Introduction
Several infectious agents (e.g. the bacterium
Helicobacter pylori, the human papilloma viruses and the
hepatitis B and C viruses) are considered to be causes of cancer in
humans (1). Of the 12.7 million
new cancer cases that occurred in 2008, 16.1% were attributed to
infections (2). Among parasites, a
carcinogenic role is recognized to Schistosoma haematobium
(leading to bladder cancer) and to Clonorchis sinensis or
Opisthorchis viverrini (which cause cholangiocarcinoma)
(3). By contrast, there is
increasing evidence indicating that certain parasitic infections
may induce antitumor activity against cancer. For instance,
infection of animals with the protozoans Trypanosoma
cruzi(4) or Plasmodium
yoelii(5) as well as the
nematode Trichinella spiralis(6), protected from tumor growth. In the
latter case, the tumor protection was also obtained in mice
immunized with crude extracts from adult parasites (6). Interestingly, an epidemiological
investigation on patients with cancer demonstrated a negative
correlation between cancer and hydatic cyst infection (7). It has been recently reported that
hydatid cyst protoscolices inhibited the proliferation of
fibrosarcoma cells in vitro(8). Collectively the evidence suggests
that some helminths may have antitumor properties.
It has been recently hypothesized that chronic
parasite infections may raise non-specific innate immune responses
that might induce anticancer activity (9). In this context, dendritic cells
(DCs), among other cells, recognize pathogens through a variety of
pattern recognition receptors (PRR) including Toll-like receptors
(TLR), C-type lectins (CLR) and intracellular Nod-like receptors
(NLR) (10). Thus, DCs can trigger
innate lymphocyte-functions, including natural killer (NK) cells
and regulate T and B cell responses (11). In particular, several molecules
produced by helminths have been described to interact with DCs
through TLRs or CLRs, modulating their maturation or function
(12–15).
Certain helminth parasites express O-glycans
and mucin-like molecules, the major carriers of O-glycans
(16–18). They participate in attachment and
invasion of host cells among other processes, by providing
proteases resistance or avoiding immune recognition (18,19).
In particular, the presence of tumor-associated antigens, such as
Tn (GalNAc-O-Ser/Thr), has been reported in larval and adult
tissues of Echinococcus granulosus, as well as circulating
Tn antigen in hydatid patients (20).
Aberrantly glycosylated mucins, carrying Tn among
others, are overexpressed in a wide range of epithelial human
cancers (21,22) and have functional importance in
cell adhesion, invasion and metastasis (23,24).
These properties make them relevant targets for the prevention of
metastasis and recurrence of cancers by therapeutic vaccination
(25,26). A number of antitumor vaccines based
on mucins have been developed and tested in preclinical models
(25). However, several clinical
trials using this type of vaccines together with different carriers
and/or adjuvant, did not exhibit sufficient efficacy, except for
some specific settings (27–29).
The low efficacy of these trials could be attributed to a poor
capacity to break immune-tolerance to mucin epitopes, which are
usually self-antigens.
Taking into account the data reporting that the
helminth E. granulosus induce antitumor cell activity and
that human mucins play main roles in tumor invasiveness, we sought
to evaluate whether peptides derived from a mucin-like protein from
this parasite would induce antitumor immunity. During a
characterization of the transcriptome of E. granulosus
larval stages (30) a cDNA clone
coding for a mucin-like protein has been isolated. The predicted
protein, denominated Egmuc, has a total of 58 amino acids, 23 of
which are threonine or serine residues that constitute potential
O-glycosylation sites. The fact that Egmuc comes from an
evolutionary distant organism should overcome tolerance issues
encountered with human-mucin based cancer therapeutic
approaches.
In the present study, we show that the Egmuc
peptide, as well as its CD4+ T cell epitope, provide
splenocytes with the capacity to mediate killing of tumor cells. We
also show that this property is correlated with an increase of
activated NK cells in spleen. We demonstrate that Egmuc peptides
induce, together with LPS, maturation of DCs by increasing the
production of IL-12 and IL-6 and that Egmuc-treated DCs may
activate NK cells, as judged by an increased expression of CD69. In
addition, the obtained results were compared to the ones induced by
a glycosylated peptide form carrying two consecutive Tn antigens.
We show that glycosylation of Egmuc does not increase the antitumor
activity induced by non-glycosylated Egmuc peptides. Our data may
contribute to the design of tumor vaccines and open new horizons in
the use of parasite-derived molecules to fight against cancer.
Materials and methods
Mice
Six- to 8-week-old female C57BL/6 or BALB/c mice
were obtained from DILAVE Laboratories (Uruguay). Animals were kept
in the animal house (URBE, School of Medicine, UdelaR, Uruguay)
with water and food supplied ad libitum and handled in
accordance with institutional guidelines for animal welfare
[Comisión Honoraria de Experimentación Animal (CHEA), Uruguay].
Egmuc peptides and glycopeptide
A 58-amino acid sequence of a E. granulosus
mucin like-protein, named Egmuc and 15 mer-peptides, overlapping by
five amino acids and spanning the Egmuc sequence were synthesized
as previously described (31) or
by SBS Bio Beijing SBS Genetech Co. Ltd. (China) or Peptide 2.0
Inc. (USA). The amino acid sequences of the peptides are detailed
in Fig. 1B.
Egmuc33-47-2Tn glycopeptide was produced by solid-phase
synthesis as previously described (32). All the (glyco)peptides were
quantified by amino acid analysis and characterized by electrospray
mass spectrometry. Atto-647N labeling of peptides and glycopeptide
with NHS ester was performed according to the instructions of the
manufacturer (Fluka, USA).
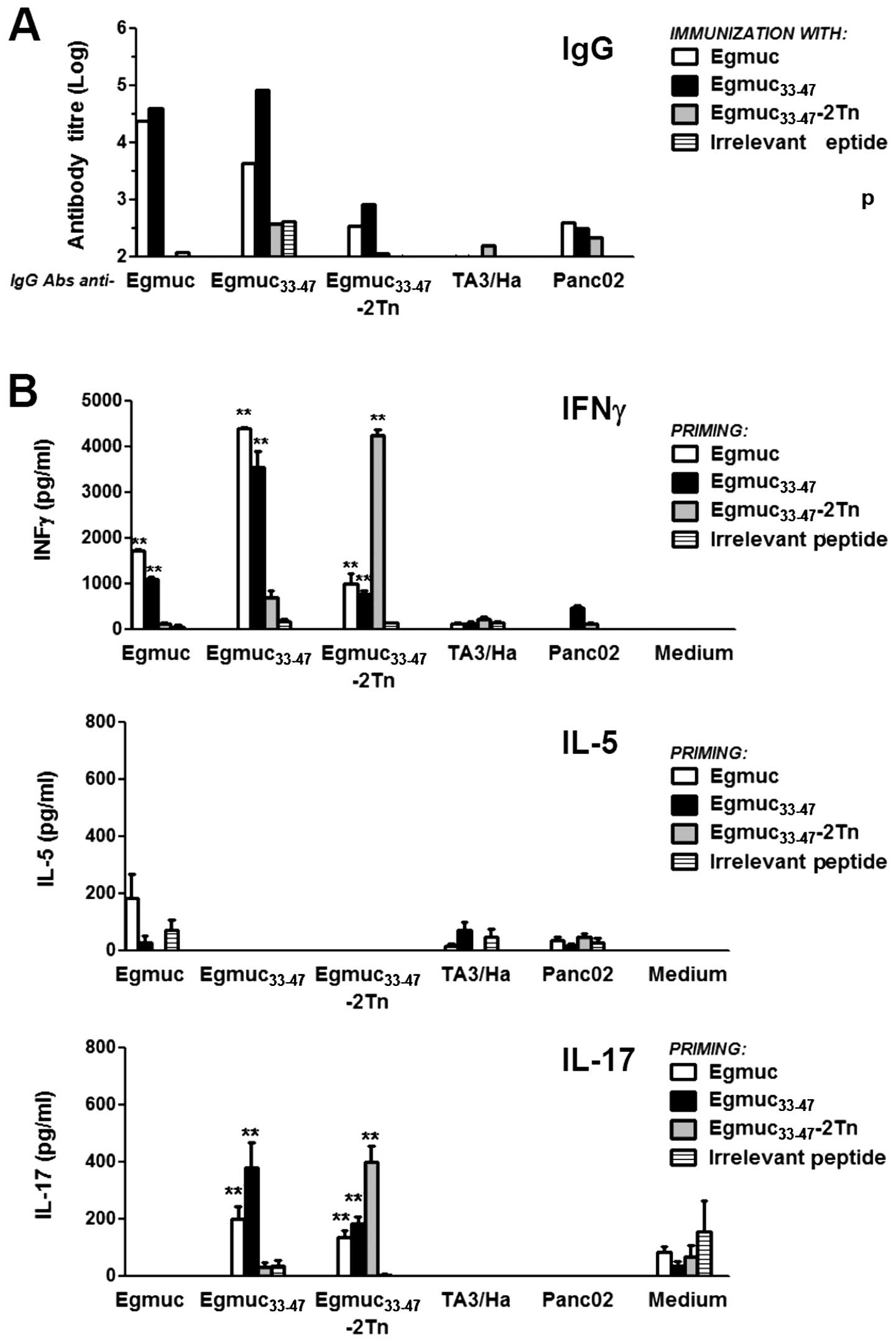
 | Figure 1Egmuc and the immunodominant peptide
Egmuc33-47 induce high production of IFNγ. (A) C57BL/6
mice (5 per group) were immunized subcutaneously with 20 μg of
Egmuc in CFA. After 10 days, inguinal lymph node cells were
cultured in triplicates in the presence of 10 μM of Egmuc, an
irrelevant peptide or medium alone for 72 h at 37°C. Cell
proliferation was evaluated according to the incorporation of
[3H]-thymidine (CPM). Culture supernatants were
collected and analyzed by ELISA for IFNγ, IL-5 and IL-17. Results
are expressed as mean value of triplicates (± SD, indicated by
error bars) and are representative of three different experiments.
