
| Antisense |
5′-TTT-TCA-GAG-CCT-TGG-AGG-AG-3′ |
| TIMP-2 | Sense |
5′-GTC-AGT-GAG-AAG-GAA-GTG-GAC-TCT-3′ |
| Antisense |
5′-ATG-TTC-TTC-TCT-GTG-ACC-CAG-TC-3′ |
| MMP-2 | Sense |
5′-GGC-CCT-GTC-ACT-CCT-GAG-AT-3′ |
| Antisense |
5′-GGC-ATC-CAG-GTT-ATC-GGG-GA-3′ |
| MMP-9 | Sense |
5′-CGG-AGC-ACG-GAG-ACG-GGT-AT3′ |
| Antisense |
5′-TGA-AGG-GGA-AGA-CGC-ACA-GC-3′ |
| GAPDH | Sence |
5′-CGG-AGT-CAA-CGG-ATT-TGG-TCG-TAT-3′ |
| Antisense |
5′-AGC-CTT-CTC-CAT-GGT-GGT-GAA-GAC-3′ |
Gelatin zymographic analysis of secreted
MMPs
After incubation with SVB for 24 h, cell culture
supernatants were collected and centrifuged at 400 × g for 5 min.
The cell-free supernatant was mixed with 2X sample buffer
(Invitrogen) and zymography was performed using precast gels (10%
polyacrylamide and 0.1% gelatin). Following electrophoresis, the
gels were washed twice at room temperature for 30 min in 2.5%
Triton X-100, subsequently washed in buffer containing 50 mM
Tris-HCl, 150 mM NaCl, 5 mM CaCl2, 1 μM
ZnCl2, 0.02% NaN3 at pH 7.5 and incubated in
this buffer at 37°C for 24 h. Thereafter, the gels were stained
with 0.5% (w/v) Coomassie brilliant blue G-250 (Bio-Rad) for 1 h,
then lightly de-stained in methanol:acetic acid:water (3:1:6).
Clear bands appear on the Coomassie stained blue background in
areas of gelatinolytic activity. Gels were scanned and images were
processed by extraction of the blue channel signal, converting it
to black and white and inverting it in order to quantify the
gelatinolytic activities from the integrated optical density.
Statistical analysis
Data are expressed as the means ± SD. Statistical
comparisons were performed using One-way ANOVA followed by a
Fisher’s test. Significant differences between the groups were
determined using an unpaired Student’s t-test. A p<0.05 was
considered significant.
Results
Growth inhibition and apoptosis induction
by SVB in A549 cells
In order to determine whether SVB inhibits cell
viability and proliferation, A549 cells were treated with various
