Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent cancers in the world, characterized by high mortality
rate and poor prognosis (1). Lack
of effective treatment options and late diagnosis are major reasons
for the high mortality rate in HCC (2). A new therapeutic strategy to address
these challenges cannot be overstated.
Green tea, compared with other teas, has a higher
concentration of catechin. Epigallocatechin-3-gallate (EGCG) is a
water-soluble catechin, which suppresses the multiplication of
cancer cells and induces apoptosis (3–5).
EGCG suppressed adhesion and invasion of hepatocarcinoma cells
through antioxidant activity (6).
EGCG inhibited the growth and metastasis of pancreatic cancer
(7,8) and colorectal cancer (9), reduced the incidence of esophageal
cancer (10) and improved the
prognosis of breast cancer (11).
It also inhibited the growth of HepG2 cells (12).
The EGCG anticancer effect is mediated by the
PI3K/AKT signaling pathway. EGCG inhibited PI3K/AKT/mTOR signaling
and promoted the apoptosis of B lymphocytes (5). It decreased the transcription of
FoxO1 via the PI3K/AKT and MEK/ERK pathways in 3T3-L1
differentiation (13). EGCG
inhibited PI3K/AKT activation, which enhanced apoptosis of T24
human bladder cancer cells (14).
The role of EGCG in proliferation or apoptosis via
AKT pathway in HCC has yet to be reported. In this study, we
reported on the ability of EGCG to inhibit cell growth in HepG2,
SMMC7721 and SK-hep1 cell lines, in vitro. We also
discovered that EGCG induced apoptosis and S phase arrest by
affecting the PI3K/AKT pathway in SMMC7721 cells, in
vitro.
Materials and methods
Cell lines and cell culture
All human hepatocellular carcinoma cell lines were
obtained from Shanghai Institutes for Biological Sciences, Chinese
Academy of Science, China. HepG2, SMMC7721 and SK-hep1 cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100
μg/ml streptomycin (all from Hyclone, USA) at 37°C in a
humidified incubator containing 5% CO2. Cells were
subcultured every two days when the density of cells reached
80%.
MTT assay
The cell growth inhibition effect of EGCG (purity
≥95%, Sigma-Aldrich, USA) was investigated using 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT)
assay. In brief, HepG2, SMMC7721 and SK-hep1 cells were seeded in
96-well culture plates at a density of 4×103 cells per
well overnight. After culture in serum-free DMEM for 2 h, cells
were treated with 0, 40, 80 and 120 μg/ml EGCG in DMEM with
10% FBS for 24, 48 or 72 h. To evaluate the cell viability, 20
μl MTT (5 μg/ml in culture medium, Sigma Chemical
Co., USA) was added to each well and incubated at 37°C for 4 h.
After removing the supernatants, 150 μl DMSO was added to
each well. The optical density (OD) was measured at 492 nm using a
microplate reader (Thermo Multiskan MK3, USA). The growth
inhibition rate was calculated as follows: Growth inhibition rate
(%) = [1 - (OD of treatment cells/OD of control cells)] × 100%.
Apoptosis assay
HepG2, SMMC7721 and SK-hep1 cells were seeded in
6-well plates at a density of 2×104 cells per well
overnight. HepG2, SMMC7721 and SK-hep1 were then treated with EGCG
at concentration of 74.7, 59.6 and 61.3 μg/ml respectively.
Later, cells were stained with acridine orange (AO) and ethidium
bromide (EB) using Normal/Apoptosis/Necrosis identification kit
(KeyGen, China), following the manufacturer’s instructions.
Apoptosis of HCC cells was observed under an IX51 inverted
fluorescent microscope (Olympus, Japan).
Flow cytometry
SMMC7721 cells were treated with or without 59.6
μg/ml EGCG in 6-well culture plates for 48 h. Approximately
1×106 cells were collected, washed with 1X PBS and fixed
with 70% ethanol at −20°C overnight. Cells were then centrifuged
and washed with PBS three times, re-suspended in 100 μl
RNase A, incubated at 37°C for 30 min, followed by staining with
400 μl propidium iodide (PI) staining solution (BD Sciences,
USA) and incubated at 4°C in the dark. Finally, DNA contents in
stained nuclei were analyzed with a flow cytometer (Beckman coulter
Epics XL, USA).
Annexin V-FITC analysis
SMMC7721 cells were treated with or without 59.6
μg/ml EGCG for 48 h. Cells were then washed twice with PBS.
Then they were stained with Annexin V and PI (BD Sciences) in
binding buffer. Cells were then analyzed using flow cytometry (BD
Caliber, USA).
RNA extraction and reverse transcriptase
PCR
Total RNA was extracted from SMMC7721 cells treated
with or without 59.6 μg/ml EGCG for 48 h, using TRIzol
reagent according to the manufacturer’s instructions (Invitrogen,
USA) and quantified by NanoDrop2000 (Thermo Scientific, USA). The
cDNA was generated from 0.5 μg total RNA using a ReverTra
Ace® qPCR RT kit (Toyobo, Japan) following the
manufacturer’s protocol and stored at −20°C.
