Introduction
Colon cancer has an important position among
malignant disorders in Japan, the United States and Europe and is
treated by a range of therapies including surgery, chemotherapy and
radiotherapy (1–8).
Polysaccharide K (PSK), a protein-bound
polysaccharide, is a widely used, non-specific immunotherapeutic
agent that is obtained from Coriolus versicolor fungi
(9). In structure, it includes a
protein-bound polysaccharide with a molecular weight of
approximately 94,000, containing approximately 38% protein, with
the sugar portion being a β-glycan glucose that contains around 75%
glucose in addition to mannose, xylose and galactose.
To date, the major known mechanisms of action of PSK
include: i) apoptosis induction, inhibition of cell invasion and
enhanced expression of MHC class I; ii) enhanced activation of
natural killer, cytotoxic T, lymphokine-activated killer and other
cells as an effect of cytokine induction involved with immunocyte
and host defense regulation, as well as regulation of cytokine
production; and iii) inhibition of TGF-β production and alleviation
of oxidative stress by inhibition of immunosuppressive substances,
and it possesses a diverse range of immunostimulating effects as a
biochemical response modifier (10–13).
In terms of clinical knowledge, Torisu et al compared a
group of patients given PSK as monotherapy after colon cancer
curative resection with a group not given PSK, and found that the
PSK-treated group had a significantly better survival rate
(14). Sakamoto et al,
Ohwada et al and Yoshitani et al also reported that,
when PSK was combined with anticancer drugs after colon cancer
curative resection, patients who received PSK had significantly
better survival rates than did those who did not receive it
(15–17). Other reports describe the efficacy
of PSK administration with respect to gastric, esophageal, breast
and lung cancer (18–22). That is, PSK has been shown to
contribute to the treatment of a variety of malignant tumors from
the perspectives of both basic science experiments and clinical
results.
Few reports, however, have addressed the genetic
changes that occur in colon cancer cells themselves as a result of
PSK administration. In the present study, we investigated which
genes in colon cancer cells themselves are acted on by PSK and what
kind of action it exerts.
Materials and methods
Cell viability
Apoptosis was detected by cytometry using
Annexin-V-Fluos staining kit (Roche, Germany). Briefly, cells were
incubated with Annexin-V-Alexa 568 for 15 min. After cells were
washed thrice in PBS, we detected red cells under a fluorescent
microscope.
Cell culture
The human colorectal cancer cell lines: HCT116, HT29
and DLD-1 (obtained from European Collection of Cell Cultures, UK)
were cultured at 37°C in 5% CO2 in RPMI-1640 medium
containing 10% fetal bovine serum (23).
We attest that the research was performed in
accordance with the humane and ethical rules for human
experimentation that are stated in the Declaration of Helsinki.
Cell treatment and cell morphology
The cells were plated in 10-cm tissue culture dish
and co-cultured with 300 μg/ml PSK (Kureha Chemical
Industry, Co., Ltd., Tokyo, Japan). After 16 h, the cell morphology
was digitally photographed at ×200 magnification.
Cell adhesion assay
CytoSelect 48-Well cell adhesion assay (ECM Array:
Cell Biolabs Inc., San Diego, CA, USA) was performed for 10 min at
room temperature. An aliquot (5×104 cells) of the
prepared cell suspension was added into the well, as an adhesive
substrate for 8 h at 37°C in a cell culture incubator. Adherent
cells were stained and quantified at OD560 after extraction
according to the manufacturer’s instructions.
RT2 Profiler™ PCR array and
real-time PCR
Total-RNA was extracted from colon cancer cells
using guanidinium-thiocyanate (23,24).
Real-time PCR was performed according to the User Manual of
RT2 Profiler PCR array system (Extracellular Matrix and
Adhesion Molecule PCR array: Catalog no. PAHS-013A) (SABioscience,
Frederick, MD, USA). The data were analyzed using Excel-based PCR
Array Data Analysis Templates.
Tumor cell-endothelium adhesion
assays
Tumor cell-endothelium adhesion assays were carried
out according to the manufacturer’s instructions (Cell Biolabs,
Inc.). In brief, human venous endothelial cells (HUVEC)
(5×104) were seeded onto the wells and were incubated at
37°C for 48 h in a tissue culture incubator. Harvest colon cancer
cells (1×106 cells) were incubated in serum-free media
with CytoTracker™ at 37°C for one hour. Colon cancer cells
(1×105 cells) were seeded to the wells and were
incubated at 37°C for 8 h. After washing cancer cells, the adhesive
cell number were counted at ×100 magnification.
Statistical considerations
Characteristics of the two treatment arms were
compared using the χ2 test. Values of P<0.05 were
considered as statistically significant.
Results
Cell viability
Colon cancer cells (HCT116, HT29 and DLD-1) analyzed
under fluorescence microscope using Annexin-V-FLUOS staining assay
showed no increased cell apoptosis and death in samples treated
with PSK (average; 100 μg/ml: staining cells 1.5% or 300
μg/ml: staining cells 2.3%) compared with untreated cells
(staining cells 1.3%). Cells treated with 500 μg/ml showed
slightly increased cell apoptosis and death (staining cells
7.1%).
