Introduction
Colorectal carcinogenesis is generally a slow
process spanning decades from cancer initiation to diagnosis. Thus,
this time span provides considerable opportunity to focus on the
discovery and identification of dietary agents and drugs that might
prevent or inhibit tumor development (1). Since about one-third of the overall
risk of cancer in human may be related to diet, a large number of
dietary compounds have been tested to determine their potential
chemopreventive properties in various experimental cancer models
(2–4). Many vegetables and their bioactive
compounds exhibit strong chemopreventive ability against several
neoplasms, including colorectal cancer, as evidenced by
epidemiological and experimental studies (5–7).
Asparagus officinalis L. is a popular vegetable dish
consumed in most part of the world. Asparagus is rich in many
bioactive phytochemicals such as steroidal saponins (8–10),
flavonoids (11,12), dietary fibre (13) and oligosaccharides (14). Several asparagus constituents have
been reported to possess antioxidant (12,15)
anti-inflammatory (16), antitumor
(10), hypolipidaemic (17) and antifungal (18) activities. However, there is a lack
of information regarding the antitumor properties of asparagus
constituents especially on colorectal cancer.
The aim of the present study was to gain more
insight into the anti-proliferative mechanisms of a methanolic
extract of white Asparagus officinalis shoots on human colon
carcinoma cells, and to evaluate its anti-carcinogenic potential in
a preclinical rat model of colon carcinogenesis. Until now,
activation of cancer cell death by constituents isolated from
asparagus was only shown for human promyelocytic leukemia cells
(HL-60) (19), liver
hepatocellular cells (HepG2) (20)
and a reduction of cell viability associated with elevated
expression of some apoptotic markers in colon carcinoma HCT116
cells (21).
In order to address both primary and metastatic
colorectal cancer cells, we used the human colon cancer SW480 cells
and their derived metastatic SW620 cells as a model for colorectal
cancer progression. The SW480 cell line is isolated from a primary
human colon adenocarcinoma, and the SW620 cell line is derived from
the primary tumor but isolated from a mesenteric lymph node
metastasis of the same patient. These two cell lines have been
validated as an in vitro model of colon cancer progression
from a primary tumor to its metastatic spreading (22).
Anticancer activity of asparagus shoots has not been
demonstrated in vivo. In order to evaluate the
chemopreventive effects of asparagus shoot extract we used the
well-known azoxymethane (AOM)-induced colon cancer model in rat
(23–25). In this model, one of the earliest
recognizable precancerous lesions is the appearance of hyper
proliferative aberrant crypt foci (ACF) about 5 weeks after cancer
initiation by AOM (26–28). These foci are putative precancerous
lesions that indicate the initiation of a carcinogenic process. ACF
have been used as surrogate biomarkers to screen numerous potential
chemopreventive agents (26), also
highlighting the importance of the rat-AOM model in the screening
for new drugs designed for preventive and/or therapeutic activity
against colorectal cancer. Here, we extended these investigations
in order to determine the effects of an oral administration of a
methanolic extract of white Asparagus officinalis L. shoots
(Asp) on the development of AOM-induced ACF formation, and on the
expression of several biomarkers involved in the inflammatory and
apoptotic responses in the early post-initiation phases of colon
carcinogenesis.
Materials and methods
Plant material
The shoots of Asparagus officinalis L. (var.
Gijnlim) were provided by a local asparagus producer
(Spargelhof Böser, Bruchsal, Germany). The crushed shoots (about 13
kg) were freeze-dried (912 g after freeze-drying) and then
extracted successively with CH2Cl2 and
CH3OH. Extraction was performed three times with
CH2Cl2 (150 ml for 15 g) for 6 h (about 25–30
extraction cycles) using a Soxhlet apparatus (150 ml, Behr,
Germany). The extracts were combined and evaporated under vacuum at
40°C. The asparagus residue was then extracted three times by
maceration under stirring for 2 h with CH3OH (150 ml for
15 g). All three methanolic extracts were then combined and
concentrated under vacuum at 40°C. The crude methanolic extract
(PE) was freeze-dried and subsequently purified on a C18 Sep-Pak
cartridge (10 g, 35 cc, Waters, Dachstein, France). About 2 g of
freeze-dried PE was dissolved in 10 ml water and loaded onto the
cartridge, previously activated using 50 ml CH3OH + 0.1%
formic acid, then conditioned with 50 ml H2O + 1% formic
acid. The washing step was performed using 75 ml of H2O
+ 1% formic acid to remove sugars and organic acids. Fraction of
interest (Asp) was then eluted from the cartridge using 75 ml of
CH3OH + 0.1% formic acid, concentrated under vacuum and
freeze-dried.
Cell culture
SW480 and SW620 cells were obtained from the
European Collection of Animals Cell Culture (Salisbury, UK). They
were maintained in Dulbecco’s modified Eagle’s medium (DMEM)
containing 25 mM glucose and supplemented with 10% heat-inactivated
(56°C) horse serum, 100 U/ml penicillin, 100 μg/ml
streptomycin and 1% non-essential amino acids (Invitrogen Corp.,
Cergy Pontoise, France) and kept at 37°C in a humidified atmosphere
with 5% CO2. For experiments, after trypsinization (0.5%
trypsin/2.6 mM ethylenediamine tetra-acetic acid), cells were
seeded at 1×106 cells in culture dishes (100 mm internal
diameter) or at 2×105 cells in culture dishes (25 mm
internal diameter). The culture medium was DMEM supplemented with
3% heat-inactivated horse serum, 100 U/ml penicillin, 100
μg/ml streptomycin, 5 μg/ml transferrin, 5 ng/ml
selenium, 10 μg/ml insulin and 1% non-essential amino acids
(Invitrogen Corp.).
Cell growth rate
Cells were exposed 24 h after seeding to different
concentrations of Asp varying from 0 to 80 μg/ml. The final
concentration of DMSO in the culture medium was 0.1%. Control cells
were treated with DMSO 0.1%. Culture medium was replaced every 48
h. At different time points, cell culture was stopped by the
addition of trichloroacetic acid (50% v/v), and cell proteins were
determined by staining with 200 μl Sulforhodamine B (0.4%
w/v) (Sigma-Aldrich, Saint Quentin Fallavier, France). The
relationship between cell number (protein content per well) and
absorbance is linear from 0 to 2×105 cells/well.
Cell death analysis by flow cytometry and
treatments
Cells (2×105) were seeded on culture
dishes (25 mm diameter) and were incubated with TRAIL (50 ng/ml)
(Alexis Biochemicals, Lausen, Switzerland) or with Asp (80
μg/ml) or with a combination TRAIL + Asp (80 μg/ml).
Cells were centrifuged and fixed with 1 ml methanol: PBS (9:1 v/v),
washed twice in PBS and re-suspended in 200 μl PBS
containing 0.25 μg/ml RNase A and 0,1 mg/ml propidium iodide
(Sigma-Aldrich). After incubation in the dark at 37°C for 30 min,
the fluorescence of 10,000 cells was analyzed by flow cytometry and
CellQuest software (FACScan, BD Biosciences, Erembodegem,
Belgium).
