Introduction
Prospective follow-up studies are effective methods
of answering research questions on disease aetiological mechanisms
and serve as data sources for estimating incidence and survival
rates of diseases within a defined population (1,2). In
addition, they provide information on potential influence of
changing environmental and life style factors on disease risk
through comparison of the same individual at different time points
(2). This allows estimation of
within-individual variations, which is useful for drawing
conclusions on effects of an intervention (3,4).
Nevertheless, large scale prospective studies are often difficult
to undertake because subjects drop out over time, potentially
leading to bias results and erroneous conclusions, particularly if
the loss of subjects over time is related to aetiological factors
that are associated with the outcomes of interest (5).
Dropout, including withdrawal, loss to follow-up
(LTFU) and death can be as high as losing up to two-third of the
original samples particularly in studies that require participants
to make several rounds of visits for routine follow-up specimen and
data collection (6,7). Apart from the duration and number of
follow-up, other factors that have been linked to the dropout rate
include characteristics of the participants such as age, gender,
social status, life style and health condition (5,7,8).
Whilst a number of studies has examined dropout in population-based
or focused group longitudinal studies (6), to the best of our knowledge no such
result has been reported amongst lung cancer high-risk populations.
Given that lung cancer is a disease of the aged (increased
incidence with ageing), there is a possibility of linkage between
dropout and factors related to outcome amongst high-risk
individuals. Knowledge of such results may inform strategies for
optimal designing, planning and implementation of future lung
cancer follow-up studies.
The aim of this study is to investigate factors that
are associated with continued participation of selected high-risk
individuals in the Liverpool Lung Project (LLP) follow-up during
the 2005–2009. In addition, we examined the performance of the
criteria previously used to stratify individuals as high-risk in
terms of the outcome event, and discuss the potential use of the
developed LLP risk model for the selection of individuals into
future ‘high-risk’ follow-up.
Materials and methods
Study population
The Liverpool Lung Project (LLP) is a population
based study conducted in a defined geographical area of Merseyside,
which has contiguous electoral wards with a high incidence of lung
cancer (9). The LLP study design
includes a case-control study, 579 newly diagnosed cases of lung
cancer and 1,157 age-gender merged population controls were
recruited between 1998–2005, a prospective cohort study targeting
approximately 7,500 participants recruited between 1998 and 2008,
and a follow-up study of high-risk group selected from the
prospective cohort population. The prospective cohort subjects
consist of people aged 45–79 living within the study area and
randomly selected from the population through the Central
Operations Group database of patients registered with the General
Practices (GPs). A detailed description of patient approached and
recruitment have already been described (9).
Selection of high-risk group
The follow-up study started in 2005 and ran through
to 2009 utilising predefined set of epidemiological criteria
involving age, smoking duration and previous respiratory disease
[history of bronchitis, emphysema or pneumonia referred to as ‘BEP’
(10)] to identify 1,486 high-risk
group that were alive, lung cancer free and active participants of
the prospective cohort population. In addition to these
self-reported risk factors, clinical measures of health status such
as systolic and diastolic blood pressure for coronary disease, and
lung function test (spirometric) for chronic obstructive pulmonary
disease (COPD) were also measured at each annual visit. Individuals
were considered ‘high-risk’ if they were ≥65 years with >40
years smoking duration history; <65 years with >20 years of
smoking and a history of BEP; current smokers with >30 years
smoking history or ex-smokers who had quit within 5 years and with
>40 years of smoking history. Although, this selection criterion
reflects the best available model prior to emergence of lung cancer
risk prediction model, it only includes few of the currently
identified risk factors and predictors of lung cancer risk
(11,12).
The annual follow-up of selected ‘high-risk’
individuals started in 2005 when all high-risk participants were
recalled for follow-up collection of serial specimen samples
(blood, sputum and bronchus swab) and further data on their
epidemiological, life style and disease diagnosis history. The
follow-up strategies include regular appointments, telephone calls
to the participants, reminder letters and regular ascertainment of
the event outcome (development of lung cancer) and vital status
(dead or alive and well). Home visits were also offered to subjects
that were unable to attend the clinic due to poor health taking
into consideration the ageing nature of the study subjects. All
individuals in the cohort were followed up through the Office of
National Statistics (ONS) National Health Service (NHS) information
service to obtain up to date information on their vital status,
including date and cause of death and documentation of multiple
cause of death as recorded on the death certificates. In addition,
the LLP participant’s database was linked with the North West
Cancer Intelligence Services (NWCIS) to acquire information on the
disease outcome recording cases of lung cancer occurrence, date and
details of diagnosis, morphology subtype and treatment history.
