Introduction
Gastric cancer is one of the most common cancers
worldwide, and mortality due to gastric cancer is second next to
lung cancer (1). Although surgery
is the standard treatment of localized gastric cancer, the results
are often disappointing, with recurrence rates as high as 70% after
successful complete (R0) resection. Attempts to improve outcome
with adjuvant therapy have yielded only modest success (2).
Emerging evidence indicates that cancer stem cells
(CSCs) may be involved in tumor maintenance, therapy resistance,
tumor progression, and distant metastasis (3,4).
CSCs are defined as a subpopulation of cells within a tumor that
possess the capacity for self-renewal and that can cause the
heterogeneous lineage of cancer cells that constitute the tumor
(5). The most important issue in
the research of CSCs is how to isolate and identify CSCs. Some
research groups have reported that gastric CSC fractions could be
successfully enriched by some cell surface phenotypes, specifically
CD44, epithelial cell adhesion molecule (EpCAM) (6,7).
Nevertheless, these markers, are not specific for identifying
gastric CSCs (6). So far, no
markers for putative gastric CSCs have yet been generally accepted,
and further study is needed to explore the isolation method for
gastric CSCs.
As a functional approach, spheroid body formation is
particularly useful to enrich the potential CSC subpopulations when
the specific CSC makers have not been defined, as is the case for
most CSCs (8–10). Therefore, in the present study we
developed spheroid body-forming cells in gastric cancer cell line
MKN-45 and determined whether these cells acquired CSCs
characteristics, including self-renewing capacity, chemoresistance
and tumorigenic capacity.
Materials and methods
Culture of parental, spheroid
body-forming cells
The human gastric cancer cell line MKN-45 obtained
from the Cell Bank of the Chinese Academy of Sciences, Shanghai,
China was cultured in RPMI-1640 medium containing 10% fetal bovine
serum (FBS) and plated at the density of 1×106 live
cells per 75-cm2 flask. When the cells attached, we
passaged them upon confluence. Spheroid bodies were derived by
placing the parental cells into serum-free RPMI-1640 culture medium
containing 1% N-2 supplement, 2% B-27 supplement (Invitrogen), 1%
antibiotic mixture (Gibco), 20 ng/ml human FGF-2 and 100 ng/ml EGF
(Chemicon). The parental cells were plated in 96-well ultra-low
attachment plate (Corning) at 100 cells per well. Two weeks later,
plates were analyzed for spheroid body formation and were
quantified using an inverted microscope (Olympus) at ×40 and ×100
magnification. After primary spheroid body reached the size of
approximately 200–500 cells per spheroid body, the spheroid bodies
were dissociated at the density of 1,000 cells per milliliter and
100 single cell suspension (100 μl) was seeded in each well
of a 96-well ultra-low attachment plate (Corning) in serum-free
medium described above. Two weeks later, wells were analyzed for
subspheroid body formation.
Quantitative real-time PCR
Total-RNA was extracted from the parental cells,
spheroid body-forming cells using Qiagen RNeasy mini kit (Qiagen)
according to the manufacturer’s instructions. RNA was treated with
DNase I (Qiagen) to eliminate genomic DNA contamination. The
integrity and purification of RNA samples were monitored by agarose
gel electrophoresis. The concentration of RNA was determined by
repeated OD measurements of aliquots at a wavelength of 260 nm.
Reverse-transciption reaction to transcript 1 μg total-RNA
into complementary DNA was performed with reagents of synthesis
system (Qiagen).