concentrations of SVB and the relative cell proliferation and
viable cell number were then measured by the MTT assay and trypan
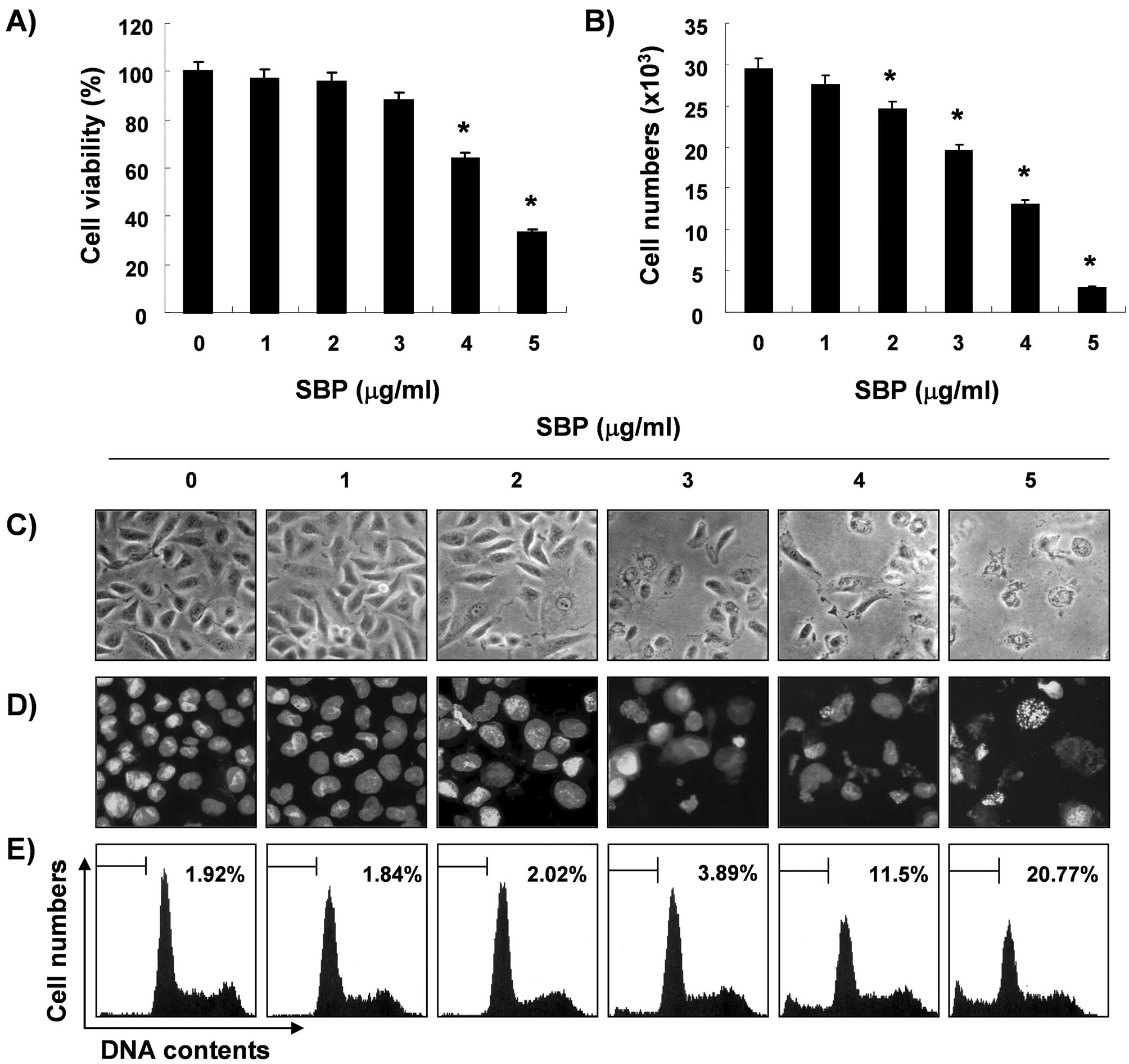
blue exclusion method, respectively. As shown in Fig. 1A and B, treatment with SVB resulted
in a significant reduction in cell proliferation and viability, and
these effects occurred in a concentration-dependent manner. Next,
experiments were performed in order to determine whether the
inhibitory effects of SVB on cell viability and proliferation are
the result of apoptotic cell death. Direct observation using an
inverted microscope showed many distinct morphological changes in
cells treated with SVB, compared with control cells (Fig. 1C). In particular, cell shrinkage,
cytoplasm condensation, and formation of cytoplasmic filaments with
protuberances resulted in more of a spindle shape, membrane
shrinkage, and cell rounding up appeared in a
concentration-dependent manner after SVB treatment. Using
morphological analysis with DAPI staining, nuclei with chromatin
condensation and formation of apoptotic bodies were observed in
cells cultured with SVB in a concentration-dependent manner. In
contrast, very few were observed in the control culture (Fig. 1D). We further analyzed the amount
of sub-G1 DNA, which contained less DNA than G1 cells, in order to
quantify the degree of apoptosis induction of A549 cells by SVB
treatment using a flow cytometer. Flow cytometric analysis
indicated that SVB treatment caused significant increases in
apoptotic cell percentages, as compared with control cells (20% of
apoptotic cells in cells with 5 μg/ml of SVB for 24 h, Fig. 1E). These results demonstrated an
association of the cytotoxic effects observed in response to SVB
with induction of apoptosis in A549 cells, and there was a good
correlation between the extent of apoptosis and inhibition of cell
viability and proliferation.
Effects of SVB on the mitochondrial
pathway
To investigate the association with the
mitochondria-mediated intrinsic pathway in SVB-induced apoptosis,