Asterisks (**) represent differences statistically significant
(p<0.01) with respect to medium. (B) C57BL/6 mice (5 per group)
were immunized subcutaneously with Egmuc (20 μg/mouse) or an
irrelevant peptide in CFA. Ten days later, cells obtained from
draining LN were pooled and cultured in triplicate in the presence
of a set of synthetic overlapping peptides contained within the
Egmuc sequence (panel in the top), Egmuc or the irrelevant peptide
(not shown). INFγ production was evaluated by ELISA. (C) C57BL/6
mice (5 per group) were immunized subcutaneously with
Egmuc33-47 or Egmuc33-47-2Tn (20 μg/mouse) in
CFA. After 10 days, cells from inguinal LN were cultured in
triplicates for 72 h in the presence of the respective peptide or
glycopeptide, as well as an irrelevant peptide (10 μM) or medium
alone. Cell proliferation was evaluated according to the
incorporation of [3H]-thymidine (CPM). Culture
supernatants were collected and analyzed by ELISA for INFγ, IL-5
and IL-17 production. Results are expressed as mean value of
triplicates (± SD, indicated by error bars) and are representative
of three different experiments. Asterisks (**) represent
statistically significant differences (**p<0.01,
*p<0.05) with respect to medium. |
Endotoxin level determination
The endotoxin level of synthetic peptides and
glycopeptide was determined according to the instructions of the
manufacturer, using the Limulus Amebocyte Lysate kit Pyrochrome
(Associates of Cape Cod). Egmuc peptides and glycopeptide showed
very low levels of endotoxins (<1.6 EU/mg protein).
Cell lines
The C57BL/6 syngeneic pancreatic tumor cell line
Panc02 (given by Dr R. Hernandez-Alcoceba, University of Navarra,
Pamplona, Spain) and the BALB/c syngeneic mammary adenocarcinoma
cell line TA3/Ha (provided by Professor C. Leclerc, Institut
Pasteur, Paris, France) were cultured in complete culture medium,
consisting of RPMI-1640 with glutamine (PAA Laboratories, Austria)
supplemented with 10% heat-inactivated fetal bovine serum, 50 μM
2-mercaptoethanol, 100 U/ml penicillin (Sigma, St. Louis, MO, USA),
100 mg/ml streptomycin (Sigma), at 37ºC and 5% CO2.
T cell response
Mice were s.c. immunized at the base of the tail
with Egmuc, Egmuc33-47 or Egmuc33-47-2Tn (20
μg or 10 nmoles/mouse depending on the experiment) in complete
Freund adjuvant (CFA). Inguinal lymph nodes (LN) from control or
Egmuc, Egmuc33-47 or Egmuc33-47-2Tn immunized
mice were removed after 10 days and the cells were dispersed
manually and centrifuged at 1,500 rpm for 5 min. Cells
(1×106/well) were suspended in complete culture medium
and cultured for 72 h at 37°C and 5% CO2 in 96-well
plates with Egmuc peptide, overlapping 15 amino acids peptides or
glycopeptide (10 μM) and with TA3/Ha or Panc02 lysates (250 μg/ml).
They were then pulsed with [3H]-thymidine (American
Radiolabeled Chemicals Inc., St. Louis, MO, USA) for the last 18 h
of culture and harvested by an automated cell harvester (Tomtec).
Proliferation was determined by incorporation of the radioactivity
by the cells and the results (expressed in counts per minute, cpm)
represent the means of triplicate determinations. Controls were
incubated either with culture medium alone or with concanavalin A
(10 μg/ml). The negative control group consisted of mice immunized
with an irrelevant peptide corresponding to a sequence of a human
mucin MUC6 (PLITVTTSRTSQVHS). Secreted cytokines (IFNγ, IL-5 and
IL-17) levels were tested on culture supernatants by interleukin
specific sandwich ELISA assays (BD Bioscience, NJ, USA). Results
are expressed in pg/ml.
Cytotoxic in vitro assays
Mice were immunized i.p. with Egmuc,
Egmuc33-47, Egmuc33-47-2Tn (10 nmoles/mouse)
or PBS in complete or incomplete Freund's adjuvant, at days 0, 14
and 28. Splenocytes from Egmuc peptides or glycopeptide and control
immunized mice were removed after 35 days and the cells were
dispersed manually and centrifuged at 1,500 rpm for 5 min. Effector
splenocytes (E) and target (T) TA3/Ha or Panc02 cells were cultured
in different ratios for 18 h at 37°C and 5% CO2 in
96-well plates in complete culture medium. Then, 10 μl/well of
WST-8
(2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
(Sigma-Aldrich, St. Louis, MO, USA) were added to the media and
incubated for 3 h at 37°C and 5% CO2. Absorbance at 450
nm was measured each hour using an ELISA autoscan (Labsystems
Multiskan MS). Each condition was analyzed in triplicates. Controls
were carried out by culturing tumor cells alone, splenocytes alone
and tumor cells in presence of 1% Triton X-100 containing media
(100% of expected cytotoxicity). Percentage of cytotoxicity was
calculated as: % cytotoxicity = [Ealone +
Talone − (E+T)] / (Talone − TTriton
X-100), where E corresponds to splenocytes and T are tumor
cells.
Generation of bone marrow-derived
dendritic cells (BMDCs)
BMDCs were generated from bone marrow precursors
from C57BL/6 mice. Briefly, bone marrow cells from femurs and
tibias were harvested and plated at a density of 2×105
cells/ml in complete culture medium supplemented with
GM-CSF-containing supernatant. After 3 days of culture at 37°C, the
medium was replaced. Cells were recovered on day 6 or 7 and
analyzed for the expression of CD11c by flow cytometry.