For caspase inhibition, cells were pre-treated with
the specific pan-caspase-inhibitor Z-VAD-FMK at 20 μM (MBL
International Corporation, Japan) for 1 h 30 min before treatment
with ASP. Cells were harvested after 24 h and processed as
described above.
For the study on the role of DR4 and DR5 death
receptors, Human recombinant DR4/Fc and DR5/Fc chimera proteins
were purchased from R&D Systems (Lille, France) and used at 100
ng/ml with a pre-treatment time of 30 min. Cells were harvested
after 24 h and processed as described above.
Measurement of caspase-3 and caspase-8
activities
Caspase activity was measured by colorimetric assay
kits (Sigma-Aldrich) according to the manufacturer’s instructions.
Briefly, 20 μl of cell or tissue lysates were added to a
buffer containing a p-nitroaniline (pNA)-conjugated substrate for
caspase-3 (Ac-DEVD-pNA) or -8 (Ac-IETD-pNA) to a total reaction
volume of 100 μl. Incubation was carried at 37°C. The
concentration of the released pNA was calculated from the
absorbance values at 405 nm and the calibration curve of defined
pNA solutions. Data were adjusted according to the protein
content.
Expression of TRAIL death receptors DR4
and DR5
Cells were treated with Asp (80 μg/ml) and
harvested by trypsinization at 24 and 48 h. Cell pellets were
washed with PBS and incubated with FITC-conjugated mouse anti-human
TRAIL receptors DR4 and DR5 (Alexis Biochemicals), or
FITC-conjugated mouse IgG1 monoclonal isotype control antibody (BD
Biosciences) for 30 min at 4°C in the dark. After washing with PBS,
cells were re-suspended in PBS and the fluorescence (515 nm) of
10.000 events per sample were analyzed by FACScan and CellQuest
Software (BD Biosciences).
Real-time quantitative
reverse-transcriptase polymerase chain reaction analysis
Total RNA was isolated, using the RNeasy Plus mini
kit (Qiagen, Austin, TX, USA), from SW480 and SW620 cells after 24
and 48 h treatment with Asp, or from the scraped proximal colon
mucosa of NaCl-injected rats, AOM-injected rats and of Asp-treated
AOM-injected rats. The High Capacity cDNA Reverse Transcription kit
(Applied Biosystems, Foster City, CA, USA) was used for cDNA
synthesis as recommended by the supplier. RT-PCR was performed by
using ABI TaqMan gene expression assays for rats: MMP-7,
MMP-9 (assay ID: Rn00563467; Rn00579162), IL1β,
TNF-α (assay ID: Rn99999009; Rn99999017), DEF-5,
LCN2 (RN01478512; RN00590612) and Bcl-2, Bax,
(assay ID: Rn99999125; Rn02532082), FAS (CD95),
FASL, DR5, TRAIL (RN00685720; RN00563754;
RN01753393; RN00686175); and for human SW40 and SW620 cells:
DR4 and DR5 (assay ID: Hs00269492 and Hs00366272)
according to the manufacturer’s instructions. All samples were run
in triplicate in 25 μl reaction volume. Quantitative
real-time RT-PCR was performed by using TaqMan Universal PCR Master
mix (Applied Biosystems) and the ABI Prism 7500 Sequence Detection
System (Sequence detector; Applied Biosystems) in triplicate wells.
The data were analyzed by a comparative threshold cycle (Ct)
method. CT values were calculated using the 7500 SDS software
(Applied Biosystems). The corresponding mRNA level from colonic
mucosa of NaCl-injected control rats was used as an external
reference. The level of β-actin mRNA (assay ID: Rn00667869
or assay ID: Hs99999903) of each sample was used as an internal
reference to normalize the data. For the in vivo
experiments, the fold-changes of each mRNA (mRNA relative
expression) were expressed relative to the mean value of the
corresponding mRNA found in the mucosa of the NaCl-injected control
rats and was calculated using the 2ΔΔCT method (29).
Animals and treatments
All animal experiments were performed in accordance
with the institutional guidelines of the French Ethics Committee
(authorization no. A67-480, French Ministry of Agriculture). Male
Wistar rats (n=24) obtained from C.E.R. Janvier (Le Genest St Isle,
France) and weighing 300 g were housed under standardized
conditions (22°C, 60% relative humidity, 12 h light/12 h dark
cycle, 20 air changes/h) and fed a standard chow with free access
to drinking water. Sixteen rats received intra-peritoneal
injections of azoxymethane (AOM) (Sigma-Aldrich), at a
concentration of 15 mg/kg body weight, once a week for 2 weeks. One
week after the last injection of AOM (post-initiation), rats were
randomly separated into two groups. One group (n=8) received daily
at 5 pm a solution of 0.01% Asp (14 mg/kg body weight) in drinking
water. The AOM-treated control rats (n=8) received drinking water.
One group of rats (n=8) injected with 0.9% NaCl (saline) once a
week for 2 weeks receiving drinking water was used as reference.
Rats consumed daily about 40 ml of the drinking fluid during the
whole experimental period. All animals were sacrificed 7 weeks
after AOM or saline injection.
Assessment of aberrant crypts in the
colon
The determination of hyperproliferative aberrant
crypts was performed on a segment of 6 cm in length, corresponding
to the distal part of the colon. The segment was washed with
physiological saline, cut open, pinned out flat and fixed in 10%
buffered formalin. The colon was stained with 0.2% methylene blue
for 5 min, rinsed in Krebs-Ringer buffer, placed onto a glass slide
and examined microscopically using a low-power objective (×5) to
assess hyperproliferative crypts and aberrant crypt foci (ACF). The
criteria for the identification of hyperproliferative aberrant
crypts were: i) increased size; ii) thicker epithelial cell lining;
and iii) increased pericryptal zone relative to normal crypts.
Mucosal samples of the distal colon of NaCl-injected rats,
AOM-injected control rats and Asp-treated AOM-injected rats were
scraped off with a glass slide and immediately frozen in liquid
nitrogen for biological assays.
