Introduction
Breast cancer is one of the common malignant tumors
of females. According to recent statistics, breast cancer occupies
the first place among newly diagnosed tumor of American women. In
China, the incidence of breast cancer has increased steadily and
the patients tend to be younger in the past decade. Surgical
resection in combination with systemic chemotherapy in breast
cancer patients is effective, but the effect is not satisfactory.
Gene therapies of cancer are unique in offering the possibility of
treatments that eradicate tumors and metastases without damaging
normal tissues. The system combined with chemotherapy of breast
cancer in vitro and in vivo show that this vector
system treatment with targeting TRE (tetracycline-responsive
element) promoter combined with appropriate chemotherapy are more
effective than either of them alone.
The multisubstrate deoxyribonucleoside kinase of
Drosophila melanogaster (Dm-dNK) has been used as a novel
suicide gene and evaluated for its effectiveness in a number of
cancer cell lines. Based on previous findings, expression of Dm-dNK
in cancer cells increases the sensitivity to several cytotoxic
nucleoside analogs (1–4), rendering the enzyme a candidate for
possible use as a suicide gene with combined gene therapy and
chemotherapy.
Aligned with other deoxyribonucleoside kinases,
homologous amino acid patches are distributed over the entire amino
acid sequence of Dm-dNK, except for the C-terminal part. The last
10 amino acids of the very C-terminal part are unique and not only
influence catalytic efficiency for thymidine but also involve
nuclear localization signals. The role of the C-terminal part of
the enzyme was investigated in detail by construction and
expression of a 10 amino-acid deletion mutant. The Δ10 Dm-dNK
mutant has an even higher catalytic rate for deoxyribonucleosides
compared with the wild-type enzyme (5). To further explore the phosphorylation
capacity of mutagenesis, we designed and constructed mutated Dm-dNK
(Dm-dNKmu) with the last 10 amino acids randomly alternated at the
sites of 244E, 245S, 251S and 252R in the amino-acid sequence. We
wished to study its enzymatic properties in a functional assay
after cellular expression.
Human telomerase reverse transcriptase (hTERT) is
expressed in cells of telomerase activity during immortalization
process, telomerase is activated in most human malignant cells but
cannot be detected in normal somatic cells. Activation of hypoxia
response element (HRE) promoter causes specific changes of solid
tumors, it leads to exogenous gene expression in tumor cells, but
minimal expression in normal cells (6–8).
Accordingly it can be used as the tumor-specific targeting
promoter. hTERT and HRE are added, respectively, to early
transcription units E1A and E1B of conditionally replicating
adenovirus SG500 (9). Like
ONYX-015, SG500 can replicate in p53-disfunctional tumor cells and
kill them. Containing cloning sites, SG500 can insert exogenous
gene to form SG500-gene, while ONYX-015 cannot. So as a carrier,
the SG500 enables exogenous gene targeting transcription in tumor
cells achieving multiple targeting.
BVDU and DFDC is known to enhance cell killing
activity by phosphorylation of enzymes (10,11).
However, our use of mutants combined with them is yet to be studied
in suicide gene therapy, or in an in vivo animal model.
Therefore, we constructed the dNKmu/BVDU or DFDC system to evaluate
the efficacy of our approach in the prevention of breast cancer. In
this study, we took advantage of the conditionally replicative
adenovirus (CRAd) SG500 vector. Conditionally replicating
adenovirus SG500 introduced Dm-dNK mutants (dNKmu) into breast
cancer cell lines MDA-MB-231 and MCF-7, recording tumor cell
killing efficiency after adding different concentrations of
nucleoside and testing killing effect and safety of SG500-dNKmu at
different levels. Then we performed the tumor-bearing mice tests
in vivo to observe the safety and sensitivity of SG500-dNKmu
nucleotide analogs in animal experiments, and investigated the
breast cancer cell killing effect of this treatment system to
provide new ideas for breast cancer treatment.
Materials and methods
Cells and cell cultures
The human breast cancer cell line, MDA-MB-231 and
MCF-7, were both purchased from the Institute of Cell Biology,
Chinese Academy of Sciences (Shanghai, China). MRC-5 and WI-38, the
human embryo lung fibroblast cell line and HEK293, the human
embryonic kidney cell line, were both purchased from American Type
Culture Collection (Manassas, VA, USA). MDA-MB-231 and MCF-7 were
cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand
Island, NY, USA) supplemented with 10% heat-inactivated fetal
bovine serum (FBS, Gibco) and incubated at 37°C, 5% CO2.
WI-38, MRC-5 and HEK293 were cultured in MEM (Gibco) or RPMI-1640
(Gibco) supplemented with 10% heat-inactivated FBS and incubated at
37°C, 5% CO2.
Construction of recombinant
adenovirus
The complete cDNA sequence of Dm-dNKmu gene was
released from plasmid pGEM-T-dNKmu with endonucleases EcoRI and
BamHI (New England Biolabs, Beverley, MA, USA) and ligated into
plasmid pENTR13 [made by ourselves, which contains the sequence of
mouse cytomegalovirus (CMV) promoter + His-tag(N)+ multiple clone
site + SV40 polyA] to generate pENTR13-dNKmu. pENTR13-dNKmu was
digested with endonucleases AgeI and NotI (New England Biolabs), a
1317 bp fragment expression cassette of dNKmu containing mouse CMV
promoter + dNKmu gene + SV40 poly A was excised and inserted into
the AgeI and NotI sites of pSG500 (Sinogenomax Inc., Beijing,
China) to generate pSG500-dNKmu. Plasmid pSG500-dNKmu was
individually transfected into HEK293 cells using Lipofectamine 2000
(Life Technologies) combined with adenovirus packaging plasmid
pBGHlox (Microbix Biosystems). After homologous recombination, we
obtained a replicative adenovirus known as SG500-dNKmu, in which
the viral E1B55-kDa gene was deleted. Replication-defective
adenovirus Ad-GFP is used to compare infection rate differences
between the cell lines.
