Contents
Introduction
Structural and functional features of VIP/PACAP and
VIPRs
VIP receptors as potential targets for imaging and
therapy
Targeting VIPRs for molecular imaging
123I-labeled VIP for receptor imaging
99mTc-labeled VIP analogs for receptor
imaging
PET molecular imaging of the VIP receptor
Targeting VIP receptor for cancer molecular
therapy
Prospective and Conclusion
Introduction
Malignant tumors have become one of the most lethal
diseases in humans, and the incidence, diagnostic studies and
therapeutic options for tumor treatment have undergone major
changes in recent years. Currently, the standard treatment for a
malignancy includes surgical resection followed by the delivery of
chemotherapeutic agents. Although surgical resection is the
preferred choice for early-stage solid tumors, it is not a suitable
treatment for invasive, metastatic or hematologic malignancies. The
overall toxicity and the side effects of chemotherapy also limit
the dose regimen, thus requiring a relatively narrow therapeutic
index, which can lead to insufficient and/or unpredictable
responses. Furthermore, during the course of therapy, multidrug
resistance can severely limit the effectiveness of chemotherapy and
is also associated with tumor recurrence (1–4).
Therefore, it is desirable to target therapeutic molecules to
primary tumors and their metastases, which represents a major
challenge for improving current cancer therapy (5). Another significant challenge is the
development of strategies that can guide the use of these targeted
molecules and enable the specific delivery of therapeutic agents to
malignant tissues (6). Targeted
therapies generally take advantage of biological molecules that are
uniquely expressed or significantly overexpressed in tumors.
Hormone receptors were some of the earliest targets utilized for
the targeted treatment of many cancers, such as breast, prostate
and thyroid cancers (7,8). Therefore, the molecular imaging of
the expression of tumor-related receptors is advantageous because
it provides information for receptor-targeted therapy (9).
Molecular imaging has been defined as the
visualization, characterization and measurement of biological
processes at the molecular and cellular levels (10). Tumor receptor imaging is an
important type of molecular imaging and can be used to assess
receptor expression for the entire disease burden to facilitate
early diagnosis, suggest personalized therapy options, and monitor
the in vivo effects of a drug on its target. Different
receptors are overexpressed in specific tumor types. Several tumor
receptors have been utilized for imaging and therapy, including
somatostatin receptors, human epidermal growth factor receptor 2
(HER2), integrin receptors, epidermal growth factor receptor
(EGFR), and vasoactive intestinal peptide receptors (VIPRs).
Vasoactive intestinal peptide receptors (VIPRs) are highly
overexpressed in human tumors and metastases. VIPRs also play a
major role in the progression of a number of malignancies, thus
suggesting that these receptors may serve as molecular targets for
cancer diagnosis and treatment. Herein, we describe the biology of
VIPRs and VIPR-targeting agents and discuss the potential
application of VIPRs for the diagnosis and treatment of cancer.
Structural and functional features of
VIP/PACAP and VIPRs
Vasoactive intestinal peptide (VIP) and pituitary
adenylate cyclase-activating polypeptide (PACAP) are two members of
a structurally related family of peptides that includes mammalian
peptide histidine methionine amide (PHM), secretin, glucagon, and
growth hormone-releasing factor (GRF) (11). VIP was isolated from the small
intestine of a pig and was characterized in the early 1970s by Mutt
and Said. VIP exhibits various physiological effects, including
increased vasodilatation and reduced arterial blood pressure
(12), smooth muscle relaxation
(13), stimulation of electrolyte
secretion (14),
immunosuppression, hormonal secretion, cell proliferation and
increased gastric motility (15,16).
The human VIP gene is located on chromosome 6 at band q25 (17,18)
and encodes the 170-amino acid precursor protein, prepro-VIP, which
undergoes post-translational modification to produce the 28 amino
acid VIP peptide (12,19), in addition to the 27 aa peptide
histidine methionine (PHM) in humans or the peptide histidine
isoleucine (PHI) in rodents (20).
These two peptides are co-synthesized with VIP as part of the same
precursor peptide, which is encoded by an adjacent exon in the
human genome, suggesting that the family of peptides has evolved
via exon duplication coupled with gene duplication (21). The N-terminus of VIP plays an
important role in this protein’s biological activity, and several
studies have shown that the N-terminus is essential for receptor
activation; however, it is not involved in the recognition of the
receptor-binding site, which seems to involve amino acids in the
C-terminal domain (22,23). The human PACAP gene is located on
chromosome 18p11 and encodes a 176-amino acid precursor protein
(preproPACAP) that contains PACAP and PACAP-related peptide (PRP)
in the C-terminal domain and a 24 amino acid signal protein in the
N-terminal domain (23,24).
These two peptides share a similar sequence and
exert their functions through the binding of vasoactive intestinal
peptide receptors (VIPRs), which are functional receptors for VIP
and PACAP. VIPRs are members of the G-protein-coupled receptor
(GPCR) family, which is comprised of three classes of receptors:
VPAC1, VPAC2 and PAC1. VPAC1 and VPAC2 respond to VIP and PACAP
with comparable affinity, whereas PAC1 displays a high affinity for
PACAP and a low affinity for VIP (25–27).
The VIPRs share a similar structural organization; they contain
seven transmembrane domains (TM), a large N-terminal segment that
includes the binding site of VIP/PACAP, and an intracellular
C-terminal region that is associated with signal transduction
(28). Upon ligand binding, these
receptors mediate their signal transduction activity via the
binding of heterotrimeric G-proteins to the receptor’s
intracellular loop, which ultimately results in cAMP production
(29,30), protein kinase A (PKA) pathway
activation (25,26), inositol phosphate (IP) turnover
(23), the activation of MAPKs
(mitogen-activated protein kinases) (31,32),
and the activation of the NF-κB pathway (Fig. 1) (33).