Western blot analyses of protein
expression
Mucosal samples were homogenized in a RIPA lysis
buffer composed of 150 mM NaCl, 50 mM Tris (pH 8.0), 5 mM EDTA,
using a polytron homogenizer. After ultra-centrifugation for 30 min
at 10,000 × g at 4°C, the protein content was measured by the Lowry
method. Equal amounts of total protein were separated by 15% sodium
dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis for 2 h 30
min at 65 V. Proteins were then transferred to a nitrocellulose
membrane (Bio-Rad Laboratories, Marnes-la-Coquette, France). The
membrane was blocked with a solution containing bovine serum
albumin (BSA) 3%, Tween-20 0.1%, Tris-HCl 10 mM (pH 7.5), and 0.1%
NaCl, for 1 h and, after stripping for 30 min at 50°C with a buffer
containing 100 mM β-mercaptoethanol, 2% SDS and 62 mM Tris-HCl (pH
6.7), was incubated overnight at 4°C with one of the following
primary monoclonal antibodies: rabbit anti-rat DR5 at 1:500 (BD
Biosciences, Erembodegem, Belgium), goat anti-rat LCN2 at 1:5,000
(Calbiochem, Merck Biosciences), rabbit anti-rat MMP-7 at 1:500
(Santa Cruz Biotechnology, Santa Cruz, USA) or mouse anti-rat
β-actin at 1:2,000 (Chemicon International, Hampshire, UK). The
membranes were washed and incubated with 0.02 μg/ml
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
(Calbiochem, Merck Biosciences) or donkey anti-goat IgG
(Calbiochem, Merck Biosciences) for 1 h and visualized using the
Super Signal West Pico Chemiluminescent Substrate System
(Pierce).
Statistical analysis
Data are reported as the mean ± SE. Statistical
differences between control and treated groups were evaluated using
the Student’s t-test or the Student-Newman-Keuls multiple
comparison test.
Results
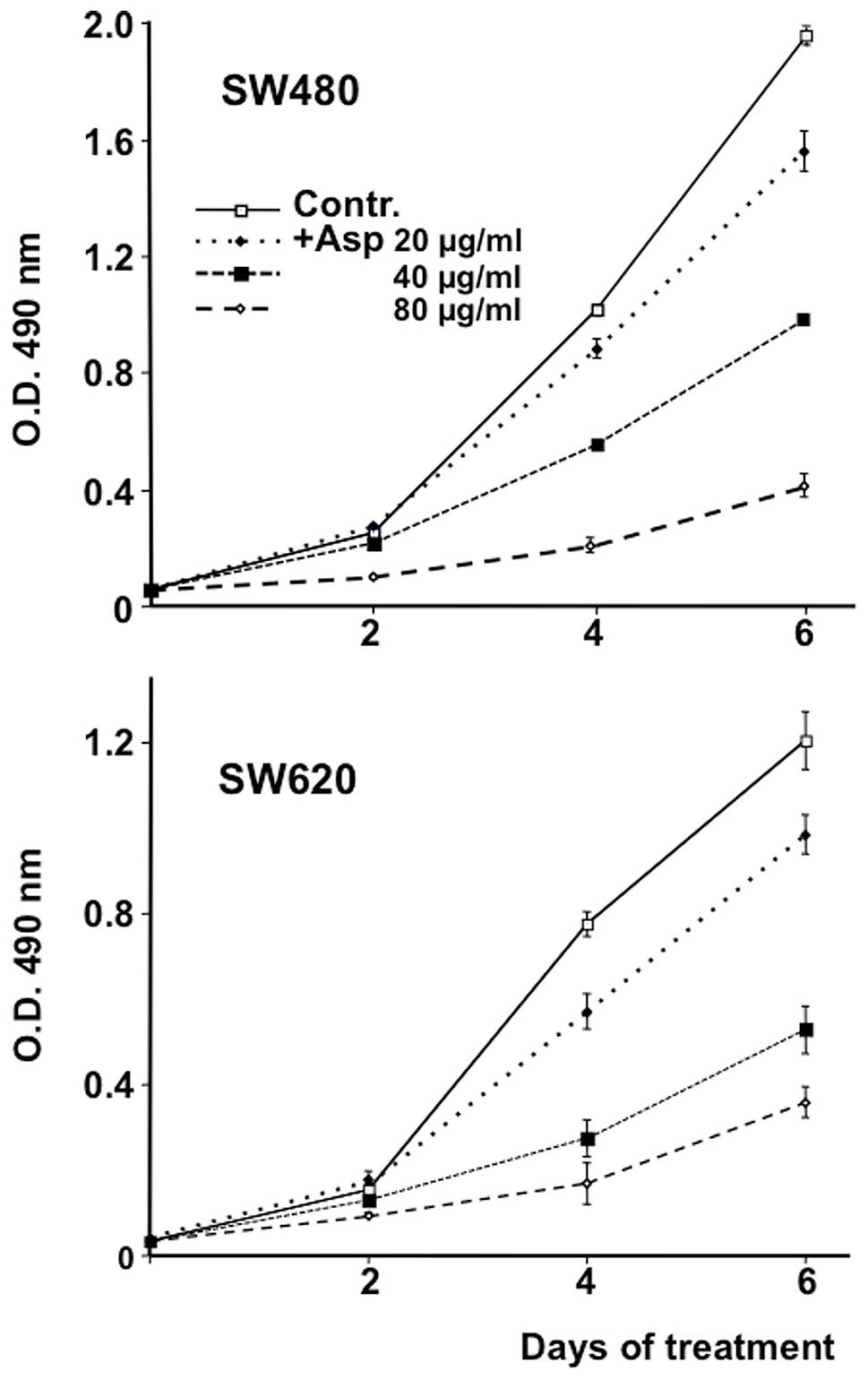
Effect of Asp on SW480 and SW620 cell
growth
SW480 and SW620 cells were exposed for 2 to 6 days
to a methanolic extract of Asparagus officinalis L. shoots
(Asp) at concentrations varying from 20 to 80 μg/ml. As
shown in Fig. 1 cell growth
inhibition was observed in a dose-dependent manner. After 4 days of
treatment, Asp at a concentration of 80 μg/ml induced an 80%
inhibition of cell growth in both cell lines.
Propidium iodide allows the study of cell
distribution in each phase of the cell cycle by measurement of
cellular DNA content. After induction of cell death, DNA is
degraded leading to formation of hypodiploid cells. These cells are
detected by flow cytometric analysis in the sub-G0/G1 region
(30). The amount of hypodiploid
cells was measured 24 and 48 h after treatment with Asp. As shown
in Fig. 2, the sub-G0/G1
population of SW480 and SW620 cells increased progressively with
time after treatment with Asp. The percentage of hypodiploid cells
was always higher in SW620 cells when compared to SW480 cells,
indicating a higher sensibility of the metastatic cells to Asp
treatment.
Effects of Asp on the expression of
apoptotic-related proteins
To further explore the underlying mechanisms induced
by Asp, we measured the expression of death receptor DR4 and DR5 of
the TRAIL signaling pathway that tightly control apoptosis
progression. We determined the protein and mRNA expression levels
of DR4 and DR5 by flow cytometry and qRT-PCR. After treatment with
Asp for 24 and 48 h, we observed upregulation of DR4 and DR5
protein expression at the cell surface of SW480 and SW620 cells
(Fig. 3). The upregulation of DR4
and DR5 was higher in the metastatic SW620 cells when compared to
the SW480 cells. Similarly, in Asp-treated SW480 cells, the amount
of DR4 and DR5 transcripts remained significantly lower when
compared with the level detected in SW620 cells (Fig. 4A). The DR4 and DR5 mRNA level
increased significantly in SW620 cells in a time-dependent manner,
respectively, by about 2-fold at 24 h and by 4-fold at 48 h
compared with untreated control cells.