To determine fold changes in each gene, real-time
qPCR was performed using the Eppendorf Mastercycler ep realplex
(2S; Eppendorf, Hamburg, Germany). EvaGreen (Biotium Inc., Hayward,
CA) served as a dye that binds to amplified DNA to emit
fluorescence during reactions. EvaGreen is an optimal green
fluorescent DNA dye for qPCR. The reaction mixture of 25 μl
contained 12.5 μl of EvaGreen™ qPCR Master mix (Biotium
Inc.), 1 μl of primers (10 mM) and 1 μl of template
cDNA, and 10.5 μl of double distilled water (ddwater). The
EvaGreen (Biotium Inc.) served as a dye that binds to amplified DNA
to emit fluorescence during reactions. The glyceraldehyde-3′
phosphate dehydrogenase (GAPDH) gene served as an internal control
for expression levels of target apotosis genes. The primer
sequences and PCR conditions are summarized in Table I. After an initial incubation for 2
min at 96°C, the reactions were carried out for 40 cycles at 96°C
for 15 sec and 60°C for 45 sec (fluorescence collection).
Fluorescent readings were taken during the extension step of each
cycle. Melting curve analysis was performed to ensure the
amplification of a single PCR production. Reactions with no
template was included as negative control. By setting the threshold
at the level at the middle steady portion of reaction cycles versus
florescence curve, the Ct values of target genes were calculated
using customized software, and the 2(−ΔΔC(T)) method was used.
Finally, the PCR products were separated on 1.5% agarose gel
electrophoresis in the presence of ethidium bromide, and visualized
on an ultra-violet illuminator to verify product sizes, and
recorded. The qPCRs were performed three times in triplicate.
 | Table I.The base sequences of primers for
quantitative real-time PCR. |
Table I.
The base sequences of primers for
quantitative real-time PCR.
| Primer name | Sequence |
|---|
| Oct4 | |
| Forward |
AACGACCATCTGCCGCT |
| Reverse |
CGATACTGGTTCGCTTTCTCT |
| Sox2 | |
| Forward |
GAAAAACGAGGGAAATGGG |
| Reverse |
GCTGTCATTTGCTGTGGGT |
| Nanog | |
| Forward |
CCTCCTCCCATCCCTCATA |
| Reverse |
TGATTAGGCTCCAACCATACTC |
| CD44 | |
| Forward |
CATCCCAGACGAAGACAGTCC |
| Reverse |
TGATCAGCCATTCTGGAATTTG |
| GAPDH | |
| Forward |
GGCATCCTGGGCTACACT |
| Reverse |
CCACCACCCTGTTGCTGT |
Immunofluorescence staining for stem cell
markers
In brief, cells plated onto poly-L-lysine-coated
glass coverslips were fixed with 4% paraformaldehyde and then
washed with PBS. Cells were permeabilized with 0.1% Triton
X-100/PBS for 10 min. Consequently, the cells were incubated with
primary antibodies (Oct-4, Nanog, Sox2 or CD44; Santa Cruz
Biotechnology). Cells were further probed with fluorescein
isothiocyanate or Rhodamine-tagged secondary antibodies. The
fluorescence was recorded by inverted fluorescence microscope
(Leika).
Western blot analysis
For western blot analyses, protein was harvested
from cells plated to 70 to 80% confluence. Spheroid body-forming
cells or parental cells were lysed directly in lysis buffer to
collect whole cell extracts. Protein samples for western blot
analysis were prepared by boiling after the addition of denaturing
sample buffer. Then, proteins were separated using SDS-PAGE on an 8
or 15% gel, transferred onto PVDF membranes. Membranes were
incubated at 4°C overnight with primary antibody, and subsequently
incubated with horseradish peroxidase-conjugated secondary
antibodies for 1 h at room temperature. Finally, protein bands were
visualized using chemiluminescence (Santa Cruz Biotechnology)
exposure on BioMax film (Kodak). Concentrations used for primary
antibodies were: anti-CD44 1:200, anti-Oct4 1:200, anti-Sox2 1:200,
anti-Nanog 1:200 (all from Santa Cruz Biotechnology).