Quantitative PCR
The mRNA levels of PI3K, AKT and NF-κB were
determined by qPCR using GoTaq®qPCR Master Mix (Promega,
USA) following the manufacturer’s instructions. The GAPDH mRNA was
used as an internal control to normalize the amount of the above
mRNA in each sample. The primers designed were as follows: GAPDH
forward, 5′-AAG GTG AAG GTC GGA GTC AAC-3′, reverse: 5′-GGG GTC ATT
GAT GGC AAC AAT A-3′; PI3K forward: 5′-TGG AAG CAG CAA CCG AAA
C-3′, reverse: 5′-CAT TGA GGG AGT CGT TGT-3′; AKT forward: 5′-GGC
AAG GTG ATC CTG GTG AA-3′, reverse: 5′-GGG ACA GGT GGA AGA ACA
GC-3′; NF-κB forward: 5′-GTC ACT GCC CAG ACT TTA CT-3′, reverse:
5′-GCT TCT CCA CTG AAA ATC CT-3′. All assays were performed in
triplicate and calculated on the basis of 2−ΔΔCt
method.
Western blot analysis
SMMC7721 cells were pretreated with EGCG, LY294002
(Cell Signaling Technology, USA), IGF-1 (R&D Systems, USA)
alone or with the combination of EGCG and LY294002 or IGF-1 for 48
h. Protein extracts were prepared by RIPA lysis buffer [50 mM Tris
(pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate,
0.1% SDS, 2 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM
EDTA, 1 mM Na3VO4] adding 0.5 μg/ml
leupeptin, 1 mM PMSF and 10 mM NaF. Protein concentration was
quantified by BCA kit (Beyotime, China) according to the