To demonstrate whether the observed Asp-induced cell
death was mediated through the TRAIL DR4/DR5 death receptors, we
used human recombinant DR4/Fc and DR5/Fc chimerical protein
exhibiting a dominant-negative effect by blocking the endogenous
receptors. As shown in Fig. 4B,
the addition of DR4/Fc and DR5/Fc chimera (@DR4/DR5) led to a
significant reduction of cell death in Asp-treated SW480 and SW620
cells.
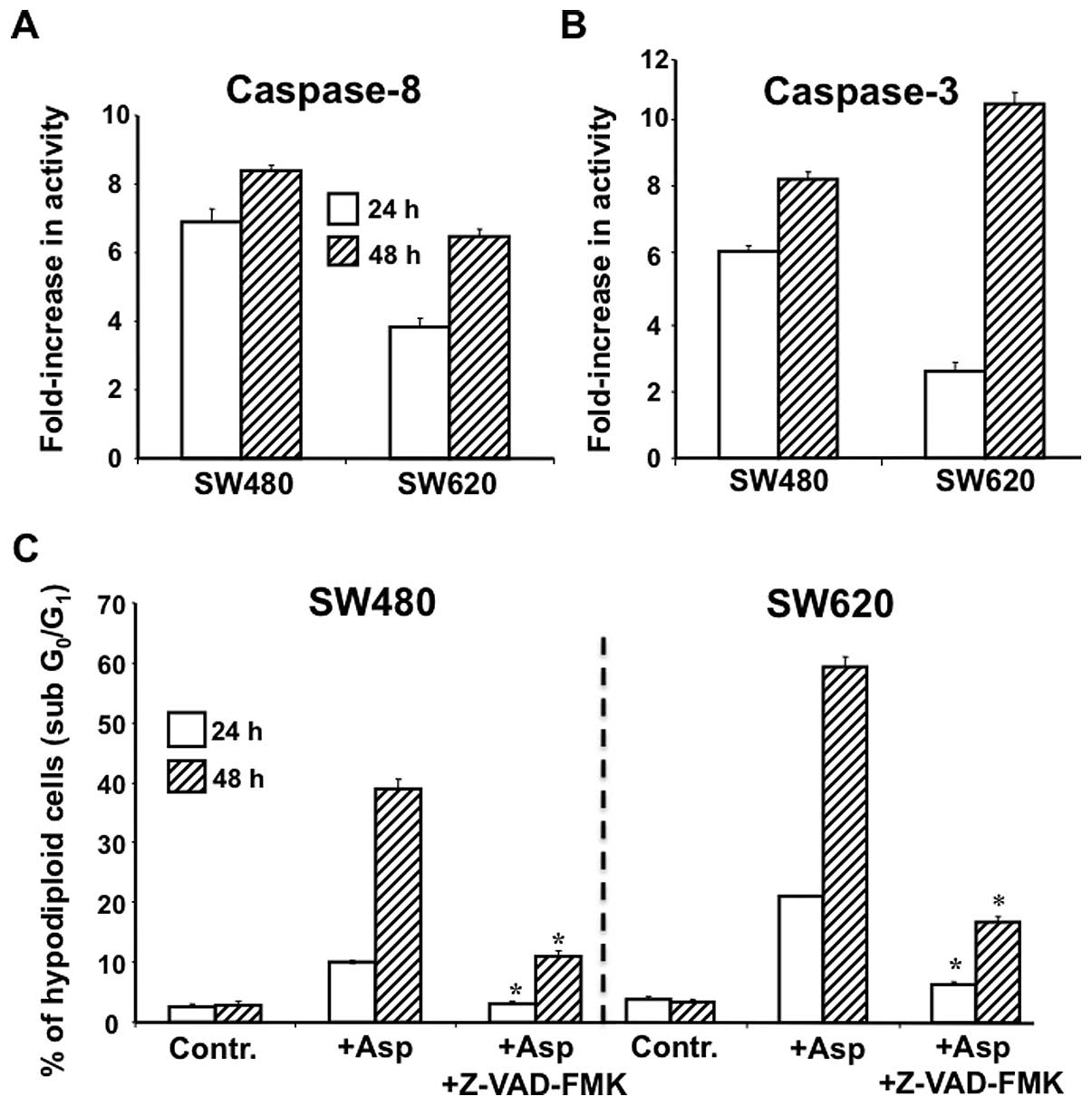
Caspase-8 and caspase-3 activities
In most cases, activation of the TRAIL death
receptor pathway targets the caspase activation cascade (31). We showed that the increased
expression of DR4/DR4 death receptors triggered by Asp was
associated with caspase-8 (Fig.
5A) and caspase-3 (Fig. 5B)
activation in both SW480 and SW620 cells. To further assess the
role played by caspases in the cell death induced by Asp, we used
the pan-caspase-inhibitor Z-VAD-FMK, and measured cell death after
24 and 48 h (Fig. 5C). When SW480
and SW620 cells were treated with the pan-caspase inhibitor,
Asp-induced cell death was blocked.
Sensitization of Asp-treated cancer cells
to TRAIL
The human colon cancer cell line SW480 is known to
be TRAIL-sensitive while its derived metastatic cell line SW620 is
TRAIL-resistant (32). Here, we
investigated the effects of exogenous recombinant human TRAIL
alone, or in combination with Asp. As shown in Fig. 6, TRAIL as a single drug exhibited
only a week activity on SW480 cells and remained ineffective on
SW620 cells. However, TRAIL potentiated the effects of Asp on both
SW480 and SW620 cell death enhancing significantly the effects of
Asp on cell death in both cell lines (95% of cell death after 48 h
of treatment).