RT-PCR analysis
MDA-MB-231, MCF-7, WI-38 and MRC-5 cells were
infected with SG500-dNKmu or SG500 at a multiplicity of infection
(MOI) rate of 10. Two days after infection, cells were purified
with a TRIzol (Sigma, St. Louis, MO, USA) method and then assayed
for Dm-dNKmu gene expression using the RT-polymerase chain reaction
(PCR) kit (Takara Bio Inc., Japan). This manipulation was done
according to the manufacturer’s instructions. A cDNA equivalent of
1 ng of RNA was amplified by PCR using primers specific for the
target genes. The thermal cycles were: 94°C for 1 min, 55°C for 1
min, 72°C for 1.5 min for 35 cycles for dNKmu (785 bp); and 94°C
for 1 min, 55°C for 1 min, 72°C for 1.5 min for 35 cycles for GAPDH
(452 bp). Nucleotide sequences of dNKmu primers were as follows:
sense 5′-AAGGACTGATGGCGGAGGCA-3′; antisense
5′-TTGTCGTACCTGGCGACCCTCTGGCT-3′. Nucleotide sequences of GAPDH
primers were as follows: sense 5′-ACCA CAGTCCATGCCATCAC-3′;
antisense 5′-TCCACCACCCTG TTGCTGTA-3′. The amplification products
were separated by 2% agarose gel electrophoresis and visualized by
SYBR-Green staining.
Immunoblot analysis
MDA-MB-231, MCF-7, WI-38 and MRC-5 cells were seeded
in 24-well plates at a density of 5×104 cells/well and
infected with SG500 or SG500-dNKmu at a MOI of 1. After 2 days,
cells were rinsed 3 times with phosphate-buffered saline (PBS) and
fixed in 4% paraformaldehyde for 25 min. Immunoblotting was
performed by incubation, first with mouse anti-human His-tag
antibody (Merck, Darmstadt, Germany) at 4°C for 1 h, followed by
incubation with goat anti-mouse IgG (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) at a dilution of 1:100.
Enzyme assay
MDA-MB-231, MCF-7, WI-38 and MRC-5 cells were seeded
in 6-well plates at a density of 5×105 cells/well and
cultured for 24 h, followed by infection with SG500-dNK and SG500
at a MOI of 1. Two days later, cell protein extracts were prepared
as described (12). The assays
were performed in 50 mM of Tris-HCl, pH 7.6, 5 mM of
MgCl2, 5 mM of ATP, 2 mM of dithiothreitol, 15 mM of
NaF, 100 mM of KCl, 0.5 mg/ml bovine serum albumin, and 0.6 mg of
protein extract in a total volume of 35 ml. We used 2.5 mM of
methyl-3H-dThd (Moravek Biochemicals, Brea, CA, USA) in the assays
and mixed with equivalent amounts of unlabeled substrates. Aliquots
of the reaction mixture were spotted on Whatman DE-81 filters after
10, 20 and 30 min incubation at 37°C. The filters were washed 3
times in 5 mM of ammonium formate. The nucleoside monophosphates
were eluted from the filter with 0.5 M of KCl and the radioactivity
was determined by scintillation counting.
Cell killing assay
Cells were dispensed in 96-well plates (Corning
Inc., Corning, NY, USA). The culture solution was removed on the
second day and the following were added to each well: 1 ml of
serum-free DMEM and virus SG500-dNKmu, SG500 and mock at a MOI of
10. After 2 days of incubation, the nucleoside pro-drug BVDU or
DFDC was added at a concentration of 0, 0.001, 0.01, 0.1, 1 and 10
μM (mock was the drug-treated only group). After another 3
days, and a total of 5 days of incubation, cell viability was
measured by the tetrazolium salt
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay (Promega, Madison, WI, USA) to determine the combined
cytotoxic effect. Absorbance was measured with an Enzyme
Immunoassay Instrument at 570 nm, which serves as a measure of cell
viability. Each data point was generated from triplicate samples
and repeated 3 times.
Flow cytometry apoptosis assays
For further clarification of the mechanism and
effect of the combined suicide therapy, the apoptotic cells were
detected by an Annexin V-FITC/PI double staining kit (Genmed
Bioscience, China). Briefly, MDA-MB-231 and MRC-5 cells were
treated with 1 μM of DFDC for 3 days after being infected
with SG500-dNKmu, SG500 and Mock for 48 h, then trypsinized,
pelleted and washed in PBS. Cells were rinsed with a binding buffer
and then resuspended with 200 μl of the binding buffer.
After adding 5 μl of Annexin V-FITC (20 μg/ml) and 10
μl of propidium iodide (PI, 50 μg/ml), the mixture
was incubated at room temperature for 10 min in the dark. The
apoptosis ratio was analyzed using a FACScan flow cytometer
[equipped with Cellquest and ModFITLT for Mac V1.01 software
(Becton-Dickinson, San Jose, CA, USA)].
Adenovirus replication assay
MDA-MB-231, MCF-7 and MRC-5 were cultured in 24-well
dishes overnight and infected with SG500-dNKmu, SG500 and wild-type
Ad at an MOI of 10, then divided into two groups. One group was
treated with 1 μM of DFDC for 3 days. Two days after
infection, a second group was only virus-treated for 5 days. Cells
and media were then collected, respectively, freeze-thawed and
titered on HEK293 cells by the limiting dilution method (TCID50).
Each assay was repeated 2 to 3 times, averaged, and expressed as
pfu/cell ± SD.
Western blot analysis
Subconfluent cells were infected with viruses in the
presence of BVDU or DFDC as described above. MDA-MB-231 and MRC-5
cells were harvested 5 days post-infection and lysed in buffer (50
mmol/l herpes, at pH 7.4, 250 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l
DTT, 1 mmol/l NaF, 1% Triton X-100) containing protease inhibitors.
