Introduction
High-risk cutaneous melanoma, a highly aggressive
form of skin cancer, is minimally susceptible to current treatment
strategies for systemic disease and thus these strategies have not
yielded any appreciable survival benefit. Chemotherapeutic
refractoriness of advanced cutaneous melanoma is attributable to
several resistance mechanisms resulting from the outgrowth of
resistant clones, subsequently promoting tumor recurrence and
metastasis. Recently, therapy-resistant clones are implicated as
melanoma initiating cells (MICs) also known as melanoma stem cells
(MSCs). CD133 is now recognized as an important cancer stem
cell-associated marker in cutaneous melanoma.
The existence of cancer cells with stem cell-like
characteristics has been reported in a variety of human
malignancies (1–5) including melanoma (6–10).
The identification and characterization of stem cells in normal
tissue has expedited isolation of cancer stem cells (CSC) and
provided biomarkers for identification of a candidate CSC
population (11). In addition, the
prospective isolation of CSC and comparison to normal stem
cells/progenitors sheds light on the origin, regulation and
association with disease pathogenesis attributable to CSCs
(12). The variability in
frequency of CSCs within the tumor and the existence of
tumor-specific stem cell markers has been a subject of
controversy.
Several reports have implicated the expression of
different markers of melanoma stem cells, including the
self-renewal transcriptional factor, Bmi-1, in primary and
metastatic melanoma. Four subpopulations of cells have been
identified in melanoma tissues, including CD20-positive,
CD133-positive, label-retaining cells and side-population (SP)
cells. These cell populations possess stem-cell characteristics
with defined self-renewal capacity, differentiation potential, and
high tumorigenicity (13). In
addition, ABCB5 chemoresistance mediator (14), CD166 activated leukocyte cell
adhesion molecule (15), and
Nestin intermediate filament protein expressed in the cytoplasm of
intraepithelial stem cells (16),
have also been reported in various stages of cutaneous melanoma
(9,10). Although CD133 has recently been
recognized as a potential stem cell marker capable of identifying a
tumor-initiating population in solid tumors, the consistent
overexpression of CD133 cells in conjunction with other melanoma
stem cell markers among patients with high-risk primary or
recurrent melanoma has not yet been clearly established and no
preferential CD133 ligands or connections with signaling pathways
have been elucidated.
The present study examines relative risk analysis on
clonal dominance of CD133+ MSCs in tissues that could be
applied to early diagnosis, prognosis, or to the development of
more effective strategies have or disease monitoring and treatment
modalities. The expression levels of CD133 were thus assessed in
tissues from patients with or without recurrent disease and
compared with melanoma thickness as a prognostic factor. In
addition, CD133 expression in tissues from patients with primary
melanoma successively progressed to lymph node metastasis and
distant metastasis were compared, utilizing immunohistochemistry
(IHC) and quantitative real-time reverse transcription-PCR
(qRT-PCR).
Materials and methods
Immunohistochemical staining of CD133,
ABCB5, CD166 and Nestin in melanoma tissue sections
Formalin-fixed, paraffin-embedded (FFPE) tissues
were selected from 3 groups of consenting patients, after
Institutional Review Board protocol approval. Group I consisted of
melanoma patients who did not develop disease recurrence or
metastases (n=18). Group II was comprised of melanoma patients who
developed metastatic lesions (n=18). Group III included patients
with disease progression from primary melanoma to lymph node (LN)
metastasis and subsequently to distant metastasis (n=8). Tissue
sections measuring 5 μm were subjected to
immunohistochemical staining using monoclonal antibodies to a
distinct subset of MSCs including CD133/prominin-1 (Abcam), a CSC
and neural stem cell marker; ABCB5 (Genway), a chemoresistance
mediator; CD166 (BD Biosciences), an adhesion molecule; and Nestin
(Abcam); a lineage marker. The tissue sections were immunostained
using a Bond-Max automated staining system (Leica). The reactions
were developed using a biotin-free bond polymer refine detection
kit (Leica) and visualized with 3′3-diaminobenzidine (DAB)
substrate. Immunoexpression of stem cell markers was evaluated with
a Zeiss Axioscope microscope using a semi-quantitative scoring
system for intensity of staining of stem cell markers on tumor
cells. Fisher’s exact test, Odds ratio analysis and relative risk
analysis were performed to determine statistical significance.