alterations in MMP (ΔΨm) were determined
using the fluorescent dye, JC-1. As shown in Fig. 2, treatment with SVB resulted in
markedly induced mitochondrial membrane hyperpolarization in a
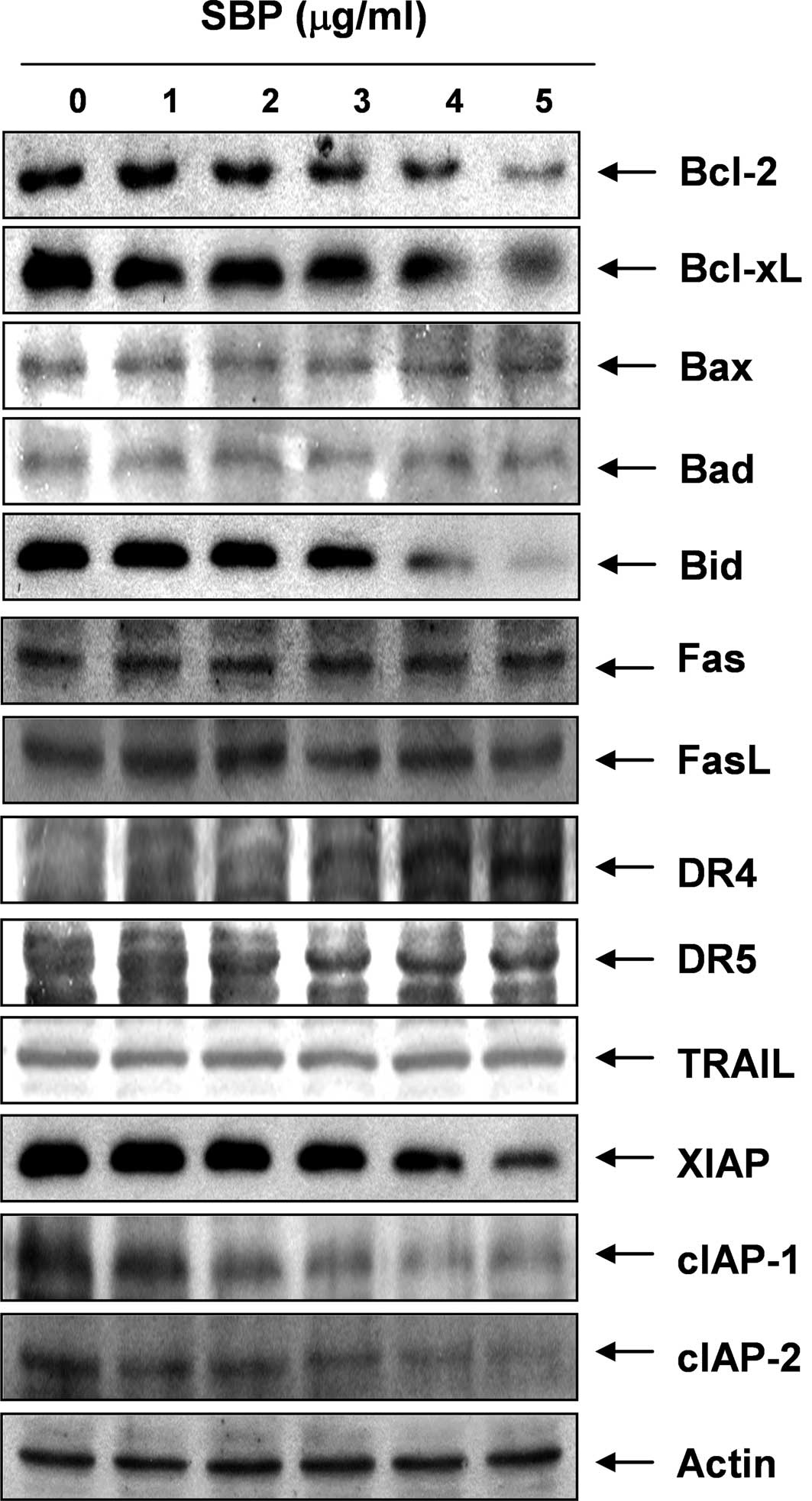
concentration-dependent manner. We next examined the expression
levels of Bcl-2 family proteins directly interacting with
mitochondria. Western blot analyses data revealed that the levels
of Bax and Bad expression, pro-apoptotic proteins, remained
virtually unchanged in response to SVB treatment, whereas the
levels of Bcl-2 and Bcl-xL, anti-apoptotic proteins, were
significantly down-regulated by SVB treatment, suggesting that SVB
alters the Bax (Bad):Bcl-2 and Bax (Bad):Bcl-XL ratio in A549 cells
in a concentration-dependent fashion (Fig. 3). These results suggested that SVB
induced apoptotic cell death in A549 cells through the intrinsic
mitochondrial pathway, as evidenced by an increase in the ratio of
Bax (Bad)/Bcl-2 (Bcl-xL) expression and mitochondrial dysfunction.
In addition, although the truncated form of pro-apoptotic protein
Bid, a BH3-only protein member of the Bcl-2 family, was not
detected, SVB treatment resulted in a decrease of the whole form of
Bid proteins. These data indicate the possibility that both the
extrinsic and intrinsic pathways might be involved in SVB-induced
apoptosis in A549 cells.
Activation of caspases and degradation of
PARP and β-catenin proteins by SVB treatment
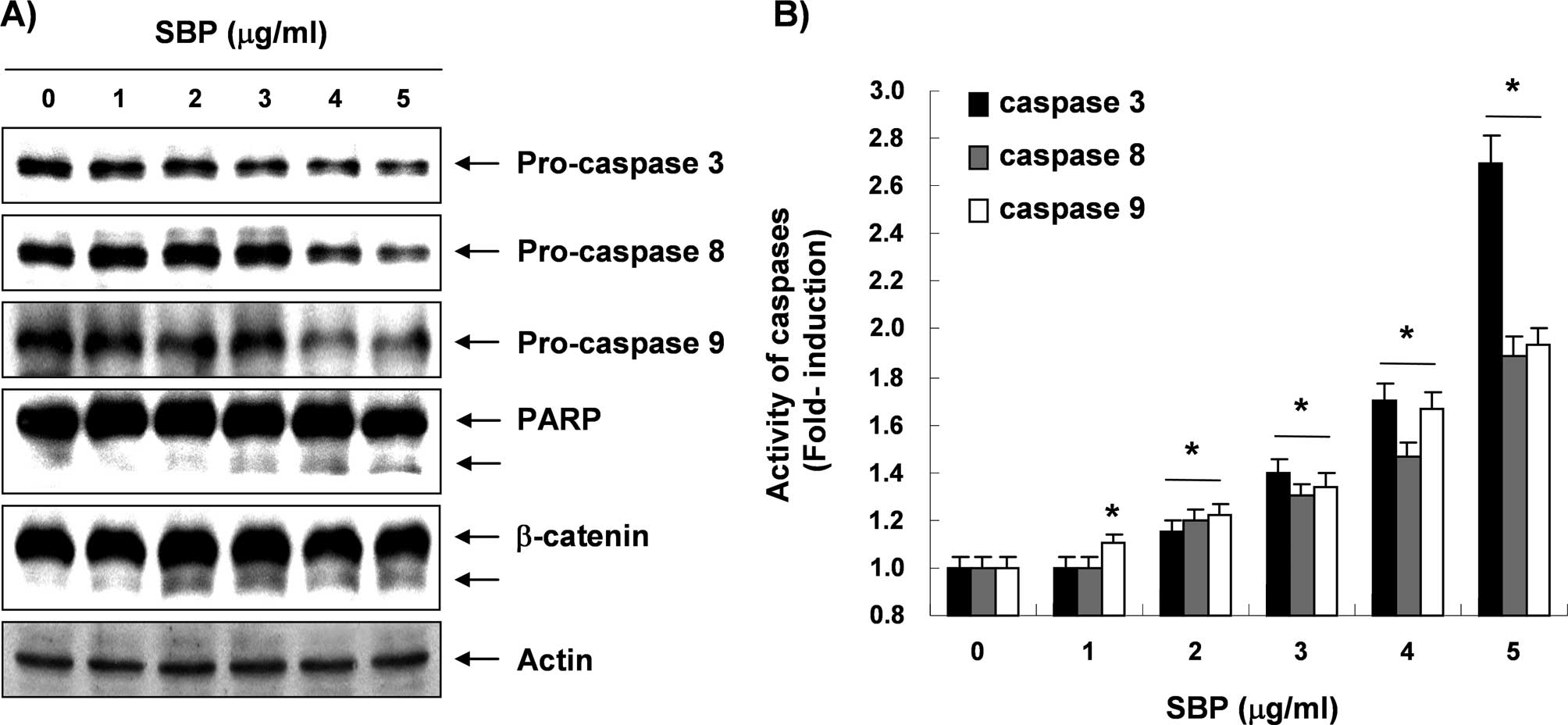
Next, experiments were performed in order to
characterize the role of caspase activation in SVB-mediated
apoptosis in A549 cells. Immunoblotting results showed markedly
decreased levels of pro-caspase-3, -8, and -9 proteins in a
concentration-dependent manner in SVB-treated A549 cells (Fig. 4A). Furthermore, in order to monitor
the enzymatic activity of these enzymes during SVB-induced
apoptosis, in vitro caspase activity was measured following
treatment with SVB using specific fluorogenic peptide substrates
for each caspase. As shown in Fig.