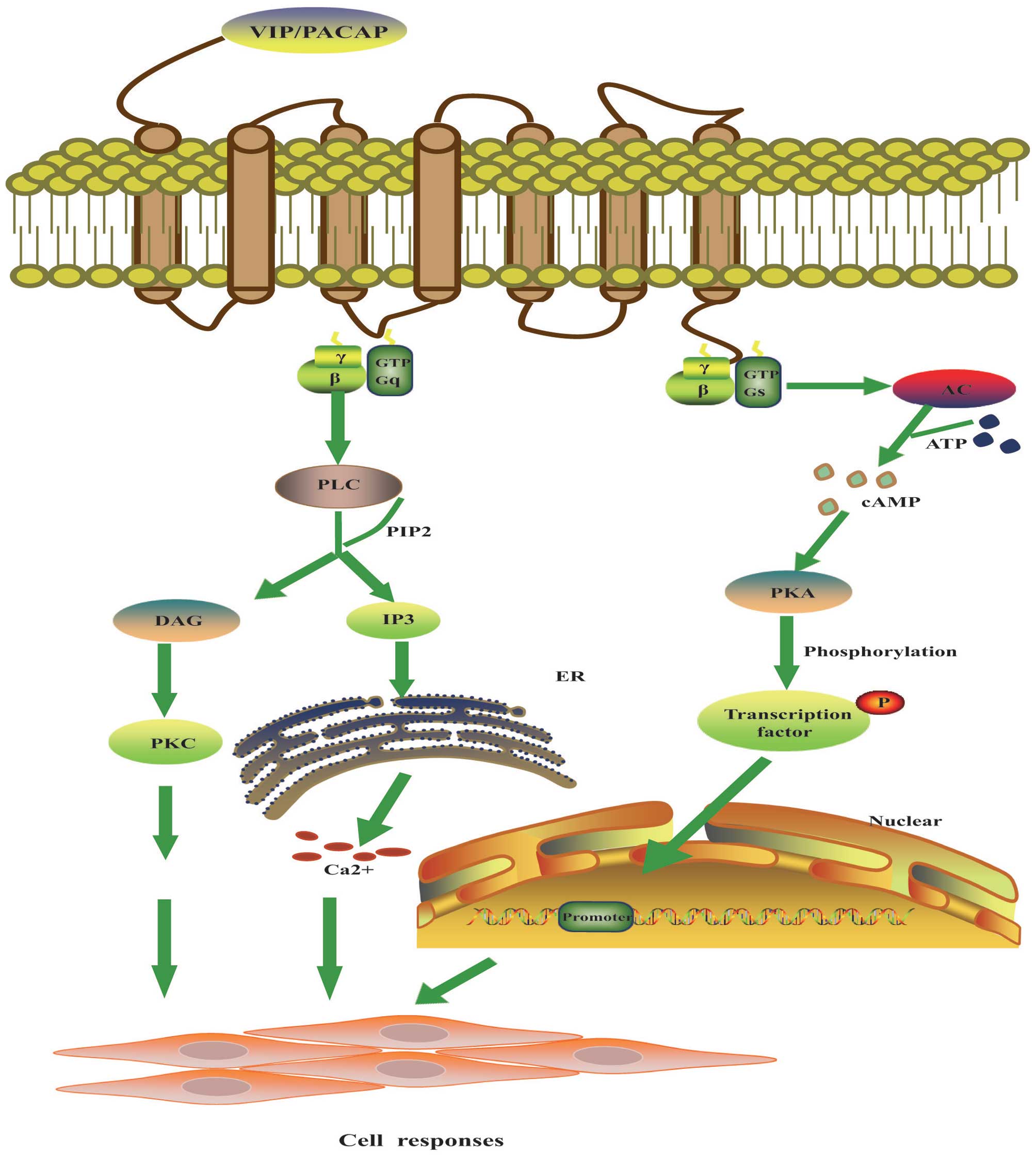 | Figure 1.Molecular mechanism of VIP signal
transduction. The main signals of VIP are mediated through
VIP/PACAP receptors via coupling to adenylate cyclase
(AC)-stimulation G protein and PI signal transduction pathways.
AC-cAMP pathway: VIP and receptor binding triggers the GDP-GTP
exchange of the Gs protein and activate AC. AC catalyzes the
synthesis of cAMP from ATP, and cAMP elicits its cellular responses
by the phosphorylation of a number of cellular proteins or
transcription factors via cAMP-dependent protein kinase (PKA). PI
pathway: VIP and receptor binding is coupled to Gq protein and
activates phospholipase C (PLC), which cleaves PIP2 into DAG and
IP3. DAG activates PKC which in turn phosphorylates a number of
cellular proteins and exerts biological functions. IP3
causes the release of Ca2+ from endoplasmic reticulum
(ER) which leads to exocytosis and various other cellular
responses. cAMP, cyclic adenosine 5′-monophosphate; GTP, guanosine
5′-triphosphate; DAG, diacylglycerol; IP3,
inositol-1,4,5-triphosphate; PIP2,
phosphatidylinositol-4,5-bisphosphate. |
The effects of VIP and PACAP are mediated by VIPRs
and have many overlapping functions. These peptides play roles in
the gastrointestinal, immune, reproductive, respiratory,
cardiovascular and endocrine systems (34,35).
For example, VIP is involved in gut motility and acts as a potent
vasoregulatory hormone, whereas PACAP is associated with the
nervous system and exerts a hypophysiotropic effect on pituitary
hormone secretion (11,23). In addition to the crucial roles
that VIPRs play in many physiological processes, VIPRs are involved
in the progression of a number of malignancies and can stimulate
tumor growth and angiogenesis through the transactivation of
epidermal growth factor receptor (EGFR) and the expression of
vascular endothelial growth factor (VEGF) (36,37).
VIP receptors as potential targets for
imaging and therapy
VIPRs have been identified in several normal tissues
and in various types of tumors. In normal human tissues, VPAC1
receptors are abundant in the brain, T lymphocytes, and most
peripheral tissues including the liver, lungs and intestines
(38,39). VPAC2 receptors are mainly expressed
in the hippocampus, the brainstem, the spinal cord, and most smooth
muscle tissues (39,40). PAC1 receptors are predominantly
found in the adrenal medulla and on the surface of neuroendocrine
neoplasms (41,42). However, VIPRs are highly
overexpressed in the majority of human tumors and are particularly
common in frequently occurring cancers. Reubi et al
(43) reported that VPAC1
receptors are overexpressed in most frequently occurring malignant
epithelial neoplasms, such as cancers of the colon, breast, lungs
and prostate, in a pattern similar to that found in normal tissues.
In contrast to the ubiquitous expression of VPAC1 receptors in most
human tumors, tumors that express VPAC2 receptors are rare, and
this phenomenon is only observed in some leiomyomas and
gastrointestinal stromal tumors (43,44).
A high incidence of PAC1 receptor expression was found in
neuroendocrine neoplasms located in the brain and the endocrine and
neuroendocrine systems and included gliomas, neuroblastomas, and
pituitary adenomas, as well as endometrial cancers (43). The VIPRs are significantly
overexpressed in most human tumors, and the differences in the cell
surface receptor profiles between cancer cells and their normal
counterparts can be utilized as a molecular signature for targeted
imaging. Thus, the VIPRs may be potential targets for molecular
imaging.
It is well documented that excessive VIPR signaling,
which arises from receptor overexpression and autocrine stimulation
by VIP/PACAP or other factors, is a hallmark of a wide variety of
tumors. Previous research has shown that the serum levels of VIP in
several tumors are significantly increased, which may serve as an
indicator of the presence of certain tumors and could become a part
of their diagnosis (45). Since
most tumors overexpress VIPRs, VIP/PACAP can bind to these VIPRs
and activate them, leading to an interaction with a stimulatory
guanine nucleotide binding protein (Gs) and ultimately resulting in
the stimulation of adenylate cyclase. Research has shown that the
addition of VIP to lung cancer cells elevated the cAMP levels and
led to the activation of a series of transcription factors that
promoted the expression of nuclear oncogenes and growth factors
(46,47). In addition, VIP activation
increased the secretion of VEGF and facilitated tumor angiogenesis
via the cAMP/PKA and PI3K signaling pathways (36). The involvement of VIPRs in
malignant transformation and angiogenesis renders them potentially
valuable targets for cancer therapy.