Investigation of colon cancer cell
morphology after PSK stimulation
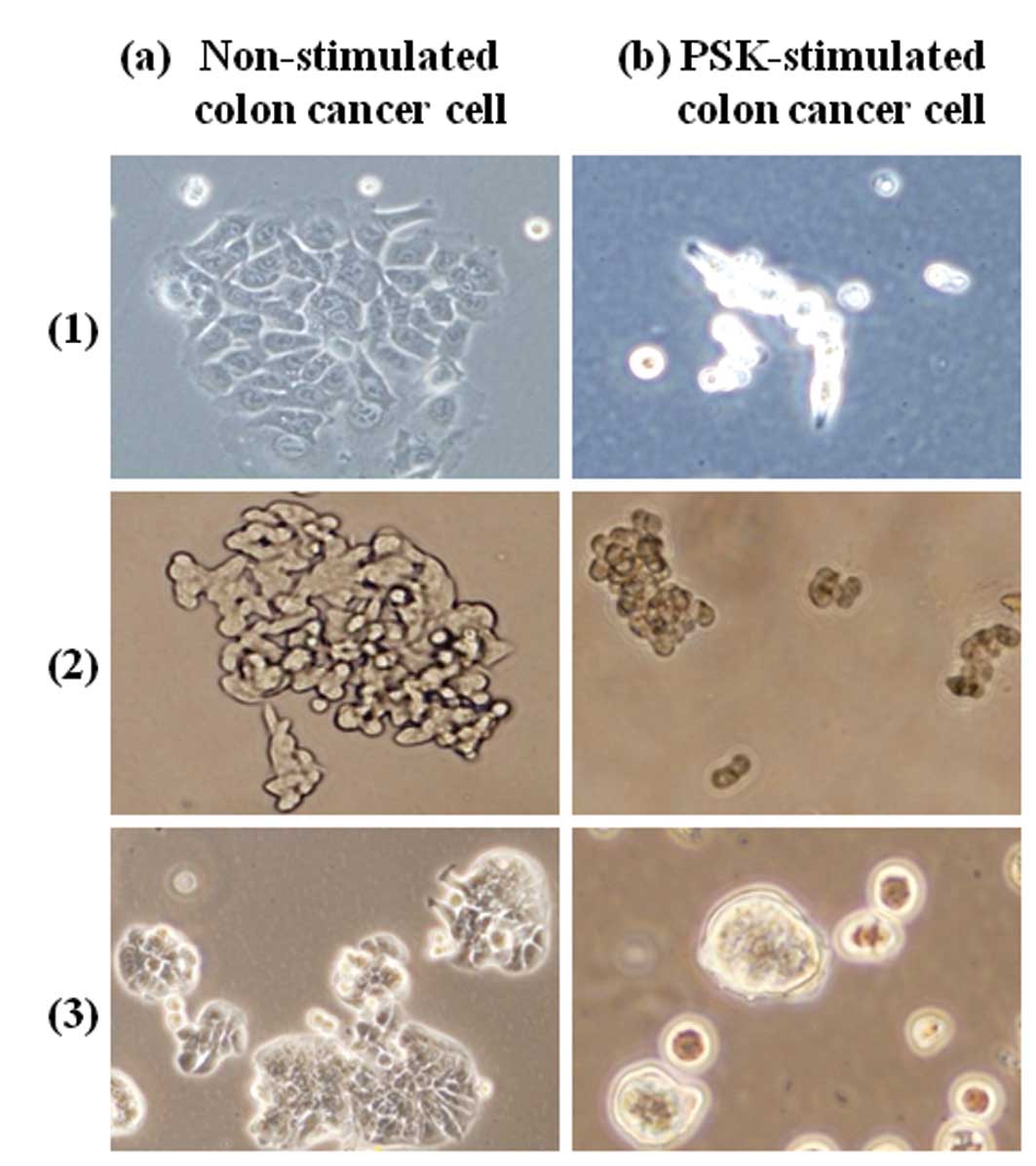
We investigated the changes in cell morphology that
occurred as a result of exposing colon cancer cells (HCT116, HT29,
DLD-1) to PSK. The results are shown in Fig. 1. Non-stimulated colon cancer cells
were fusiform in shape and exhibited intercellular adhesion,
proliferating in sheet form, whereas PSK-stimulated colon cancer
cells were spherical and exhibited reduced adhesion between
cells.