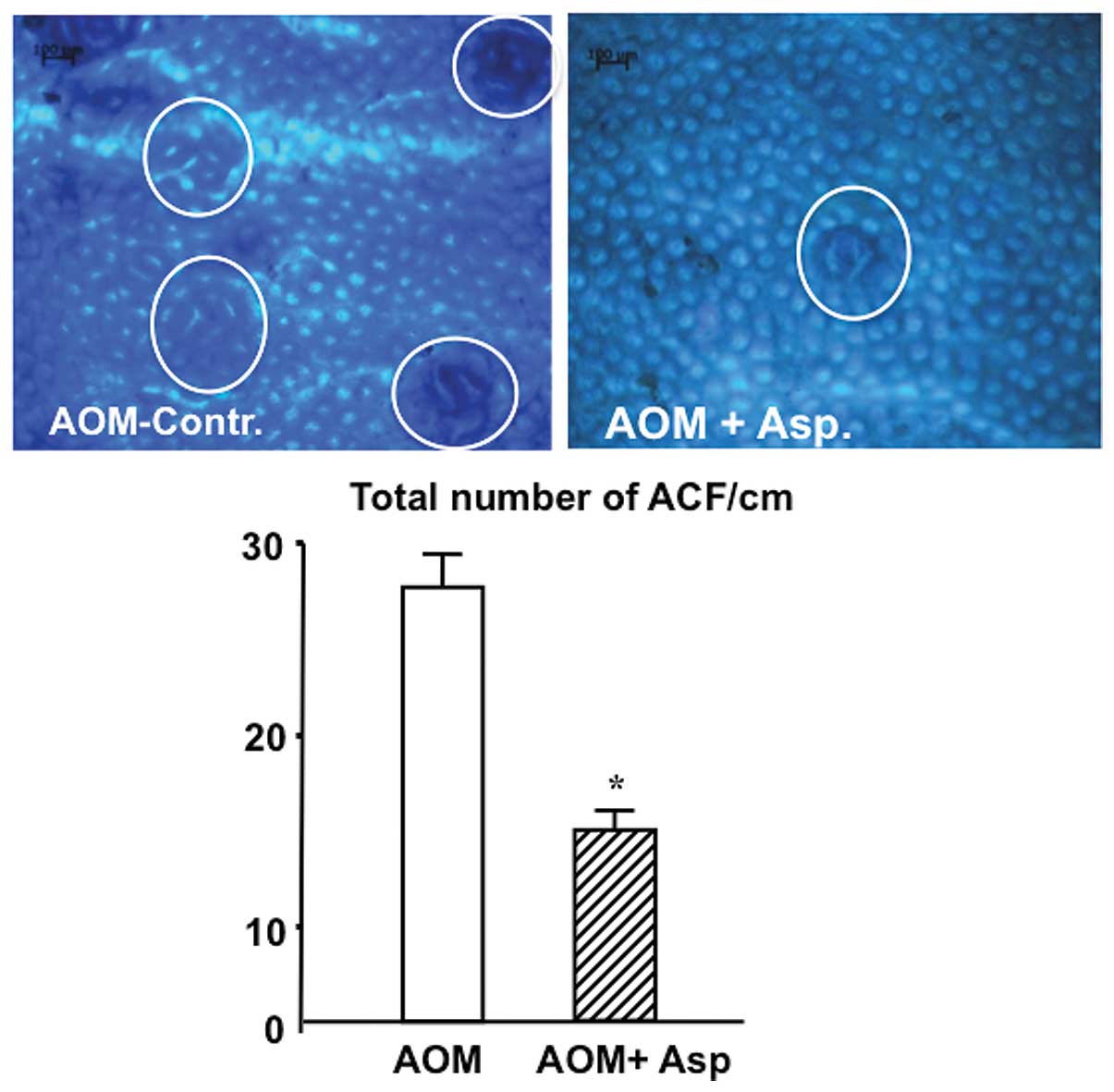
Asp inhibits the development of
preneoplastic lesions in the colon of rats
Based on our results showing strong anticancer
effects of Asp on colon adenocarcinoma SW480 and meta-static SW620
cells in culture, we examined the efficacy of Asp in a rat model of
colon carcinogenesis using azoxymethane (AOM)-initiated colon
cancer. Colon carcinogenesis was induced in rats by
intra-peritoneal injections of the chemical carcinogen AOM once a
week for 2 weeks. Starting one week after the last injection rats
received 0.01% of daily freshly prepared Asp (14 mg/kg body weight)
in their drinking fluid. After 7 weeks of intervention, the colon
of NaCl-injected rats exhibited no preneoplastic lesions (i.e.,
aberrant crypts or ACF) in contrast to the colonic mucosa of
AOM-injected rats that always exhibited preneoplastic lesions.
AOM-injected rats receiving Asp showed a 2-fold reduction of
preneoplastic lesions when compared with the AOM control group
(Fig. 7).
Modulation by Asp of inflammatory and
host-defense gene expression in colonic mucosa
To gain more insight into the mechanisms underlying
the in vivo antitumor efficacy of Asp, mucosa tissues were
analyzed by quantitative real-time RT-PCR for the differential
expression of inflammatory (IL1β, MMP-7 and
MMP-9) and innate immunity components (DEF-5,
LCN2). In the colonic mucosa of AOM-injected rats treated
with Asp, we found a significant downregulation of both
MMP-7 and MMP-9, close to levels measured in the
mucosa of the saline-injected rats (Fig. 8A). Moreover, the amount of
MMP-7 transcript was directly correlated with the amount of
MMP-7 protein (Fig. 8B). It was
reported that the transcription of MMP genes is positively
regulated by cytokines and growth factors such as interleukins
(IL1β) or TNF-α, (33,34) both suspected to be associated with
the formation of colorectal adenoma in humans (35,36).
Accordingly, firstly we observed a significant upregulation of both
IL1β (more than 2-fold) and TNF-α (more than 4-fold)
in the colonic mucosa of AOM-injected rats compared to the
expression profiles of these genes in the colonic mucosa of
saline-injected rats. Secondly, the treatment of AOM-injected rats
with Asp inhibited the expression of these inflammatory cytokines
close to the level measured in the mucosa of saline-injected rats
(Fig. 8C).
We also found that two biomarkers of the innate
immune system, α-defensin-5 (DEF-5) and lipocalin-2
(LCN2), were upregulated at the mRNA level by 5- and
11-fold, respectively, in the colon of Asp-treated AOM-injected
rats compared to AOM-controls or saline-injected rats (Fig. 8D). Furthermore, the levels of the
LCN2 transcripts were correlated with an increased amount of
LCN2 protein (Fig. 8B). These data
were consistent with the reported negative correlation between the
expression levels of host-defense genes and those implicated in the
inflammatory response (37).
Asp activates apoptotic cell death in
colonic mucosa
Since we observed that Asp exerted potent
pro-apoptotic effects in human adenocarcinoma and metastatic cancer
cells in vitro, we also investigated the effect of Asp on
the expression of apoptotic molecules in the colonic mucosa of
AOM-injected rats by RT-PCR quantitative analyses and western blot
analysis.
Our data showed that Asp upregulated by 6-fold the
level of TRAIL-death receptor DR5 and of TRAIL ligand
(Fig. 9A) when compared to AOM or
saline-injected rats. Furthermore, the levels of DR5 and
TRAIL transcripts were correlated with an increased amount
of DR5 and TRAIL protein (Fig.
9B). Asp targeted specifically the TRAIL apoptotic signaling
pathway since a treatment with Asp did not significantly modify the
expression level of FAS, FAS ligand, Bax or
Bcl-2.
In most cases, activation of the extrinsic TRAIL
death receptor pathway leads to the activation of effector
caspase-3. Accordingly, we found a 5-fold increase of caspase-3
activity in the mucosa of Asp-treated AOM-Injected rats when
compared to AOM-injected controls (Fig. 9C). These data are in accordance
with in vitro data showing that Asp activated
caspase-dependent apoptotic signaling pathway in human colon cancer
SW480 and derived metastatic SW620 cells.