Establishment and characterization of
human melanoma cell lines
Human melanoma cell lines were established from
fresh metastatic tumor tissues of consenting patients comprising
two groups. Group A (n=4) consisted of patients (FS-4, FS-5, FS-7
and FS-9) with poor clinical outcomes and short overall survival
(<10 months), and group B (n=4) was comprised of melanoma
patients (FS-11, FS-12, FS-13 and FS-14) with good clinical
outcomes and longer overall survival (24 months). The majority of
cell lines were derived from lymph node metastases.
Single cell suspensions were prepared from freshly
resected tumor tissue specimens by mechanical mincing; no enzymatic
dissociation was used. Viable tumor cells were cultured in Iscove’s
medium supplemented with 10% fetal bovine serum (FBS) and
antibiotics. After overnight incubation at 37°C with 5%
CO2, floating debris was discarded and fresh complete
medium was added. Cultures were fed 2–3 times per week, replacing
about half of the spent medium. Melanoma cell lines were split when
near confluence and sub-cultured at 4×104 viable cells
per cm2 surface area in polystyrene culture flasks.
Cultures were shown to be free of mycoplasma contamination using
the MycoProbe™ mycoplasma detection kit (R&D Systems,
Minneapolis, MN, USA). To insure that each cultured cell line
consisted of melanoma cells, each cell line was stained and
analyzed by flow cytometry for melanoma-specific antigens MART-1,
gp100, TRP75, or melanoma-associated chondroitin sulfate
proteoglycan (MCSP). At least one of these four melanoma antigens
was observed in order to include the cell line in the study. All
cell lines were early passages of less than 20.
Isolation of CD133+ and
CD133− subsets of MSCs from early passages (<20) of
cell cultures of metastatic melanoma
CD133+ or CD133− cells were
isolated from cell culture suspensions per kit instructions using
magnetically labeled CD133 Micro Beads and a MACS®
Column (Miltenyi Biotech, Auburn, CA). To increase the purity, the
positively selected cell fraction containing the CD133+
cells were further separated over a second MACS®
column.
In vivo assessment of CD133+
vs. CD133− tumorigenicity in athymic NCr-nu/nu mouse
xenotransplantation assay
CD133+ or CD133− cells were
cultured in DMEM/F-12 (1:1) plus 1% penicillin/streptomycin, 20
μg/ml each of epidermal growth factor (EGF) and fibroblast
growth factor (FGF), and 1X B-27 supplement (Invitrogen). Culture
plates were coated in 10 mg/ml 2-hydroxyethly methacrylate
(polyHEMA; Sigma, St. Louis, MO) in order to ensure a spheroid
suspension. Flow cytometry was used to confirm the populations as
CD133+ or CD133− using the monospecific mouse
mAb CD133/2 (Miltenyi Biotech). Athymic NCr-nu/nu mice were
purchased from Harlan Laboratories (IN). Purified CD133 cells were
counted using a Coulter Counter (Beckman Coulter Brea, CA) and then
serially diluted. Cells were re-suspended in a mixture of growth
media containing Matrigel (BD, Bedford, MA), and injected
subcutaneously into each hind flank. Animals were each injected
with 2×105 or 1×103 of either
CD133+ or CD133− cells; each group of cells
was injected in replicates of four. Animals were allowed to recover
from anesthesia and monitored daily to check for the presence of
palpable tumors. Tumor growth was measured using a digital
micrometer and calculated using the modified ellipsoid formula (1/2
length x width2) for tumor volume as described
previously (17,18). Tumor volumes were recorded each
week for a period of eight weeks. All animal studies were performed
in compliance of regulations of Georgetown University Animal Care
and Use Committee (GUACUC; Protocol 10-051; expiration 10/18/2013)
Washington DC, USA.
CD133 gene transcripts by qRT-PCR
Total RNA was prepared from 8 melanoma cell lines
established as described above using Trizol (Invitrogen) and an
RNeasy Mini Kit (Qiagen). From 1 μg RNA, cDNA was
synthesized using an iScript™ cDNA synthesis kit (Bio-Rad)
according to the manufacturer’s instructions. Absolute quantitation
of CD133 gene transcripts was performed using qRT-PCR analysis on a
7500 real-time PCR system using the SYBR Green method (Applied
BioSystems). The following human-specific intron spanning primer
pairs for CD133 were used: forward, CATCCACAGATGCTCCTAAGGC;
reverse, GCTTTATGGGAGTCTTGGGTC. Cycle conditions were as follows: 1
cycle at 95°C for 10 min, 40 cycles at 95°C for 15 sec, and 60°C
for 1 min. The specificity of the PCR product was verified by
melting curve analysis. Threshold cycles of primer probes were
normalized to those of 18S rRNA. Absolute values of transcripts
were calculated using the standard curve method.