Investigation of the adhesion rate of
colon cancer cells after PSK stimulation
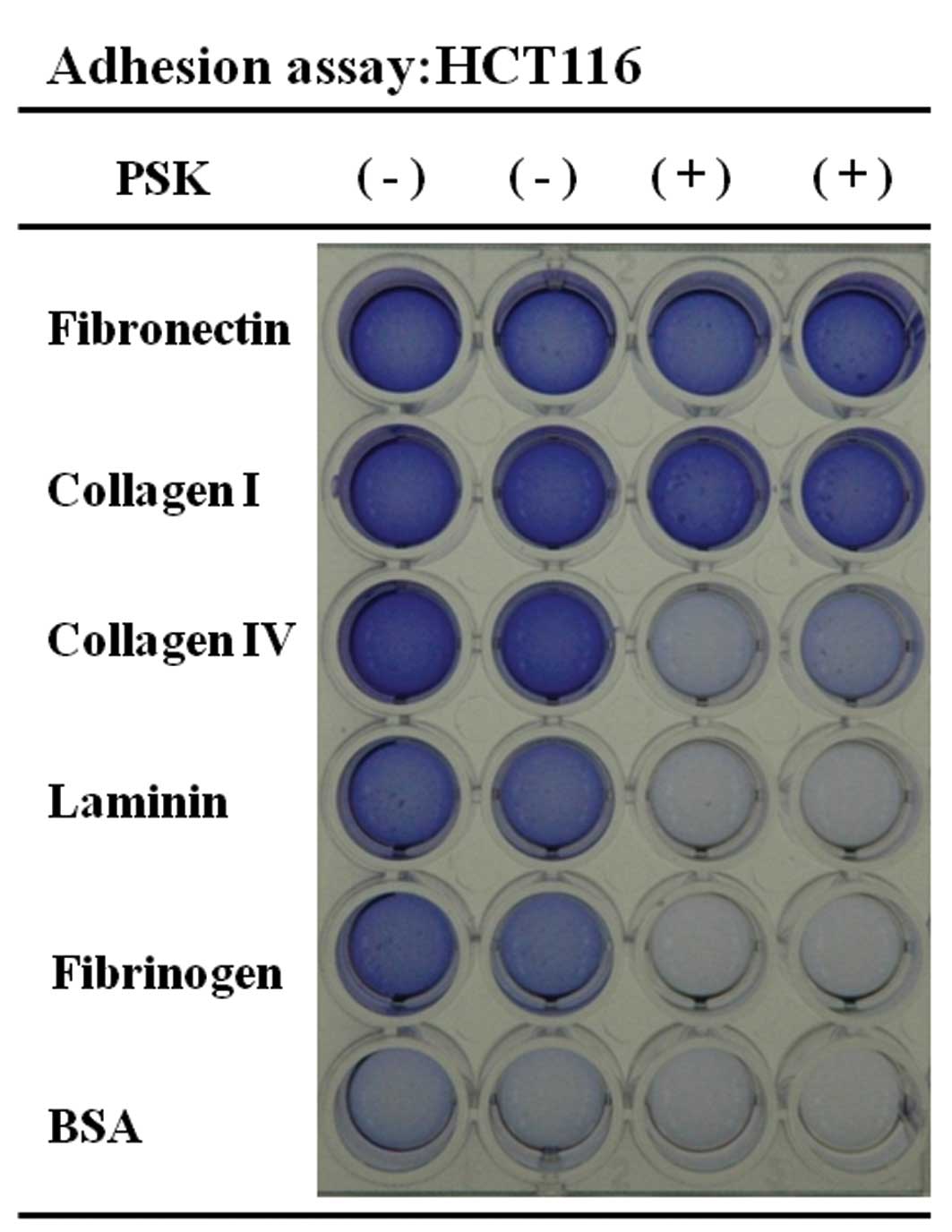
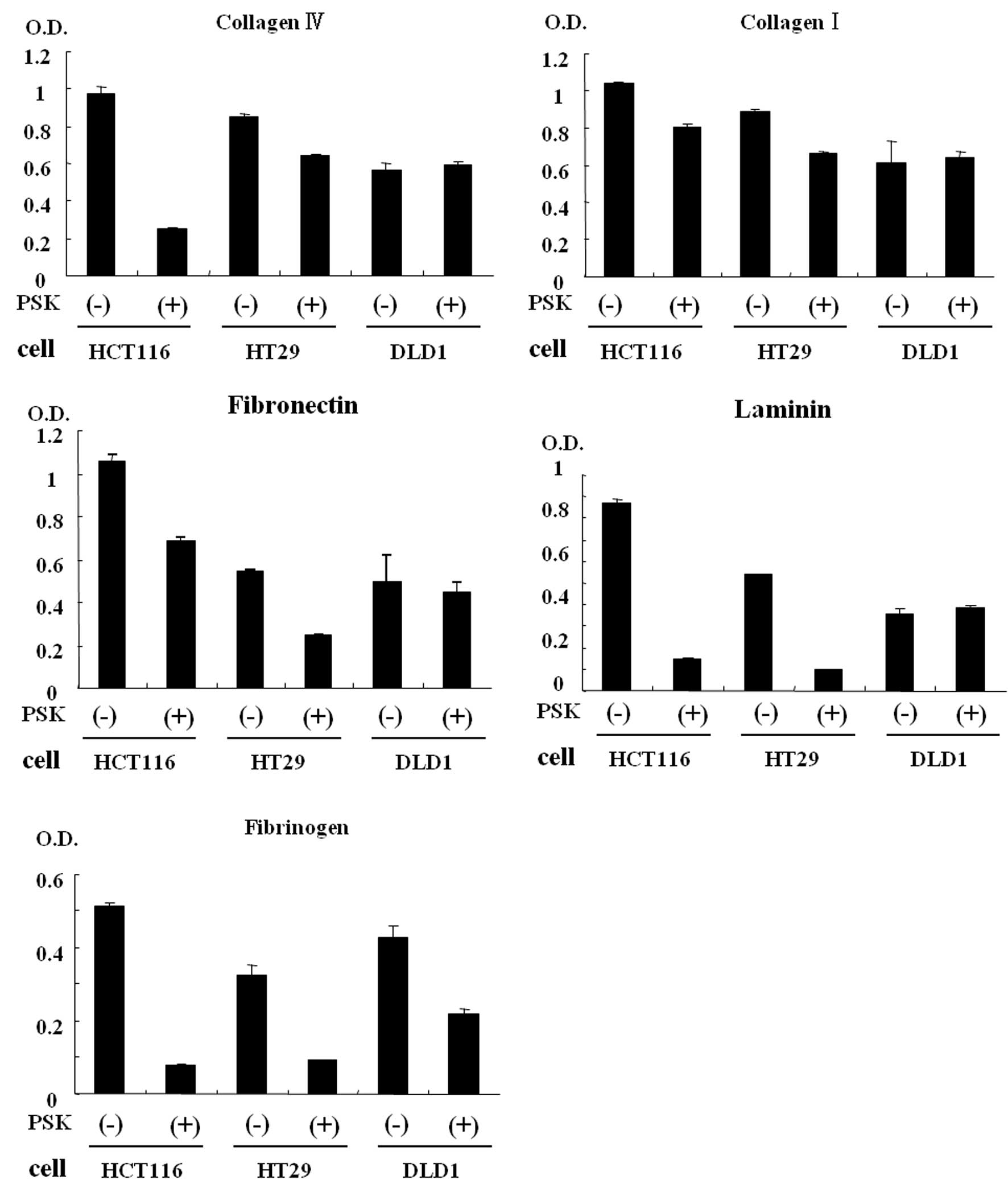
We investigated the cellular adhesion rate of colon
cancer cells after PSK stimulation. The results are shown in
Figs. 2 and 3. A comparison of non-stimulated colon
cancer cells (HCT116 and HT29) and PSK-stimulated HCT116 or HT29
colon cancer cells showed reduced adhesion rates for laminin,
fibrinogen, collagen IV, collagen I and fibronectin. A comparison