In vitro internalization assay
The in vitro internalization of Egmuc
peptides or glycopeptide was analyzed by confocal microscopy and
flow cytometry. BMDCs were incubated (2.5 ×105/well)
with Atto-647N-labeled antigens for 1 h at 37°C in complete medium
(to assess uptake), or at 4°C in complete medium (to assess
binding). Cells were then washed twice and analyzed by flow
cytometry. Alternatively, cells were fixed with 1% formaldehyde and
dried over glass slides. Cells were then stained with anti-mouse
Lamp-1 (eBioscience, CA, USA) followed by an anti-rat IgG
TRITC-conjugated antibody (eBioscience) and with anti-mouse CD11c
antibody FITC-conjugated. All images were taken using laser
confocal microscope Leica TCS SP5 and a 63× oil objective. Four
optical sections scanned at intervals of 0.5 μm were projected
using Maximun Intensity Model using LASAF Lite. 2.6.0v
software.
BMDC maturation
BMDCs were incubated (2.5×105/well) at
37°C and 5% CO2 in 96-well plates with Egmuc,
Egmuc33-47, Egmuc33-47-2Tn (10 μM) or medium
alone for 1 h. After 2 h, cells were stimulated with LPS (1 μg/ml)
and incubated overnight at 37°C and 5% CO2. Cells were
centrifuged at 1,500 rpm for 5 min at 4°C and supernatants were
then collected. Cytokine (IL-12p40p70, IL-10 and IL-6) levels were
tested on culture supernatants by interleukin specific sandwich
ELISA assays (BD Bioscience). Results are expressed in pg/ml.
Phenotypic analyses of loaded BMDC were evaluated by
flow cytometry. Briefly, cells were incubated for 15 min at 4°C
with fluorochrome-conjugated antibodies: CD86 (GL-2), CD40
(HM40-3), CD80 (16-10A1) and MHC II (M5/114.15.2) (eBioscience) and
fixed with 0.1% formaldehyde.
Analysis of splenocytes by flow
cytometry
Splenocytes, from Egmuc peptides or glycopeptide and
control-immunized mice obtained as described above, were washed
twice with PBS containing 2% FBS and 0.1% sodium azide. Splenocytes
were then stained with an anti-NK1.1-PE (PK136), anti-CD69-FITC
(H1.2F3) and CD49b-APC (DX5), to identify activated NK cells. Cells
were then washed twice with PBS containing 5% FBS and 0.1% sodium
azide and fixed with 1% formaldehyde. Cell populations were
analyzed using a CyAn ADP Analyzer (Beckman Coulter). Other
antibodies used in this study included: CD11b (M1/70), CD11c
(N418), CD3 (17A2), CD103 (2E7), CD4 (RM4-5), CD8α (53-6.7), CD40
(HM40-3), I-A/I-E (2G9), F4/80 (BM8), MHC II (m5/114.15.2), Ly-6G
(RB6-8C5), Ly-6C (HK1.4), CD25 (PC61.5), FoxP3 (FJK-16s), CD19
(eBio1 D3), obtained from eBioscience.
In vitro analysis of the activation of NK
cells by Egmuc-treated BMDCs
BMDCs were incubated (2.5×105/well) at
37°C and 5% CO2 in 96-well plates with Egmuc,
Egmuc33-47, Egmuc33-47-2Tn or medium alone
for 1 h. After 2 h, cells were stimulated with LPS (1 μg/ml) and
incubated overnight at 37°C and 5% CO2. Cells were then
centrifuged at 1,500 rpm for 5 min at 4°C and supernatants were
then collected. Splenocytes (1×106/well) from naïve
animals were co-cultured with Egmuc-treated BMDC, or incubated only
with 100 μl of supernatants from Egmuc-treated BMDC, for 4 days at
37°C. IL-12p40p70 and IFNγ levels were tested on culture
supernatants by interleukin specific sandwich ELISA assays. Results
are expressed in pg/ml. Activated NK cells were detected by flow
cytometry after staining cells with an anti-NK1.1-PE (PK136),
anti-CD69-FITC (H1.2F3) and CD49b-APC (DX5), as described
above.
Results
Egmuc peptides induce a Th1-like
response
We first analyzed the cellular immunogenicity of a
mucin-like peptide derived from E. granulosus (Egmuc) in
mice immunized once with Egmuc in Freund's adjuvant. After 10 days
of the immunization, cells were obtained from the draining LN and
stimulated in vitro with Egmuc, medium or an irrelevant
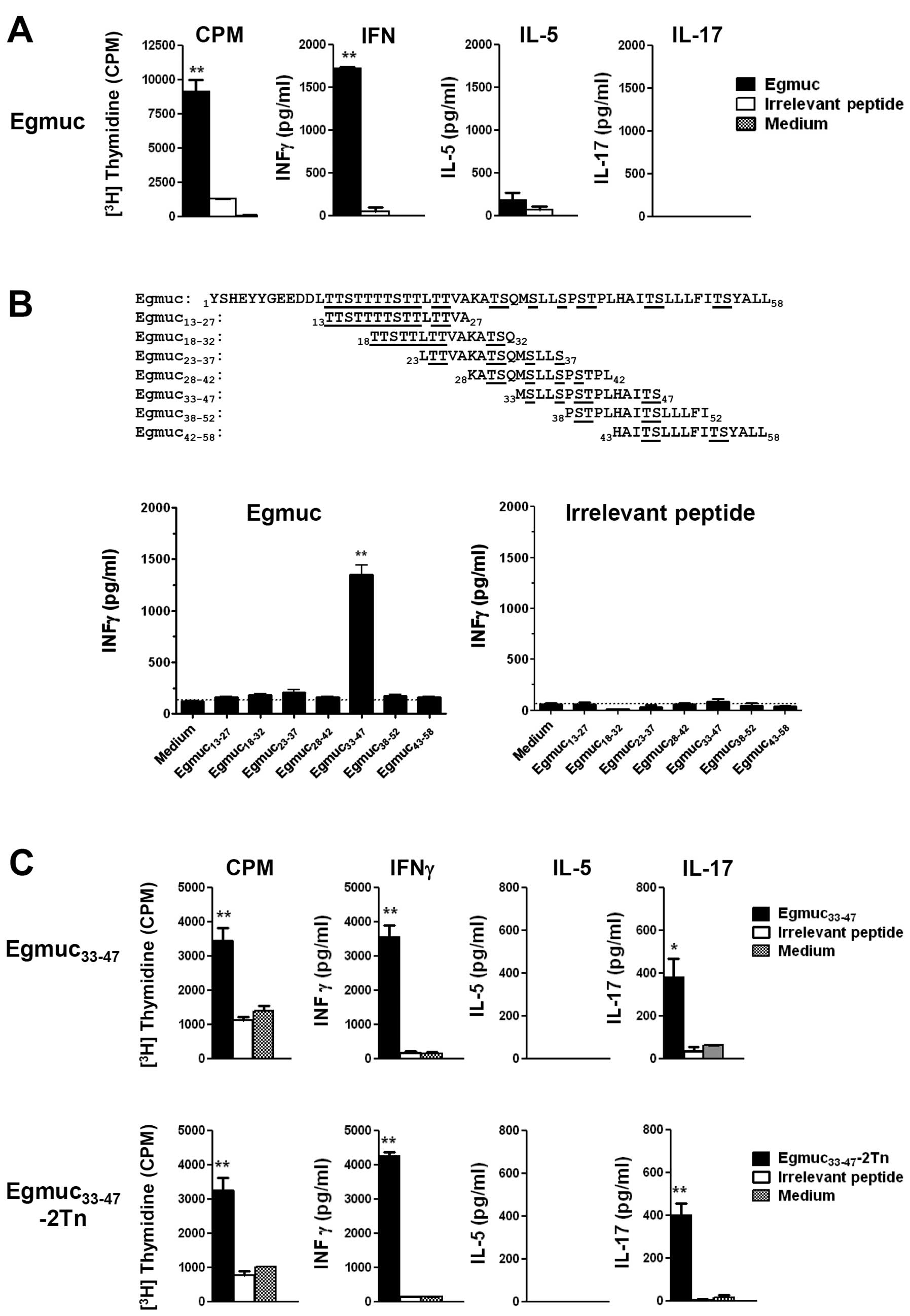
peptide derived from the human mucin MUC6. As depicted in Fig. 1A, immunization with Egmuc induced a
strong proliferative T cell response evaluated by the incorporation
of [3H]-thymidine (CPM). This response was associated to
a high production of IFNγ, while neither IL-5 nor IL-17 was
detected.