4B, activities of these caspases were significantly increased
in a concentration-dependent fashion, as compared with untreated
control cells. Subsequent Western blot analyses showed progressive
proteolytic cleavage of poly(ADP ribose) polymerase (PARP) and
β-catenin proteins, and accumulation of their cleavage forms, which
are substrate proteins of caspase-3 (34,35)
in A549 cells after SVB treatment (Fig. 4A). The data suggested that
activation of caspases is clearly involved in the SVB-induced
apoptotic pathway.
Effects of SVB on levels of the
death-receptor pathway and IAP family proteins
In order to determine whether the extrinsic
apoptotic pathway was involved in SVB-induced apoptosis, we used
Western blot analyses for measurement of expression of death
receptors and corresponding pro-apoptotic ligands. As shown in
Fig. 3, no significant changes of
Fas, Fas ligand (FasL), necrosis factor-related apoptosis-inducing
ligand (TRAIL), and death receptor (DR) 5 protein levels were noted
in A549 cells treated with SVB; however, SVB induced markedly
increased expression levels of DR4 proteins in a
concentration-dependent manner, suggesting that the extrinsic
apoptotic pathway is also engaged in SVB-induced apoptotic cell
death. Furthermore, expression levels in SVB-treated A549 cells
were also examined in order to determine whether SVB induces A549
cell death through a change in expression of IAP family proteins,
which binds caspases and leads to caspase inactivation for an
anti-apoptotic effect (36). As
shown in Fig. 3, all of the IAP
family proteins examined in this study, including X-linked
inhibitor of apoptosis protein (XIAP), cellular
inhibitor-of-apoptosis protein (cIAP)-1, and cIAP-2, were
concentration-dependently down-regulated in A549 cells treated with
SVB.
Inhibition of SVB-induced apoptosis by
caspase-3 inhibitor
To show that activation of caspase-3 is a key step
in the SVB-induced apoptotic pathway, A549 cells were pretreated
with z-DEVD-fmk (50 μM), a cell-permeable caspase-3 inhibitor, for
1 h, followed by treatment with 5 μg/ml of SVB for 24 h. Blockade
of caspase-3 activity by pretreatment of cells with z-DEVD-fmk
prevented SVB-induced chromatin condensation, growth inhibition,
and the increase in the sub-G1 population (Fig. 5). These results clearly
demonstrated an association of SVB-induced apoptosis with
activation of caspase-3 and that activation of caspase-3 plays an
important role in SVB-induced apoptosis in A549 cells.