manufacturer’s protocol. Proteins (40 μg) were
electrophoresed on 10% SDS-polyacrylamide gels and transferred to
PVDF membranes. Membranes were probed with antibodies to AKT, p-AKT
(Ser473) and β-actin. All antibodies were purchased from Cell
Signaling Technology. Immunoreactive bands were visualized using
the DyLight®680 Conjugate-anti-rabbit IgG (Cell
Signaling Technology). Bands were then scanned and analyzed by
Odyssey two-color infrared imaging system (LI-COR, USA).
Statistical analysis
Differences between the two groups were analyzed
with the Student’s t-test unless indicated otherwise. Results were
considered statistically significant at a P<0.05.
Results
EGCG inhibits the growth of human
hepatocellular carcinoma cells in vitro
To investigate the effect of EGCG on hepatocellular
carcinoma cells, HepG2, SMMC7721 and SK-hep1 cells were treated
with EGCG at different concentrations and time-points. The
inhibition rate was calculated, based on the cell viability,
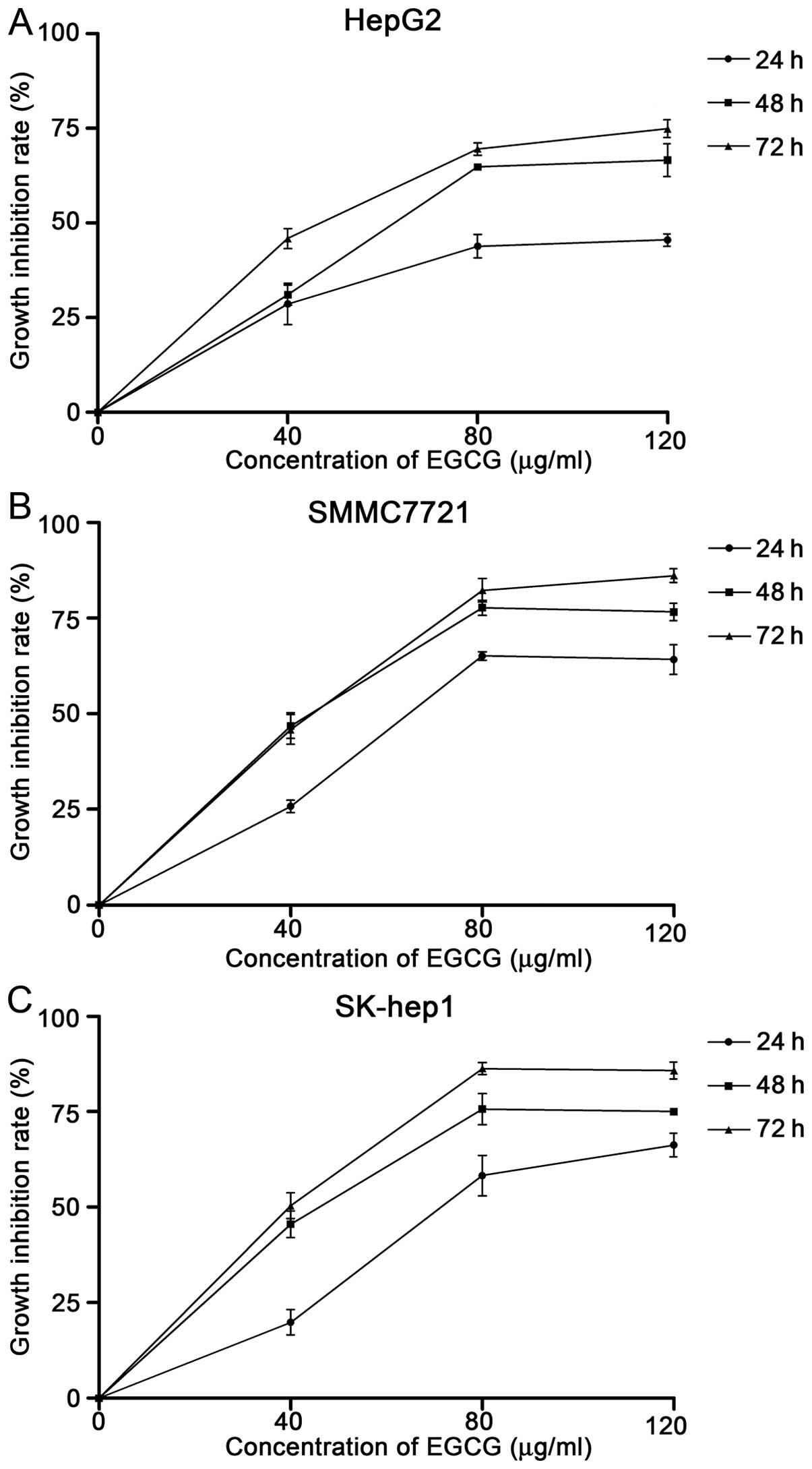
determined by MTT assay. As shown in Fig. 1, the inhibition rate increased with
the increase in concentration of EGCG across all three cell lines
(Fig. 1). The cell growth
inhibition rates at 48 h were significantly higher compared with