We sought to determine potential CD4+ T
cell epitopes in the Egmuc peptide. Epitope-based vaccines have the
ability to focus the immune response on highly antigenic epitopes
and are of great importance in the design of cancer vaccines
(33). Thus, CD4+ T
cell epitopes on Egmuc would be responsible for the type and
intensity of the specific immune response generated. We immunized
mice with Egmuc in Freund's adjuvant and after 10 days, cells
obtained from the draining LN were stimulated in vitro with
a panel of different overlapping peptides spanning the Egmuc
sequence (Fig. 1B). The peptides
were synthesized as 15-mers and overlapped by 5 residues. The
identification of the CD4+ T cell epitopes was performed
by the analysis of the proliferation and IFNγ production of cells
from draining LN, obtained from C57BL/6 mice immunized with Egmuc
or an irrelevant peptide (negative control). The
Egmuc33-47 stimulated a specific response of these LN
cells. In contrast, LN cells from mice immunized with a non-related
peptide did not produce IFNγ following stimulation with these Egmuc
peptides (Fig. 1B).
Following identification of the immunodominant
region of Egmuc, the corresponding glycosylated Egmuc peptide
carrying the Tn antigen was prepared by solid phase synthesis. We
introduced two GalNAc moieties at Ser39 and Thr40, since Tn
clusters have been shown to play a critical role in improving both
the intensity and the efficiency of the immune response (34). Both Egmuc33-47 peptide
and glycopeptide were capable of inducing a strong proliferative T
cell response as evaluated by the incorporation of
[3H]-thymidine (CPM) on cells derived from draining LN
of immunized mice. These responses were characterized by a high
production of IFNγ and low levels of IL-17, in the absence of IL-5
(Fig. 1C).
The Egmuc peptide was also shown to be immunogenic
in BALB/c mice, but in this case, the CD4+ T cell
epitopes identified belonged to the sequences spanned by
Egmuc23-37 Egmuc33-47 and
Egmuc38-52 (not shown).
Egmuc-induced antibodies or primed
T-cells do not recognize antigens from tumor cell lines
In order to mediate tumor cell killing, antibodies
or cytotoxic T cells must recognize antigens expressed on cancer
cells. Thus, we analyzed the ability of Egmuc peptides to induce
humoral and cellular immune response that recognize tumor antigens
or tumor cells.
The capacity of generated antibodies to recognize
native tumor antigens was evaluated on ELISA plates coated with
Egmuc peptides or, alternatively, with tumor lysates from the tumor
cell lines TA3/Ha and Panc02. The mammary adenocarcinoma cell line
TA3/Ha strongly expresses a membrane-associated mucin carrying the
Tn antigen (35), while the
pancreatic adenocarcinoma cell line Panc02 is known to be reactive
to GalNAc-specific lectins (36).
IgG antibodies induced by Egmuc peptides or glycopeptide did not
recognize antigens from TA3/Ha or Panc02 tumor cell lines nor the
glycosylated-Egmuc form, although they were capable of recognizing
both the Egmuc or Egmuc33-47 peptides (Fig. 2A). Unexpectedly, the glycosylated
peptide Egmuc33-47-2Tn, did not induce antibodies
recognizing the peptides or the glycopeptide (Fig. 2A).
We also analyzed the capacity of primed T-cells to
respond to stimulation with tumor antigens. When LN cells from
Egmuc-, Egmuc33-47- or
Egmuc33-47-2Tn-immunized mice were incubated with tumor
antigens, very low levels of IFNγ and undetectable levels of IL-5
or IL-17 were found on culture supernatants, while they recognized
Egmuc peptides, as shown by the high levels of IFNγ when stimulated
in vitro with the corresponding antigen (Fig. 2B).
Overall, these results indicated that Egmuc
peptides, although inducing high levels of specific antibodies,
hardly recognize tumor-derived antigens. Moreover, in
vivo-primed splenocytes cross-reacted at very low levels with
antigens present on tumor cells, while they produced very high
levels of IFNγ in response to stimulation with Egmuc peptides.
Thus, Egmuc-specific antibodies or T-cells unlikely would