of non-stimulated DLD-1 colon cancer cells and PSK-stimulated DLD-1
colon cancer cells showed that fibrinogen was the protein that
showed the reduction in cellular adhesion.
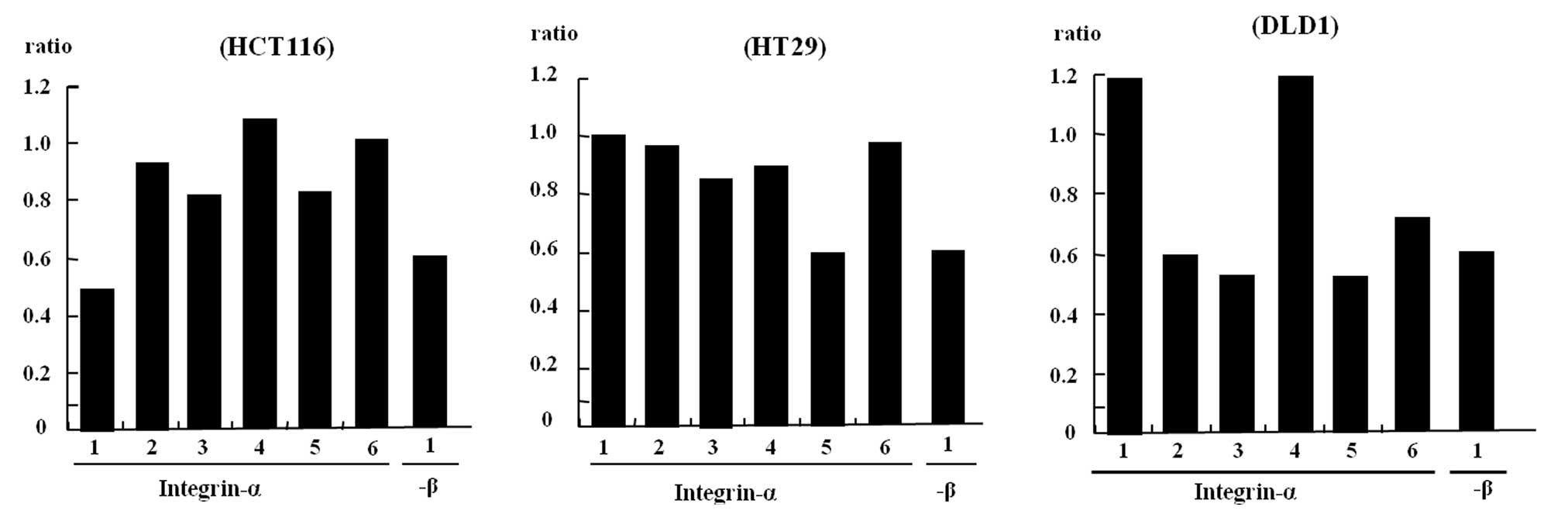
Investigation of adhesion molecule
integrin mRNA of colon cancer cells after PSK stimulation
The results mentioned above indicate the involvement
of integrin mRNA, which are believed to be ligands for the
substrates; therefore, the expression of each of these mRNAs was
investigated (Fig. 4).