rates at 24 h, at each concentration in all cell lines. However,
the difference between rates at 48 and 72 h following treatment was
not significant. As a result, we chose 48-h pretreatment in the
following experiments. The half maximal (50%) inhibitory
concentrations (IC50) of EGCG at 48 h for HepG2,
SMMC7721 and SK-hep1 were 74.7, 59.6 and 61.3 μg/ml,
respectively (Fig. 1).
EGCG induces apoptosis
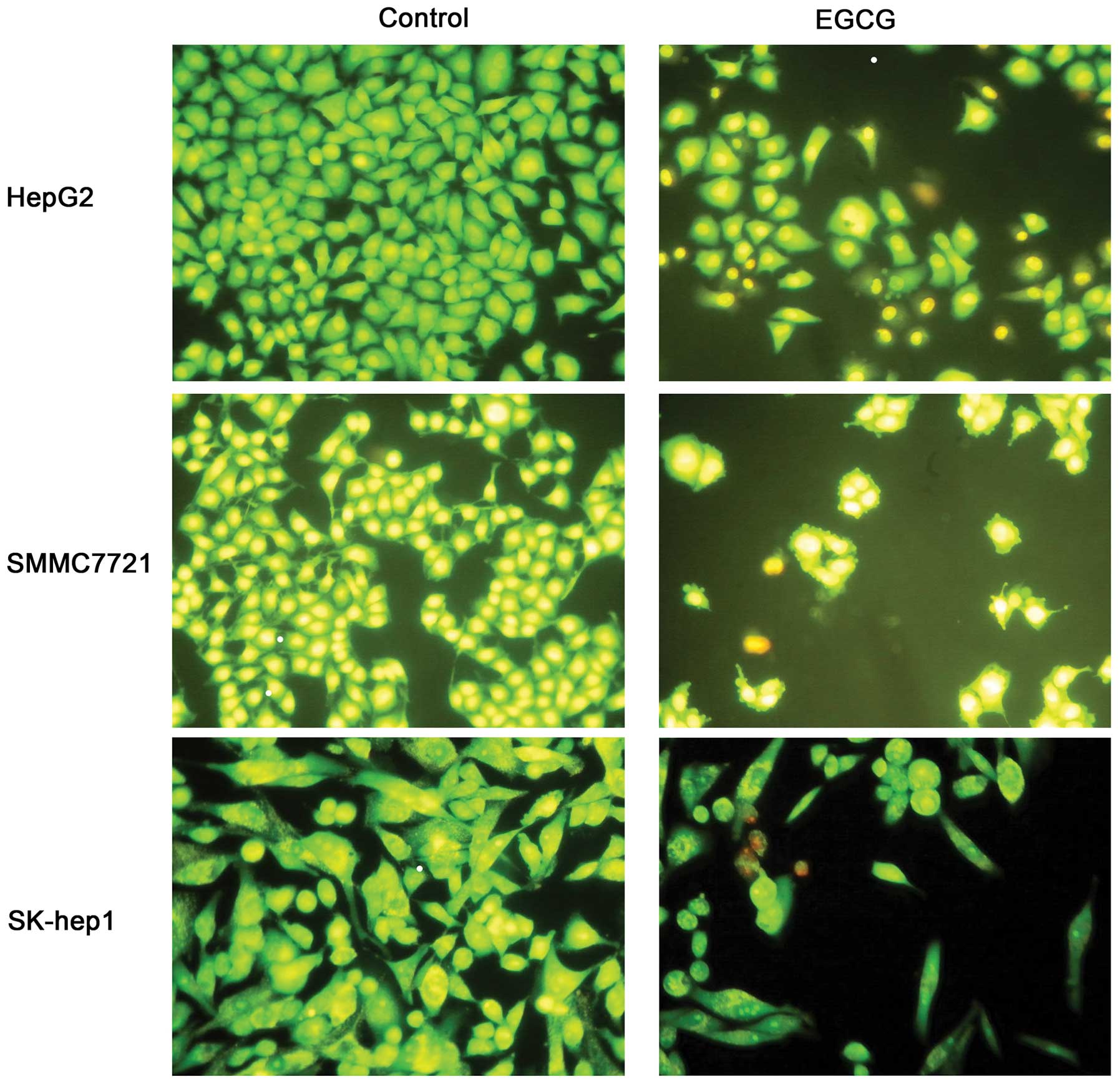
We next measured apoptosis in the presence of EGCG,
as apoptosis was closely related to cell growth. Each cell line was
treated with EGCG at IC50 concentration for 48 h. The
cell numbers of EGCG-treated group were significantly less than the
control group (Fig. 2). Untreated
cells (control) showed normal structure without prominent apoptosis
and necrosis. The AO/EB staining indicated late apoptosis in 48-h
incubated cells (Fig. 2),
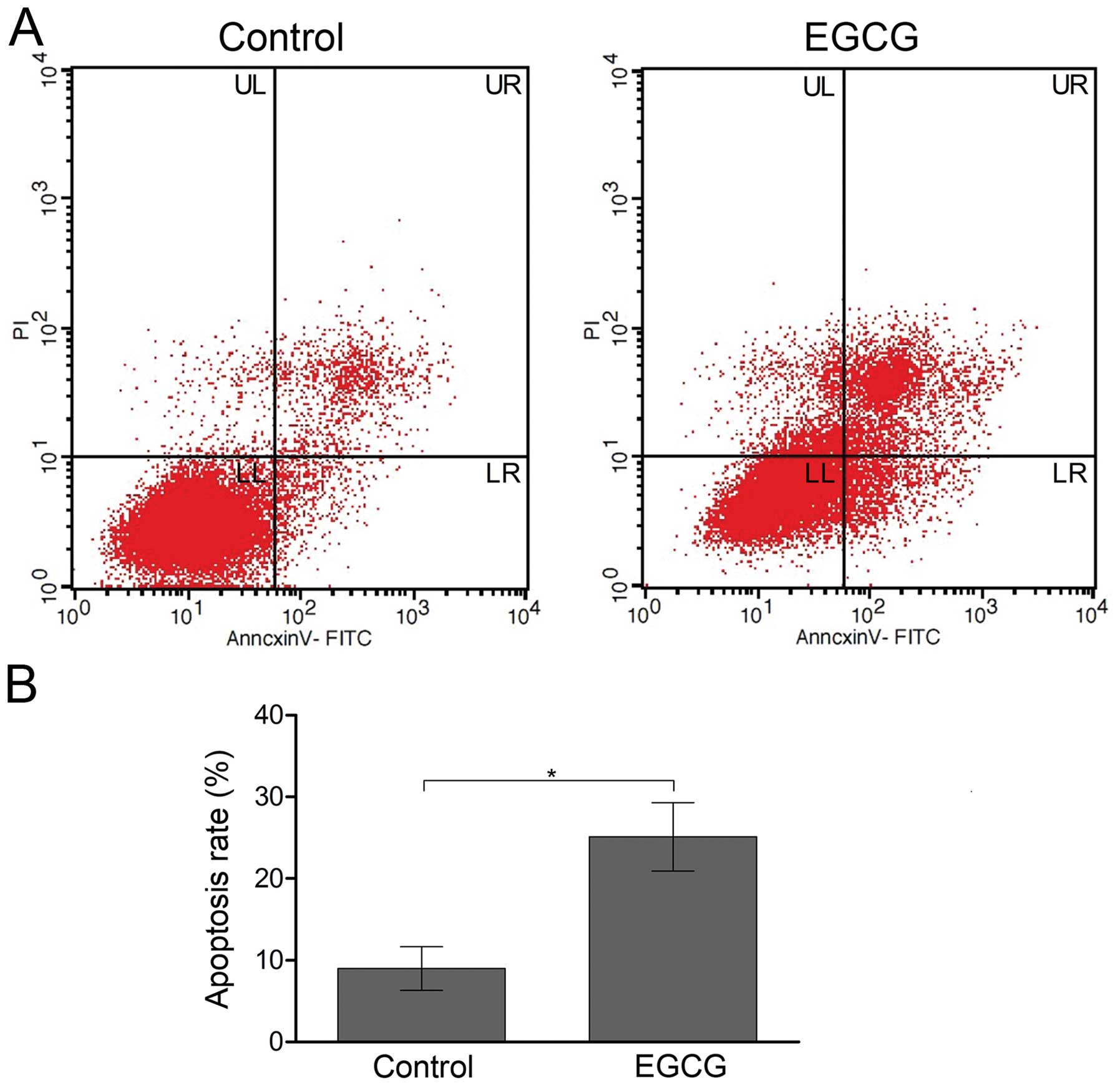
represented by orange staining. Flow cytometry also confirmed
induction of apoptosis by EGCG in SMMC7721 cells. In EGCG-treated
SMMC7721 cells, increased early and late apoptosis were observed
(Fig. 3A). The total percentage of
apoptosis (sum up of early stage and late stage) was 25.1±4.2% in
EGCG-treated cells, compared with 9.0±2.7% in the non-treated
control cells (Fig. 3B).