participate in tumor recognition.
In vivo primed-splenocytes with Egmuc
peptides contain increased levels of activated NK cells and induce
tumor cell cytotoxicity in vitro
Tumor cell killing can be mediated by NK cells or
cytotoxic T CD8+ lymphocytes, as well as by macrophages
(37) or CD4+ T cells
(38), which can also display
cytotoxic properties. In order to study whether Egmuc peptides
could modify the levels of these or other cell types, we carried
out an extensive phenotyping of splenocytes from immunized mice.
The number of CD11c+,
CD11b+F4/80+, Ly6G+
Ly6C+/CD11b+, Ly6G−
Ly6C+/CD11b+, CD19+
CD3−, CD3+ CD4+ CD19−,
CD3+ CD8+ CD19−, CD3+
CD4+/CD25+ FoxP3+ or
NK1.1+ CD49b+ cells was not modified in
Egmuc-primed mice as compared to spleens of mice immunized only
with the adjuvant (data not shown). However,
CD69+/NK1.1+ CD49b+ cells were
augmented in spleens of Egmuc-immunized mice (Fig. 3A).
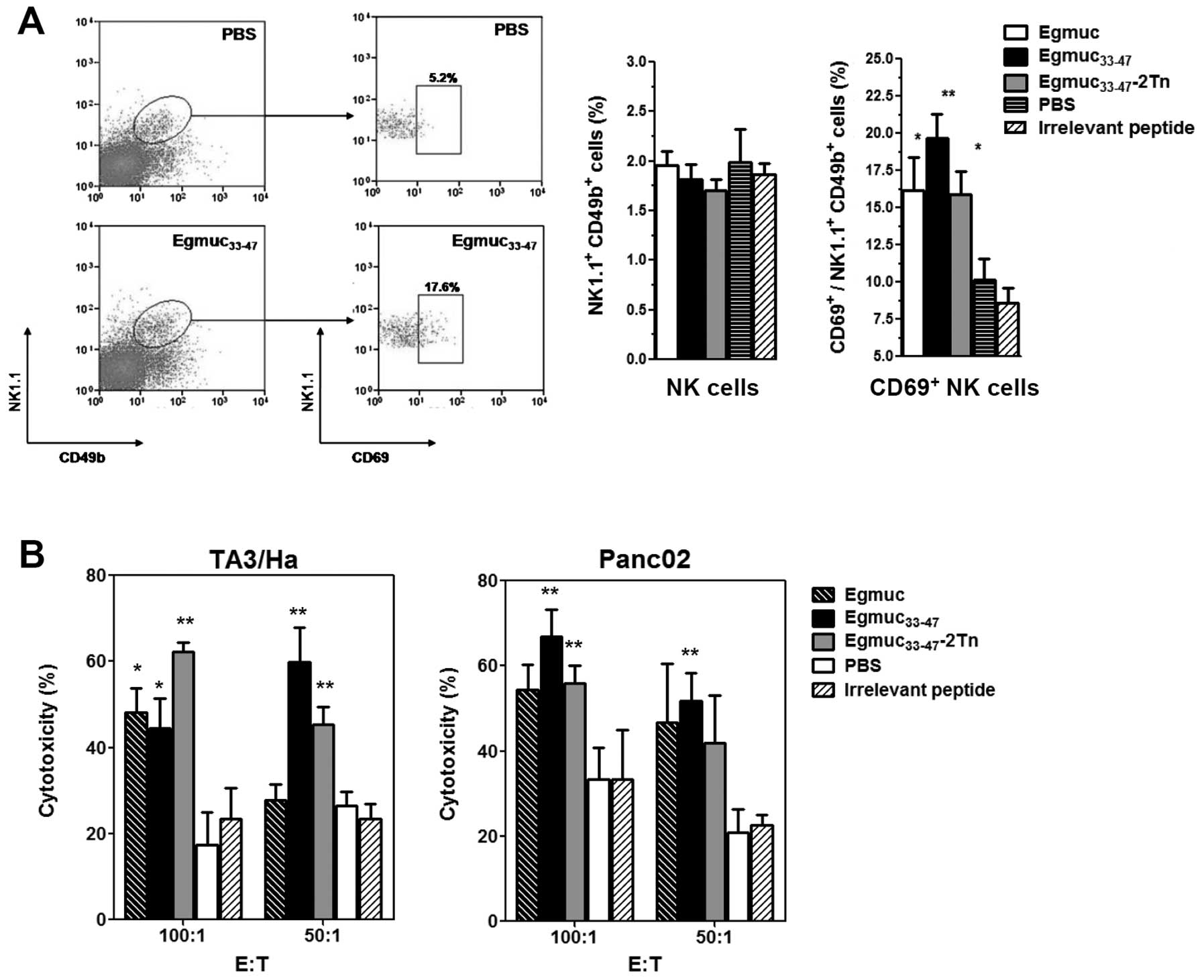 | Figure 3In vivo primed-splenocytes
with Egmuc peptides show augmented levels of activated NK cells and
mediate cytotoxicity in vitro. (A) C57BL/6 mice (4 per
group) were immunized i.p, at days 0, 14 and 28 with Egmuc,
Egmuc33-47 or Egmuc33-47-2Tn (20 μg/mouse),
as well as PBS (control group) in Freund's adjuvant. At day 35,
spleen cells were stained with anti-CD69, -NK1.1 and -CD49b
fluorochrome-conjugated specific antibodies and analyzed by flow
cytometry. Thirty thousand events were collected and gated on FSC
vs SSC dot plot. NK cells are shown as the percentage of cells in
the spleen, while activated NK cells are expressed as the
percentage of NK cells co-expressing the CD69+
activation marker. Percentage of cells is expressed as mean value
of triplicates (± SD, indicated by error bars) and are
representative of two different experiments. (B) C57BL/6 or BALB/c
mice (4 per group) were immunized i.p. with Egmuc,
Egmuc33-47 or Egmuc33-47-2Tn (10
nmoles/mouse) in CFA. After 10 days, splenocytes (E, effector
cells) of each group were cultured overnight in triplicates in the
presence of different ratios of TA3/Ha or Panc02 tumor cells (T,
target cells). Then, 10 μl/well of WST-8
(2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium)
was added and incubated for 3 h. WST-8 binding to NADPH was
followed by absorbance measurement at 450 nm. Results are expressed
as mean value of triplicates (± SD, indicated by error bars) and
are representative of two different experiments. Asterisks
represent statistically significant differences
(*p<0.05, **p<0.01) with respect to
mice injected with PBS (control). |
Then, we investigated whether in vivo-primed
splenocytes can mediate killing of tumor cells. Splenocytes
(effector cells) from mice immunized at days 0, 14 and 28 were
co-cultured with TA3/Ha or Panc02 tumor cells (target cells) at
different cell ratios. Egmuc peptide or glycopeptide-primed
splenocytes were able to mediate TA3/Ha or Panc02 cell cytotoxicity
(Fig. 3B). This result together
with the increase of activated-NK cells in the spleen of immunized
mice, suggests that activated NK cells might be responsible for
mediating tumor cell killing.
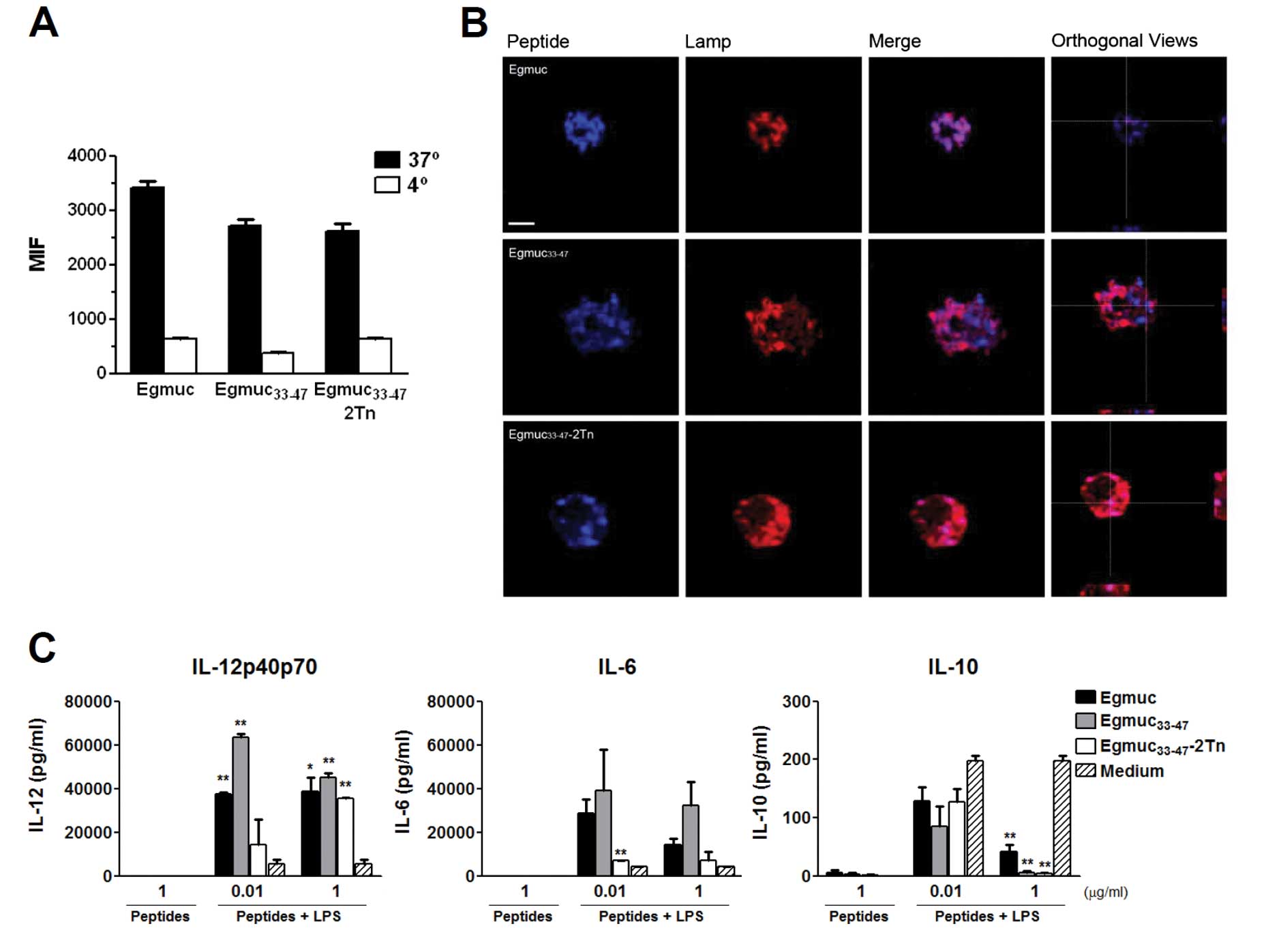
Egmuc peptides are internalized and
synergize with LPS to induce maturation of DCs
Due to their unique capacity to trigger
innate-lymphocyte functions, such as NK cells, we investigated
whether DC could be responsible of the increase in CD69+
NK cells in the spleens of immunized mice. We first evaluated
whether DC uptake of the Egmuc peptides occurs at different levels.
To this end, we subjected BMDCs to 1 h pulses with
Atto-647N-labeled Egmuc peptides. Internalization was analyzed by
either flow cytometry or confocal microscopy. Egmuc peptides and
glycopeptide were similarly taken up by DCs (Fig. 4A) and colocalized with the late
endosome/lysosome marker Lamp-1, suggesting that the three
evaluated peptides are delivered into the cell endosomal
compartments for antigen degradation and presentation with MHC
class II molecules (Fig. 4B).