A comparison of non-stimulated HCT116 colon cancer
cells with PSK-stimulated HCT116 colon cancer cells showed that
expression of the integrin α-1, 3, 5 and β-1 mRNA was significantly
reduced in PSK-stimulated HCT116 colon cancer cells.
Non-stimulated HT29 colon cancer cells compared with
PSK-stimulated HT29 colon cancer cells showed that expression of
the integrin α-5 and β-1 mRNA was significantly reduced in
PSK-stimulated HT29 colon cancer cells.
A comparison of non-stimulated DLD-1 colon cancer
cells with PSK-stimulated DLD-1 colon cancer cells showed that
expression of the integrin α-2, 3, 5, 6 and β-1 mRNA was
significantly reduced in PSK-stimulated DLD-1 colon cancer
cells.
Investigation of the adhesion rate of
PSK-stimulated colon cancer cells to human vascular endothelial
cells
We investigated the extent to which non-stimulated
colon cancer cells and PSK-stimulated colon cancer cells adhered to
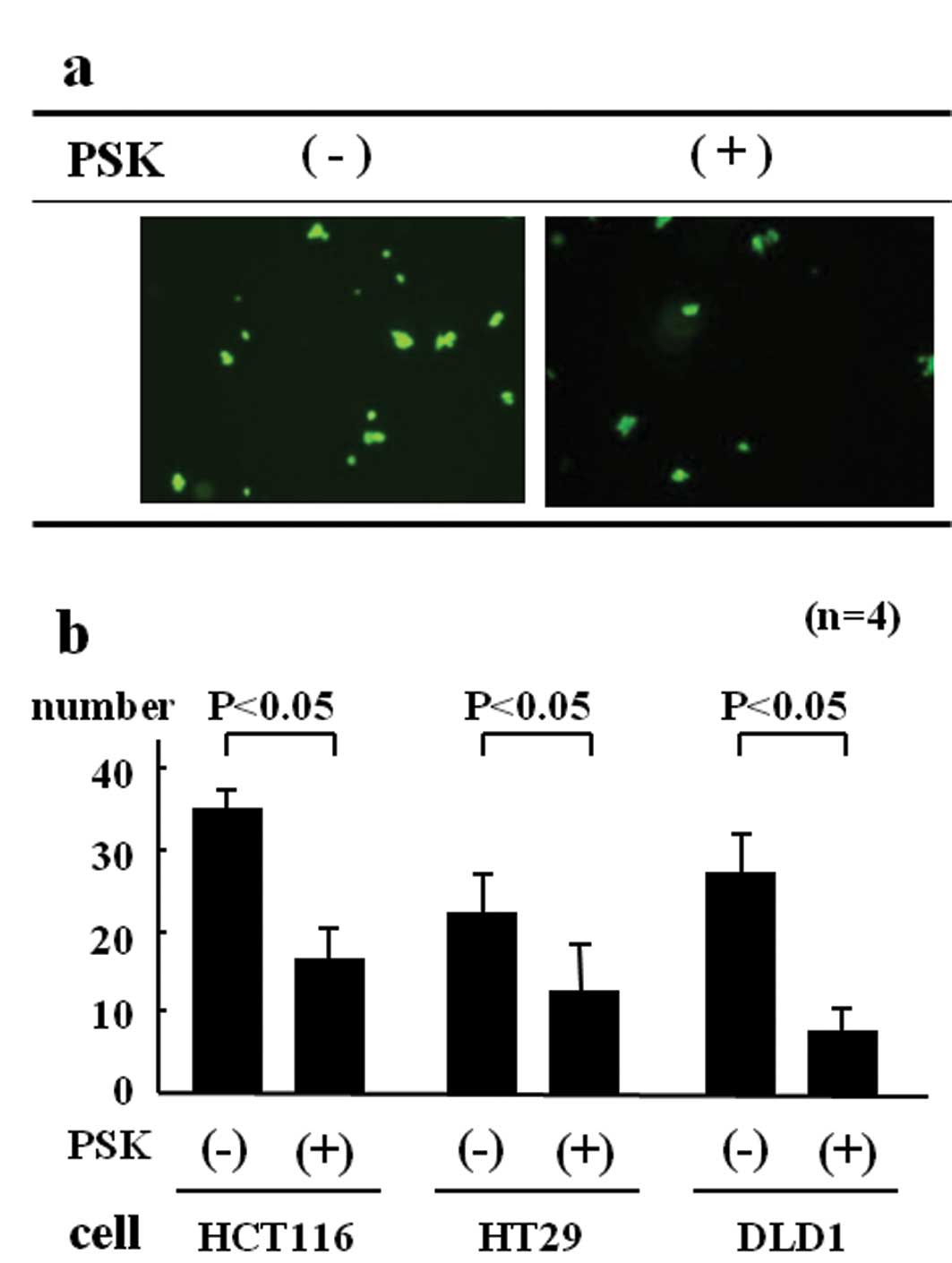
human vascular endothelial cells. The results are shown in Fig. 5. The cell adhesion rate for
non-stimulated HCT116 colon cancer cells was 34.5 cells/field of
view, whereas for PSK-stimulated HCT116 colon cancer cells, it was
17.3 cells/field of view, a significantly lower rate of adhesion to
HUVEC (Fig. 5b).