EGCG treatment arrests SMMC7721 cells at
S phase
To further investigate the underlying mechanism, we
analyzed distribution of the cell cycle by flow cytometry (Fig. 4). After 48-h treatment, the
percentage of EGCG-treated SMMC7721 cells in S phase was
significantly higher compared with non-treated cells (49.7±1.2 vs.
22.1±1.5%, Fig. 4). This result
suggested that EGCG arrested SMMC7721 cells at S phase.
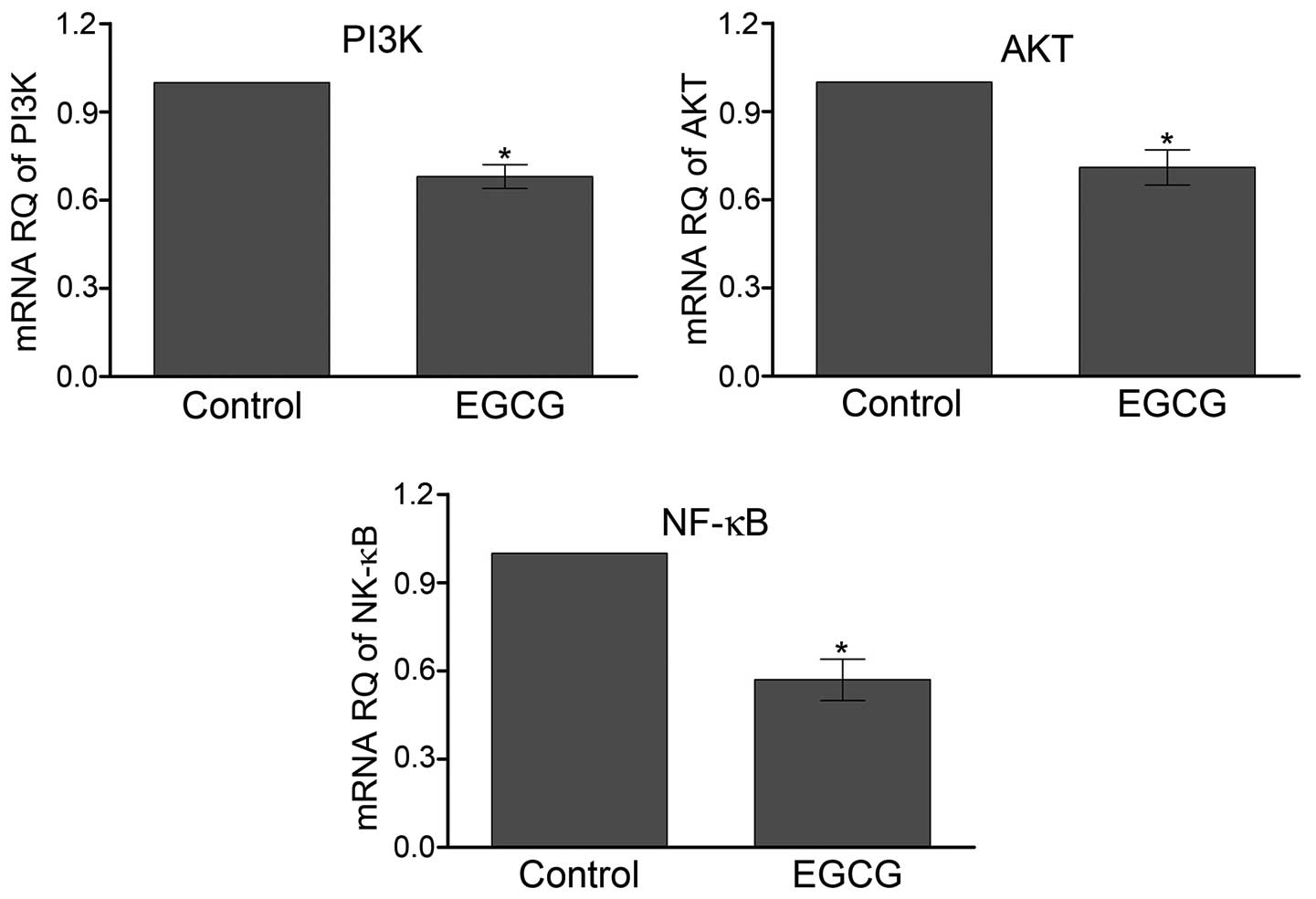
EGCG decreases the transcription of PI3K
and AKT
We further investigated the role of AKT pathway
mediating EGCG function in SMMC7721 cells. The mRNA level of AKT
was quantified by qPCR (Fig. 5).