Chemoresistance assay
Rates of resistance to drugs were assessed using MTT
assay. Briefly, 2,000 healthy spheroid body-forming cells or
parental cells per well were plated in 96-well plates in 100
μl RPMI-1640 medium (4 wells per group) with
chemotherapeutic drugs (5-Fu, DDP) or control PBS. At each time
point (24 and 48 h), 10 ml MTT solution was added to each well and
the plate was incubated for 4 h at 37°C, then the medium was
replaced by 150 ml DMSO. To assess the effect of drug resistance of
spheroid body-forming cells or parental cells, we treated the
dissociated cells with 5-Fu (50 μg/ml, 100 μg/ml, 200
μg/ml) alone, DDP (10 μg/ml, 20 μg/ml, 40
μg/ml) alone, 5-Fu plus DDP (50 μg/ml + 10
μg/ml; 100 μg/m + 20 μg/ml; 200 μg/ml +
40 μg/m) or control PBS for 24 and 48 h. MTT assay is based
on mitochondrial conversion of MTT to yellowish formazan, being
indicative of the number of viable cells. The number of viable
cells was evaluated by absorbance OD450 nm (Abs) using Model 680
microplate reader.
In vivo tumorigenicity experiments
Male athymic nude mice (nu/nu), 6 to 8 weeks old,
were obtained from Shanghai Laboratory Animal Center, Chinese
Academy of Sciences, Shanghai, China, and were housed under
pathogen-free conditions in the barrier animal facility. All animal
procedures were carried out with the approval of the Animal Ethics
Committee of Nantong University.
For in vivo tumorigenicity experiments, equal
number (1×104, 2×104, 2×105,
2×106) of freshly dissociated cells was suspended in 200
μl PBS, the spheroid body-forming cells were injected
subcutaneously into the right rear flank of each mouse (6 mice per
group) and the parental cells were injected subcutaneously into the
left rear flank of each mouse, we examined the tumorigenic capacity
of spheroid body-forming cells and parental cells. The mice were
observed for tumor growth every 10 days over 8 weeks and then
sacrificed by cervical dislocation. The grafts were removed, fixed
with 10% buffered formalin, and stained with hematoxylin and eosin
(H&E).
Statistical analysis