Inhibition of cell motility and cell
invasion by SVB in A549 cells
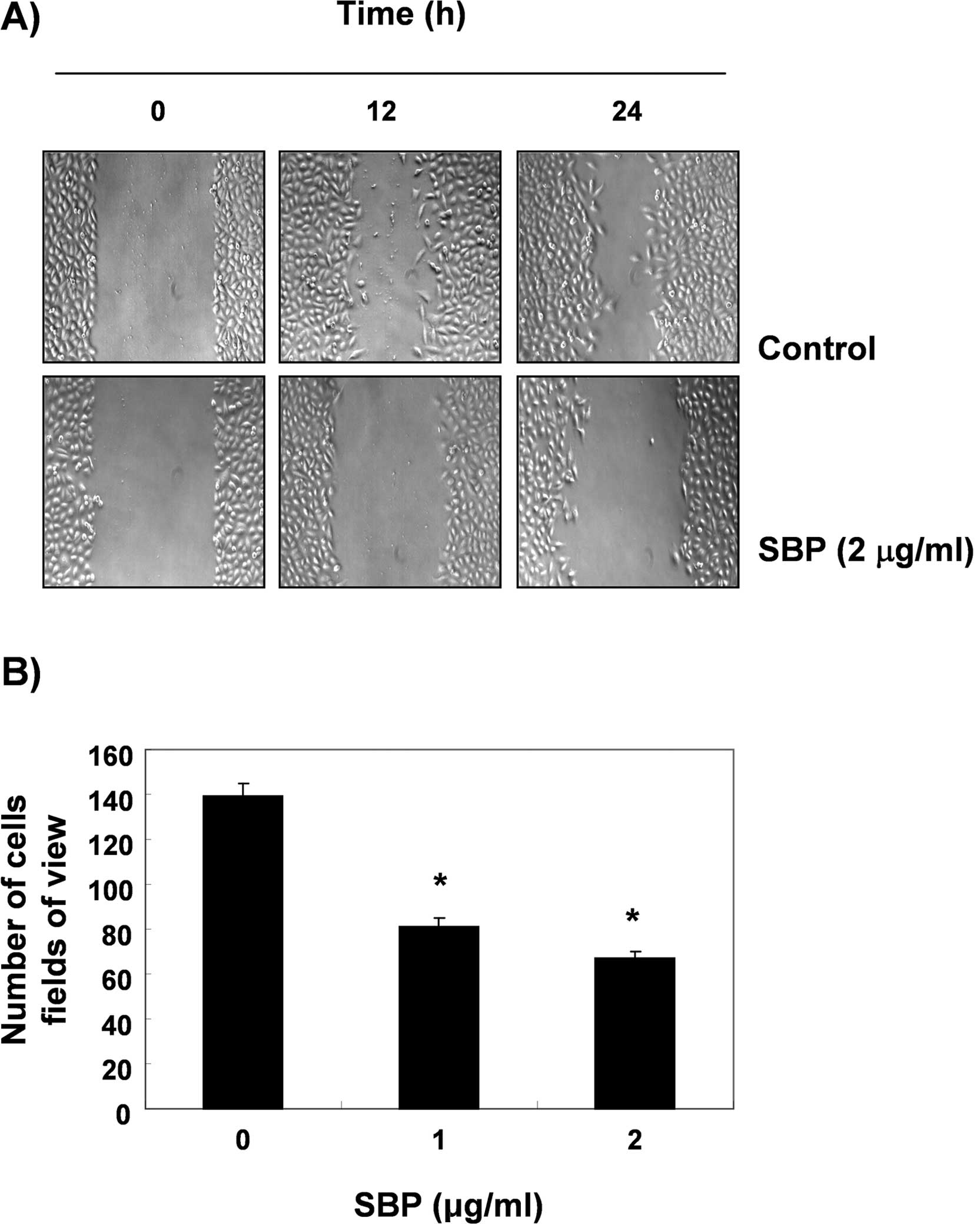
A wound healing experiment was performed. In order
to determine whether SVB inhibits the cell motility of A549 cells.
The results, as shown in Fig. 6A,
demonstrated that 2 μg/ml of SVB, which was not cytotoxic, as shown
by the MTT assay, resulted in time-dependent delay of the cell
motility of A549 cells, as compared with control cells. Using a
Boyden chamber invasion assay, we next examined the question of
whether or not SVB decreases the activity of cell invasion. As
shown in Fig. 6B, SVB treatment
resulted in reduced cell invasion through the Matrigel chamber in a
concentration-dependent manner, suggesting that inhibition of cell
motility by SVB was associated with inhibition of cell invasion in
A549 cells.
Down-regulated activities and expression
of MMPs by SVB in A549 cells
Cell migration plays an important role in the
process of metastasis, and invasion of the basement membrane is
primarily mediated by gelatinase MMPs (10,11);
therefore, we tested the effects of SVB on TIMP and MMP mRNA levels
by RT-PCR. As shown in Fig. 7A,
SVB treatment resulted in slightly increased TIMP-1 and -2 mRNA
levels; however, MMP-2 and -9 levels were decreased in a
concentration-dependent manner. We next investigated the effects of
SVB on protein levels and activities of MMPs using Western blot
analysis and gelatin zymography. Data indicated that activities of
MMP-2 and -9 in A549 cells were decreased by SVB treatment, which
was connected with a concurrent down-regulation of their mRNA and
protein levels and up-regulation of the levels of TIMP-1 and -2
(Fig. 7B and C). These results
suggest an association of the anti-invasive effect of SVB with
increased TIMP-1 and -2 levels, as well as inhibition of MMP-2 and
-9 mRNA, protein expression, and activity in A549 cells.
Discussion
Recent studies have reported that the extracts of
Chan Su or its components can cause cell cycle arrest and apoptosis
in various human cancer cell lines, which suggests that their
growth inhibitory effect occurred through blockade of the G1/S or
G2/M phase, and that these cancer cells do not enter cell cycle
progression and die through apoptosis (24–30,37–40).
However, the mechanisms responsible for the apoptotic and
anti-invasive effects of the whole skin of V. bufonis (SVB) have
yet to be determined. Therefore, the aim of this study was to
determine the capacity of SVB to induce apoptosis and inhibit
invasion, and to identify the biochemical mechanisms in the NSCLC
A549 cell line. Our present results demonstrated that SVB
significantly inhibits A549 cell growth by induction of apoptotic
cell death through modulation of several apoptosis related proteins
and activation of caspase. In addition, SVB exhibited anti-invasive
activity of A549 cells through inhibition of MMPs activity.
Apoptosis can be triggered by various stimuli,
including death receptor-mediated signaling (the death
receptor/extrinsic pathway) and intracellular stress (the
mitochondrial/intrinsic pathway). In addition to the energy source,
mitochondria are known as major regulators of extrinsic as well as
intrinsic apoptosis pathways, and they undergo a series of
consequential changes during apoptosis. Mitochondrial function is
controlled by several factors, such as the Bcl-2 and IAP family
proteins, and activity of caspases (1,2,6).