Total protein (50–80 μg) was separated on SDS-polyacrylamide
gels under reducing conditions, transferred to polyvinylidene
fluoride membranes (Millipore), and detected by the following
antisera: rabbit anti-Ad2/Ad5 E1A at 1:1,000 dilution (Santa Cruz
Biotechnology) and goat anti-β-actin at 1:1,000 dilution (Santa
Cruz Biotechnology), mouse anti-human His-tag at 1:500 dilution
(Merck). Detection was by horseradish peroxidase-conjugated
secondary IgG-antibodies (Santa Cruz Biotechnology) as appropriate,
and chemiluminescence reagent (Amersham Pharmacia Biotech) followed
by autoradiography (BioMaxfilm, Kodak).
In vivo tumor growth
Female BALB/C nude mice 4- to 6-week-old were bred
and maintained under specific pathogen-free conditions, and all
studies conducted under the protocols approved by the China Medical
University of Shenyang Animal Research Committee. Mice were
subcutaneously injected with breast cancer cells (MDA-MB-231,
107 cells suspended in 100 μl PBS) into the right
flank of each of the 48 mice. When the tumors had grown to about 90
mm3 in size, the mice were randomly divided into 6
treatment groups: Group 1, DFDC with SG500-dNKmu and Group 2, SG500
infected; Group 3, SG500-dNKmu and Group 4, SG500 virus alone;
Group 5, DFDC alone and PBS (control) group. All of the mice in the
adenovirus treatment groups were intratumoraly injected with a
total dose of 109 pfu 3 times at 48-h intervals and DFDC
at 5 mg/kg i.p. twice on days 2 to 8 after virus injection. Tumor
growth was monitored 5 days once and tumor volume (V) was
calculated using the formula: V (mm3) = 1/2 length (mm)
× width (mm)2. Survival analysis expressed as time to
progression was performed according to the method of Kaplan-Meier
(log-rank test for statistical significance). Tumor growth curves
were compared using one-way ANOVA for significance.
Results
Construction of recombinant adenovirus
with Dm-dNKmu
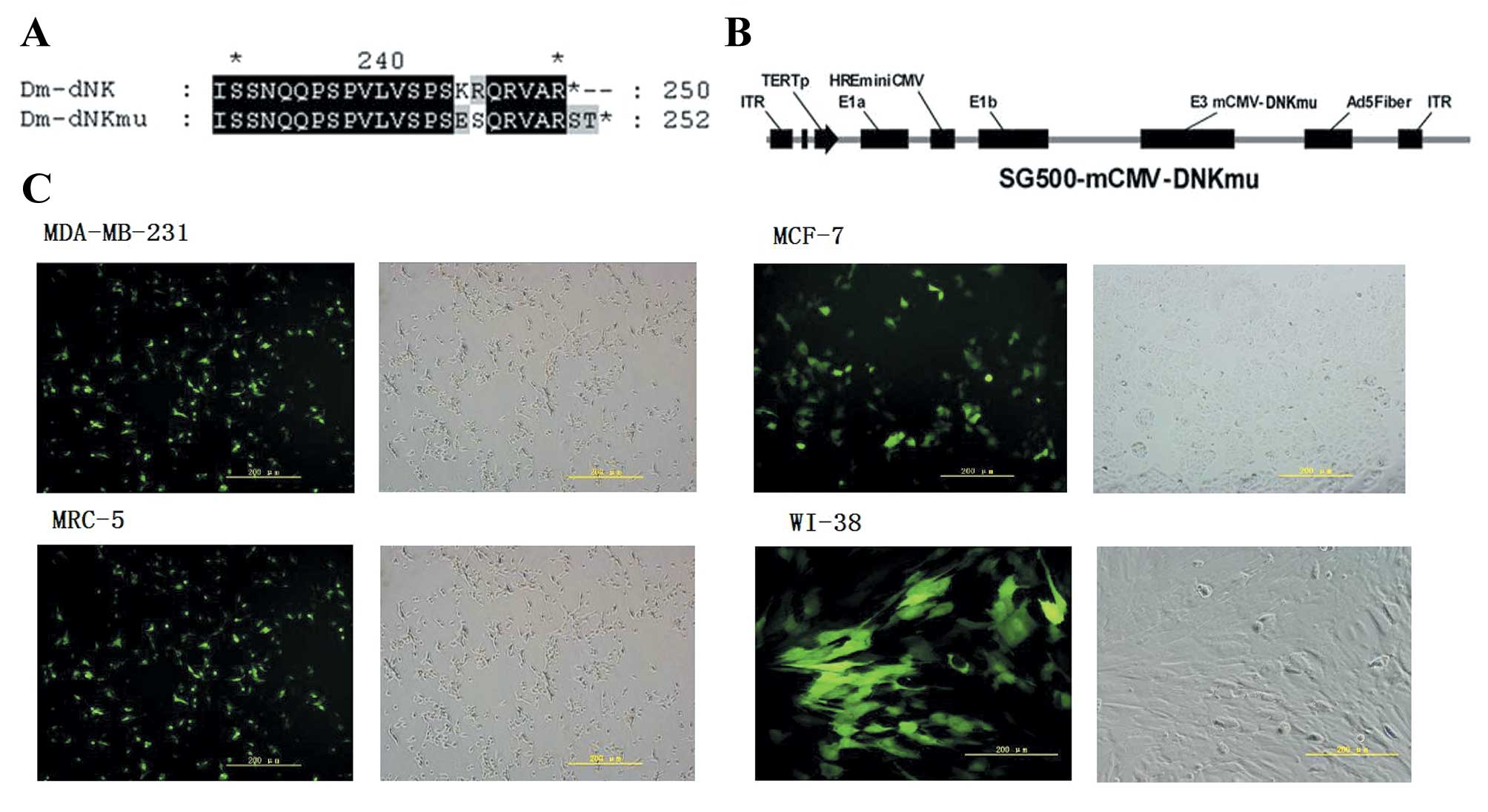
Mutations of Dm-dNK at the last 10 amino acids in
the sequence may increase the sensitivity of cells to other purine
nucleoside analogs as well as altering the expression location of
Dm-dNK. The Dm-dNK mutants were created by site-directed
mutagenesis in the amino-acid sequence at sites of 244E, 245S, 251S
and 252R alternated to evaluate the enzyme activity (Fig. 1A). The viral E1B55-kDa gene was
replaced by an expression cassette encoding dNKmu containing the
sequence of CMV promoter + multiple clone site + SV40 poly A into
plasmid SG500 to generate plasmid SG500-dNKmu (Fig. 1B). The infectivity of the cell
lines were determined by the wild-type 5 adenovirus with expression
of green fluorescence (Ad5-GFP), as shown in Fig. 1C. The cancer cell lines MDA-MB-231
and MCF-7 and the normal cell line WI38 and MRC-5 exhibited nearly
the same infectivity.