We then evaluated whether the Egmuc (glyco)peptides
influenced DCs maturation induced by LPS. For this purpose, BMDCs
were pulsed with different concentrations of the three Egmuc
peptides and we evaluated the expression of co-stimulatory markers
and the production of IL-6, IL-10 or IL-12p40p70 in the culture
supernatants. When incubated alone, Egmuc peptides did not modify
either the expression of CD40, CD80, CD86 MHC class II molecules
(not shown), nor the production of the evaluated cytokines
(Fig. 4C). Nevertheless, when
incubated 2 h before LPS, the level of IL-6 and specially the level
of IL-12p40p70, were considerable augmented, while a decrease of
IL-10 was detected (Fig. 4C),
suggesting that Egmuc peptides synergize with LPS to induce BMDC
maturation.
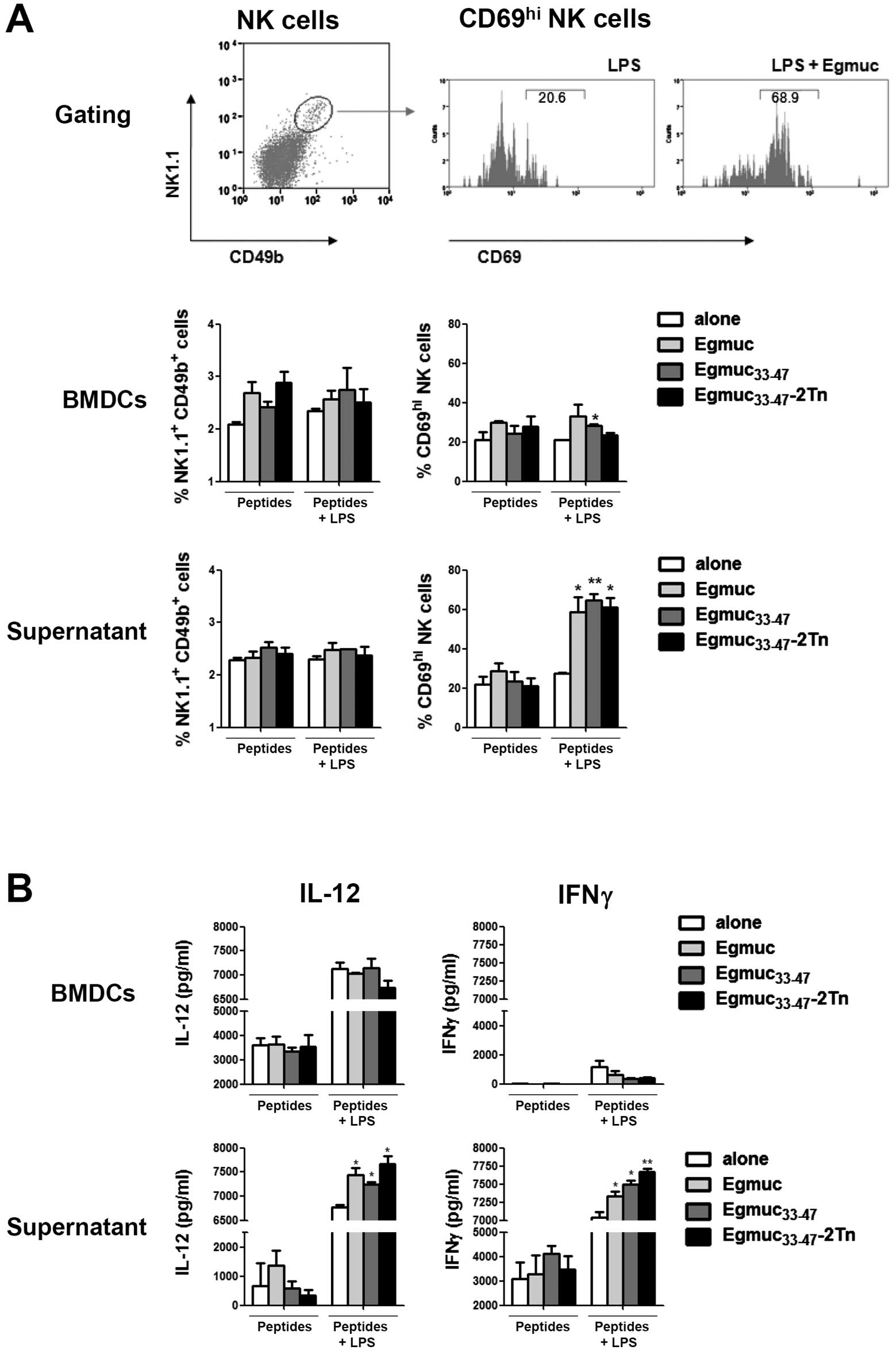
Soluble products from DCs activated with
Egmuc peptides plus LPS induce in vitro activation of NK cells
The activation of NK cells by DCs represents a
pathway which may be important in directly mediating NK
cell-dependent antitumor effects. In order to determine whether DCs
activated with Egmuc peptides together with LPS, as well as their
soluble products, could induce activation of NK cells, we
co-cultured BMDCs previously incubated with Egmuc peptides followed
by LPS, with splenocytes, or with their derived products. We found
a considerable increase of
CD69hi/CD49b+NK1.1+ cells in
splenocytes incubated with culture media derived from BMDCs loaded
with Egmuc, Egmuc33-47, Egmuc33-47-2Tn
peptides, as compared to culture media from BMDC incubated with LPS
alone (Fig. 5A). In addition, in
these conditions, we observed increased levels of IL-12p40p70 and
IFNγ (Fig. 5B). On the other hand,
no significant difference was detected in splenocytes stimulated
with BMDCs, or BMDC-derived products, previously loaded with
peptides in absence of LPS (Fig.
5). These results suggest that soluble factors produced by
BMDCs are sufficient for NK activation in vitro.
Discussion
Data reporting the antitumor effects of parasite
infections are increasing. Indeed, several reports have
demonstrated the induction of antitumor immunity by parasites and a
prolonged survival of tumor-bearing mice. Recent results indicated
that tumor protection can be achieved through the induction of both
innate and adaptive antitumor responses, as has been shown for the
malaria parasite infection (5) or
by intratumoral injection of attenuated Toxoplasma
gondii(39). In the present
study we evaluated and compared the capacity of an E.
granulosus-derived mucin-like peptide to induce antitumor
immune activity. We show that, when administered into mice, the
three Egmuc peptides conferred splenocytes the ability to kill
tumor cells. Also, this cytotoxic activity was correlated with an
increased number of activated-NK cells in the spleen of immunized
mice, suggesting that these peptides from E. granulosus
would activate the innate arm of the immune response that may be
efficient for eliminating tumor cells.
NK cells have been shown to have an important role
in antitumor immune response. For instance, patients with lung
cancer who have a high level of NK cell infiltration in the tumor,
have better prognosis than those with a low level of NK cells
(40). NK-cell activation is
controlled by the dynamic balance between activating and inhibitory
signals. The battery of receptors possessed by NK cells helps
mediate the detection of tumor or infected cells and embark on the
signaling pathways necessary to eliminate them (41). NK-cell effector functions can also
be stimulated through DC-derived signals via cytokines and cell
contact (42). Indeed, several
studies have already shown the activation, both phenotypically and
functionally, of NK cells by DCs in response to different microbial
stimuli, such as reovirus-infected human melanoma cells (43), inactivated influenza virus
(44), adenovirus encoding
melanoma tumor antigens (45), as
well as TLR ligands (44), by
mechanisms involving cell contact and secretion of DC-derived
cytokines.