Results
Immunohistochemical expression of
MSCs
As shown in Fig. 1,
CD133 and CD166 specific mAb stained the membrane as well as the
cytoplasm to a lesser degree, while Nestin and ABCB5 mAbs
predominantly stained cytoplasm of MSCs. Tables I–III summarize the IHC expression levels of
CD133, ABCB5, CD166 and Nestin in tissues derived from patients
with different clinical outcomes.
 | Table I.Immunoexpression of MSC markers in
patients with or without disease recurrence. |
Table I.
Immunoexpression of MSC markers in
patients with or without disease recurrence.
| Disease stage | Immunoexpression of
MSC markers
|
|---|
| CD133 (%) | ABCB5 (%) | CD166 (%) | Nestin (%) |
|---|
| Group I: no
recurrence (n=18) | 5/18 (27) | 8/18 (44) | 10/18 (55) | 16/18 (88) |
| Group II:
recurrence (n=18) | 11/18a(61) | 9/17 (52) | 9/18 (50) | 15/18 (83) |
 | Table III.Expression of MSC markers in patients
with primary melanoma successively progressed to lymph node and
distant organ metastasis. |
Table III.
Expression of MSC markers in patients
with primary melanoma successively progressed to lymph node and
distant organ metastasis.
| Immunoexpression of
melanoma stem cell markers
|
|---|
| Disease stage | CD133 (%) | ABCB5 (%) | CD166 (%) | Nestin (%) |
|---|
| Primary melanoma
(n=8) | 8/8 (100) | 8/8 (100) | 3/8 (37) | 8/8 (100) |
| Lymph node
metastasis (n=8) | 8/8 (100) | 8/8 (100) | 0/8 (0) | 8/8 (100) |
| Distant organ
metastasis (n=6) | 5/6a(83) | 4/6a(66) | 2/6 (33) | 6/6 (100) |
Melanoma patients with disease recurrence
or non-recurrence
Significantly greater expression of CD133 in tissues
was seen in melanoma patients who developed metastatic
lesions/recurrence of the disease, when compared with the group of
melanoma patients who did not develop any metastatic lesions or
recurrence (p<0.02, Fisher’s exact test; Table I). This significant difference was
also confirmed by Odds ratio analysis (p=0.025). Further, relative
risk analysis of these two groups of melanoma patients suggested
CD133-immunopositivity was 2-fold higher in tissues from patients
with recurrence or metastasis. No significant differences in
expression levels of ABCB5, CD166 or Nestin were observed in these
groups.
Primary melanocytic lesions with
different thickness
Comparison of immunoexpression of CD133, ABCB5,
CD166 and Nestin in tissues with different primary lesion
thicknesses revealed greater expression of CD133 and CD166 in
primary melanomas that demonstrated >1.00 mm thickness compared
to those that were less than ≤1.00 mm. However, this difference was
not statistically significant (p>0.05; Table II).
 | Table II.Immunoexpression of MSC markers vs.
primary human melanoma thickness. |
Table II.
Immunoexpression of MSC markers vs.
primary human melanoma thickness.
| Immunoexpression of
melanoma stem cell markers
|
|---|
| Thickness of
primary lesion | CD133 (%) | ABCB5 (%) | CD166 (%) | Nestin (%) |
|---|
| ≤1.00 mm (n=8) | 2/8 (25) | 5/8 (62) | 3/8 (37) | 8/8 (100) |
| >1.00 mm
(n=32) | 16/32a(50) | 15/31 (48) | 18/32 (56) | 27/32 (84) |
Patients with primary melanoma
successively progressed to lymph node metastasis and distant
metastasis
Consistent high levels of CD133 and ABCB5 marker
expression were observed in 100% tissues from patients with primary
melanoma that successively progressed to lymph node metastasis
(100%) and distant metastasis (83% and 66%, respectively; Table III). The levels and incidence of
CD166 expression was low in all stages of disease and also not
altered significantly (p>0.05). Nestin expression levels were
found to be conserved in all tissues of the study groups (100%).