Expression and enzyme activity of
Dm-dNKmu
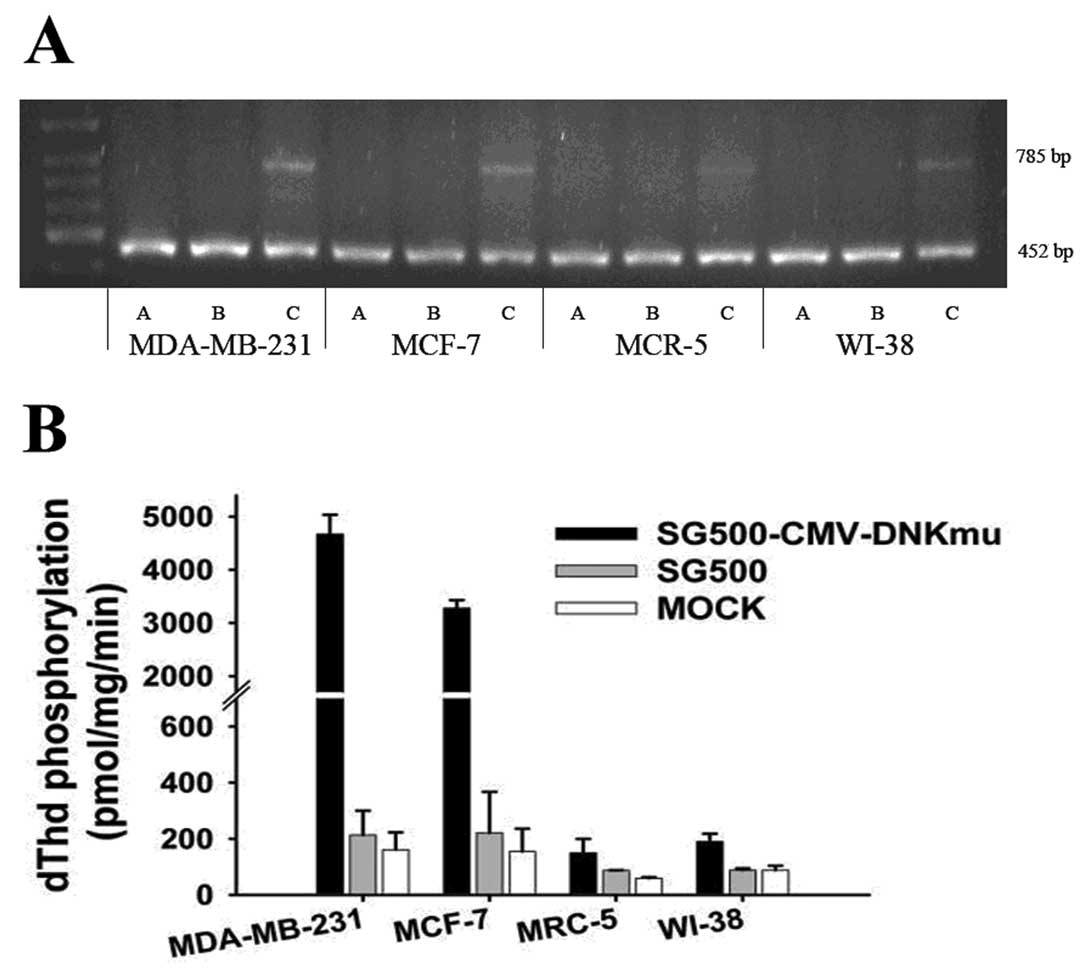
SG500-dNKmu expressed the Dm-dNKmu gene with a
higher efficiency and activity in MDA-MB-231 and MCF-7 cancer cells
compared with the replication-deficient adenovirus SG500. Our
RT-PCR study results showed the selective expression of SG500-dNKmu
at a MOI of 10 after a 2-day infection. The dNKmu gene of
SG500-dNKmu was somewhat higher than that of SG500 in MDA-MB-231
and MCF-7 cancer cells; however, in MRC-5 cells, SG500-dNKmu showed
lower levels than that of SG500. (Fig.
2A). Enzyme activity was determined by the phosphorylation of
dThd. The uninfected or infected with SG500 vector adenovirus
showed low levels of dThd phosphorylation, whereas the MDA-MB-231
or MCF-7 cancer cells infected with SG500-dNKmu exhibited nearly
30-fold higher enzymatic activity than the parent cell line; in the
normal cell lines MRC-5 or WI-38, activity of cells infected by
SG500-dNKmu was 10-fold less than that in cancer cells (Fig. 2B).