Considering the role of DCs in stimulating NK cell
activation, we sought to evaluate whether DCs treated with Egmuc
peptides could activate NK cells. First, we found that Egmuc
peptides and Tn-glycopeptide synergize with LPS-induced maturation
of DCs, inducing the production of higher levels of IL-12 and IL-6,
in the absence of the regulatory cytokine IL-10. Then, we evaluated
whether cytokines produced by Egmuc-treated-BMDCs (such as IL-12),
or DC-NK contact interactions might activate NK cells in
vitro. We co-cultured Egmuc-treated BMDCs with splenocytes, or
alternatively, splenocytes cultured in the presence of BMDC-derived
soluble factors only. The NK cells from splenocytes incubated with
soluble factors from loaded-BMDCs presented a more activated
phenotype, defined by an increase in CD69 expression, as compared
to splenocytes incubated with BMDCs stimulated with LPS only,
indicating that DC-derived products, are sufficient for NK
activation. Whether NK activation detected by an increase of CD69
expression, is responsible for the in vitro cell
cytotoxicity by splenocytes from Egmuc-immunized mice, or for the
increase of activated NK cells in vivo, remains to be
explored. Additional cytotoxicity experiments with purified NK
cells from immunized mice will give direct evidence of their role
in eliminating tumor cells in vivo.
Importantly, the results reported here indicate that
the synergic effect of Egmuc peptides on LPS-maturation of DCs, as
well as the increase of activated NK cells and cytotoxic activity
in the spleens from immunized mice, would rather rely on the Egmuc
peptide sequence from E. granulosus that could act as a
PAMP. Molecules from different pathogens that induce maturation of
DCs have been identified. For example, Schistosoma mansoni
adult worm glycolipids interact with DCs and skew T cell responses
towards a Th1 profile, upregulating the production of
pro-inflammatory cytokines in an interaction that is mediated by
DC-SIGN and TLR4 (46). Also, DCs
exposed to Gram-negative bacteria respond by making IL-6, IL-12 and
IL-23, which promote the development of naive Th cells into Th1 or
Th17 cells (47). Interestingly,
several TLR-ligand components from parasites, such as the
glycosylphosphatidylinositol of Plasmodium
falciparum(48) or T.
cruzi(49) might be important
factors in the induction of pro-inflammatory responses and could be
used as adjuvant components to enhance antitumor immune responses
(50).
One possibility that could explain the observed
synergism is that the interaction of Egmuc peptides with DCs may
upregulate TLR4 expression on DCs, leading to an enhanced
production of pro-inflammatory cytokines when incubated with LPS.
Indeed, it has been reported that many microbial products induced
upregulation of several TLRs (51). Thus, we are currently evaluating
the expression of TRL4 in BMDC stimulated with Egmuc peptides.
Moreover, experiments using TLR knockout mice would bring evidence
regarding activation of DCs by Egmuc peptides together with
LPS.
Our results indicate that the introduction of the
cluster of two Tn moieties displayed on the Egmuc glycopeptides did
not confer an advantage for antigen uptake by dendritic cells or
for induction of antitumor antibodies. Despite this apparent
contradiction, it might be possible that clusters of more than
three Tn antigens are needed to induce antibodies capable of
recognizing tumor cells (32).
Also, the Egmuc33-47 -2Tn glycopeptide induced a cell
immune response that did not cross-react with the Egmuc
non-glycosylated peptides and very low specific-antibody titres,
suggesting that GalNAc linkage to the Egmuc33-47 peptide
modified its B and T cell immunogenicity. These data, together with
other already published (52),
indicate that the introduction of glycosylation sites on peptides
must be attentively selected since their immunological properties
can be markedly modified.
In conclusion, we present data suggesting that
mucin-like peptides from parasitic origin might be useful targets
for antitumor immunotherapy, since they confer splenocytes the
capacity to eliminate tumor cells, through a mechanism that could
involve activation of NK cells, in a process mediated soluble
DC-derived factors. This constitute, to our knowledge, the first
report showing that a single purified peptide of helminth parasite
origin induces antitumor activity.
Acknowledgements
This study was supported by grants from Comisión
Honoraria de Lucha contra el Cáncer (Uruguay), International Centre
for Genetic Engineering and Biotechnology (Italy), Programme
Transversal de Recherche No. 262 Institut Pasteur (France) and
Programa Grupos de Investigación (CSIC, Universidad de la
República, Uruguay). V.N. was supported by Agencia Nacional de
Investigación e Innovación (Uruguay). We thank F. Groh for the
amino acid analyses and F. Bonhomme for the mass spectrometry
analyses.
Abbreviations:
|
APC
|
antigen presenting cells
|
|
BMDCs
|
bone marrow derived dendritic
cells
|
|
DCs
|
dendritic cells
|
|
NK
|
natural killer
|
|
LN
|
lymph node
|
|
PRR
|
pattern recognition receptors
|
|
TLR
|
Toll-like receptor
|
|
NLR
|
Nod-like receptor
|
|
PAMP
|
pathogen-associated molecular
patterns
|
|
CFA
|
complete Freund adjuvant
|
|
CPM
|
counts per minute
|
|
Ig
|
immunoglobulin
|
|
MHC
|
major histo-compatibility complex
|
|
MGL
|
macrophage Gal/GalNAc lectin
|
|
SD
|
standard deviation
|
|
nd
|
not done
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Martel C, Ferlay J, Franceschi S, et
al: Global burden of cancers attributable to infections in 2008: a
review and synthetic analysis. Lancet Oncol. 13:607–615.
2012.PubMed/NCBI
|
|
3
|
Benamrouz S, Conseil V, Creusy C, Calderon
E, Dei-Cas E and Certad G: Parasites and malignancies, a review,
with emphasis on digestive cancer induced by Cryptosporidium parvum
(Alveolata: Apicomplexa). Parasite. 19:101–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oliveira EC, Leite MS, Miranda JA, et al:
Chronic Trypanosoma cruzi infection associated with low
incidence of 1,2-dimethylhydrazine-induced colon cancer in rats.
Carcinogenesis. 22:737–740. 2001.
|
|
5
|
Chen L, He Z, Qin L, et al: Antitumor
effect of malaria parasite infection in a murine Lewis lung cancer
model through induction of innate and adaptive immunity. PLoS One.
6:e244072011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang XL, Fu BQ, Yang SJ, et al:
Trichinella spiralis - a potential antitumor agent. Vet
Parasitol. 159:249–252. 2009. View Article : Google Scholar
|
|
7
|
Akgül H, Tez M, Unal AE, Keşkek M, Sayek I
and Ozçelik T: Echinococcus against cancer: why not? Cancer Immunol
Immunother. 98:1999–2000. 2003.
|
|
8
|
Darani HY and Yousefi M: Parasites and
cancers: parasite antigens as possible targets for cancer
immunotherapy. Future Oncol. 8:1529–1235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yousofi Darani H, Soozangar N, Khorami S,
Taji F, Yousofi M and Shirzad H: Hydatid cyst protoscolices induce
cell death in WEHI-164 fibrosarcoma cells and inhibit the
proliferation of baby hamster kidney fibroblasts in vitro. J
Parasitol Res. 304183:January 31–2012. View Article : Google Scholar
|
|
10
|
Medzhitov R: Recognition of microorganisms
and activation of the immune response. Nature. 449:819–826. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palucka AK, Ueno H, Fay JW and Banchereau
J: Taming cancer by inducing immunity via dendritic cells. Immunol
Rev. 220:129–150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correale J and Farez M: Helminth antigens
modulate immune responses in cells from multiple sclerosis patients
through TLR2-dependent mechanisms. J Immunol. 183:5999–6012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elliott DE and Weinstock JV: Helminth-host
immunological interactions: prevention and control of
immune-mediated diseases. Ann NY Acad Sci. 1247:83–96. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Vliet SJ, van Liempt E, Saeland E, et
al: Carbohydrate profiling reveals a distinctive role for the
C-type lectin MGL in the recognition of helminth parasites and
tumor antigens by dendritic cells. Int Immunol. 17:661–669.