The results showed that relative mRNA levels of PI3K and AKT
decreased ∼31 and 29% respectively, after treatment with EGCG
compared with control. NF-κB was the major downstream effector of
AKT. The transcription of NF-κB was 43% lower in the SMMC7721 cells
treated with EGCG than control cells (P<0.05).
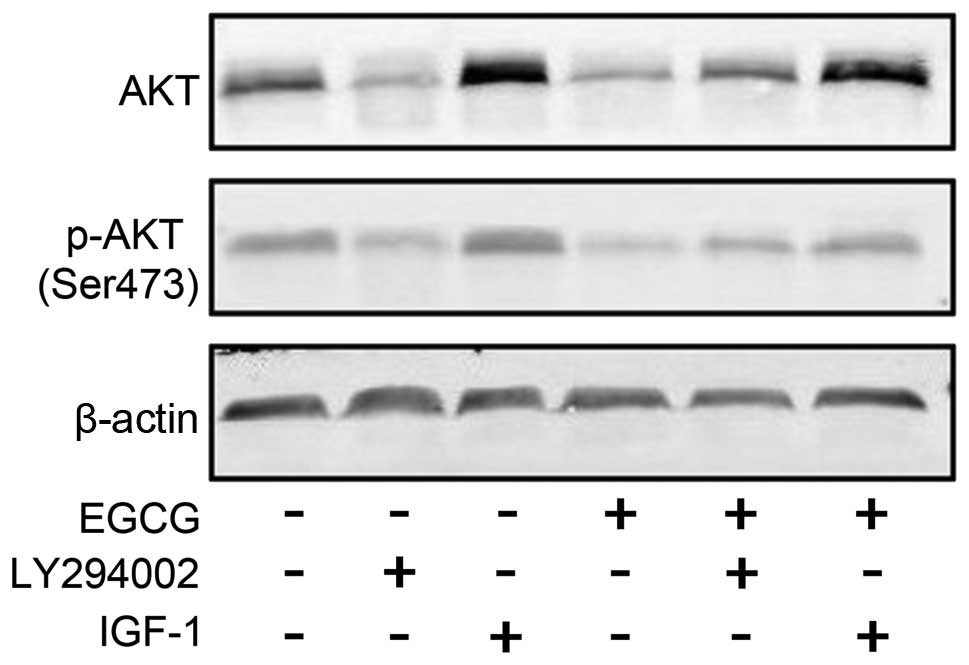
EGCG downregulates the expression of AKT
and its phosphorylation
EGCG downregulated the activity of AKT. We also
verified the phosphorylation of AKT at Ser473 in SMMC7721 cells
treated with EGCG using western blotting. As expected, the
expression and phosphorylation of AKT were both reduced following
treatment (Fig. 6).
Discussion
HCC is characterized by high mortality rate and poor
prognosis (1). HepG2, SMMC7721 and
SK-hep1 are classic cell lines for studying HCC. Studies have shown
that EGCG induced tumor apoptosis, and prevented tumor invasion and
metastasis (6,8,15–17).
We therefore, used these cell lines to investigate the effect of
EGCG on inhibition of cell growth and the underlying mechanism. We
observed that EGCG halted cell growth and induced apoptosis of
SMMC7721 cells, which was also confirmed in other HCC cell lines
(Figs. 1–3). Further, we observed that the
IC50 of these three HCC cell lines at 48 h ranged from
60 to 75 μg/ml (Fig. 1),
which was in accordance with previous studies (17–19).
Progression through each phase of the cell cycle is
regulated carefully to avoid proliferation under adverse
conditions. Cells are arrested in G1, S and G2/M phases to prevent
replication of damaged DNA or to prevent aberrant mitosis. Previous
studies reported that EGCG blocked progression of the cell cycle at
G1 phase in HCC lines (12), which
is reportedly related to the activation of AMPK (12). However, we found that EGCG caused S
phase but not G1 arrest in SMMC7721 cells (Fig. 4), which might be attributed to the
unique features of HCC cell lines.
PI3K/AKT pathway was reported to play an important
role in cancer proliferation and migration. Previous studies
indicated the role of PI3K/AKT pathway in the development and
progression of HCC cells, mainly reflected in the mechanism of
liver cancer cell proliferation, differentiation and apoptosis
(20–22). The effect of EGCG on PI3K/AKT
pathway in carcinogenesis, progression and metastasis in various
type of tumors, have been widely studied in breast cancer (23), pancreatic cancer (8), endometrial cancer (24) and thyroid carcinoma (25). Here, we first detected the mRNA
level of major components of AKT pathway, and found that EGCG
downregulated the PI3K, AKT and NF-κB (Fig. 4). Phosphorylation at Ser473 and
Thr308, AKT activates the downstream signaling molecules including
the NF-κB and regulates cancer cell apoptosis (26–28).