Cell cytotoxic activity of Dm-dNKmu with
chemotherapy
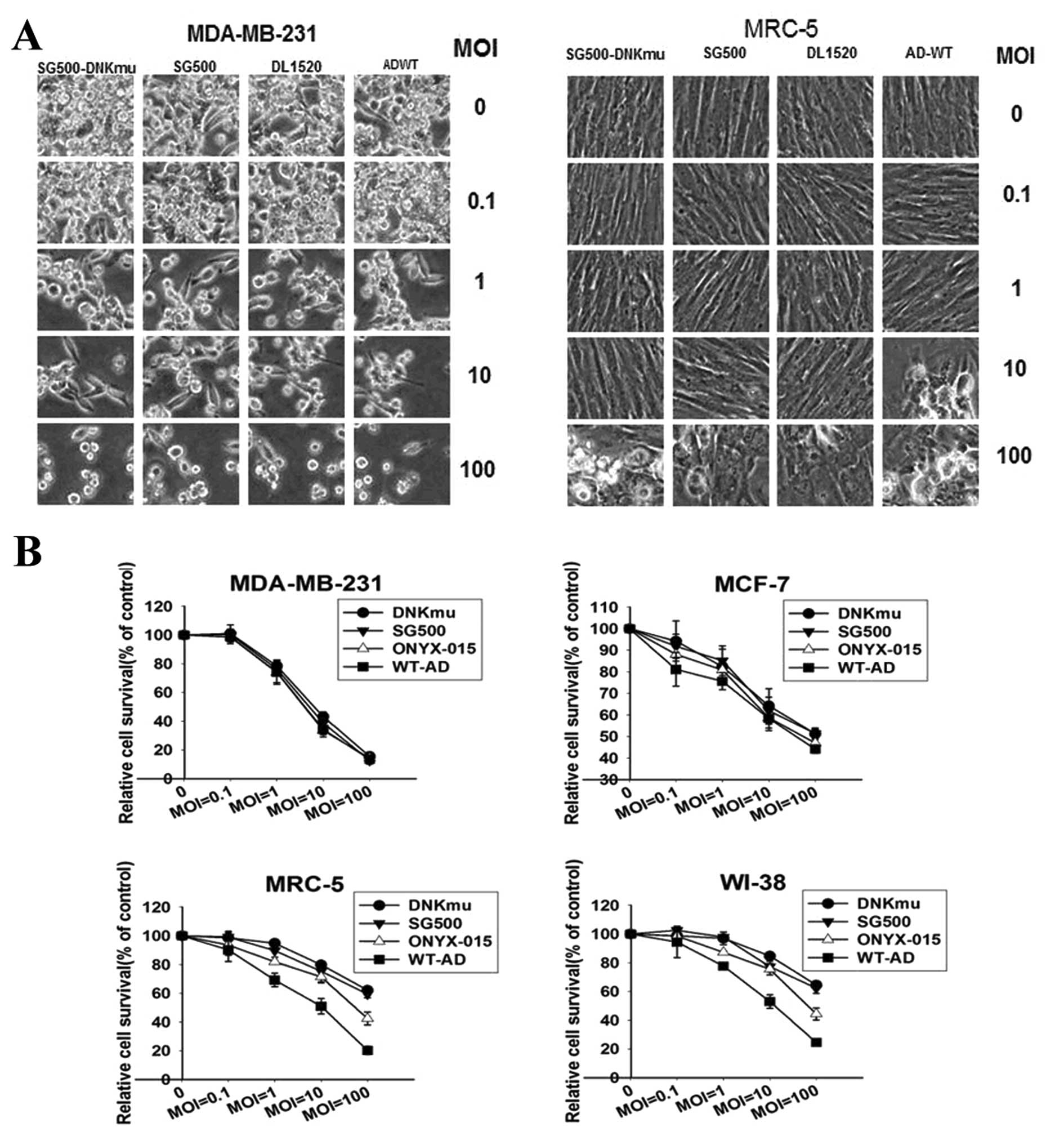
SG500-dNKmu virus alone showed greater killing
activity on cell lysis than DL1520 (ONYX-15) and WtAd in MDA-MB-231
cells, and almost the same protective as SG500 and DL1520 in MRC-5
cells (Fig. 3A). SG500-dNKmu
induced much more rapid cell death in MDA-MB-231 cells than in
normal MRC-5 cells 5 days after infection. Compared with DL1520 and
WtAd infection, SG500-dNKmu demonstrated no more apparent cytolysis
against MDA-MB-231 and MCF-7, but less profound cytotoxicity than
DL1520 and WtAd5 against normal cells.
We investigated Dm-dNKmu for its potential use as a
suicide gene in combination with the nucleoside analog BVDU or
DFDC. As shown by the MTT assay results, the treatment of cells
with SG500-dNKmu and SG500 resulted in a dose-dependent reduction
of cell viability within 5 days. With different concentrations of
BVDU or DFDC, SG500-dNKmu and SG500 were able to kill MDA-MB-231
and MCF-7 cancer cells more effectively than the SG500 and mock.
This occurred even at the very low concentration of 0.001 μM
DFDC (p<0.05). The nucleoside analog dose used was much lower
than IC50. Cell killing rates of SG500-dNKmu was
slightly less than that of SG500 in MDA-MB-231 and not less than
that of SG500 in the MCF-7 cell line. The difference may be because
of the different enzyme activity and sensitivity in various cell
lines. However, in the normal MRC-5 and WI-38 cells, SG500-dNKmu
with drugs showed less cytotoxic than the other virus (Fig. 4A), with cell killing rates reaching
a maximum of 50% lower than SG500, even when drugs was added. The
FACS assay also supported the results of MTT (Fig. 4B). The SG500-dNKmu and SG500
(MOI=10) with BVDU or DFDC induced the most cell apoptosis reaching
as high as 61 and 82% (right upper and lower quadrant) in the
MDA-MB-231 cancer cells, which was 60% higher than the SG500 with
DFDC. On the other hand, in the MRC-5 cells, with the presence of 1
μM of DFDC, 16% of apoptosis was induced by SG500-dNKmu,
which was far less than SG500 and SG500. The above data suggest
that SG500-dNKmu can sensitize cancer cell lines to DFDC without
causing significant toxicity to normal cells.