Members of the Bcl-2 family are significantly involved in
regulation of apoptosis, either as an activator (e.g., Bax, and
Bad) or as an inhibitor (e.g., Bcl-2, and Bcl-xL); therefore, it
has been suggested that the Bax/Bcl-2 ratio is a key factor in
regulation of the apoptotic process (41,42).
Members of the IAP family function through binding to and
inhibition of several caspases (43,44).
Activation of the intrinsic/mitochondrial apoptosis pathway leads
to disruption of MMP (ΔΨm) and release of
apoptogenic proteins, such as cytochrome c, which removes
IAP blockage of caspase activation (45,46).
The present data showed that SVB-induced apoptosis was related to
down-regulation of pro-apoptotic Bcl-2 and Bcl-xL proteins without
alteration of pro-apoptotic Bax and Bad expression (Fig. 3). Furthermore, exposure of A549
cells to SVB resulted in a loss of MMP
(ΔΨm) (Fig.
2) and down-regulation of IAP family proteins, including XIAP,
cIAP-1 and cIAP-2 (Fig. 3). The
data suggest that SVB induced an increase in the Bax (or Bad)/Bcl-2
(or Bcl-xL) ratio and induced mitochondrial dysfunction, leading to
apoptosis in A549 cells.
Cell surface death ligand/receptor systems, such as
Fas/FasL and TRAIL/DRs, are key signaling transduction pathways of
the extrinsic pathway of apoptosis in cells. Binding FasL to Fas
receptors and/or TRAIL to DRs leads to receptor oligomerization and
formation of the death-inducing signaling complex, followed by
activation of caspase-8, and then cleavage of Bid (tBid). tBid can
translocate to mitochondria and bind to Bax, leading to a
conformational change of Bax and to activation of caspase-9, and
concomitant activation of caspase-3 (45,47).
Thus, caspase-3 is the most important executioner of apoptosis.
Significant evidence has indicated that caspase-3 is either
partially or totally responsible for proteolytic cleavage of many
key proteins, including PARP and β-catenin, which are marker
proteins for apoptosis (34,35).
Thus, the levels of death receptor-related proteins, the catalytic
activity of caspases, and the levels of Bid were next examined in
order to further gain mechanical insights into SVB-induced
apoptosis of A549 cells. The data demonstrated that SVB induced an
increase in the levels of DR4, the enzymatic activity of extrinsic
and intrinsic caspase cascades, such as caspase-8 and -9, and
decreased the levels of total Bid expression (Figs. 3 and 4). In addition, SVB-induced apoptosis was
associated with increased activities of caspase-3 in a
concentration-dependent fashion and a concomitant degradation of
PARP and β-catenin, and cleavage fragments of both proteins showed
a gradual increase in SVB-treated A549 cells (Fig. 4). However, under the same
conditions, a specific caspase-3 inhibitor, z-DEVD-fmk, was able to
prevent SVB-induced apoptosis (Fig.
5) showing that activation of caspase-3 contributed to
SVB-induced apoptosis. Thus, the results of this study demonstrate
that SVB triggers apoptosis of A549 cells through activation of the
intrinsic caspase pathway along with the death receptor-mediated
extrinsic pathway.
Metastasis is the process of spread of cancer cells
to tissues and organs beyond where the tumor originated and
formation of new tumors. The process ultimately leads to outgrowth
in a different organ from which it had originated (5,7,8).
Cancer cell invasion and migration are critical steps during
metastasis; therefore, their inhibition is an important mechanism
for anti-cancer drugs. Endopeptidase MMPs play important roles in
cancer invasion and metastasis; therefore, tumor metastasis can be
inhibited by blockade of MMP synthesis and activity (14,48).
Many researchers have reported that the anti-metastatic actions of
natural products, including phytochemical agents, were associated
with a reduction in MMP-2 and MMP-9 activity (49–53).
MMP activity is tightly controlled by transcriptional activation,
by a complex proteolytic activation cascade, and by an endogenous
system of TIMPs. TIMPs inhibit MMPs by formation of 1:1
stoichiometric complexes for regulation of matrix turnover
(15,16). Treatment with <2 μg/ml of SVB,
which was not cytotoxic, resulted in markedly inhibited cell
motility and invasive activity in AGS cells (Fig. 6); therefore, the question of
whether or not the inhibitory effects of SVB were associated with
modulation of TIMP and MMP expression or their activities was
investigated. Our results indicated that SVB induced marked
inhibition of MMP-2 and MMP-9 mRNA and protein levels, as well as
their enzymatic activities in a concentration-dependent manner
(Fig. 7). However, the
transcriptional levels of both TIMP-1 and TIMP-2 showed
concentration-dependent up-regulation in response to SVB treatment,
demonstrating that SVB-induced inhibition of cell motility and
invasion is related to down-regulation of MMP-2 and MMP-9
activities through elevation of TIMP expression. Therefore, the
results suggested that SVB may induce an increase in the TIMPs/MMPs
ratio as a key factor in regulation of the anti-invasive process,
which subsequently blocks degradation of ECM and leads to inhibited
cell invasion.
In conclusion, the present results indicate that SVB
induces significant suppression of proliferation of A549 cells by
induction of apoptosis through activation of the mitochondrial
mediated-intrinsic caspase pathway along with the death
receptor-mediated extrinsic pathway. The present data also revealed
that SVB has an anti-invasive property, which is accompanied by
repression of MMPs activities while concurrently inducing TIMPs
expression. Although it is still unclear whether SVB can induce
apoptosis and inhibit metastasis through other pathways, the
results provide new information on possible mechanisms for the
anti-cancer activity of SVB; Chan Su is a promising candidate for
cancer chemoprevention and/or chemotherapy as well as decreasing
the risk of development of cancer.