The cell adhesion rate for non-stimulated HT29 colon
cancer cells was 21.4 cells/field of view, whereas for
PSK-stimulated HT29 colon cancer cells, it was 11.8 cells/field of
view, a significantly lower rate of adhesion to HUVEC. The cell
adhesion rate for non-stimulated DLD-1 colon cancer cells was 27.2
cells/field of view, whereas for PSK-stimulated DLD-1 colon cancer
cells, it was 8.8 cells/field of view, a significantly lower rate
of adhesion to HUVEC.
Discussion
Polysaccharide K (PSK), a protein-bound
polysaccharide, is a widely used, non-specific immunotherapeutic
agent that is obtained from Corillus versicolor fungi
(9). It basically acts as a
biochemical response modifier and possesses a diverse range of
immunostimulating effects, including activating lymphocyte
proliferation and enhancing lymphokine production (25–27).
In research on mice, Kobayashi et al implanted Lewis lung
carcinoma subcutaneously and found that metastasis to the lung was
suppressed by peritoneal administration of PSK (28), while Hosokawa et al
administered PSK before or after surgery on mice with induced
autologous tumor lineages and reported that survival time was
extended compared with mice that had undergone the surgical
procedure only (29). A number of
reports have also indicated its clinical efficacy for malignant
tumors of the digestive tract, particularly gastric and colon
cancers (14–17). In terms of clinical results with
respect to human colon cancer, Torisu et al(14), Sakamoto et al(15) and Ohwada et al(16) have reported significantly improved
survival rates as a result of PSK administration to patients
undergoing curative resection, compared with patients who did not
receive PSK. It is thus clear that PSK acts effectively as a
therapeutic agent, but most reports concerning its mechanism of
action have focused on normal cells other than cancer cells,
examining how it acts on the immune response in a variety of
healthy mice or human tissues (5,6) and
there have been almost no studies of the morphological changes to
cancer cells themselves or of their molecular biology. In the
present study, we investigated the way in which PSK stimulation
acts on colon cancer cells themselves. As shown in Fig. 1, clear changes in cellular
morphology were observed, leading to the discovery that PSK has an
effect on the skeletal system of the cells.
Liver metastasis is generally regarded as a
prognostic factor in colon cancer. The mechanism whereby liver and
hematogenous metastases of colon cancer occur involves cancer cells
breaking off the primary lesion, after which they penetrate the
capillaries and spread throughout the body via the portal and
greater circulatory systems. They then adhere to vascular
endothelial cells at the target organ, escape from the blood
vessel, infiltrate the area outside and proliferate at the
metastatic site. As a result of the elucidation of this mechanism
of metastasis in recent years, it has been reported that molecules
such as cellular adhesion molecules and angiogenic growth factors
are important (30).
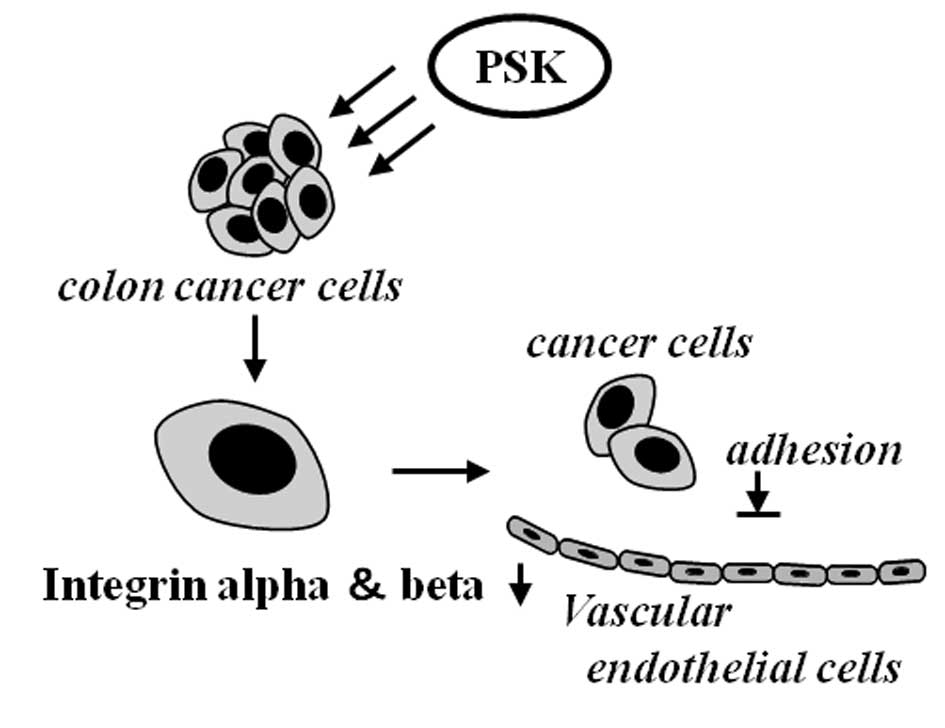
We therefore focused on adhesion to vascular
endothelial cells at target organs as the first stage in metastasis
to a distant organ and investigated what changes occurred to
adhesion molecules and adhesion of colon cancer cells after PSK
stimulation. The reduction in the adhesion rate was observed for
laminin, fibrinogen, collagen IV, collagen I and fibronectin. These
proteins are a constituent of basement membranes together with
nidogen and heparan sulfate proteoglycan, and reported to be
involved in neurite growth and cell adhesion, proliferation,
differentiation and migration (31,32).