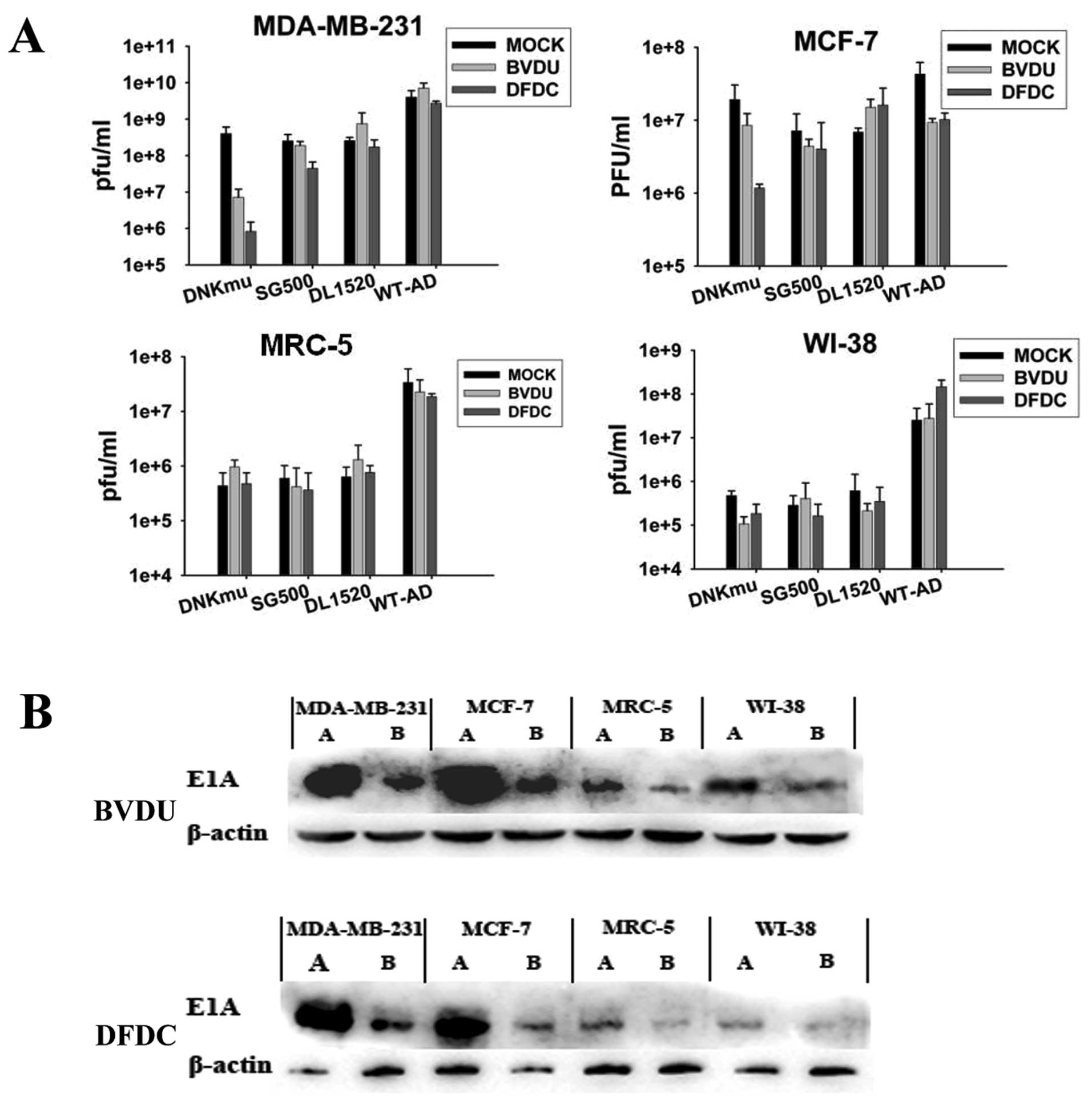
Viral replication is inhibited by
Dm-dNKmu with drugs
Surprisingly, in all the cell lines, viral
replication was detected at very low levels in the presence of 1
μmol/l of DFDC for the combination with SG500-dNKmu, but not
with SG500 without Dm-dNKmu. MDA-MB-231 cells supported higher
levels of 109 pfu/ml replication of the virus when SG500
was used alone, but in the presence of 1 μM DFDC,
replication was detected at low levels of <106 pfu/ml
for the SG500-dNKmu (Fig. 5A).
Despite a clear increase in replication over time without the drug,
low doses of DFDC (1 μM) added 48 h after infection can lead
to replicative inhibition of the virus for the combination
SG500-dNKmu. Attenuation was also observed to varying degrees in
normal cell lines. The normal cells did not support replication of
SG500 vectors, but in combination with 1 μmol/l DFDC, viral
replication was potently attenuated. The E1A protein levels in
western blot experiments verify that DFDC activated by Dm-dNKmu led
to inhibition of adenovirus replication. The E1A protein produced
from the combination of SG500-dNKmu with DFDC were at the lowest
levels in both MDA-MB-231 and MRC-5 cells (Fig. 5B), not obvious in that with BVDU.
SG500-dNKmu followed by the addition of DFDC attenuated E1A
expression of adenovirus obviously in cancer cell lines, but no
clear lane of E1A was detected in MRC-5 cells, except for wild-type
Ad. These results show that viral E1A expression is not sufficient
to overcome the DFDC-induced arrest in response to DNA damage
causing attenuation of replication. As expected, viral replication
was decreased in the presence of this potent DNA-polymerase
inhibitor, even at low concentrations.