Targeting VIPRs for molecular imaging
Tumor receptor imaging plays an important role in
molecular imaging, the characterization of receptor expression,
tumor biology, the identification of molecular targets, and the
application of targeted cancer therapy (48). The advantages of tumor receptor
imaging include its noninvasive nature, the ability to assess
receptor expression, and the ability to evaluate the in vivo
effects of the drug on the tumor. Several types of imaging agents
may be used for the imaging of receptors that are overexpressed in
tumors, including antibodies or their fragments, natural peptide
ligands or their analogs, and non-peptide small molecules. Although
the introduction of antibodies as specific agents to target
malignant tumors dates back almost 35 years, these proteins are
insufficient for tumor receptor imaging due to their high molecular
weight, slow targeting of the tumor, slow clearance from the blood
and normal tissues, and relatively low tumor to non-tumor ratios
when used in tumor imaging and therapy (49,50).
In general, natural ligands and small ligand analogs for targeted
imaging are the most common imaging agents in use because small
peptide ligands are small and readily diffusible and have rapid
kinetics (51). Another important
molecular imaging tool is the use of a tracer that accumulates at
the sites of targeted receptors. Most molecular imaging tracers
include a reporter moiety that emits a signal that can be detected
by a special external device and a linker joining the reporter
moiety to the targeted ligands. Some of the most commonly used
tracers are radionuclide-labeled probes and optical imaging probes.
However, the tissue penetration of optical signals is limited to a
few millimeters and thus limits the clinical application of optical
imaging probes. Therefore, radionuclide-based tracers dominate in
clinical molecular imaging techniques due to their high sensitivity
and increased tissue penetration. Currently, there are two major
radionuclide-based imaging methods used in the pre-clinical or
clinical settings: single photon emission computed tomography
(SPECT) and positron emission tomography (PET). For the reasons
stated above, we focus on radionuclide-labeled peptide ligands and
small molecule analogs used for SPECT and PET imaging in this
review.
123I-labeled VIP for receptor
imaging
Virgolini et al (52) first reported that
123I-labeled VIP could be successfully used in
vivo to scan patients with gastrointestinal adenocarcinomas,
carcinoids and insulinomas. After the intravenous injection of the
123I-labeled VIP, ∼45% of the radioactivity was found in
the lungs within 30 min, and the activity decreased rapidly.
Visualization of the primary tumors and metastases was possible
within an hour of injection and was still apparent at 24-h
post-injection. Most importantly, radiolabeled VIP-receptor
scanning is more advantageous than CT scanning because receptor
imaging is based on the receptor expression pattern rather than the
macroscopic morphology (52).
Subsequently, the same group performed a series of clinical studies
in which 123I-labeled VIP was used to image various
tumors, such as colorectal cancers (53), pancreatic cancers (54), VIPomas (55), and endocrine tumors (56). These imaging results exhibited a
high sensitivity, which was an advantage over CT scanning,
particularly in patients with tumors of a small size. Raderer et
al (57) compared the in
vitro and in vivo binding of 123I-VIP and an
111In-labeled monoclonal antibody specific to the TAG-72
protein in patients with intestinal adenocarcinomas in a
single-blinded prospectively randomized trial. The results indicate
that VIP receptor scanning is more sensitive than
immunoscintigraphy for the localization of intestinal
adenocarcinomas and metastatic spread (57). Although 123I-VIP has
promising potential to localize even small tumors expressing VIPRs
and is useful for the early diagnosis and treatment of tumors,
naturally occurring peptides like VIP exhibit limitations for in
vivo tumor imaging because they have a short biological
half-life and are rapidly degraded in the liver and kidneys. Due to
this metabolic instability, native VIP peptides require further
chemical modifications to improve their bioavailability for
receptor binding prior to their use in tumor receptor imaging. In
addition, the radionuclide 123I is generated by an
expensive cyclotron instrument, which increases the cost of
treatment. Furthermore, radiolabeled VIP requires further isolation
and purification, which greatly limits its clinical applications.
Therefore, novel stable analogs of VIP and applicable radionuclides
are required for VIPR imaging.
99mTc-labeled VIP analogs for
receptor imaging
In contrast to 123I, 99mTc has
excellent physical characteristics for scintigraphic imaging, is
very inexpensive, and is produced by a 99Mo generator
system (Table I) (58). In an initial attempt to employ
99mTc labeling, the N terminus (His1) of VIP was
conjugated to one of two well-known bi-functional chelating agents
(BFCAs): CPTA [4-(1,4,8,11-tetraazacyclotetradec-1-yl) methyl]
benzoic acid or MAG3[N-[N[N-(benzylthio) acetyl]-glycyl]
glycyl] glycine]. These compounds have two major drawbacks:
radiochemical impurity and a loss of biological function. It was
found that the histidine residue in the number one position of VIP
played an important role in the biological activity of VIP
(59).
 | Table I.Radionuclides for labeling of
molecular imaging and therapeutic probes. |
Table I.
Radionuclides for labeling of
molecular imaging and therapeutic probes.
| Nuclide | Half-life (h) | Production | Energy (keV) | Application |
|---|
|
99mTc | 6.01 | Generator | γ 140 | SPECT imaging |
|
123I | 13.27 | Cyclotron | γ 159 | SPECT imaging |
| 18F | 1.8 | Cyclotron | β+ 634 γ
511 | PET imaging |
|
64Cu | 12.7 | Cyclotron | β+ 655
β− 573 | PET imaging |
|
111In | 67 | Cyclotron | γ 171 Auger e-
30 | SPECT imaging |
|
68Ga | 1.1 | Generator | β+ 1899
γ 511 | PET imaging |
| 90Y | 65 | Generator | β−
2964 | Therapy |
|
131I | 192 | Cyclotron | β+ 607 γ
364 | Therapy |
|
177Lu | 161 | Cyclotron | β− 498 γ
208 | Therapy |
To improve the radiolabeling efficiency, Thakur
et al (60) further
modified VIP at the carboxy terminus of Asn28. The
research group chose to use 4-aminobutyric acid (Aba) as the spacer
and extended the molecule to include Gly-Gly-(D)-Aba-Gly, which
resulted in an N4 configuration that could be used for
the chelation of 99mTc (Fig. 2). This modified analog was referred
to as TP3654 (VIP labeled with 99mTc). The biological
activity of 99mTc-TP3654 was equivalent to that of the
native VIP peptide, and the 24-h tumor uptake of
99mTc-TP3654 was higher than that of
123I-VIP. These results implied that a simple and
efficient hybrid peptide technique had been developed for labeling
peptides with 99mTc. Subsequently, Thakur et al
(58,60,61)
evaluated the pharmacokinetics and imaging characteristics of
99mTc-TP3654 in normal volunteers and patients with a
history of cancer. No adverse reactions or changes were noted in
the imaging process. Furthermore, ∼70% of the injected
radioactivity was eliminated in the urine, and 20% was cleared
through the liver. The tumor uptake of 99mTc-TP3654 was
significantly higher than that of 111In-octreotide,
which is concordant with the higher VIP receptor density observed
in most tumors when compared to the density of somatostatin
receptors. This VIP analog promises to be a nontoxic and reliable
agent for imaging human tumors that overexpress VIPRs.
Kothari et al (62) synthesized three VIP analogs
functionalized at the N terminus with histidine [VP05 (28 residue),
VD4 (20 residue), VD5 (19 residue)] by solid phase synthesis and
radiolabeled the VIP analogs with 99mTc via a novel
tricarbonyl synthon. All of the radiolabeled complexes were stable
at room temperature and were produced with high yields.