All experiments were repeated at least three times
and representative results are presented. All values in the figures
and text are the means ± SD. Statistical analyses were performed
using the SPSS statistical software package (SPSS/PC+, SPSS Inc.,
Chicago, IL, USA). Any significant differences among mean values
were evaluated by the Student’s t-test. A two-sided p<0.05 was
accepted as significant.
Results
Gastric cancer cells form
anchorage-independent spheroid bodies
MKN-45 parental cells were cultured in serum-free
medium described in the methods section. In this condition, cells
grew as non-adherent, three-dimensional spheroid clusters, called
spheroid body. The self-renewing capacity of these spheroid
body-forming cells was assessed by dissociating them into single
cell and growing in serum-free medium described in the methods
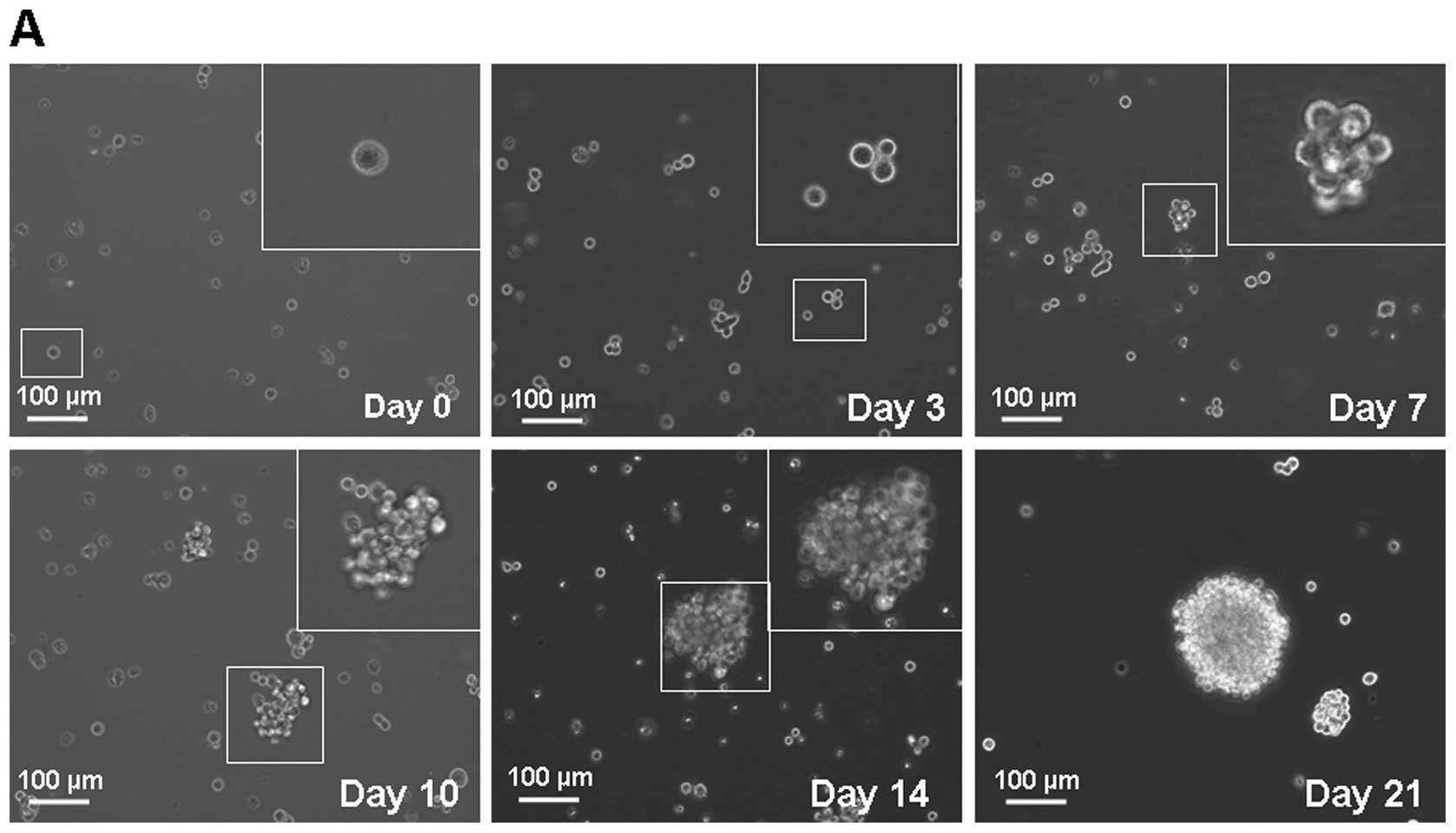
section. Fig. 1A shows the process
of single MKN-45 cell forming a spheroid body. After 2 weeks, these
cells derived from spheroid body-forming cells generated
sub-spheroid bodies again at 29.70±6.21% compared with 3.30±1.49%
of parental cells (Fig. 1B).
Spheroid body-forming cells possess the
ability of resistance to conventional chemotherapy in vitro
The MKN-45 spheroid body-forming cells exhibited
general resistance to 5-Fu and DDP in the treatment of 24 h
(Fig. 2A). Compared with the
parental MKN-45 cells, the survival rates of spheroid body-forming
cells were higher under the treatment of 50 μg/ml, 100
μg/ml and 200 μg/ml 5-Fu (1.3-fold, 1.4-fold and
1.7-fold, respectively); 10 μg/ml, 20 μg/ml and 40
μg/ml DDP (1.7-fold, 3.2-fold and 3.4-fold, respectively).
Whereas, under the treatment for 48 h (Fig. 2B), the relative survival rates were
not significantly increased, neither 5-Fu nor DDP. But in the
treatment of 5-Fu combined with DDP, the survival rates of MKN-45
spheroid body-forming cells were increased 2.6-fold, 2.9-fold and
2.7-fold, respectively, under the treatment for 24 h; and 5.1-fold,
4.8-fold and 5.4-fold, respectively, for 48 h.