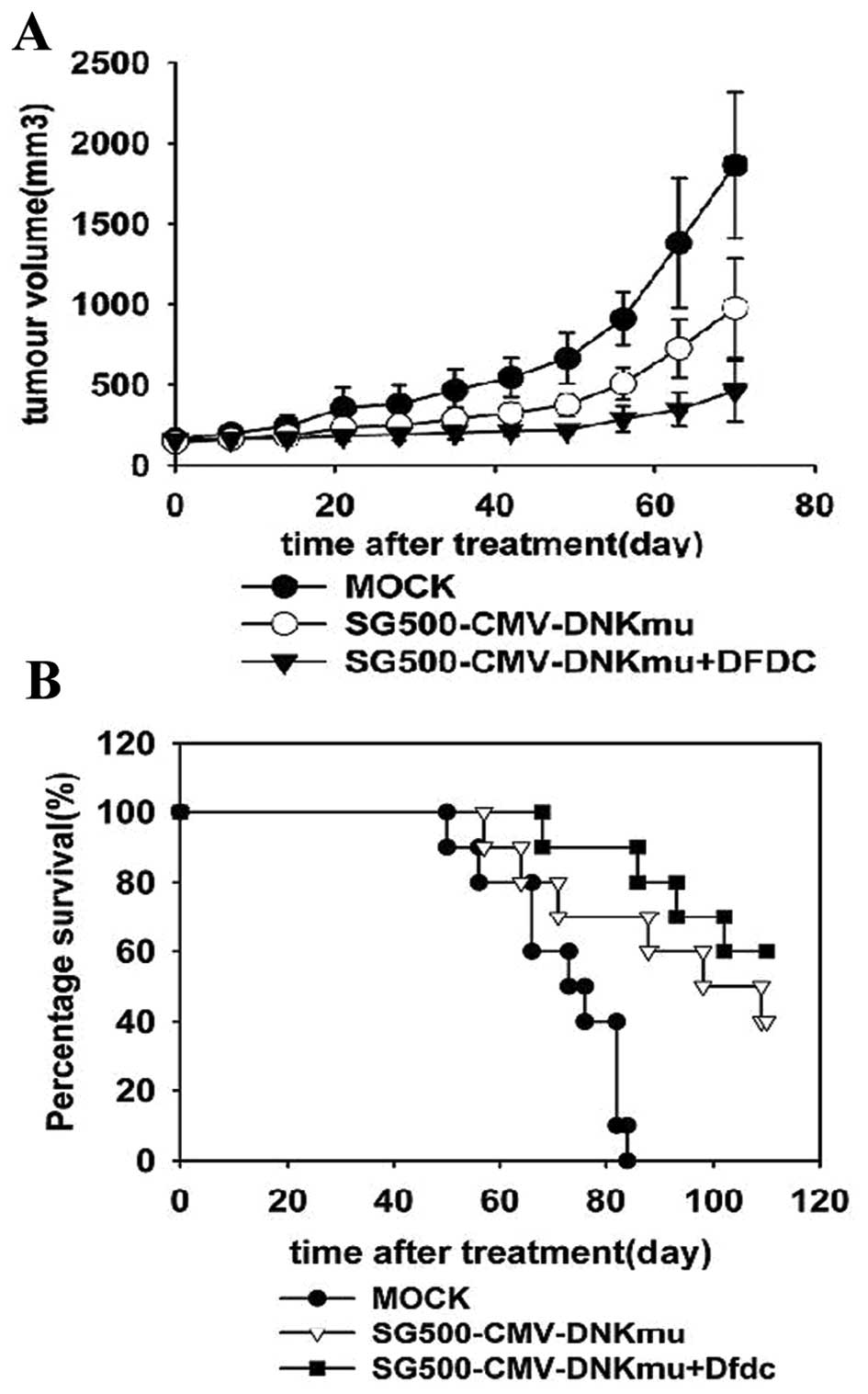
Antitumoral efficacy of Dm-dNKmu with
DFDC in MDA-MB-231 xenograft in vivo
The potency of the combination treatments was
evaluated in an in vivo MDA-MB-231 xenograft model with
subcutaneous tumors treated with suboptimal doses of SG500-dNKmu,
SG500 and other control viruses with or without DFDC (Fig. 6A and B). In animals treated with a
virus dose at 1×109 pfu, median survival was 40 days for
SG500-dNKmu. However, when SG500 and SG500-dNKmu in combination
with 5 mg/kg of DFDC was studied, time to progression was prolonged
to >60 days. Statistically significant antitumoral efficacy in
terms of tumor size was observed in SG500-dNKmu and SG500 with
DFDC-treated groups (1,032.2±613.1, 844.6±165.3 mm3)
compared with the virus (2,033.3±410.2 and 2,754.6±332.9
mm3) and mock control group (2,766.76±431.54
mm3), respectively, p<0.05. The SG500-dNKmu-treated
group appeared to be the most efficient in retarding tumor growth
with the longer survival period.
Discussion
We found that the replication capacity of
SG500-dNKmu could specifically replicate and cause significant CPE
in breast cancer cells, while showing a very low effect in normal
cells. In addition, SG500-dNKmu showed markedly strong enzyme
activity in breast cancer cells and significantly weak in normal
cells, suggesting that this oncolytic adenovirus can target
malignancy with high specificity. In the MTT assay, the nucleoside
analogs show low toxicity to untransduced cells, but the cells
expressing Dm-dNKmu are highly sensitive to the compound,
especially for DFDC, and the FACSCalibur confirmed that the
cytotoxic effect of SG500-dNKmu combined with DFDC was mainly due
to apoptotic events.
SG500-dNKmu was supposed to express enzymatical
activity of dNKmu increased with the adenovirus genome
amplification. Interestingly, there was no obviously potent
increase in cell death in response to the combined treatment
depended on virus, even attenuation of replication with SG500-dNKmu
in gastric cancer cells combined with 1 μM of DFDC was
observed 5 days post-infection. We considered that the DFDC
conversion to its triphosphates form (TP) phosphorylated by dNKmu
may lead to more apoptosis in the cancer cells and the adenovirus,
as shown in our western blot analysis of adenovirus E1A protein and
titer assays. The replication of adenovirus could be controlled, to
some extent, by certain types of dNKmu that possess nucleoside
analogs to protect the normal cells. DFDC itself also could block
replication of viruses due to DFDC-induced delay in G1/S-cell cycle
progression, with repression of cyclin E and cdc25A (13–15).
In a more recent publication by Morris and Wildner, using wild-type
replicating adenoviruses containing an HSV-TK transgene, no
augmentation of killing was observed after the addition of GCV
(16). The controversial
conclusion could be explained by the GCV-TP inhibiting the virus
vector expression of HSV-TK continuously through inducing the death
of virus, as is our finding.
Although SG500 combined with DFDC showed no less
killing activity than SG500-dNKmu in the breast carcinoma cell line
MDA-MB-231 or the tumor xenograft mouse model, the activated DFDC
played an important role in inhibiting viral replication. Activated
DFDC actually is limited by low selectivity toward the target cells
and low transduction efficiency. Futhermore, severe side-effects
resulting from excessive replication were the key obstacles of
CRCAds in the clinical treatment of cancer gene therapy (17–19).