2005.PubMed/NCBI
|
|
15
|
Vanhoutte F, Breuilh L, Fontaine J, et al:
Toll-like receptor (TLR)2 and TLR3 sensing is required for
dendritic cell activation, but dispensable to control
Schistosoma mansoni infection and pathology. Microbes
Infect. 9:1606–1613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cummings RD and Nyame AK: Schistosome
glysoconjugates. Biochim Biophys Acta. 1455:363–374. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doedens A, Loukas A and Maizels RM: A cDNA
encoding Tc-MUC-5, a mucin from Toxocara canis larvae identified by
expression screening. Acta Trop. 79:211–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theodoropoulos G, Hicks SJ, Corfield AP,
Miller BG and Carrington SD: The role of mucins in host-parasite
interactions: Part II - helminth parasites. Trends Parasitol.
17:130–135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas PG and Harn DAJ: Immune biasing by
helminth glycans. Cell Microbiol. 6:13–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alvarez Errico D, Medeiros A, Míguez M, et
al: O-glycosylation in Echinococcus granulosus:
identification and characterization of the carcinoma-associated Tn
antigen. Exp Parasitol. 98:100–109. 2001.
|
|
21
|
Brockhausen I: Mucin-type O-glycans
in human colon and breast cancer: glycodynamics and functions. EMBO
Rep. 7:599–604. 2006.
|
|
22
|
Schietinger A, Philip M, Yoshida BA, Azadi
P, Liu H, Meredith SC and Schreiber H: A mutant chaperone converts
a wild-type protein into a tumor-specific antigen. Science.
314:304–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baldus SE, Engelmann K and Hanisch FG:
MUC1 and the MUCs: a family of human mucins with impact in cancer
biology. Crit Rev Clin Lab Sci. 41:189–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carraway KL III, Funes M, Workman HC and
Sweeney C: Contribution of membrane mucins to tumor progression
through modulation of cellular growth signaling pathways. Curr Top
Dev Biol. 78:1–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freire T and Osinaga E: The sweet side of
tumor immunotherapy. Immunotherapy. 4:719–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kufe DW: Mucins in cancer: function,
prognosis and therapy. Nat Rev Cancer. 9:874–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beatson RE, Taylor-Papadimitriou J and
Burchell JM: MUC1 immunotherapy. Immunotherapy. 2:305–327. 2010.
View Article : Google Scholar
|
|
28
|
Quoix E, Ramlau R, Westeel V, et al:
Therapeutic vaccination with TG4010 and first-line chemotherapy in
advanced non-small-cell lung cancer: a controlled phase 2B trial.
Lancet Oncol. 12:1125–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Slovin SF, Ragupathi G, Musselli C, et al:
Fully synthetic carbohydrate-based vaccines in biochemically
relapsed prostate cancer: clinical trial results with
alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J
Clin Oncol. 21:4292–4298. 2003. View Article : Google Scholar
|
|
30
|
Fernandez C, Gregory WF, Loke P and
Maizels RM: Full-length-enriched cDNA libraries from
Echinococcus granulosus contain separate populations of
oligo-capped and trans-spliced transcripts and a high level of
predicted signal peptide sequences. Mol Biochem Parasitol.
122:171–180. 2002.PubMed/NCBI
|
|
31
|
Coïc YM, Lan CL, Neumann JM, Jamin N and
Baleux F: Slightly modifying pseudoproline dipeptides incorporation
strategy enables solid phase synthesis of a 54 AA fragment of
caveolin-1 encompassing the intramembrane domain. J Pept Sci.
16:98–104. 2010.
|
|
32
|
Lo-Man R, Vichier-Guerre S, Perraut R, et
al: A fully synthetic therapeutic vaccine candidate targeting
carcinoma-associated Tn carbohydrate antigen induces tumor-specific
antibodies in nonhuman primates. Cancer Res. 64:4987–4994. 2004.
View Article : Google Scholar
|
|
33
|
Rosa DS, Ribeiro SP and Cunha-Neto E:
CD4+ T cell epitope discovery and rational vaccine
design. Arch Immunol Ther Exp. 58:121–130. 2010.
|
|
34
|
Lo-Man R, Vichier-Guerre S, Bay S, Deriaud
E, Cantacuzene D and Leclerc C: Antitumor immunity provided by a
synthetic multiple antigenic glycopeptide displaying a tri-Tn
glycotope. J Immunol. 166:2849–2854. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singhal AK: Histo-blood group antigens in
cancer. Semin Cancer Biol. 2:379–388. 1991.PubMed/NCBI
|
|
36
|
Singh SK, Streng-Ouwehand I, Litjens M, et
al: Characterization of murine MGL1 and MGL2 C-type lectins:
distinct glycan specificities and tumor binding properties. Mol
Immunol. 46:1240–1249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hubert P, Heitzmann A, Viel S, et al:
Antibody-dependent cell cytotoxicity synapses form in mice during
tumor-specific antibody immunotherapy. Cancer Res. 71:5134–5143.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Quezada SA, Simpson TR, Peggs KS, et al:
Tumor-reactive CD4(+) T cells develop cytotoxic activity and
eradicate large established melanoma after transfer into
lymphopenic hosts. J Exp Med. 207:637–650. 2010.
|
|
39
|
Baird JR, Byrne KT, Lizotte PH, et al:
Immune-mediated regression of established B16F10 melanoma by
intratumoral injection of attenuated Toxoplasma gondii protects
against rechallenge. J Immunol. 190:469–478. 2013. View Article : Google Scholar
|
|
40
|
Villegas FR, Coca S, Villarrubia VG, et
al: Prognostic significance of tumor infiltrating natural killer
cells subset CD57 in patients with squamous cell lung cancer. Lung
Cancer. 35:23–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Malhotra A and Shanker A: NK cells: immune
cross-talk and therapeutic implications. Immunotherapy.
3:1143–1166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walzer T, Dalod M, Robbins SH, Zitvogel L
and Vivier E: Natural-killer cells and dendritic cells: ‘l'union
fait la force’. Blood. 106:2252–2258. 2005.
|
|
43
|
Prestwich RJ, Errington F, Steele LP, et
al: Reciprocal human dendritic cell-natural killer cell
interactions induce antitumor activity following tumor cell
infection by oncolytic reovirus. J Immunol. 183:4312–4321. 2009.
View Article : Google Scholar
|
|
44
|
Gerosa F, Gobbi A, Zorzi P, et al: The
reciprocal interaction of NK cells with plasmacytoid or myeloid
dendritic cells profoundly affects innate resistance functions. J
Immunol. 174:727–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Blalock LT, Landsberg J, Messmer M, et al:
Human dendritic cells adenovirally-engineered to express three
defined tumor antigens promote broad adaptive and innate immunity.
Oncoimmunology. 1:287–357. 2012. View Article : Google Scholar
|
|
46
|
van Die I, van Stijn CM, Geyer H and Geyer
R: Structural and functional analysis of glycosphingolipids of
Schistosoma mansoni. Methods Enzymol. 480:117–140.
2010.PubMed/NCBI
|
|
47
|
Truchetet ME, Beven L, Renaudin H, et al:
Potential role of Mycoplasma hominis in interleukin
(IL)-17-producing CD4+ T-cell generation via induction
of IL-23 secretion by human dendritic cells. J Infect Dis.
204:1796–1805. 2011.PubMed/NCBI
|
|
48
|
Gowda DC: TLR-mediated cell signaling by
malaria GPIs. Trends Parasitol. 23:596–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Campos MA, Almeida IC, Takeuchi O, et al:
Activation of Toll-like receptor-2 by glycosylphosphatidylinositol
anchors from a protozoan parasite. J Immunol. 167:416–423. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Junqueira C, Guerrero AT, Galvão-Filho B,
et al: Trypanosoma cruzi adjuvants potentiate T
cell-mediated immunity induced by a NY-ESO-1 based antitumor
vaccine. PLoS One. 7:e362452012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tarang S, Kumar S and Batra SK: Mucins and
toll-like receptors: kith and kin in infection and cancer. Cancer
Lett. 321:110–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Freire T, Lo-Man R, Bay S and Leclerc C:
Tn glycosylation of the MUC6 protein modulates its immunogenicity
and promotes the induction of Th17-biased T cell responses. J Biol
Chem. 286:7797–7811. 2011. View Article : Google Scholar : PubMed/NCBI
|