Acknowledgments
This work was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education, Science, and Technology
(2010-0001730), and Blue-Bio Industry RIC at Dong-Eui University as
a RIC (08-06-07) program of KIAT under Ministry of Knowledge
Economy, Republic of Korea.
References
|
1
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
2
|
Wesche-Soldato DE, Swan RZ, Chung CS and
Ayala A: The apoptotic pathway as a therapeutic target in sepsis.
Curr Drug Targets. 8:493–500. 2007. View Article : Google Scholar
|
|
3
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 6:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stennicke HR and Salvesen GS: Properties
of the caspases. Biochim Biophys Acta. 1387:17–31. 1998. View Article : Google Scholar
|
|
5
|
Makin G and Dive C: Apoptosis and cancer
chemotherapy. Trends Cell Biol. 11:S22–S26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hart IR, Goode NT and Wilson RE: Molecular
aspects of the metastatic cascade. Biochem Biophys Acta. 989:65–84.
1989.PubMed/NCBI
|
|
8
|
Jiang WG, Puntis MCA and Hallet MB: The
molecular and cellular basis of cancer invasion and metastasis and
its implications for treatment. Br J Surg. 81:1576–1590. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duffy MI, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vihinen P, Ala-aho R and Kähäri VM: Matrix
metalloproteinases as therapeutic targets in cancer. Curr Cancer
Drug Targets. 5:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gibbs DF, Warner RL, Weiss SJ, Johnson KJ
and Varani J: Characterization of matrix metalloproteinases
produced by rat alveolar macrophages. Am J Respir Cell Mol Biol.
20:1136–1144. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cockett MI, Murphy G, Birch ML, O’Connell
JP, Crabbe T, Millican AT, Hart IR and Docherty AJ: Matrix
metalloproteinases and metastatic cancer. Biochem Soc Symp.
63:295–313. 1998.PubMed/NCBI
|
|
14
|
Matrisian LM: The matrix-degrading
metalloproteinases. Bioessays. 14:455–463. 1992. View Article : Google Scholar
|
|
15
|
Uzui H, Harpf A, Liu M, Doherty TM, Shukla
A and Chai N: Increased expression of membrane type 3-matrix
metalloproteinase in human atherosclerotic plaque: role of
activated macrophages and inflammatory cytokines. Circulation.
106:3024–3030. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lambert E, Dasse E, Haye B and Petitfrere
E: TIMPs as multifacial proteins. Crit Rev Oncol Hematol.
49:187–198. 2004. View Article : Google Scholar
|
|
17
|
Chen KK and Kovarikova A: Pharmacology and
toxicology of toad venom. J Pharm Sci. 56:1535–1541. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clarke BT: The natural history of
amphibian skin secretions, their normal functioning and potential
medical applications. Biol Rev. 72:365–379. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steyn PS and van Heerden FR:
Bufadienolides of plants and animal origin. Nat Prod Rep.
15:397–413. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nogawa T, Kamano Y, Yamashita A and Pettit
GR: Isolation and structure of five new cancer cell growth
inhibitory bufadienolides from the Chinese traditional drug Ch’an
Su. J Nat Prod. 64:1148–1152. 2001.PubMed/NCBI
|
|
21
|
Bhuiyan MB, Fant ME and Dasgupta A: Study
on mechanism of action of Chinese medicine Chan Su: dose-dependent
biphasic production of nitric oxide in trophoblastic BeWo cells.
Clin Chim Acta. 330:179–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye M and Guo DA: Analysis of
bufadienolides in the Chinese drug Chan Su by high-performance
liquid chromatography with atmospheric pressure chemical ionization
tandem mass spectrometry. Rapid Commun Mass Spectrom. 19:1881–1892.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang SW, Lin H and Tsai SC: Effects of
methanol extract of Chansu on hypothalamic-pituitary-testis
function in rats. Metabolism. 47:1211–1216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watabe M, Kawazoe N, Masuda Y, Nakajo S
and Nakaya K: Bcl-2 protein inhibits bufalin-induced apoptosis
through inhibition of mitogen-activated protein kinase activation
in human leukemia U937 cells. Cancer Res. 57:3097–3100. 1997.
|
|
25
|
Kawazoe N, Watabe M, Masuda Y, Nakajo S
and Nakaya K: Tiam1 is involved in the regulation of
bufalin-induced apoptosis in human leukemia cells. Oncogene.
18:2413–2421. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giri B and Gomes A, Debnath A, Saha A,
Biswas AK, Dasgupta SC and Gomes A: Antiproliferative, cytotoxic
and apoptogenic activity of Indian toad (Bufo melanostictus,
Schneider) skin extract on U937 and K562 cells. Toxicon.