However, no statistical significance (p>0.05) was observed in
the overexpression of CD133 or ABCB5 in this study group.
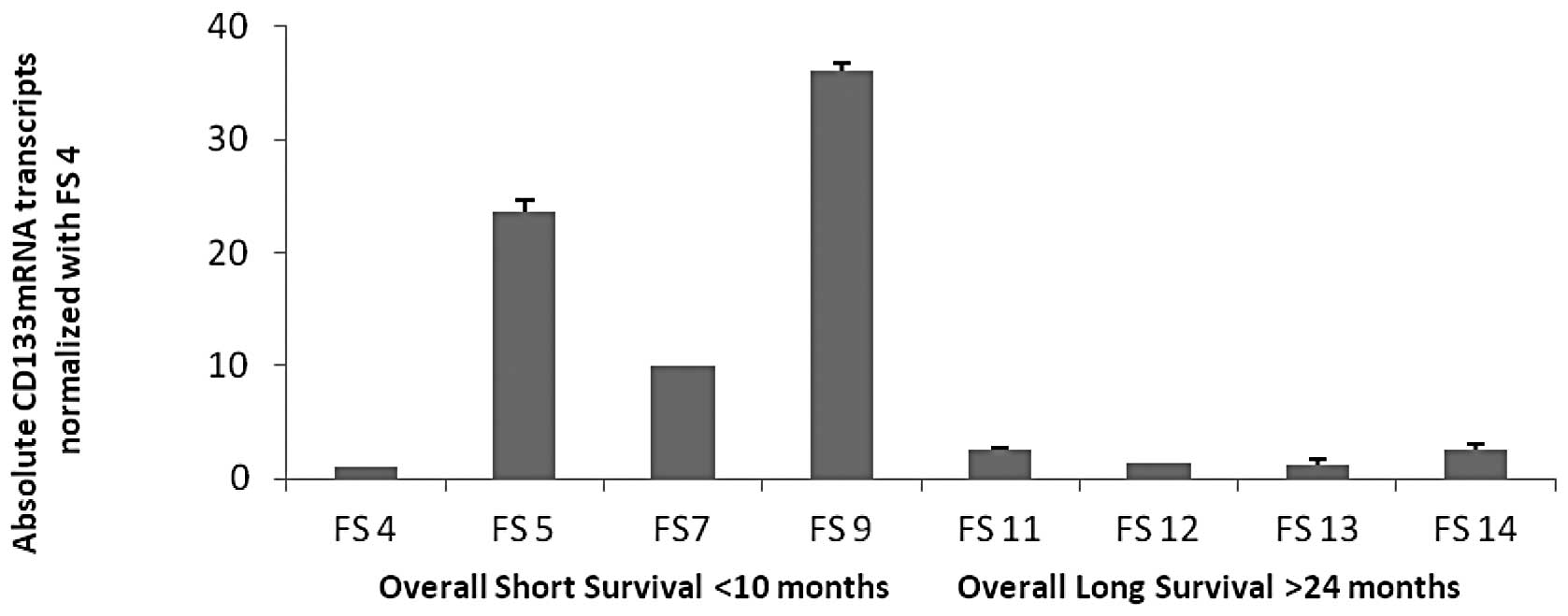
Expression of CD133+ mRNA
transcripts in cell cultures established from tissues of patients
with poor outcome and short overall survival, good outcome and long
overall survival
To investigate the potential of CD133 as a molecular
biomarker of melanoma progression and disease recurrence, we
assessed CD133 mRNA transcript levels by qRT-PCR in low passage
(<20) primary cell lines established from fresh tissues of
patients with either poor outcome and short overall survival, or
from patients with good outcome and long overall survival status.
Using 18S rRNA as a control, CD133 mRNA expression levels in each
individual cell line derived from patients with poor clinical
outcome and short overall survival (FS-4, FS-5, FS-7 and FS-9) were
compared with four cell lines derived from melanoma patients with
good clinical outcome and longer overall survival (FS-11, FS-12,
FS-13, FS-14; Fig. 2).
CD133+ mRNA transcripts were expressed at levels 15–30
times higher in the latter group, suggesting that CD133 transcripts
strongly and negatively correlated with the clinical outcome, and
were thus a potential predictor of poor prognosis in high-risk
melanoma (p<0.04).