Phosphorylation of AKT at Ser473 in SMMC7721 cells was reduced by
EGCG (Fig. 5), which was
consistent with a previous study of HepG2 cells, reporting that
EGCG induced apoptosis and caused a decrease in the p-IGF-1R
protein and its downstream signaling molecules including the p-ERK,
p-Akt, p-Stat-3, and p-GSK-3β proteins (29).
EGCG affects both upstream and downstream targets of
AKT. Previous reports showed that EGCG inhibited the
thrombin-PAR1/PAR4-p42/p44 MAPKinase invasive signaling axis in
hepatocellular carcinoma cells (30). HIF-1α/VEGF function was also a
therapeutic target for EGCG in cancer chemoprevention and
anticancer therapy (15,22). EGCG also delayed HCC cell growth
through inhibition of Bcl-2 family (16,19)
or induced apoptosis in HCC cells via downregulation of COX-2 and
Bcl-2, and consequently activated caspase-9 and caspase-3 (31). The anti-metastatic effects of EGCG
were associated with the inhibition of MMP-2 and MMP-9 activity
(32,33).
In conclusion, our study provides evidence that EGCG
inhibited HCC cell growth by affecting cell cycle and inducing
apoptosis via downregulation of PI3K/AKT activity. The results
suggested that EGCG is a potential anticancer agent in HCC
therapy.
Acknowledgements
This study was supported by grants
from the Youth Science Foundation of Guangxi Medical University
(no. 02602211011) and Guangxi Natural Science Foundation (no.
2013GXNSFBA0191865).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Shek FH, Fatima S and Lee NP: Implications
of the use of eukaryotic tanslation initiation factor 5A (eIF5A)
for prognosis and treatment of hepatocellular carcinoma. Int J
Hepatol. 2012:7609282012.PubMed/NCBI
|
|
3.
|
Hagen RM, Chedea VS, Mintoff CP, Bowler E,
Morse HR and Ladomery MR: Epigallocatechin-3-gallate promotes
apoptosis and expression of the caspase 9a splice variant in PC3
prostate cancer cells. Int J Oncol. 43:194–200. 2013.PubMed/NCBI
|
|
4.
|
Li JJ, Gu QH, Li M, Yang HP, Cao LM and Hu
CP: Role of Ku70 and Bax in epigallocatechin-3-gallate-induced
apoptosis of A549 cells in vivo. Oncol Lett. 5:101–106.
2013.PubMed/NCBI
|
|
5.
|
Liu D, Li P, Song S, et al: Pro-apoptotic
effect of epigallo-cate-chin-3-gallate on B lymphocytes through
regulating BAFF/PI3K/Akt/mTOR signaling in rats with
collagen-induced arthritis. Eur J Pharmacol. 690:214–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zhang G, Miura Y and Yagasaki K:
Suppression of adhesion and invasion of hepatoma cells in culture
by tea compounds through antioxidative activity. Cancer Lett.
159:169–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Shankar S, Ganapathy S, Hingorani SR and
Srivastava RK: EGCG inhibits growth, invasion, angiogenesis and
metastasis of pancreatic cancer. Front Biosci. 13:440–452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Shankar S, Marsh L and Srivastava RK: EGCG
inhibits growth of human pancreatic tumors orthotopically implanted
in Balb C nude mice through modulation of FKHRL1/FOXO3a and
neuropilin. Mol Cell Biochem. 372:83–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Inaba H, Nagaoka Y, Kushima Y, et al:
Comparative examination of anti-proliferative activities of
(-)-epigallocatechin gallate and (--)-epigallocatechin against
HCT116 colorectal carcinoma cells. Biol Pharm Bull. 31:79–84. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Li ZG, Shimada Y, Sato F, et al: Promotion
effects of hot water on N-nitrosomethylbenzylamine-induced
esophageal tumorigenesis in F344 rats. Oncol Rep. 10:421–426.
2003.PubMed/NCBI
|
|
11.
|
Kushima Y, Iida K, Nagaoka Y, et al:
Inhibitory effect of (-)-epigallocatechin and (-)-epigallocatechin
gallate against heregulin beta1-induced migration/invasion of the
MCF-7 breast carcinoma cell line. Biol Pharm Bull. 32:899–904.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY
and Way TD: EGCG inhibits protein synthesis, lipogenesis, and cell
cycle progression through activation of AMPK in p53 positive and
negative human hepatoma cells. Mol Nutr Food Res. 53:1156–1165.