Biodistribution studies revealed good tumor uptake kinetics;
however, the soft tissue uptake was also high due to the lipophilic
nature of the peptide. Thus, more research is required to modify
the reported analogs and create novel radiolabeled analogs to
enhance their ability to target VIP receptors.
PET molecular imaging of the VIP
receptor
Although PET is not as widely available as SPECT,
PET is advantageous for tumor diagnosis because it exhibits higher
resolution and sensitivity than computed tomography (CT), magnetic
resonance imaging (MRI) and SPECT. Therefore, labeling VIP with
positron emission radionuclides is important for PET molecular
imaging of tumors expressing VIP receptors. Moody et al
(63) used 18F to
radiolabel VIP analogs (at Arg15/Arg21) and
obtained a labeled complex (18F-RR) VIP that bound to
breast cancer cells with high specificity and affinity and acted as
an agonist. The tumor imaging results showed that 4 h after
injection, the density of (18F-RR) VIP was 4-fold
greater in the tumor than in the normal tissue with the highest
uptake (intestinal) and was ∼15-fold greater than in the normal
breast, indicating that (18F-RR) VIP could localize to
breast tumors in vivo. However, a comparison of
(18F-RR) VIP and 18F-FDG showed that FDG
exhibited a 2- to 3-fold greater tumor accumulation and
target-to-non-target ratio relative to (18F-RR) VIP
(64). To further improve the
stability and biodistribution characteristics of
18F-labeled VIP, Cheng et al (65,66)
synthesized (R8,15,21, L17) VIP by replacing
Asp8, Lys15, and Lys21 with Arg and Met17 with Leu in the amino
acid sequence of VIP. The radiolabeled
18F-(R8,15,21, L17) VIP was
obtained with high radio-chemical purity, high specific
radioactivity and good stability in vitro. Biodistribution
data showed higher tumor-to-muscle and tumor-to-blood ratios,
indicating its potential application as a PET imaging agent for
tumors overexpressing VIPRs.
The radionuclide 64Cu has a longer
half-life and a wealth of known chemistry and provides nearly
quantitative yields so that the radiolabeled compound can be
prepared without further purification (Table I).
64Cu-1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic
acid (TETA) VIP was the first reported 64Cu-VIP analog,
but no further preclinical or clinical data have been reported
(67). Subsequently, Thakur et
al (68) synthesized the VIP
analog TP3982 to harbor a carboxy-terminal lysine residue separated
from asparagine by 4-aminobutyric acid (Aba) as a spacer. The
biological activity of 64Cu-TP3982 was not compromised.
It also exhibited in vivo stability and a 74-fold increase
in tumor uptake relative to 99mTc-TP3654, which had
previously been successfully used to image human tumors. Thus,
64Cu-TP3982 is a desirable PET imaging agent for the
imaging of cancers overexpressing VIPRs. Zhang et al
(69,70) used the same method to synthesize
the VIP analog TP3939 and label it with 64Cu. The
imaging data obtained for 64Cu-TP3939 in experimental
and spontaneous prostate cancer indicated that with its
uncompromised biological activity, 64Cu-TP3939 could
detect xenografts and cases of occult prostate cancer that were not
detectable with 18F-FDG or CT. Thus,
64Cu-TP3939 is worthy of further investigation for the
PET imaging of human tumors and their metastases or recurrent
lesions and for determining the efficacy of tumor therapy.
Targeting VIP receptor for cancer molecular
therapy
VIP antagonist-based cancer therapy
Since VIP is thought to have growth promoting
properties through VIP receptor activation (71,72),
the treatment of human tumors with a VIP antagonist may lead to the
inhibition of tumor growth (Table
II). The VIP-receptor antagonist VIPhyb was synthesized as a
hybrid peptide of neurotensin and VIP consisting of an N-terminal
Lys-Pro-Arg-Arg-Pro-Tyr (designed to increase membrane
permeability), followed by the C-terminal 22 amino acids of VIP
(73). This broad spectrum VIP
receptor antagonist inhibited non-small cell lung cancer (73), breast cancer (74), and colon cancer (75) growth in vitro and in
vivo. In addition, a further modification of VIPhyb was the
addition of a stearyl group at the N-terminus and the exchange of
the methionine at position 17 with norleucine. These modifications
resulted in an antagonist with improved affinity (SN)VIPhyb
(76). (SN)VIPhyb bound the VPAC1
receptor with an ∼10-fold greater affinity than VIPhyb and acted as
a cytostatic agent in non-small cell lung cancer (77) and pancreatic cancer (Table II) (78). Moreover, (SN)VIPhyb enhanced the
anti-proliferative effects of chemotherapeutic agents on cancer
cell lines (79).
 | Table II.Some analogs of VIP and their
therapeutic effects. |
Table II.
Some analogs of VIP and their
therapeutic effects.
| Analogs | Selectivity | Affinity
(IC50:nM) | Application |
|---|
| VIPhyb | PAC antagonist | 300 | Inhibition of colon
and breast cancer |
| (SN)VIPhyb | VPAC1
antagonist | 30 | Inhibition of
non-small lung cancer |
| [K15,
R16, L27]-VIP(1–7)/GRF (8–27) | VPAC1
antagonist | 10 | Inhibition various
cancers |
| VIP-LALA-E | VPAC1 agonist | 100 | Inhibition of
breast cancer |
| Ro 25-1553 | VPAC2 agonist | 1 | Therapeutic for
bronchial athma |
| BAY 55-9837 | VPAC2 agonist | 60 | Stimulation of
insulin secretion |
|
stearyl-[Nle17]-VIP | Nonselective
agonist | 5 | Inhibition of
breast cancer |
| [R15, 20,
21, L17]-VIP-GRR | Nonselective
agonist | 1.4 | Treatment of asthma
and COPD |
| [R15, 20,
21, L17]-VIP(1–23) | Nonselective
agonist | 48 | Relaxation of
muscle |
To avoid the side effects of the broad-spectrum VIP
receptor antagonists, new analogs selectively inhibiting each VIP
receptor subtype may significantly prevent undesirable side
effects, such as the inhibition of VPAC2 activation, which has been
associated with glucose-dependent insulin secretion and
bronchodilation (80). Recently, a
VPAC1 antagonist conjugated to a high-molecular-weight PEG was
synthesized, and the pharmacokinetic characteristics were improved
without compromising functional activities. The results showed that
the selective inhibition of the VPAC1 receptor is sufficient to
prevent tumor proliferation, which suggests that a VPAC1-selective
antagonist may be a safe and effective tool for treating tumors
(unpublished).
Cytotoxic peptide conjugate-based cancer
therapy
Conventional chemotherapy is limited by multidrug
resistance of tumor cells, toxicity to normal cells and a lack of
tumor specificity. The more specific delivery of chemotherapeutic
drugs to cancer cells can produce a higher drug concentration in
tumors and reduce the toxicity to normal cells (81). Therefore, ‘targeted therapy’ was
introduced with the aim of enhancing the specificity of
chemotherapy and reducing the non-specific toxicity to normal cells
(82). Since high-affinity VIP
receptors are overexpressed on many tumors, they can be targeted
using cytotoxic VIP conjugates.