Integrin is the best-known ligand for the proteins of basement
membranes (33) and has been
implicated by a number of reports in liver metastasis of colon
cancer (34). We investigated the
changes that are caused by PSK stimulation to the expression of
integrin mRNA in colon cancer cells. As shown in Fig. 4, the expression of integrin mRNA
decreased. We also investigated the adhesiveness between vascular
endothelial cells and PSK-stimulated colon cancer cells. Compared
with cells not exposed to PSK, their adhesiveness was significantly
lower, indicating that PSK acts on colon cancer cells, lowering
their adhesion to vascular endothelial cells by reducing cell
adhesion molecules (Fig. 6).
References
|
1
|
Japanese Society for Cancer of the Colon
and Rectum: Multi-institutional registry of large bowel cancer in
Japan, cases treated in 1995–1998.
17:1999.18:2000.21:2001.24:2003.
|
|
2
|
Heald RJ, Husband EM and Ryall RD: The
mesorectum in rectal cancer surgery - the clue to pelvic
recurrence? Br J Surg. 69:613–616. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah SA, Haddad R, Al-Sukhni W, Kim RD,
Greig PD, Grant DR, Taylor BR, Langer B, Gallinger S and Wei AC:
Surgical resection of hepatic and pulmonary metastases from
colorectal carcinoma. J Am Coll Surg. 202:468–475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004. View Article : Google Scholar
|
|
5
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G,
Landi B, Colin P, Louvet C and de Gramont A: FOLFIRI followed by
FOLFOX6 or the reverse sequence in advanced colorectal cancer: a
randomized GERCOR study. J Clin Oncol. 22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, Couture F, Sirzén F and Cassidy J: Bevacizumab in
combination with oxaliplatin-based chemotherapy as first-line
therapy in metastatic colorectal cancer: a randomized phase III
study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M and Bedenne
L: Preoperative radiotherapy with or without concurrent
fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD
9203. J Clin Oncol. 24:4620–4625. 2006.PubMed/NCBI
|
|
9
|
Tsukagoshi S, Hashimoto Y, Fujii G,
Kobayashi H, Nomoto K and Orita K: Krestin (PSK). Cancer Treat Rev.
11:131–155. 1984. View Article : Google Scholar
|
|
10
|
Araya S, Nio Y, Hayashi H, Masai Y,
Tsubono M, Ishigami S and Imamura M: Various plant-derived
polysaccharides augment the expression of HLA on Colo205 human
colonic cancer line. J Jpn Soc Cancer Ther. 29:1965–1973. 1994.
|
|
11
|
Hirose K, Zachariae CO, Oppenheim JJ and
Matsushima K: Induction of gene expression and production of
immunomodulating cytokines by PSK in human peripheral blood
mononuclear cells. Lymphokine Res. 9:475–483. 1990.PubMed/NCBI
|
|
12
|
Algarra I, Collado A, Garcia Lora A and
Garrido F: Differential effect of protein-bound polysaccharide
(PSK) on survival of experimental murine tumors. J Exp Clin Cancer
Res. 18:39–46. 1999.PubMed/NCBI
|
|
13
|
Harada M, Matsunaga K, Oguchi Y, Iijima H,
Tamada K, Abe K, Takenoyama M, Ito O, Kimura G and Nomoto K: Oral
administration of PSK can improve the impaired anti-tumor
CD4+ T-cell response in gut-associated lymphoid tissue
(GALT) of specific-pathogen-free mice. Int J Cancer. 70:362–372.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torisu M, Hayashi Y, Ishimitsu T, Fujimura
T, Iwasaki K, Katano M, Yamamoto H, Kimura Y, Takesue M, Kondo M,
et al: Significant prolongation of disease-free period gained by
oral polysaccharide K (PSK) administration after curative surgical
operation of colorectal cancer. Cancer Immunol Immunother.
31:261–268. 1990. View Article : Google Scholar
|
|
15
|
Sakamoto J, Morita S, Oba K, Matsui T,
Kobayashi M, Nakazato H and Ohashi Y; Meta-Analysis Group of the
Japanese Society for Cancer of the Colon Rectum: Efficacy of
adjuvant immunochemotherapy with polysaccharide K for patients with
curatively resected colorectal cancer: a meta-analysis of centrally
randomized controlled clinical trials. Cancer Immunol Immunother.