Spheroid body-forming cells overexpress
gastric CSC-related genes and proteins
Quantitative real-time PCR and western blot analysis
were performed on spheroid body-forming cells and parental cells.
The results showed that the cells expressing Oct-4, Sox2, Nanog and
CD44 were significantly more in spheroid body-forming cells than in
parental cells (Fig. 3).
Intracellular localization of Oct-4,
Sox2, Nanog and CD44 in spheroid body-forming cells
To examine the subcellular localization of Oct-4,
Sox2, Nanog and CD44 in spheroid body-forming cells,
immunofluorescent staining of Oct-4, Sox2, Nanog and CD44 was
performed. Oct-4, Sox2 and Nanog proteins were positively stained
within the perinuclear and cytoplasm of the spheroid body-forming
cells, and CD44 was positively stained mainly in the membrane. Dual
staining of Oct-4/CD44, Sox2/CD44, and Nanog/CD44 indicated that
the embryonal proteins (Oct-4, Sox2 and Nanog) were colocalized
with CD44 in the spheroid body-forming cells (Fig. 4).
Spheroid body-forming cells exhibit high
tumorigenicity in vivo
The tumorigenicity experiments in vivo showed
that as few as 2×104 cells from the MKN-45 spheroid body
were able to form a tumor when subcutaneously injected into nude
mice, while 2×106 parental cells were needed (Fig. 5A and B). This was 100 times higher
than that of spheroid body-forming cells. Moreover, spheroid
body-forming cells generated subcutaneous tumors with larger volume
and shorter time compared with those generated from parental cells.
H&E examination of xenografts derived from MKN-45 spheroid
body-forming cells showed that these tumors closely resembled
tumors from the parental cells, mainly containing differentiated
cells (Fig. 5C and D).
Discussion
The hypothesis that cancers are maintained by a
subpopulation of stem cells while non-stem cells have a finite life
span raises the possibility that targeting cancer stem cells could
provide a means of cancer control. As a preliminary step to
investigate whether this hypothesis is applicable to gastric
cancers, it is necessary to identify and isolate gastric cancer
stem cells, which could provide new insight into the gastric cancer
tumorigenic process and possibly bear great therapeutic
implications.
Three distinct methodologies based on the properties
of CSC have been successfully used for isolation of CSC from solid
tumors (5,11,12).
i) Fluorescence-activated cell sorting (FACS) according to
CSC-specific cell surface markers such as CD44 or CD133 is made
possible for isolation of CSCs (4,13,14).
ii) The side populations (SP) of tumor cells, which display
intracellular Hoechst 33342 exclusion in vitro, also may
cause chemoresistance is isolated and characterized as CSCs
(15–17). iii) The spheroid body formation
assay in which cells are cultured in non-adherent condition in a
serum-free medium supplemented with basic fibroblast growth factor
(bFGF) and epidermal growth factor (EGF) is a practical approach
for individual solid tumor tissues or cancer cells (18,19).
There have been some reports on the isolation and
identification of gastric CSCs by FACS based on CD44, which is a
proposed marker for gastric CSCs (6,7),
however, the sensitivity and specificity for identifying gastric
CSCs are being challenged. For example, Rocco et al(20) reported although CD133(+) and
CD133(+)/CD44(+) were detectable in human primary gastric cancers,
they neither expressed stem-like properties nor exhibited
tumor-initiating properties in xenograft transplantation
experiments. The sorting of SP cells is another type of method for
the isolation and identification of gastric CSCs (21). However, some studies have indicated
that there was not a significant association between the SP
fraction and CSCs. Patrawala et al(22) reported that glioma cell lines which
expressed ABCG2, an ATP-binding cassette half-transporter that is
associated with SP cells, had a similar tumorigenicity as
ABCG2-negative cells. Burkert et al(23) also reported that among four colon
cancer cell lines examined, SP and non-SP cells were similarly
clonogenic in vitro and tumorigenic in vivo and
displayed equivalent multipotential differentiation potential. They
also showed that SP and non-SP populations are interconvertible,
each giving rise to the other in culture. Takaishi et
al(6) found in their study
that human gastric cancer MKN-45 cells have a significant SP
fraction, but the SP and non-SP cells both possess tumorigenic
ability in vitro and in vivo.