A new cytotoxic analog of VIP-ellipticine was
prepared that consisted of tetra- and pentapeptide ellipticine (E)
attached to the C-terminus of VIP. The VIP-E conjugates functioned
as VPAC1 receptor agonists, bound to VPAC1 receptors with high
affinity and retained their anti-proliferative activity. The VIP-E
conjugates were internalized by cancer cells expressing the VPAC1
receptor and were subsequently metabolized by proteolytic enzymes,
leading to the release of ellipticine and cellular cytotoxicity.
The VIP-E derivatives exhibited significant cytotoxicity toward
breast cancer cells and lung cancer cells in vitro (83,84).
Subsequently, Moody et al (85) extended these studies and
synthesized VIP-camptothecin (CPT) conjugates that contain a novel
carbamate linker with a built-in nucleophile associated releasing
group, L2. The obtained conjugate (A-NL-K)-VIP-L2-CPT is
metabolized by cytochrome P450 enzymes, releases CPT and exhibits
cytotoxic effects on breast cancer cells.
Peptide-receptor radionuclide therapy
(PRRT)
As mentioned previously, VIP receptor-positive
tumors can be targeted with radiolabeled VIP and its analogs
(52,61). It is theoretically possible to
treat such tumors selectively with therapeutic nuclide-labeled VIP
analogs. This peptide-receptor radio-nuclide therapy is based on
the presence of high levels of VIP receptors in tumors and on their
ability to form ligand-receptor complexes, which allows for the
internalization and accumulation of the radiopharmaceuticals inside
the tumors. Although there are many studies regarding somatostatin
receptor-based radiotherapy (51,86),
there are currently no reports on VIP receptor radiotherapy for
human tumors. One reason may be the lack of appropriate
radiolabeled ligands. A second reason is certainly the inadequate
tumor-to-non-tumor ratio for radiotherapy. In this case, VIP
receptor-based PRRT could be highly radiotoxic to surrounding
normal tissues, especially to radiosensitive tissues such as
immune, lung, kidney and liver cells (51). Therefore, the development of new
peptide analogs with increased binding affinity and specificity may
lead to a higher accumulation of radioactivity in tumors and
improve the efficacy of peptide receptor radionuclide therapy.
Prospective and Conclusion
VIP receptors are very important in the biology of
many malignancies and are overexpressed in many tumors; thus, VIP
receptors can be targeted by receptor-specific molecules. The
generation of radiolabeled peptides has opened new avenues for the
molecular imaging and therapy of tumors. Due to their low molecular
weight, high-affinity, and good tissue/cell penetration, they are
becoming ideal candidates for molecular imaging techniques and
therapeutic interventions. To date, radiolabeled peptides specific
for VIP receptors have evolved from the initial native VIP peptide
into peptide analogs or peptide antagonists with improved
pharmacokinetic profiles. Although a series of preclinical studies
on VIP receptor-based imaging and therapy using
radionuclide-labeled VIP and its analogs, antagonists and cytotoxic
peptide conjugates have shown promising results, the therapeutic
potential of VIP receptor-based radionuclide therapy must be
refined and optimized.
Under the circumstances, it is necessary to develop
novel peptide analogs with improved binding affinity, specificity
and stability to result in a higher accumulation of radioactivity
inside tumor cells. In a recent study, our research group
identified a novel dodecapeptide that could specifically bind to
the VPAC1 receptor with high affinity using a phage display peptide
library. The results imply that the peptide may serve as a
potential molecular imaging probe and therapeutic agent (87). Increasing the therapeutic window,
which can be achieved by reducing the radiation toxicity to normal
organs, could significantly enhance the therapeutic effects through
increased injected radioactivity. Since the kidney has been one of
the dose-limiting organs in some clinical studies, several standard
procedures to reduce renal uptake have been developed and followed
(88). Most importantly, the
combination of radionuclide imaging and therapy with other
diagnosis and treatment modalities, such as CT, MRI and
chemotherapy, may greatly increase the efficiency of early
diagnosis and treatment for the heterogeneous tumors.
Recently, the most common somatostatin
receptor-based agent 111In-DTPA-pentetreotide
(Octreoscan; Mallinckrodt) was approved by the US Food and Drug
Administration for clinical somatostatin receptor imaging (89). Moreover, several
90Y-labeled octreotides were tested in different phase-I
and phase-II clinical trials (90), and a major breakthrough in the
tumor receptor-targeted imaging and therapy field may be on the
way. The clinical success of somatostatin receptor imaging and
therapy will provide insight into the exploration and development
of VIP receptor-based imaging and therapy. Although there are still
many other drawbacks to overcome in VIP receptor-based imaging and
therapy, we believe that major progress will be made in preclinical
settings. The importance of VIP receptors in tumor biology and the
ability to predict responses to targeted therapy and monitor drug
interventions suggest that VIP receptor imaging will be critical
for oncologic molecular imaging and will play a key role in cancer
management in the future.
Abbreviations:
|
VIP
|
vasoactive intestinal peptide;
|
|
PACAP
|
pituitary adenylate cyclase-activating
polypeptide;
|
|
PHM
|
peptide histidine methionine
amide;
|
|
GRF
|
growth-hormone-releasing-factor;
|
|
PHI
|
peptide histidine isoleucine;
|
|
GPCR
|
G-protein-coupled receptor;
|
|
SPECT
|
single photon emission computed
tomography;
|
|
PET
|
positron emission tomography;
|
|
CT
|
computed tomography;
|
|
MRI
|
magnetic resonance imaging;
|
|
FDG
|
fluorodeoxyglucose;
|
|
PRRT
|
peptide-receptor radionuclide
therapy
|
References
|
1.
|
Ozben T: Mechanisms and strategies to
overcome multiple drug resistance in cancer. FEBS Lett.
580:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Xie X, Tang B, Zhou J, Gao Q and Zhang P:
Inhibition of the PI3K/Akt pathway increases the chemosensitivity
of gastric cancer to vincristine. Oncol Rep. 30:773–782.
2013.PubMed/NCBI
|
|
3.
|
Pluchino KM, Hall MD, Goldsborough AS,
Callaghan R and Gottesman MM: Collateral sensitivity as a strategy
against cancer multidrug resistance. Drug Resist Updat. 15:98–105.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Garanger E, Boturyn D and Dumy P: Tumor
targeting with RGD peptide ligands-design of new molecular
conjugates for imaging and therapy of cancers. Anticancer Agents
Med Chem. 7:552–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tolmachev V: Imaging of HER-2
overexpression in tumors for guiding therapy. Curr Pharm Des.
14:2999–3019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kaklamani V and O’Regan RM: New targeted
therapies in breast cancer. Semin Oncol. 31:20–25. 2004. View Article : Google Scholar
|
|
8.
|
Mankoff DA, Link JM, Linden HM,
Sundararajan L and Krohn KA: Tumor receptor imaging. J Nucl Med.
49:S149–S163. 2008. View Article : Google Scholar
|
|
9.
|
Nunn AD: Molecular imaging and
personalized medicine: an uncertain future. Cancer Biother
Radiopharm. 22:722–739. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mankoff DA: A definition of molecular
imaging. J Nucl Med. 48:N18–N21. 2007.
|
|
11.
|
Sherwood NM, Krueckl SL and McRory JE: The
origin and function of the pituitary adenylate cyclase-activating
polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 21:619–670.