55:404–411. 2006. View Article : Google Scholar
|
|
16
|
Ohwada S, Ikeya T, Yokomori T, Kusaba T,
Roppongi T, Takahashi T, Nakamura S, Kakinuma S, Iwazaki S,
Ishikawa H, Kawate S, Nakajima T and Morishita Y: Adjuvant
immunochemotherapy with oral Tegaful/Uracil plus PSK in patients
with stage II or III colorectal cancer: a randomized controlled
study. Br J Cancer. 90:1003–1010. 2004. View Article : Google Scholar
|
|
17
|
Yoshitani S and Takashima S: Efficacy of
postoperative UFT (Tegafur/Uracil) plus PSK therapies in elderly
patients with resected colorectal cancer. Cancer Biother
Radiopharm. 24:35–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shibata M, Nezu T, Fujisaki S, Andou K,
Tomita R and Fukuzawa M: Clinical potential of biological response
modifiers combined with chemotherapy for gastric cancer. Japanese
experience Dig Surg. 19:255–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oba K, Teramukai S, Kobayashi M, Matsui T,
Kodera Y and Sakamoto J: Efficacy of adjuvant immunochemotherapy
with polysaccharide K for patients with curative resections of
gastric cancer. Cancer Immunol Immunother. 56:905–911. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogoshi K, Satou H, Isono K, Mitomi T,
Endoh M and Sugita M: Immunotherapy for esophageal cancer. A
randomized trial in combination with radiotherapy and
radiochemotherapy Cooperative Study Group for Esophageal Cancer in
Japan. Am J Clin Oncol. 18:216–222. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morimoto T, Ogawa M, Orita K, Sugimachi K,
Toge T, Dohi K, Nomura Y, Monden Y and Ogawa N: Postoperative
adjuvant randomised trial comparing chemoendocrine therapy,
chemotherapy and immunotherapy for patients with stage II breast
cancer: 5-year results from the Nishinihon Cooperative Study Group
of Adjuvant Chemoendocrine Therapy for Breast Cancer (ACETBC) of
Japan. Eur J Cancer. 32A:235–242. 1996.
|
|
22
|
Hayakawa K, Mitsuhashi N, Saito Y,
Nakayama Y, Furuta M, Nakamoto S, Kawashima M and Niibe H: Effect
of Krestin as adjuvant treatment following radical radiotherapy in
non-small cell lung cancer patients. Cancer Detect Prev. 21:71–77.
1997.PubMed/NCBI
|
|
23
|
Sato T, Yamaguchi A, Goi T, Hirono Y,
Takeuchi K, Katayama K and Matsukawa S: Heparanase expression in
human colorectal cancer and its relationship to tumor angiogenesis,
hematogenous metastasis and prognosis. J Surg Oncol. 87:174–181.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goi T, Yamaguchi A, Nakagawara G, Urano T,
Shiku H and Furukawa K: Reduced expression of deleted colorectal
carcinoma (DCC) protein in established colon cancers. Br J Cancer.
77:466–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fisher M and Yang LX: Anticancer effects
and mechanisms of polysaccharide-K (PSK): implications of cancer
immunotherapy. Anticancer Res. 22:1737–1754. 2002.PubMed/NCBI
|
|
26
|
Asai H, Iijima H, Matsunaga K, Oguchi Y,
Katsuno H and Maeda K: Protein-bound polysaccharide K augments IL-2
production from murine mesenteric lymph node CD4+ T
cells by modulating T cell receptor signaling. Cancer Immunol
Immunother. 57:1647–1655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kato M, Hirose K, Hakozaki M, Ohno M,
Saito Y, Izutani R, Noguchi J, Hori Y, Okumoto S, Kuroda D, et al:
Induction of gene expression for immunomodulating cytokines in
peripheral blood mononuclear cells in response to orally
administered PSK, an immunomodulating protein-bound polysaccharide.
Cancer Immunol Immunother. 40:152–156. 1995. View Article : Google Scholar
|
|
28
|
Kobayashi H, Matsunaga K and Oguchi Y:
Antimetastatic effects of PSK (Krestin), a protein-bound
polysaccharide obtained from basidiomycetes: an overview. Cancer
Epidemiol Biomarkers Prev. 4:275–281. 1995.PubMed/NCBI
|
|
29
|
Hosokawa M, Mizukoshi T, Sugawara M and
Kobayashi H: Therapeutic effects of PS-K and busulfan on the
recurrent and metastatic diseases after the surgical removal of
3-methylcholanthrene-induced autochthonous tumors in C57BL/6 mice.
Jpn J Cancer Res. 76:61–67. 1985.
|
|
30
|
Fidler IJ and Ellis LM: The implications
of angiogenesis for the biology and therapy of cancer metastasis.
Cell. 79:185–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heino J and Käpylä J: Cellular receptors
of extracellular matrix molecules. Curr Pharm Des. 15:1309–1317.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kramer JM: Basement membranes. WormBook.
1:1–15. 2005.
|
|
33
|
van der Flier A and Sonnenberg A: Function
and interactions of integrins. Cell Tissue Res. 305:285–298.
2001.
|
|
34
|
Paschos KA, Canovas D and Bird NC: The
role of cell adhesion molecules in the progression of colorectal
cancer and the development of liver metastasis. Cell Signal.
21:665–674. 2009. View Article : Google Scholar : PubMed/NCBI
|