In-vivo tumorigenicity of
CD133+ and CD133− phenotypes in
xenotransplantation in athymic NCr-nu/nu mice
To validate the traditional stochastic model of
CD133+ MSCs, we derived two patient cell populations,
either enriched for, or depleted of, cell surface CD133, and found
that each population consistently maintained their CD133 status,
since 97.2% and 95% of CD133+ cells expressed this
marker in passage 2 (Fig. 3A) and
8 (Fig. 3B) as determined by flow
cytometric analysis. Likewise, CD133− cells retained
their phenotype in culture for eight passages. Passage 8
CD133+ or CD 133− cells were then
xenotransplanted into immunodeficient mice. Successful
xenotransplantation with as few as 1,000 CD133+ cells
demonstrated their ability to repopulate and form tumors
(p<0.03) in athymic NCr-nu/nu mice within a short span of
8 weeks. However, tumor formation could not be observed in the mice
injected with matched cell density of CD133− phenotype,
nor with up to 200,000 cells (Fig. 4A
and B).
Discussion
This study has demonstrated, for the first time,
that there exists a clonal dominance of the CD133 stemness marker
within the hierarchy of cells in the cutaneous tissues from
patients that have undergone successive progressive stages of
melanoma, from primary to metastatic lesions. In addition,
overexpression of CD133 correlates with the prognostic marker of
tumor thickness in primary melanoma. A correlation also exists
between the consistent overexpression of CD133 marker (p<0.02)
and CD133 gene transcript levels in cell lines from metastatic
patients with poor clinical outcome and short overall survival
status. These findings suggest that CD133 represents a
hyperpolarized cell population with tumorigenic potential at
metastatic sites and is linked to survival status of the
patient.
Although most CSCs in metastatic colon cancer
(19), non-small cell lung
carcinoma (20,21), glioblastoma (22), oral squamous cell carcinoma
(23) and metastatic melanoma
(7,14) have been detected as rare cell
populations with tumorigenic capacity in vitro and in
vivo, these studies have not been able to define a
phenotypically distinct cell population with tumorigenic potential
in target tissues with different clinical outcome or associated
with the progression of the disease.
It has been widely reported that CSC represent rare
tumor cell populations that possess unique properties making them
important for tumor initiation, growth and metastasis. As per the
CSC hypothesis, tumor initiation is regarded as an exclusive
characteristic of CSCs (24).
However, their distribution is highly variable depending on the
clinical stage and anatomic site of the tumor. Although our
previous study has demonstrated varied frequencies of expression of
melanoma stem cell markers at different stages of the disease and
site of the tumor tissue (10),
our present study clearly delineates clonal dominance of CD133 in
tissues of patients with primary melanoma who had disease
progression or disease recurrence in comparison with primary
melanoma with no recurrence, suggesting that the CD133 subset may
be a determinant of tumor metastasis/tumor relapse. Our in
vivo assessment of a CD133+ subset population
further confirmed that CD133+ MSCs contain self-renewal
and repopulating capabilities.
Although the CD133 cell population co-expresses
other stem cell markers or may represent overlapping markers,
recent reports on metastatic melanoma containing CD133 and
ABCG2-positive cells with enhanced tumorigenic potential further
support our present findings on consistent expression of stemness
marker CD133 in conjunction with ABCB5 in various progressive
stages of cutaneous melanoma (25,26).
Further the proportionately lower incidence of CD133 positive
tissues in distant metastatic melanomas that successively
progressed from primaries, are possibly associated with selective
pressure of a hypoxic niche or impact of novel mechanisms of
dormancy of melanoma stem cells within the post-metastatic tissue
micro-environment.
Our gene quantitation analysis of CD133 in cell
lines from metastatic melanoma patients with poor clinical outcomes
and short overall survival strongly supports our hypothesis that
CD133 is a reliable marker of disease recurrence and an abundance
of CD133 transcripts may play a pivotal role in progression of
human cutaneous melanoma.
Based on our encouraging leads on clonal dominance
of tumorigenic CD133+ phenotypes in tumor metastasis and
tumor recurrence as determined by immunohistochemical expression in
tissues from patients with short overall survival and poor clinical
outcome, as well as gene expression analysis by qRT-PCR, we further
validated our hypothesis by examining their in vivo
tumorigenicity by utilizing xenotransplantation experiments in
immunodeficient mice.