2000.PubMed/NCBI
|
|
12.
|
Said SI and Mutt V: Polypeptide with broad
biological activity: isolation from small intestine. Science.
169:1217–1218. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Piper PJ, Said SI and Vane JR: Effects on
smooth muscle preparations of unidentified vasoactiv peptides from
intestine and lung. Nature. 225:1144–1146. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Barbezat GO and Grossman MI: Intestinal
secretion: stimulation by peptides. Science. 174:422–424. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gozes I, Fridkinb M, Hill JM and Brenneman
DE: Pharmaceutical VIP: prospects and problems. Curr Med Chem.
6:1019–1034. 1999.PubMed/NCBI
|
|
16.
|
Gozes I and Furman S: VIP and drug design.
Curr Pharm Des. 9:483–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tsukada T, Horovitch SJ, Montminy MR,
Mandel G and Goodman RH: Structure of the human vasoactive
intestinal polypeptide gene. DNA. 4:293–300. 1985.
|
|
18.
|
Gozes I, Avidor R, Yahav Y, Katznelson D,
Croce CM and Huebner K: The gene encoding vasoactive intestinal
peptide is located on human chromosome 6p21-6qter. Hum Genet.
75:41–44. 1987.PubMed/NCBI
|
|
19.
|
Davidson A, Moody TW and Gozes I:
Regulation of VIP gene expression in general. Human lung cancer
cells in particular. J Mol Neurosci. 7:99–110. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Itoh N, Obata K, Yanaihara N and Okamoto
H: Human preprovasoactive intestinal polypeptide contains a novel
PHI-27-like peptide, PHM-27. Nature. 304:547–549. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Bodner M, Fridkin M and Gozes I: Coding
sequences for vasoactive intestinal peptide and PHM-27 peptide are
located on two adjacent exons in the human genome. Proc Natl Acad
Sci USA. 82:3548–3551. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Vandermeers A, Vandenborre S, Hou X, de
Neef P, Robberecht P, Vandermeers-Piret MC and Christophe J:
Antagonistic properties are shifted back to agonistic properties by
further N-terminal shortening of pituitary
adenylate-cyclase-activating peptides in human neuroblastoma
NB-OK-1 cell membranes. Eur J Biochem. 208:815–819. 1992.
View Article : Google Scholar
|
|
23.
|
Vaudry D, Gonzalez BJ, Basille M, Yon L,
Fournier A and Vaudry H: Pituitary adenylate cyclase-activating
polypeptide and its receptors: from structure to functions.
Pharmacol Rev. 52:269–324. 2000.PubMed/NCBI
|
|
24.
|
Fahrenkrug J: VIP and PACAP. Results Probl
Cell Differ. 50:221–234. 2010.
|
|
25.
|
Dickson L and Finlayson K: VPAC and PAC
receptors: From ligands to function. Pharmacol Ther. 121:294–316.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Vaudry D, Falluel-Morel A, Bourgault S,
Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H,
Galas L and Vaudry H: Pituitary adenylate cyclase-activating
polypeptide and its receptors: 20 years after the discovery.
Pharmacol Rev. 61:283–357. 2009.PubMed/NCBI
|
|
27.
|
Muller JM, Debaigt C, Goursaud S, Montoni
A, Pineau N, Meunier AC and Janet T: Unconventional binding sites
and receptors for VIP and related peptides PACAP and PHI/PHM: an
update. Peptides. 28:1655–1666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Laburthe M, Couvineau A and Marie JC: VPAC
receptors for VIP and PACAP. Recept Chann. 8:137–153. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Couvineau A, Lacapere JJ, Tan YV,
Rouyer-Fessard C, Nicole P and Laburthe M: Identification of
cytoplasmic domains of hVPAC1 receptor required for activation of
adenylyl cyclase. Crucial role of two charged amino acids strictly
conserved in class II G protein-coupled receptors. J Biol Chem.
278:24759–24766. 2003. View Article : Google Scholar
|
|
30.
|
Dickson L, Aramori I, McCulloch J, Sharkey
J and Finlayson K: A systematic comparison of intracellular cyclic
AMP and calcium signalling highlights complexities in human
VPAC/PAC receptor pharmacology. Neuropharmacology. 51:1086–1098.
2006. View Article : Google Scholar
|
|
31.
|
Barrie AP, Clohessy AM, Buensuceso CS,
Rogers MV and Allen JM: Pituitary adenylyl cyclase-activating
peptide stimulates extracellular signal-regulated kinase 1 or 2
(ERK1/2) activity in a Ras-independent, mitogen-activated protein
Kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol
Chem. 272:19666–19671. 1997. View Article : Google Scholar
|
|
32.
|
Lelièvre V, Pineau N, Du J, Wen CH, Nguyen
T, Janet T, Muller JM and Waschek JA: Differential effects of
peptide histidine isoleucine (PHI) and related peptides on
stimulation and suppression of neuroblastoma cell proliferation. A
novel VIP-independent action of PHI via MAP kinase. J Biol Chem.
273:19685–19690. 1998.PubMed/NCBI
|
|
33.
|
Delgado M and Ganea D: Vasoactive
intestinal peptide and pituitary adenylate cyclase-activating
polypeptide inhibit interleukin-12 transcription by regulating
nuclear factor kappaB and Ets activation. J Biol Chem.
274:31930–31940. 1999. View Article : Google Scholar
|
|
34.
|
Hashimoto H, Shintani N, Tanaka K, Mori W,
Hirose M, Matsuda T, Sakaue M, Miyazaki J, Niwa H, Tashiro F,
Yamamoto K, Koga K, Tomimoto S, Kunugi A, Suetake S and Baba A:
Altered psychomotor behaviors in mice lacking pituitary adenylate
cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci USA.
98:13355–13360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Jozsa R, Hollosy T, Nemeth J, Tamás A,
Lubics A, Jakab B, Olah A, Arimura A and Reglödi D: Presence of
PACAP and VIP in embryonic chicken brain. Ann NY Acad Sci.
1070:348–353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Valdehita A, Carmena MJ, Collado B, Prieto
JC and Bajo AM: Vasoactive intestinal peptide (VIP) increases
vascular endothelial growth factor (VEGF) expression and secretion
in human breast cancer cells. Regul Pept. 144:101–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Valdehita A, Bajo AM, Schally AV, Varga
JL, Carmena MJ and Prieto JC: Vasoactive intestinal peptide (VIP)
induces transactivation of EGFR and HER2 in human breast cancer
cells. Mol Cell Endocrinol. 302:41–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sreedharan SP, Patel DR, Huang JX and
Goetzl EJ: Cloning and functional expression of a human
neuroendocrine vasoactive intestinal peptide receptor. Biochem
Biophys Res Commun. 193:546–553. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Usdin TB, Bonner TI and Mezey E: Two
receptors for vasoactive intestinal polypeptide with similar
specificity and complementary distributions. Endocrinology.