Comprehensive comorbidity conditions of all individuals in the
study were obtained from the Health Episode Statistics (HES)
database.
Statistical analysis
Statistical analyses were conducted using dropout as
the primary dependent variable. A dropout was defined as anyone who
was not observed at a particular visit (year) and was not seen or
did not respond to subsequent annual recalls. This includes all
cases of LTFU and withdrawal through refusal to continue
participation. Separate and joint analyses were undertaken for
patient’s withdrawal and LTFU. A discrete survival analysis was
conducted to adjust the estimated rate for censoring and
individual’s time to dropout (13,14).
This analysis considers the length of follow-up before dropout and
treats deaths as censored observations. In addition, individuals
that remained active in the follow-up at the time of the analysis
were considered as right censored observations; this implies that
for these subjects the event of interest (withdrawal or LTFU) did
not occur while still being followed up, but we do not know whether
or not it will occur at some point in the future. The Kaplan-Meier
survival, which is a non-parametric estimation of survival
probability over time, was utilised for univariate exploration of
the relationship between the dropout and patient’s characteristics.
The log-rank test was used to compare the Kaplan-Meier curves
across levels of each covariate. The Cox proportional hazard model
was used to examine the relationship between the dropout rates
overtime and all explanatory factors simultaneously.
In order to evaluate the strategy used for selecting
individuals into the ‘high-risk’ group, a retrospective prediction
of the 5-year absolute risk of developing lung cancer was
undertaken for each participant using the LLP risk model (9–11).
The absolute risk calculation was based on the baseline data
collected at the time of recruitment into the cohort. Data analyses
were performed using Stata software version 12.1 (StataCorp,
College Station, TX). All statistical tests were two-tailed carried
out at 5% level of significance.
Results
Table I shows the