Our xenotransplantation studies with
CD133+ cells in nude mice clearly demonstrated that as
few as 1,000 CD133+ cells are capable of generating
rapidly growing tumors in nude mice within a short period of 8
weeks thus demonstrate positive correlation between enrichment of
more aggressive CD133+ phenotypes and tumor growth. This
further reminds us that even CSCs are susceptible to selection
pressure in aggressive tumors. This observation is a reflection of
its pre-existence in the original metastatic melanoma prior to its
resection from the patient. We believe it is likely that the
metastatic phenotype existed in unrecognized form in the
pre-metastatic lesions in the patients. Our findings sufficiently
justify the basis of identifying patients whose cancers are at
high-risk for metastases even in the absence of clinical evidence
of metastases.
It is still not clear whether melanoma stem cells
are derived from melanocytic stem cells, melanocytic progenitors,
or mature melanocytes. It is assumed that primary CSCs originate
through cell fusion with the cell population and that they have
altered intrinsic/epigenetically unstable mechanisms with an
enhanced metastatic potential and drug resistance. Further, the
pathways and signaling molecules involved in stem cell homeostasis,
de-differentiation, and transformation to melanoma stem cells
remain to be determined. These molecules or pathways associated
with melanoma stem cells would serve as potential candidates for
targeted therapies.
Substantial variation exists on the precise
definition of melanoma stem cells based on the traditional
stochastic model. However, our results uniquely provided a
rationale for the definition of a predictive factor of disease
progression and represent a significant step towards identifying a
more promising target in the clinical management of high-risk
cutaneous melanoma.
Acknowledgements
The authors would like to thank Sharon
Honts and Amy Avergas for their assistance in editing this
manuscript. This study was supported in part by a generous grant
from the Russel Fund, Intramural research funds of Georgetown
University School of Medicine, Washington, DC and funds from the
Intramural Research Program of the National Institute on Aging, NIH
Baltimore, MD, USA.
References
|
1.
|
T ReyaSJ MorrisonMF ClarkeIL WeissmanStem
cells, cancers, and cancer stem
cellsNature414105111200110.1038/3510216711689955
|
|
2.
|
IM Al-HajMS WichaA Benito-HernandezSJ
MorrisonMF ClarkeProspective identification of tumorigenic breast
cancer cellsProc Natl Acad Sci
USA10039833988200310.1073/pnas.0530291100
|
|
3.
|
SK SinghID ClarkeM TerasakiVE BonnC
HawkinsJ SquireDB DirksIdentification of a cancer stem cell in
human brain tumorsCancer Res6358215828200314522905
|
|
4.
|
GG WulfRY WangI KuehneD WeinderF MariniMK
BrennerM AndreeffMA GoodellA leukemic stem cell with intrinsic drug
efflux capacity in acute myeloid
leukemiaBlood9811661173200110.1182/blood.V98.4.116611493466
|
|
5.
|
T KondoT SetoguchT TagaPersistence of a
small subpopulation of cancer stem like cells in the C6 glioma cell
lineProc Natl Acad Sci
USA101781786200410.1073/pnas.030761810014711994
|
|
6.
|
JM GrichnikJA BurchRD SchulteisS ShanJ
LiuTL DarrowCE VervaertHF SeiglerMelanoma, a tumor based on a
mutant stem cell?J Invest
Dermatol126142153200610.1038/sj.jid.570001716417230
|
|
7.
|
D FangTK NguyenK LeishearR FinkoAN KulpS
HotzPA van BelleX XuDE ElderM HerlynA tumorigenic subpopulation
with stem cell properties in melanomasCancer
Res6593289337200510.1158/0008-5472.CAN-05-134316230395
|
|
8.
|
SE ZabierowskiM HerlynMelanoma stem cells:
the dark seed of melanomaJ Clin
Oncol2628902894200810.1200/JCO.2007.15.546518539969
|
|
9.
|
WM KleinBP WuS ZhaoH WuAJP Klein-SzantoSR
TahanIncreased expression of stem cell markers in malignant
melanomaMod Pathol20102107200710.1038/modpathol.380072017143262
|
|
10.
|
BK SharmaV ManglikEG EliasImmunoexpression
of melanoma stem cell markers in various stages of disease in human
melanomaJ Surg
Res163e11e15201010.1016/j.jss.2010.03.04320638684
|
|
11.