135:2662–2680. 1994.PubMed/NCBI
|
|
40.
|
Wei Y and Mojsov S: Tissue specific
expression of different human receptor types for pituitary
adenylate cyclase activating polypeptide and vasoactive intestinal
polypeptide: implications for their role in human physiology. J
Neuroendocrinol. 8:811–817. 1996. View Article : Google Scholar
|
|
41.
|
Moller K and Sundler F: Expression of
pituitary adenylate cyclase activating peptide (PACAP) and PACAP
type I receptors in the rat adrenal medulla. Regul Pept.
63:129–139. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Zeng N, Kang T, Lyu RM, Wong H, Wen Y,
Walsh JH, Sachs G and Pisegna JR: The pituitary adenylate cyclase
activating polypeptide type 1 receptor (PAC1-R) is expressed on
gastric ECL cells: evidence by immunocytochemistry and RT-PCR. Ann
NY Acad Sci. 865:147–156. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Reubi JC, Läderach U, Waser B, Gebbers JO,
Robberecht P and Laissue JA: Vasoactive intestinal
peptide/pituitary adenylate cyclase-activating peptide receptor
subtypes in human tumors and their tissues of origin. Cancer Res.
60:3105–3112. 2000.
|
|
44.
|
Reubi JC, Körner M, Waser B, Mazzucchelli
L and Guillou L: High expression of peptide receptors as a novel
target in gastrointestinal stromal tumours. Eur J Nucl Med Mol
Imaging. 31:803–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Gozes I and Furman S: Clinical
endocrinology and metabolism. Potential clinical applications of
vasoactive intestinal peptide: a selected update. Best Pract Res
Clin Endocrinol Metab. 18:623–640. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Whitmarsh AJ and Davis RJ: Transcription
factor AP-1 regulation by mitogen-activated protein kinase signal
transduction pathways. J Mol Med. 74:589–607. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Casibang M, Purdom S, Jakowlew S, Neckers
L, Zia F, Ben-Av P, Hla T, You L, Jablons DM and Moody TW:
Prostaglandin E2 and vasoactive intestinal peptide
increase vascular endothelial cell growth factor mRNAs in lung
cancer cells. Lung Cancer. 31:203–212. 2001.
|
|
48.
|
Mankoff DA, O’Sullivan F, Barlow WE and
Krohn KA: Molecular imaging research in the outcomes era: measuring
outcomes for individualized cancer therapy. Acad Radiol.
14:398–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Goldenberg DM, DeLand F, Kim E, Bennett S,
Primus FJ, van Nagell JR Jr, Estes N, DeSimone P and Rayburn P: Use
of radiolabeled antibodies to carcinoembryonic antigen for the
detection and localization of diverse cancers by external
photo-scanning. N Engl J Med. 298:1384–1386. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Behr TM, Memtsoudis S, Sharkey RM,
Blumenthal RD, Dunn RM, Gratz S, Wieland E, Nebendahl K,
Schmidberger H, Goldenberg DM and Becker W: Experimental studies on
the role of antibody fragments in cancer radio-immunotherapy:
influence of radiation dose and dose rate on toxicity and
anti-tumor efficacy. Int J Cancer. 77:787–795. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Reubi JC: Peptide receptors as molecular
targets for cancer diagnosis and therapy. Endocr Rev. 24:389–427.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Virgolini I, Raderer M, Kurtaran A,
Angelberger P, Banyai S, Yang Q, Li S, Banyai M, Pidlich J,
Niederle B, Scheithauer W and Valent P: Vasoactive intestinal
peptide-receptor imaging for the localization of intestinal
adenocarcinomas and endocrine tumors. N Engl J Med. 33:1116–1121.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Raderer M, Kurtaran A, Hejna M, Vorbeck F,
Angelberger P, Scheithauer W and Virgolini I:
123I-labelled vasoactive intestinal peptide receptor
scintigraphy in patients with colorectal cancer. Br J Cancer.
78:1–5. 1998. View Article : Google Scholar
|
|
54.
|
Raderer M, Kurtaran A, Yang Q, Meghdadi S,
Vorbeck F, Hejna M, Angelberger P, Kornek G, Pidlich J, Scheithauer
W and Virgolini I: Iodine-123-vasoactive intestinal peptide
receptor scanning in patients with pancreatic cancer. J Nucl Med.
39:1570–1575. 1998.PubMed/NCBI
|
|
55.
|
Virgolini I, Kurtaran A, Leimer M, Kaserer
K, Peck-Radosavljevic M, Angelberger P, Hübsch P, Dvorak M, Valent
P and Niederle B: Location of a VIPoma by iodine-123-vasoactive
intestinal peptide scintigraphy. J Nucl Med. 39:1575–1579.
1998.PubMed/NCBI
|
|
56.
|
Virgolini I, Kurtaran A, Raderer M, Leimer
M, Angelberger P, Havlik E, Li S, Scheithauer W, Niederle B and
Valent P: Vasoactive intestinal peptide receptor scintigraphy. J
Nucl Med. 36:1732–1739. 1995.PubMed/NCBI
|
|
57.
|
Raderer M, Becherer A, Kurtaran A,
Angelberger P, Li S, Leimer M, Weinlaender G, Kornek G, Kletter K,
Scheithauer W and Virgolini I: Comparison of iodine-123-vasoactive
intestinal peptide receptor scintigraphy and indium-111-CYT-103
immunoscintigraphy. J Nucl Med. 37:1480–1487. 1996.PubMed/NCBI
|
|
58.
|
Thakur ML, Marcus CS, Saeed S, Pallela V,
Minami C, Diggles L, Le Pham H, Ahdoot R and Kalinowski EA:
99mTc-labeled vasoactive intestinal peptide analog for
rapid localization of tumors in humans. J Nucl Med. 41:107–110.
2000.
|
|
59.
|
Pallela VR, Thakur ML, Chakder S and
Rattan S: 99mTc-labeled vasoactive intestinal peptide
receptor agonist: functional studies. J Nucl Med. 40:352–360.
1999.
|
|
60.
|
Thakur ML, Marcus CS, Saeed S, Pallela V,
Minami C, Diggles L, Pham HL, Ahdoot R, Kalinowski EA and Moody T:
Imaging tumors in humans with Tc-99m-VIP. Ann NY Acad Sci.
921:37–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Rao PS, Thakur ML, Pallela V, Patti R,
Reddy K, Li H, Sharma S, Pham HL, Diggles L, Minami C and Marcus
CS: 99mTc labeled VIP analog: evaluation for imaging
colorectal cancer. Nucl Med Biol. 28:445–450. 2001. View Article : Google Scholar
|
|
62.
|
Kothari K, Prasad S, Korde A, Mukherjee A,
Mathur A, Jaggi M, Venkatesh M, Pillai AM, Mukherjee R and
Ramamoorthy N: 99mTc(CO)3-VIP analogues: preparation and
evaluation as tumor imaging agent. Appl Radiat Isot. 65:382–386.