48:388–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang C, Chen A, Guo M and Yu J: Membrane
dielectric responses of bufalin-induced apoptosis in HL-60 cells
detected by an electrorotation chip. Biotechnol Lett. 29:1307–1313.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ko WS, Park TY, Park C, Kim YH, Yoon HJ,
Lee SY, Hong SH, Choi BT, Lee YT and Choi YH: Induction of
apoptosis by Chan Su, a traditional Chinese medicine, in human
bladder carcinoma T24 cells. Oncol Rep. 14:475–480. 2005.PubMed/NCBI
|
|
30
|
Yun HR, Yoo HS, Shin DY, Hong SH, Kim JH,
Cho CK and Choi YH: Apoptosis induction of human lung carcinoma
cells by Chan Su (Venenum bufonis) through activation of
caspases. J Acupunct Meridian Stud. 2:210–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryu DS, Baek GO, Kim EY, Kim KH and Lee
DS: Effects of polysaccharides derived from Orostachys japonicus on
induction of cell cycle arrest and apoptotic cell death in human
colon cancer cells. BMB Rep. 43:750–755. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yue T, Yin J, Li F, Li D and Du M: High
glucose induces differentiation and adipogenesis in porcine muscle
satellite cells via mTOR. BMB Rep. 43:140–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho SY, Lee JH, Bae HD, Jeong EM, Jang GY,
Kim CW, Shin DM, Jeon JH and Kim IG: Transglutaminase 2 inhibits
apoptosis induced by calcium-overload through down-regulation of
Bax. Exp Mol Med. 42:639–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukuda K: Apoptosis-associated cleavage of
β-catenin in human colon cancer and rat hepatoma cells. Int J
Biochem Cell Biol. 31:519–529. 1999.
|
|
36
|
De Graaf AO, De Witte T and Jansen JH:
Inhibitor of apoptosis proteins: new therapeutic targets in
hematological cancer? Leukemia. 18:1751–1759. 2004.PubMed/NCBI
|
|
37
|
Jing Y, Watabe M, Hashimoto S, Nakajo S
and Nakaya K: Cell cycle arrest and protein kinase modulating
effect of bufalin on human leukemia ML1 cells. Anticancer Res.
14:1193–1198. 1994.PubMed/NCBI
|
|
38
|
Nasu K, Nishida M, Ueda T, Takai N, Bing
S, Narahara H and Miyakawa I: Bufalin induces apoptosis and the
G0/G1 cell cycle arrest of endometriotic stromal cells: a promising
agent for the treatment of endometriosis. Mol Hum Reprod.
11:817–823. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Bufalin induces growth inhibition, cell cycle arrest
and apoptosis in human endometrial and ovarian cancer cells. Int J
Mol Med. 21:637–643. 2008.PubMed/NCBI
|
|
40
|
Choi JH, Choi AY, Yoon H, Choe W, Yoon KS,
Ha J, Yeo EJ and Kang I: Baicalein protects HT22 murine hippocampal
neuronal cells against endoplasmic reticulum stress-induced
apoptosis through inhibition of reactive oxygen species production
and CHOP induction. Exp Mol Med. 42:811–822. 2010. View Article : Google Scholar
|
|
41
|
Murphy AN, Bredesen DE, Cortopassi G, Wang
E and Fiskum G: Bcl-2 potentiates the maximal calcium uptake
capacity of neural cell mitochondria. Proc Natl Acad Sci USA.
93:9893–9898. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thees S, Hubbard GB, Winckler J, Schultz C
and Rami A: Specific alteration of the Bax/Bcl2 ratio and
cytochrome c without execution of apoptosis in the hippocampus of
aged baboons. Restor Neurol Neurosci. 23:1–9. 2005.PubMed/NCBI
|
|
43
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Galluzzi L, Larochette N, Zamzami N and
Kroemer G: Mitochondria as therapeutic targets for cancer
chemotherapy. Oncogene. 25:4812–4830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gupta S, Kass GE, Szegezdi E and Joseph B:
The mitochondrial death pathway: a promising therapeutic target in
diseases. J Cell Mol Med. 13:1004–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Javadov S and Karmazyn M: Mitochondrial
permeability transition pore opening as an endpoint to initiate
cell death and as a putative target for cardioprotection. Cell
Physiol Biochem. 20:1–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mook OR, Frederiks WM and van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
49
|
Hazgui S, Bonnomet A, Nawrocki-Raby B,
Milliot M, Terryn C, Cutrona J, Polette M, Birembaut P and Zahm JM:
Epigallocatechin-3-gallate (EGCG) inhibits the migratory behavior
of tumor bronchial epithelial cells. Respir Res. 9:332008.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takada Y, Andreeff MM and Aggarwal BB:
Indole-3-carbinol suppresses NF-κB and IκBα kinase activation,
causing inhibition of expression of NF-κB-regulated antiapoptotic
and metastatic gene products and enhancement of apoptosis in
myeloid and leukemia cells. Blood. 106:641–649. 2005.
|
|
51
|
Choi YH, Choi WY, Hong SH, Kim SO, Kim GY,
Lee WH and Yoo YH: Anti-invasive activity of sanguinarine through
modulation of tight junctions and matrix metalloproteinase
activities in MDA-MB-231 human breast carcinoma cells. Chem Biol
Interact. 179:185–191. 2009. View Article : Google Scholar
|
|
52
|
Moon DO, Choi YH, Moon SK, Kim WJ and Kim
GY: Butein suppresses the expression of nuclear factor-κB-mediated
matrix metalloproteinase-9 and vascular endothelial growth factor
in prostate cancer cells. Toxicol In Vitro. 24:1927–1934. 2010.
|
|
53
|
Shin DY, Ryu CH, Lee WS, Kim DC, Kim SH,
Hah YS, Lee SJ, Shin SC, Kang HS and Choi YH: Induction of
apoptosis and inhibition of invasion in human hepatoma cells by
anthocyanins from meoru. Ann NY Acad Sci. 1171:137–148. 2009.
View Article : Google Scholar : PubMed/NCBI
|