Spheroid body culture has been increasingly used as
a method for enriching stem cells which relies on their property of
anchorage-independent growth. Various types of potential CSC
subpopulations from primary tumors have been reported to be
isolated and enriched by the application of spheroid body culture
(24–26). The spheroid body-forming cells from
primary tumors, such as ovarian cancer and breast cancer, showed
stem-like properties and expressed their CSC markers (24,27).
To our knowledge, there have been few reports on the isolation and
characterization of gastric CSCs by the method of spheroid body
culture, therefore, we developed spheroid body cells by cultivating
human gastric cancer cell line MKN-45 in defined serum-free medium,
and demonstrated that those cells derived from spheroid body could
generate greater numbers of new spheroid bodies than the parental
cells, indicating that spheroid body-forming cells were capable of
self-renewal and proliferation, which is an important
characteristic of CSCs.
Chemoresistance is another important characteristic
of CSCs. To assess whether the self-renewing spheroid body-forming
cells possess a hypothesized CSC chemoresistant property, we
examined the sensitivity of spheroid body-forming cells to
chemotherapeutics. The MKN-45 spheroid body-forming cells exhibited
general resistance to 5-Fu and DDP, even in the treatment of 5-Fu
combined with DDP, and showed higher survival percentages compared
with its parental cells. These results support a role for these
spheroid body-forming cells in gastric cancer chemoresistance,
which may explain why current therapies fail to eradicate cancer
cells and prevent tumor re-growth.
Xenotransplantation is generally regarded as the
gold standard for evaluating tumorigenicity of tumor cells. We
tested the MKN-45 spheroid body-forming cells for their tumor
initiating capability. In vivo, as fewer as 100-fold cells
from spheroid body-forming cells could generate tumors upon
xenotransplantation than those from parental cells. Moreover,
spheroid body-forming cells generated subcutaneous tumors with
larger volume and shorter time compared with those generated from
parental cells. These data therefore indicated that the spheroid
body-forming cells represented CSCs that had tumorigenic
capacity.
To further explore the CSC properties of spheroid
body-forming cells, We evaluated the MKN-45 spheroid body-forming
cells for their stemness characteristics. Overexpression of stem
cell-specific transcription factors such as Oct4, Sox2 and Nanog is
a vital characteristics of CSCs (28). These transcription factors often
function in combinatorial complexes to regulate the expression of
gene loci which are involved in self-renewal, proliferation and
differentiation (29). CD44, which
is a type I transmembrane glycoprotein that serves as the receptor
for the extracellular matrix component, hyaluronic acid, was one of
the first markers of solid tumors that was shown to be enriched in
tumor-initiating cells (13).
Recent studies have provided support for its role as a CSC marker
(6,30). In this study we found that all
three transcription factors (Oct4, Sox2 and Nanog) and CD44 are
overexpressed in MKN-45 spheroid body-forming cells as compared
with parental cells. More importantly, we have first focused on the
question of whether there is a physical linkage between CD44 and
the three transcription factors in spheroid body-forming cells. we
found that the CD44 positive stained spheroid body-forming cells
were costained with Oct4, Sox2 or Nanog, indicating CD44 positive
cells co-expressed of the pluripotency factors Oct4, Sox2 or Nanog
in MKN-45 spheroid body might represent a kind of gastric CSCs.