2009. View Article : Google Scholar
|
|
13.
|
Kim H and Sakamoto K: (-)-Epigallocatechin
gallate suppresses adipocyte differentiation through the MEK/ERK
and PI3K/Akt pathways. Cell Biol Int. 36:147–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Qin J, Xie LP, Zheng XY, et al: A
component of green tea, (-)-epigallocatechin-3-gallate, promotes
apoptosis in T24 human bladder cancer cells via modulation of the
PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res
Commun. 354:852–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Shirakami Y, Shimizu M, Adachi S, et al:
(-)-Epigallocatechin gallate suppresses the growth of human
hepatocellular carcinoma cells by inhibiting activation of the
vascular endothelial growth factor-vascular endothelial growth
factor receptor axis. Cancer Sci. 100:1957–1962. 2009. View Article : Google Scholar
|
|
16.
|
Tsang WP and Kwok TT: Epigallocatechin
gallate up-regulation of miR-16 and induction of apoptosis in human
cancer cells. J Nutr Biochem. 21:140–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Uesato S, Kitagawa Y, Kamishimoto M,
Kumagai A, Hori H and Nagasawa H: Inhibition of green tea catechins
against the growth of cancerous human colon and hepatic epithelial
cells. Cancer Lett. 170:41–44. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Abou El Naga RN, Azab SS, El-Demerdash E,
Shaarawy S, El-Merzabani M and Ammar el SM: Sensitization of
TRAIL-induced apoptosis in human hepatocellular carcinoma HepG2
cells by phytochemicals. Life Sci. 92:555–561. 2013.PubMed/NCBI
|
|
19.
|
Nishikawa T, Nakajima T, Moriguchi M, et
al: A green tea polyphenol, epigalocatechin-3-gallate, induces
apoptosis of human hepatocellular carcinoma, possibly through
inhibition of Bcl-2 family proteins. J Hepatol. 44:1074–1082. 2006.
View Article : Google Scholar
|
|
20.
|
Bu X, Jia F, Wang W, Guo X, Wu M and Wei
L: Coupled down-regulation of mTOR and telomerase activity during
fluorouracil-induced apoptosis of hepatocarcinoma cells. BMC
Cancer. 7:2082007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tang C, Lu YH, Xie JH, et al:
Downregulation of survivin and activation of caspase-3 through the
PI3K/Akt pathway in ursolic acid-induced HepG2 cell apoptosis.
Anticancer Drugs. 20:249–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhang Q, Tang X, Lu Q, Zhang Z, Rao J and
Le AD: Green tea extract and (-)-epigallocatechin-3-gallate inhibit
hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF
expression in human cervical carcinoma and hepatoma cells. Mol
Cancer Ther. 5:1227–1238. 2006. View Article : Google Scholar
|
|
23.
|
Zhang G, Wang Y, Zhang Y, et al:
Anti-cancer activities of tea epigallocatechin-3-gallate in breast
cancer patients under radiotherapy. Curr Mol Med. 12:163–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Park SB, Bae JW, Kim JM, Lee SG and Han M:
Antiproliferative and apoptotic effect of
epigallocatechin-3-gallate on Ishikawa cells is accompanied by sex
steroid receptor downregulation. Int J Mol Med. 30:1211–1218.
2012.PubMed/NCBI
|
|
25.
|
De Amicis F, Perri A, Vizza D, et al:
Epigallocatechin gallate inhibits growth and
epithelial-to-mesenchymal transition in human thyroid carcinoma
cell lines. J Cell Physiol. 228:2054–2062. 2013.PubMed/NCBI
|
|
26.
|
Dang TP: Notch, apoptosis and cancer. Adv
Exp Med Biol. 727:199–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Roos WP and Kaina B: DNA damage-induced
cell death: from specific DNA lesions to the DNA damage response
and apoptosis. Cancer Lett. 332:237–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shimizu M, Shirakami Y, Sakai H, et al:
EGCG inhibits activation of the insulin-like growth factor
(IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells.
Cancer Lett. 262:10–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kaufmann R, Henklein P, Henklein P and
Settmacher U: Green tea polyphenol epigallocatechin-3-gallate
inhibits thrombin-induced hepatocellular carcinoma cell invasion
and p42/p44-MAPKinase activation. Oncol Rep. 21:1261–1267. 2009.
View Article : Google Scholar
|
|
31.
|
Chen XL, Wang Q, Cao LQ, et al:
Epigallocatechin-3-gallate induces apoptosis in human
hepatocellular carcinoma cells. Zhonghua Yi Xue Za Zhi.
88:2524–2528. 2008.(In Chinese).
|
|
32.
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Comparative effects of EGCG, green tea and a
nutrient mixture on the patterns of MMP-2 and MMP-9 expression in
cancer cell lines. Oncol Rep. 24:747–757. 2010.PubMed/NCBI
|
|
33.
|
Zhang Y, Owusu L, Duan W, et al:
Anti-metastatic and differential effects on protein expression of
epigallocatechin-3-gallate in HCCLM6 hepatocellular carcinoma
cells. Int J Mol Med. 32:959–964. 2013.PubMed/NCBI
|