Discussion
Alterations of the processes controlling apoptosis
extend the life span of cells and may favor cell neoplastic
expansion independently of cell division (38). In addition, failure in apoptotic
death contributes to resistance against host immune-based response
and/or anticancer drug treatments. The expression of receptors
belonging to the super family of tumor necrosis factor receptors
such as TNF-related apoptosis inducing ligand (TRAIL) receptors DR4
and DR5, are often altered in patients with colon cancer (33). The ligand (TRAIL) by interacting
with its apoptotic death receptors DR4 and DR5, selectively induces
apoptosis in a wide variety of cancer cells (39). This finding has led to the
development of anticancer strategies based on the use of TRAIL or
TRAIL agonistic antibodies (40).
However these strategies have shown only limited efficiency and the
best treatment response to TRAIL agonist obtained in cancer
patients from clinical studies is a stabilization of the disease
(41). Nevertheless it seems that
the sensitivity to TRAIL-induced apoptosis is a key factor
influencing the efficacy of TRAIL treatment. Therefore, the TRAIL
apoptotic pathway may be a valuable target with great potential for
anticancer treatment when TRAIL or TRAIL agonists are used in
combination with other anticancer therapies.
The search for dietary constituents with antitumor
activity with high effectiveness on cancer cells and low toxicity
on normal tissues has become one of the hotspots in research on
cancer prevention and treatment (42). Asparagus officinalis L. is a
popular vegetable in Western and Oriental countries alike.
Interestingly many components of asparagus are poorly absorbed when
ingested, and a substantial amount remains intact in the digestive
tract where it might exert protective effects targeting the colonic
mucosa. In the present study we investigated the chemopreventive
mechanisms of Asparagus officinalis L. shoots extract (Asp),
in vitro on human colon adenocarcinoma SW480 cells and their
derived metastatic SW620 cells, and in vivo in a preclinical
model of colon carcinogenesis in rat.
Here, we show that Asp-induced cancer cell death was
mediated through the activation of the TRAIL apoptotic pathway in
both adenocarcinoma SW480 cells and meta-static SW620 cells. In
these cells Asp initiated a significant (P<0.01) upregulation of
DR4 and DR5 transcripts and proteins. By inactivating the TRAIL
death receptors with specific blocking agents (DR4/Fc and DR5/Fc)
we were able to inhibit Asp-triggered cell death demonstrating the
key role of TRAIL receptors in this process. It is noteworthy that
Asp was able to induce the activity of both DR4 and DR5 death
receptor in the metastatic SW620 cells which normally do not
express active DR4/DR5 receptors at their cell surface and are
known to be TRAIL-resistant (43,44).
Activation of DR4/DR5 by Asp led in both cell lines to the
activation of caspase-8 and caspase-3 subsequently triggering the
apoptotic cell death. This was attested by the fact that
pancaspase-inhibitor Z-VAD-FMK inhibited the Asp-induced cell death
in these two cell lines.
From these data it can be hypothesized that
Asp-treatment might represent a way to sensitize resistant cancer
cells to TRAIL-treatment via the activation of DR4/DR5 death
receptors. This was confirmed by our present data showing that Asp
was able to potentiate the effects of TRAIL on cell death, even in
TRAIL-resistant metastatic cancer cells.
Based on our in vitro data demonstrating the
potent proapoptotic effects of Asp, we have further aimed to
evaluate its chemopreventive effects in a preclinical model of
colon carcinogenesis. We show that 0.01% Asp freshly administered
daily in drinking water (14 mg/kg body weight) to rats, for a
duration of 7 weeks starting one week after injection with a
chemical carcinogen (AOM), caused a 50% reduction in the number of
preneoplastic lesions (aberrant crypt foci) (ACF) at the surface of
the colon. AOM-induced ACF have been widely used as a useful
biomarker for colon carcinogenesis and for the evaluation of many
chemopreventive agents (23,24).
In order to gain more insight into the molecular targets involved
in the anti-neoplastic effects of Asp, the expression of several
key genes and proteins involved in the inflammatory, immune and
apoptotic responses were measured in the colonic mucosa.
It is widely reported that cytokines (IL1β, TNF-α),
matrix metalloproteinase (MMP-7, MMP-9) are involved in chronic
inflammation, which creates a microenvironment favoring colon
carcinogenesis (45). The role of
these molecules have been linked to all steps involved in
tumorigenesis, including initiation, cellular transformation,
promotion, survival, invasion, and metastasis. Matrilysin (MMP-7)
has been demonstrated to contribute to early stages of intestinal
tumor progression (46). Chronic
exposure to MMP-7 in premalignant epithelial cells can result in
the selection of a subpopulation of cells that display a decreased
sensitivity to pro-apoptotic signals. Additionally, such cells also
demonstrate a significantly reduced sensitivity to drugs that
stimulate apoptosis (46). Tissue
inhibitors of MMPs have been suggested to be useful in combination
therapy with TRAIL since it was reported that MMP inhibitors
reduced tumor growth and angiogenesis in nude mice (47). Accordingly, our data indicate that
MMP-7 and, to a lesser extent MMP-9, are
significantly enhanced in colonic mucosa of AOM-injected rats and
that their expression declined significantly (P<0.01) with Asp
treatment. Furthermore, we observed that Asp drastically reduced
IL1β and TNF-α expression, which are known to induce an
upregulation of MMPs (48,49). Furthermore, we observed an inverse
correlation between the genetic expression of these
pro-inflammatory cytokines and that of host-defense components of
the innate immune system such as α-defensin-5 (DEF-5) and
lipocalin 2 (LCN2), these genes being upregulated in
Asp-treated AOM rats. Bioactive asparagus constituents such as
saponins or fructooligosaccharides are well recognized as potent
immune stimulators (50,51). These effects might be in part
modulated by DEF-5 and LCN2 (38,52),
which are considered as active weapons against several cancer cell
types (53). Furthermore, an
impaired expression of DEF-5 and LCN2 and the over-production of
the pro-inflammatory cytokine IL1β have been closely associated
with inflammatory bowel disease (54–56).
It was also shown that DEF-5 plays an important role in the
maintenance of intestinal immune homeostasis by controlling the
production of IL1β (57).
Progressive inhibition of apoptosis has been
described during transformation of colorectal epithelium to
carcinoma. Here we showed in accordance with our in vitro
findings that Asp-treatment of AOM-injected rats induced an
important upregulation of biomarkers of the TRAIL-apoptotic pathway
like DR5 and TRAIL at both gene and protein levels. These effects
were also associated with an activation of caspase-3 in the colonic
mucosa of Asp-treated AOM-rats.
In conclusion, this study demonstrates that a
methanolic extract of white asparagus shoots activates the TRAIL
death receptor pathway in the SW480 human colon adeno-carcinoma
cells and in their derived TRAIL-resistant metastatic SW620 cells.