|
CY ParkD TsengIL WeismanCancer stem
cells-directed therapies: recent data from laboratory and clinicMol
Ther172219230200910.1038/mt.2008.254
|
|
12.
|
I WeissmanStem cell research: paths to
cancer therapies and regenerative
medicineJAMA29413591366200510.1001/jama.294.11.135916174694
|
|
13.
|
SV ShmelkovR St ChairD LydenAC133/CD133/
Prominin-1Int J Biochem Cell
Biol37715719200510.1016/j.biocel.2004.08.01015694831
|
|
14.
|
T SchattonGF MurphyNY FrankK YamauraAM
Wagga-GasserM GasserQ ZhanS JordanLM DuncanC WeishauptRC
FuhlbriggeTS KupperMH SayeghMH FrankIdentification of cells
initiating human
melanomasNature451345349200810.1038/nature0648918202660
|
|
15.
|
GW SwartPC LunterJW KilsdonkActivated
leukocyte cell adhesion molecule (ALCAM/CD166): signaling at the
divide of melanoma cell clustering and cell migration?Cancer
Metastasis Rev24223236200510.1007/s10555-005-1573-015986133
|
|
16.
|
VA FlorenesR HolmO MyklebostExpression of
the neuroectodermal intermediate filament Nestin in human
melanomasCancer Res5435435619948275467
|
|
17.
|
MM TomaykoCP ReynoldsDetermination of
subcutaneous tumor size in athymic (nude) miceCancer Chemother
Pharmacol24148154198910.1007/BF003002342544306
|
|
18.
|
DM EuhusC HuddMC La ReginaFE JohnsonTumor
measurement in the nude mouseJ Surg
Oncol31229234198610.1002/jso.29303104023724177
|
|
19.
|
IL BotchkinaRA RowehlDE RivadenneiraMS
Karpeh JrH CrawfordA DufourJ JuY WangY LeyfmanGI
BotchkinaPhenotypic subpopulations of metastatic colon cancer stem
cells: genomic analysisCancer Genomics
Proteomics61929200919451087
|
|
20.
|
V TirinoR CamerlingoR FrancoD MalangaA La
RoccaG VigliettoG RoccoG PirozziThe role of CD133 in the
identification and characterization of tumor-initiating cells in
non-small cell lung cancerEur J Cardiothorac
Surg36446453200910.1016/j.ejcts.2009.03.06319464919
|
|
21.
|
G BertoliniL RozP PeregoM TortoretoE
FontanellaL GattiG PratesiA FabbriF AndrianiS TinelliE RozR
CaseriniS Lo VulloT CameriniL MarianiD DeliaE CalabroU PastorinoG
SozziHighly tumorigenic lung cancer CD133+ cells display
stem-like features and are spared by cisplatin treatmentProc Natl
Acad Sci USA1061628116286200919805294
|
|
22.
|
S BaoQ WuRE McLendonY HaoY ShiAB
HjelmelandMW DewhirstDD BignerJN RichGlioma stem cells promote
radioresistance by preferential activation of the DNA damage
responseNature444756760200610.1038/nature0523617051156
|
|
23.
|
SH ChiouCC YuCY HuangSC LinCJ LiuTH TsaiSH
ChouCS ChienHH KuJF LoPositive correlations of Oct-4 and Nanog in
oral cancer stem-like cells and high-grade oral squamous cell
carcinomaClin Cancer
Res1440854095200810.1158/1078-0432.CCR-07-440418593985
|
|
24.
|
MF ClarkeJE DickPB DirksCJ EavesCH
JamiesonDL JonesJ VisvaderIL WeismannGM WahlCancer stem
cells-perspectives on current status and future directions: AACR
Workshop on cancer stem cellsCancer
Res6693399344200610.1158/0008-5472.CAN-06-3126
|
|
25.
|
E MonzaniF FacchettiE GalmozziE CorsiniA
BenettiC CavazzinA GrittiA PiccininiD PorroM SantinamiG InverniciE
ParatiG AlessandariCA La PortaMelanoma contains CD133 and ABCG2
positive cells with enhanced tumourigenic potentialEur J
Cancer43935946200710.1016/j.ejca.2007.01.01717320377
|
|
26.
|
G RappaO FodstadA LoricoThe stem
cell-associated antigen CD133 (Prominin-1) is a molecular
therapeutic target for metastatic melanomaStem
Cells2630083017200810.1634/stemcells.2008-060118802032
|