2007. View Article : Google Scholar
|
|
63.
|
Moody TW, Leyton J, Unsworth E, John C,
Lang L and Eckelman WC: (Arg15, Arg21) VIP:
evaluation of biological activity and localization to breast cancer
tumors. Peptides. 19:585–592. 1998.
|
|
64.
|
Jagoda EM, Aloj L, Seidel J, Lang L, Moody
TW, Green S, Caraco C, Daube-Witherspoon M, Green MV and Eckelman
WC: Comparison of an 18F labeled derivative of
vasoactive intestinal peptide and
2-deoxy-2-[18F]fluoro-D-glucose in nude mice bearing
breast cancer xenografts. Mol Imaging Biol. 4:369–379. 2002.
|
|
65.
|
Cheng D, Yin D, Zhang L, Wang M, Li G and
Wang Y: Radiosynthesis of 18F-(R8,15,21,
L17)-vasoactive intestinal peptide and preliminary
evaluation in mice bearing C26 colorectal tumours. Nucl Med Commun.
28:501–506. 2007.
|
|
66.
|
Cheng D, Yin D, Li G, Wang M, Li S, Zheng
M, Cai H and Wang Y: Radiolabeling and in vitro and in vivo
characterization of [18F]FB-[R8,15,21,
L17]-VIP as a PET imaging agent for tumor overexpressed
VIP receptors. Chem Biol Drug Des. 68:319–325. 2006.
|
|
67.
|
Chen X, Edwards WB, Anderson CJ, Mccarthy
TJ and Welch MJ: Solid phase synthesis of TETA conjugated
vasoactive intestinal peptide and in vivo behavior of copper-64
radiolabeled VIP conjugate. J Labelled Compds Radiopharm.
44:S688–S690. 2001. View Article : Google Scholar
|
|
68.
|
Thakur ML, Aruva MR, Gariepy J, Acton P,
Rattan S, Prasad S, Wickstrom E and Alavi A: PET imaging of
oncogene over-expression using 64Cu-vasoactive
intestinal peptide (VIP) analog: comparison with
99mTc-VIP analog. J Nucl Med. 45:1381–1389.
2004.PubMed/NCBI
|
|
69.
|
Zhang K, Aruva MR, Shanthly N, Cardi CA,
Rattan S, Patel C, Kim C, McCue PA, Wickstrom E and Thakur ML: PET
imaging of VPAC1 expression in experimental and spontaneous
prostate cancer. J Nucl Med. 49:112–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Zhang K, Aruva MR, Shanthly N, Cardi CA,
Patel CA, Rattan S, Cesarone G, Wickstrom E and Thakur ML:
Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase
activating peptide (PACAP) receptor specific peptide analogues for
PET imaging of breast cancer: In vitro/in vivo evaluation. Regul
Pept. 144:91–100. 2007. View Article : Google Scholar
|
|
71.
|
Collado B, Carmena MJ, Clemente C, Prieto
JC and Bajo AM: Vasoactive intestinal peptide enhances growth and
angiogenesis of human experimental prostate cancer in a xenograft
model. Peptides. 28:1896–1901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Fernández-Martínez AB, Bajo AM,
Sánchez-Chapado M, Prieto JC and Carmena MJ: Vasoactive intestinal
peptide behaves as a pro-metastatic factor in human prostate cancer
cells. Prostate. 69:774–786. 2009.PubMed/NCBI
|
|
73.
|
Moody TW, Zia F, Draoui M, Brenneman DE,
Fridkin M, Davidson A and Gozes I: A vasoactive intestinal peptide
antagonist inhibits non-small cell lung cancer growth. Proc Natl
Acad Sci USA. 90:4345–4349. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Zia H, Hida T, Jakowlew S, Birrer M, Gozes
Y, Reubi JC, Fridkin M, Gozes I and Moody TW: Breast cancer growth
is inhibited by vasoactive intestinal peptide (VIP) hybrid, a
synthetic VIP receptor antagonist. Cancer Res. 56:3486–3489.
1996.PubMed/NCBI
|
|
75.
|
Levy A, Gal R, Granoth R, Dreznik Z,
Fridkin M and Gozes I: In vitro and in vivo treatment of colon
cancer by VIP antagonists. Regul Pept. 109:127–133. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Moody TW, Jensen RT, Fridkin M and Gozes
I: (N-stearyl, norleucine 17) VIP hybrid is a broad spectrum
vasoactive intestinal peptide receptor antagonist. J Mol Neurosci.
18:29–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Moody TW, Leyton J, Coelho T, Jakowlew S,
Takahashi K, Jameison F, Koh M, Fridkin M, Gozes I and Knight M:
(Stearyl, Norleucine 17) VIP hybrid antagonizes VIP receptors on
non-small cell lung cancer cells. Life Sci. 61:1657–1666. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Zia H, Leyton J, Casibang M, Hau V,
Brenneman D, Fridkin M, Gozes I and Moody TW: (N-stearyl,
norleucine17) VIP hybrid inhibits the growth of pancreatic cancer
cell lines. Life Sci. 66:379–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Moody TW, Leyton J, Chan D, Brenneman DC,
Fridkin M, Gelber E, Levy A and Gozes I: VIP receptor antagonists
and chemotherapeutic drugs inhibit the growth of breast cancer
cells. Breast Cancer Res Treat. 68:55–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Pan CQ, Hamren S, Roczniak S, Tom I and
DeRome M: Generation of PEGylated VPAC1-selective antagonists that
inhibit proliferation of a lung cancer cell line. Peptides.
29:479–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Schally AV and Nagy A: Chemotherapy
targeted to cancers through tumoral hormone receptors. Trends
Endocrinol Metab. 15:300–310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Kim JA: Targeted therapies for the
treatment of cancer. Am J Surg. 186:264–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Moody TW, Czerwinski G, Tarasova NI and
Michejda CJ: VIP-ellipticine derivatives inhibit the growth of
breast cancer cells. Life Sci. 71:1005–1014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Moody TW, Czerwinski G, Tarasova NI, Moody
DL and Michejda CJ: The development of VIP-ellipticine conjugates.
Regul Pept. 123:187–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Moody TW, Mantey SA, Fuselier JA, Coy DH
and Jensen RT: Vasoactive intestinal peptide-camptothecin
conjugates inhibit the proliferation of breast cancer cells.
Peptides. 28:1883–1890. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Zaccaro L, Del Gatto A, Pedone C and
Saviano M: Peptides for tumour therapy and diagnosis: current
status and future directions. Curr Med Chem. 16:780–795. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Tang B, Li Z, Huang D, Zheng L and Li Q:
Screening of a specific peptide binding to VPAC1 receptor from a
phage display peptide library. PLoS One. 8:e542642013. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
de Visser M, Verwijnen SM and de Jong M:
Update: improvement strategies for peptide receptor scintigraphy
and radionuclide therapy. Cancer Biother Radiopharm. 23:137–157.
2008.PubMed/NCBI
|
|
89.
|
Balon HR, Goldsmith SJ, Siegel BA,
Silberstein EB, Krenning EP, Lang O and Donohoe KJ: Procedure
guideline for somatostatin receptor scintigraphy with
(111)In-pentetreotide. J Nucl Med. 42:1134–1138. 2001.PubMed/NCBI
|
|
90.
|
Kwekkeboom DJ, Kam BL, van Essen M,
Teunissen JJ, van Eijck CH, Valkema R, de Jong M, de Herder WW and
Krenning EP: Somatostatin-receptor-based imaging and therapy of
gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer.
17:R53–R73. 2010. View Article : Google Scholar : PubMed/NCBI
|
