This extract also sensitized these cells to TRAIL-induced apoptosis
via the upregulation of death receptors (DR4/DR5) and the
associated activation of caspase-8 and caspase-3, finally leading
to cell death. We also demonstrated that asparagus extract
administered in drinking water to AOM-injected rats exerted various
anti-carcinogenic and protective effects on the colonic mucosa at
early post-initiation stages. At the molecular level, the asparagus
extract exhibited multi-targeted effects on the preneoplastic
colonic mucosa including the inhibition of cellular
pro-inflammatory mediators such as IL1β, TNF-α, MMP-7 and MMP-9, in
association with an increased expression of the host-defense
mediators α-defensin-5 and lipocalin 2. In the colonic mucosa of
AOM-injected rats receiving the asparagus extract we confirmed the
pro-apoptotic effects observed in vitro involving the
activation of the TRAIL death receptor signaling pathway. Taken
together our data highlight the chemopreventive potential of
asparagus shoot extract on colon carcinogenesis and its ability to
promote normal cellular homeostasis.
Acknowledgements
This research was part of the
‘nutrhi.net’ project co-financed by the European Regional
Development Fund: Interreg IV Oberrhein/Rhin Supérieur.
References
|
1.
|
Kelloff GJ, Boone CW, Crowell JA, Steele
VE, Lubet R and Sigman CC: Chemopreventive drug development:
perspectives and progress. Cancer Epidemiol Biomarkers Prev.
3:85–98. 1994.PubMed/NCBI
|
|
2.
|
Kelloff GJ, Boone CW, Crowell JA, Steele
VE, Luber RA, Doody LA, Malone WF, Hawk ET and Sigman CC: New
agents for cancer chemoprevention. J Cell Biochem (Suppl). 26:1–28.
1996. View Article : Google Scholar
|
|
3.
|
Corpet DE and Taché S: Most effective
colon cancer chemo-preventive agents in rats: a systematic review
of aberrant crypt foci and tumor data, ranked by potency. Nutr
Cancer. 43:1–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tanaka T and Sugie S: Inhibition of colon
carcinogenesis by dietary non-nutritive compounds. J Toxicol
Pathol. 20:215–235. 2007. View Article : Google Scholar
|
|
5.
|
Kushi LH, Meyer KA and Jacobs DR Jr:
Cereals, legumes, and chronic disease risk reduction: evidence from
epidemiologic studies. Am J Clin Nutr. 70:451S–458S.
1999.PubMed/NCBI
|
|
6.
|
Bobe G, Barrett KG, Mentor-Marcel RA,
Saffiotti U, Young MR, Colburn NH, Albert PS, Bennink MR and Lanza
E: Dietary cooked navy beans and their fractions attenuate colon
carcinogenesis in azoxymethane-induced ob/ob mice. Nutr Cancer.
60:373–381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Steele VE, Pereira MA, Sigman CC and
Kelloff GJ: Cancer chemoprevention agent development strategies for
genistein. J Nutr. 125:713S–716S. 1995.PubMed/NCBI
|
|
8.
|
Yu S, Poobsasert O, Kennelly EJ, Chin CK,
Ho CT, Huang MT, Garrison SA and Cordell GA: Steroidal saponins
from Asparagus officinalis and their cytotoxic activity.
Planta Medica. 63:258–262. 1997.
|
|
9.
|
Huang X and Kong L: Steroidal saponins
from roots of Asparagus officinalis. Steroids. 71:171–176.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shao Y, Chin CK, Ho CT, Ma W, Garrison SA
and Huang MT: Anti-tumor activity of the crude saponins obtained
from asparagus. Cancer Lett. 104:31–36. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang M, Tadmor Y, Wu QL, Chin CK, Garrison
SA and Simon JE: Quantification of protodioscin and rutin in
asparagus shoots by LC/MS and HPLC methods. J Agric Food Chem.
51:6132–6136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Makris DP and Rossiter JT: Domestic
processing of onion bulbs (Allium cepa) and asparagus spears
(Asparagus officinalis): effect on flavonol content and
antioxidant status. J Agric Food Chem. 49:3216–3222.
2001.PubMed/NCBI
|
|
13.
|
Villanueva-Suarez MJ, Redondo-Cuenca A,
Rodriguez-Sevilla MD and Heredia-Moreno A: Postharvest storage of
white asparagus (Asparagus officinalis I.): changes in
dietary fiber (Nonstarch polysaccharides). J Agric Food Chem.
47:3832–3836. 1999.PubMed/NCBI
|
|
14.
|
Yamamori A, Onodera S, Kikuchi M and
Shiomi N: Two novel oligosaccharides formed by
1F-fructosyltransferase purified from roots of asparagus
(Asparagus officinalis L.). Biosci Biotechnol Biochem.
66:1419–1422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rodriguez R, Jaramillo S, Rodriguez G,
Espejo JA, Guillén R, Fernandez-Bolanos J, Heredia A and Jiménez A:
Antioxidant activity of ethanolic extracts from several asparagus
cultivars. J Agric Food Chem. 53:5212–5217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jang DS, Cuendet M, Fong HH, Pezzuto JM
and Kinghorn AD: Constituents of Asparagus officinalis
evaluated for inhibitory activity against cyclooxygenase-2. J Agric
Food Chem. 52:2218–2222. 2004.PubMed/NCBI
|
|
17.
|
Mei HS, Wu YQMG and Wu ZY: The effects by
eating asparagus spears on blood-lipid levels of human body. Acta
Sci Nat Univ Pekin. 26:369–372. 1990.
|
|
18.
|
Wang H and Ng TB: Isolation of a novel
deoxyribonuclease with antifungal activity from Asparagus
officinalis seeds. Biochem Biophys Res Commun. 289:120–124.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Huang J, Sun Y and Lu S: Experimental
study on apoptosis induced by ursolic acid isolated from asparagus
in HL-60 cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 19:296–298.
1999.(In Chinese).
|
|
20.
|
Liu W, Huang XF, Qi Q, Dai QS, Yang L, Nie
FF, Lu N, Gong DD, Kong LY and Guo QL: Asparanin A induces G(2)/M
cell cycle arrest and apoptosis in human hepatocellular carcinoma
HepG2 cells. Biochem Biophys Res Commun. 381:700–705. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bhutani KK, Paul AT, Fayad W and Linder S:
Apoptosis inducing activity of steroidal constituents from
Solanum xanthocarpum and Asparagus racemosus.
Phytomedicine. 17:789–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hewitt RE, McMarlin A, Kleiner D, Wersto
R, Martin P, Tsokos M, Stamp GW and Stetler-Stevenson WG:
Validation of a model of colon cancer progression. J Pathol.