Expression of adenovirus early transcription gene E1A could be
controlled, to some extent, and was associated with apoptosis in
the majority of SG500-dNKmu/DFDC-treated tumor cells, even when the
adenovirus vector SG500 amplification in the normal cells expressed
suicide gene to produce unwanted effects.
In conclusion, we have developed a replicative,
Dm-dNKmu-armed adenovirus, SG500-dNKmu, which demonstrated that
cancer-specific killing activity could be enhanced by pro-drugs
administration. The combination therapy synergistically induced
growth apoptosis in human breast cancer cells while suppressing the
replication of the adenovirus in vitro. DFDC with less toxic
doses under dNKmu catalysis enhanced cytotoxicity and had a
synergistic effect of suppressing excessive adenovirus replication.
Combining the dNKmu therapy with other antimetabolites to achieve
synergistic effects may be another approach to enhance the
efficiency of nucleoside kinase suicide gene therapy. Even though
mechanisms of synergetic effects still need to be studied and
preventive strategies are being entertained, the ultimate clinical
use of gene therapy for improving breast cancer treatment would
most likely be in combination with surgery, radiation, or
chemotherapy.
Acknowledgements
This study was supported by grants
from the Hi-Tech Research Development Program of China (863
Program, 2006AA02Z493) and the National Natural Science Foundation
of China (no. 81071900 and 81172199). We appreciate the support of
Professor Qijun Qian (Laboratory of Gene and Viral Therapy, Eastern
Hepatobiliary Surgical Hospital, Second Military Medical
University, China) in constructing the adenovirus vectors.
References
|
1.
|
Zheng X, Johansson M and Karlsson A:
Retroviral transduction of cancer cell lines with the gene encoding
Drosophila melanogaster multisubstrate deoxyribonucleoside
kinase. J Biol Chem. 275:39125–39129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Zheng X, Johansson M and Karlsson A:
Bystander effects of cancer cell lines transduced with the
multisubstrate deoxyribonucleoside kinase of Drosophila
melanogaster and synergistic enhancement by hydroxyurea. Mol
Pharmacol. 60:262–266. 2001.PubMed/NCBI
|
|
3.
|
Zheng X, Johansson M and Karlsson A:
Nucleoside analog cytotoxicity and bystander cell killing of cancer
cells expressing Drosophila melanogaster deoxyribonucleoside
kinase in the nucleus or cytosol. Biochem Biophys Res Commun.
289:229–233. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Betham B, Shalhout S, Marquez VE and
Bhagwat AS: Use of Drosophila deoxynucleoside kinase to study
mechanism of toxicity and mutagenicity of deoxycytidine analogs in
Escherichia coli. DNA Repair (Amst). 9:153–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Munch-Petersen B, Knecht W, Lenz C,
Sondergaard L and Piskur J: Functional expression of a
multisubstrate deoxyribonucleoside kinase from Drosophila
melanogaster and its C-terminal deletion mutants. J Biol Chem.
275:6673–6679. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sokkar P, Sathis V and Ramachandran M:
Computational modeling on the recognition of the HRE motif by
HIF-1: molecular docking and molecular dynamics studies. J Mol
Model. 18:1691–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lee KJ, Lee KY and Lee YM: Downregulation
of a tumor suppressor RECK by hypoxia through recruitment of HDAC1
and HIF-1alpha to reverse HRE site in the promoter. Biochim Biophys
Acta. 1803:608–616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Mikkelsen NE, Munch-Petersen B and Eklund
H: Structural studies of nucleoside analog and feedback inhibitor
binding to Drosophila melanogaster multisubstrate
deoxyribonucleoside kinase. FEBS J. 275:2151–2160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yu de B, Zhong SY, Yang M, Wang YG, Qian
QJ, Zheng S and Liu XY: Potent antitumor activity of
double-regulated oncolytic adenovirus-mediated ST13 for colorectal
cancer. Cancer Sci. 100:678–683. 2009.PubMed/NCBI
|
|
10.
|
Beltinger C, Fulda S, Kammertoens T, Meyer
E, Uckert W and Debatin KM: Herpes simplex virus thymidine
kinase/ganciclovir-induced apoptosis involves ligand-independent
death receptor aggregation and activation of caspases. Proc Natl
Acad Sci USA. 96:8699–8704. 1999. View Article : Google Scholar
|
|
11.
|
Wildner O and Morris JC: The role of the
E1B 55 kDa gene product in oncolytic adenoviral vectors expressing
herpes simplex virus-tk: assessment of antitumor efficacy and
toxicity. Cancer Res. 60:4167–4174. 2000.PubMed/NCBI
|
|
12.
|
Sandrini MP, Clausen AR, On SL, Aarestrup
FM, Munch-Petersen B and Piskur J: Nucleoside analogues are
activated by bacterial deoxyribonucleoside kinases in a
species-specific manner. J Antimicrob Chemother. 60:510–520. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jordheim LP, Galmarini CM and Dumontet C:
Gemcitabine resistance due to deoxycytidine kinase deficiency can
be reverted by fruitfly deoxynucleoside kinase, DmdNK, in human
uterine sarcoma cells. Cancer Chemother Pharmacol. 58:547–554.
2006. View Article : Google Scholar
|
|
14.
|
Zhang ZL, Zou WG, Luo CX, et al: An armed
oncolytic adenovirus system, SG500-gene, demonstrating potent
antitumoral efficacy. Cell Res. 13:481–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cappella P, Tomasoni D, Faretta M, et al:
Cell cycle effects of gemcitabine. Int J Cancer. 93:401–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Morris JC and Wildner O: Therapy of head
and neck squamous cell carcinoma with an oncolytic adenovirus
expressing HSV-tk. Mol Ther. 1:56–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Heilbronn R and Weger S: Viral vectors for
gene transfer: current status of gene therapeutics. Handb Exp
Pharmacol. 197:143–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nayak S and Herzog RW: Progress and
prospects: immune responses to viral vectors. Gene Ther.
17:295–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Büning H, Perabo L, Coutelle O,
Quadt-Humme S and Hallek M: Recent developments in adeno-associated
virus vector technology. J Gene Med. 10:717–733. 2008.
|