In summary, the study demonstrated that the
non-adherent spheroid body-forming cells from human gastric cancer
cell line MKN-45, which are cultured in stem cell conditioned
medium, possess gastric CSC properties. Further experiments using
more refined selection criteria such as a combination of two or
multiplemarkers would be useful to specifically identify and purify
CSCs. Testing on resected tumor samples or peritoneal effusion
fluid would help to clarify its applicability in clinical
settings.
Acknowledgements
This study was supported by a grant
from the Bureau of Science and Technology of Nantong City
(HS2012067).
References
|
1.
|
Yasui W, Sentani K, Sakamoto N, Anami K,
Naito Y and Oue N: Molecular pathology of gastric cancer: research
and practice. Pathol Res Pract. 207:608–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Brenner B, Hoshen MB, Purim O, David MB,
Ashkenazi K, Marshak G, Kundel Y, Brenner R, Morgenstern S, Halpern
M, Rosenfeld N, Chajut A, Niv Y and Kushnir M: MicroRNAs as a
potential prognostic factor in gastric cancer. World J
Gastroenterol. 17:3976–3985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Marx J: Cancer research: mutant stem cells
may seed cancer. Science. 301:1308–1310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells: perspectives on current status and future directions -
AACR Workshop on Cancer Stem Cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar
|
|
6.
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ,
Shim HE, Yoon S, Baek SY, Kim BS, Kang CD and Oh SO: Cancer spheres
from gastric cancer patients provide an ideal model system for
cancer stem cell research. Cell Mol Life Sci. 68:3589–3596. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
9.
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone M:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
11.
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Dean M, Fojo T and Bates S: Tumor stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
16.
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F,
Maclaughlin DT and Donahoe PK: Ovarian cancer side population
defines cells with stem cell-like characteristics and Mullerian
inhibiting substance responsiveness. Proc Natl Acad Sci USA.
103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK
and Fine HA: Tumor stem cells derived from glioblastomas cultured
in bFGF and EGF more closely mirror the phenotype and genotype of
primary tumors than do serum-cultured cell lines. Cancer Cell.
9:391–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radio-resistance by preferential activation of the
DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rocco A, Liguori E, Pirozzi G, Tirino V,
Compare D, Franco R, Tatangelo F, Palaia R, D’Armiento FP,
Pollastrone G, Affuso A, Bottazzi EC, Masone S, Persico G and
Nardone G: CD133 and CD44 cell surface markers do not identify
cancer stem cells in primary human gastric tumors. J Cell Physiol.
227:2686–2693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Fukuda K, Saikawa Y, Ohashi M, Kumagai K,
Kitajima M, Okano H, Matsuzaki Y and Kitagawa Y: Tumor initiating
potential of side population cells in human gastric cancer. Int J
Oncol. 34:1201–1207. 2009.PubMed/NCBI
|
|
22.
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2− cancer cells are
similarly tumorigenic. Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Burkert J, Otto WR and Wright NA: Side
populations of gastrointestinal cancers are not enriched in stem
cells. J Pathol. 214:564–573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
26.
|
Rappa G, Mercapide J, Anzanello F,
Prasmickaite L, Xi Y, Ju J, Fodstad O and Lorico A: Growth of
cancer cell lines under stem cell-like conditions has the potential
to unveil therapeutic targets. Exp Cell Res. 314:2110–2122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone M:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
Gifford DK, Melton DA, Jaenisch R and Young RA: Core
transcriptional regulatory circuitry in human embryonic stem cells.
Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kashyap V, Rezende NC, Scotland KB,
Shaffer SM, Persson JL, Gudas LJ and Mongan NP: Regulation of stem
cell pluripotency and differentiation involves a mutual regulatory
circuit of the NANOG, OCT4, and SOX2 pluripotency transcription
factors with polycomb repressive complexes and stem cell microRNAs.
Stem Cells Dev. 18:1093–1108. 2009. View Article : Google Scholar
|
|
30.
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|