192:446–454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Reddy BS: Studies with azoxymethane-rat
preclinical model for assessing colon tumor development and
chemoprevention. Environ Mol Mutagen. 44:26–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Corpet DE and Pierre F: How good are
rodent models of carcinogenesis in predicting efficacy in humans? A
systematic review and meta-analysis of colon cancer chemoprevention
in rats, mice and humans. Eur J Cancer. 41:1911–1922. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Bousserouel S, Gossé F, Bouhadjar M, Soler
L, Marescaux J and Raul F: Long-term administration of aspirin
inhibits tumour formation and triggers anti-neoplastic molecular
changes in a pre-clinical model of colon carcinogenesis. Oncol Rep.
23:511–517. 2010.
|
|
26.
|
Pereira MA, Barnes LH, Rassman VL, Kelloff
GV and Steele VE: Use of azoxymethane-induced foci of aberrant
crypts in rat colon to identify potential cancer chemopreventive
agents. Carcinogenesis. 15:1049–1054. 1994. View Article : Google Scholar
|
|
27.
|
Takayama T, Katsuki S, Takahashi Y, Ohi M,
Nojiri S, Sakamaki S, Kogawa K, Miyake H and Niitsu Y: Aberrant
crypt foci of the colon as precursors of adenoma and cancer. N Engl
J Med. 339:1277–1284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bird RP and Good CK: The significance of
aberrant crypt foci in understanding the pathogenesis of colon
cancer. Toxicol Lett. 112–113:395–402. 2000.
|
|
29.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
30.
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar
|
|
32.
|
Jin Z, McDonald ER III, Dicker DT and
El-Deiry WS: Deficient tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) death receptor transport to the
cell surface in human colon cancer cells selected for resistance to
TRAIL-induced apoptosis. J Biol Chem. 279:35829–35839. 2004.
View Article : Google Scholar
|
|
33.
|
Cawston TE and Wilson AJ: Understanding
the role of tissue degrading enzymes and their inhibitors in
development and disease. Best Pract Res Clin Rheumatol.
20:983–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Spinale FG: Myocardial matrix remodeling
and the matrix metalloproteinases: Influence on cardiac form and
function. Physiol Rev. 87:1285–1342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Gunter MJ, Canzian F, Landi S, Chanock SJ,
Sinha R and Rothman N: Inflammation-related gene polymorphisms and
colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 15:1126–1131.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Buck I, Morceau F, Grigorakaki C, Dicato M
and Diederich M: Linking anemia to inflammation and cancer: the
crucial role of TNFalpha. Biochem Pharmacol. 77:1572–1579. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ramasundara M, Leach ST, Lemberg DA and
Day AS: Defensins and inflammation: the role of defensins in
inflammatory bowel disease. J Gastroenterol Hepatol. 24:202–208.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Reed JC: Dysregulation of apoptosis in
cancer. J Clin Oncol. 17:2941–2953. 1999.PubMed/NCBI
|
|
39.
|
Wang S: TRAIL: a sword for killing tumors.
Curr Med Chem. 17:3309–3317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Bellail AC, Qi L, Mulligan P, Chhabra V
and Hao C: TRAIL agonists on clinical trials for cancer therapy:
the promises and the challenges. Rev Recent Clin Trials. 4:34–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Thorburn A, Behbakht K and Ford H: Trail
receptor-targeted therapeutics: resistance mechanisms and
strategies to avoid them. Drug Resist Updat. 11:17–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Wang H, Khor TO, Shu L, Su Z, Fuentes F,
Lee JH and Kong AN: Plants vs. cancer: a review on natural
phytochemicals in preventing and treating cancers and their drug
ability. Anticancer Agents Med Chem. 12:1281–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Kong CK, Lam WS, Chiu LC, Ooi VE, Sun SS
and Wong YS: A rice bran polyphenol, cycloartenyl ferulate, elicits
apoptosis in human colorectal adenocarcinoma SW480 and sensitizes
metastatic SW620 cells to TRAIL-induced apoptosis. Biochem
Pharmacol. 77:1487–1496. 2009. View Article : Google Scholar
|
|
44.
|
Kauntz H, Bousserouel S, Gossé F and Raul
F: The flavonolignan silibinin potentiates TRAIL-induced apoptosis
in human colon adenocarcinoma and in derived TRAIL-resistant
metastatic cells. Apoptosis. 17:797–809. 2012. View Article : Google Scholar
|
|
45.
|
Lu H, Ouyang W and Huang C: Inflammation,
a key event in cancer development. Mol Cancer Res. 4:221–233. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Fingleton B, Vargo-Gogola T, Crawford HC
and Matrisian LM: Matrilysin (MMP-7) expression selects for cells
with reduced sensitivity to apoptosis. Neoplasia. 3:459–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Nyormoi O, Mills L and Bar-Eli M: An
MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of
the TNF receptor superfamily in cancer cells. Cell Death Differ.
10:558–569. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Friedl P and Wolf K: Tumor-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Johansson N, Ahonen M and Kähäri VM:
Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci.
57:5–15. 2000. View Article : Google Scholar
|
|
50.
|
Rajput ZI, Hu SH, Xiao CW and Arijo AG:
Adjuvant effects of saponins on animal immune responses. J Zhejiang
Univ Sci B. 8:153–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Sabater-Molina M, Larqué E, Torrella F and
Zamora S: Dietary fructooligosaccharides and potential benefits on
health. J Physiol Biochem. 65:215–328. 2009. View Article : Google Scholar
|
|
52.
|
Chien MH, Ying TH, Yang SF, Yu JK, Hsu CW,
Hsieh SC and Hsieh YH: Lipocalin-2 induces apoptosis in human
hepatocellular carcinoma cells through activation of mitochondria
pathways. Cell Biochem Biophys. 64:177–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Papo N and Shai Y: Host defense peptides
as new weapons in cancer treatment. Cell Mol Life Sci. 62:784–790.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Lee HJ, Lee EK, Lee KJ, Hong SW, Yoon Y
and Kim JS: Ectopic expression of neutrophil gelatinase-associated
lipocalin suppresses the invasion and liver metastasis of colon
cancer cells. Int J Cancer. 118:2490–2497. 2006. View Article : Google Scholar
|
|
55.
|
Simms LA, Doecke JD, Walsh MD, Huang N,
Fowler EV and Radford-Smith GL: Reduced alpha-defensin expression
is associated with inflammation and not NOD2 mutation status in
ileal Crohn’s disease. Gut. 57:903–910. 2008.
|
|
56.
|
Friedl A, Stoesz SP, Buckley P and Gould
MN: Neutrophil gelatinase-associated lipocalin in normal and
neoplastic human tissues. Cell type-specific pattern of expression.
Histochem J. 31:433–441. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Shi J, Aono S, Lu W, Ouellette AJ, Hu X,
Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M, Dykstra C, Morrison
EE and Elson CO: A novel role for defensins in intestinal
homeostasis: regulation of IL-1beta secretion. J Immunol.
179:1245–1253. 2007. View Article : Google Scholar : PubMed/NCBI
|