pattern of follow-up outcome by annual recall visit. More than half
of subjects on annual follow-up (62%) are still active in the
study. The average follow-up visit attendance was 3 (range 1–5
visit attendants) as at the end of the study in 2009. A total of
460 subjects (31%) have dropped out of the study due to LTFU
(n=160; 11%) and withdrawal (n=300, 20%). The proportion of dropout
rate and death was higher earlier during the initial stage of the
follow-up but reduced over time.
 | Table I.Participation status of the subjects
at each annual follow-up recall. |
Table I.
Participation status of the subjects
at each annual follow-up recall.
| Follow-up status | Annual follow-up
visit
| Total |
|---|
| 1 | 2 | 3 | 4 |
|---|
| Lost to
follow-up | 75 (5.1) | 54 (4.5) | 25 (2.4) | 6 (0.6) | 160 (10.8) |
| Withdrawal | 155 (10.4) | 105 (8.7) | 29 (2.8) | 11 (1.2) | 300 (20.2) |
| Deceased | 52 (3.5)a | 22 (1.8) | 19 (1.9) | 9 (0.9) | 102 (6.9) |
| Active | 1,204 (81.0) | 1,023 (85.0) | 950 (92.9) | 924 (97.3) | 924 (62.1) |
| Cumulative
dropoutsa | 230 (15.5) | 389 (26.2) | 443 (29.9) | 460 (31.0) | |
Table II shows
distribution of the baseline characteristics by end-of-study
follow-up outcome. Subject’s age, gender, smoking status or smoking
duration, prior diagnosis of malignant disease and systolic blood
pressure were independently and statistically significantly
associated with overall follow-up outcome. The dropout rate was
slightly higher for female (33.1%) compared to male (28.3%),
current smokers (33.2%) relative to ex-smokers (27.5%), and in
individuals with no previous diagnosis of malignant disease (32.2%)
compared with individuals with previous diagnosis of malignant
disease (22.8%). Dropout rate for those with previous history of
pneumonia (31.4%) was very close to those without history of
pneumonia (30.9%). The average age of subjects dropping out was
statistically significantly higher than the overall average age for
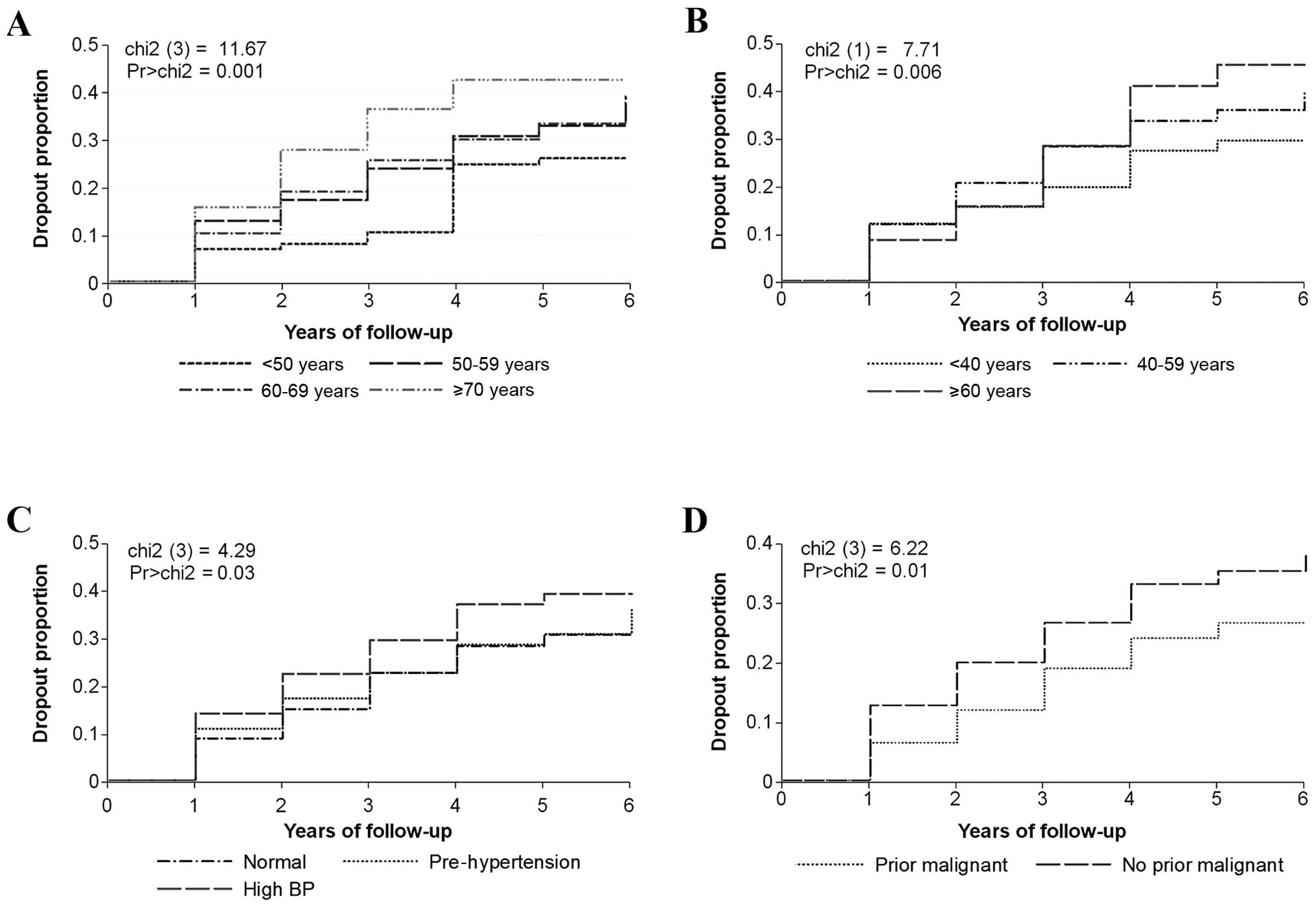
all participants or the active subjects in the follow-up. Fig. 1 shows the Kaplan-Meier curves and
log-rank test for covariates with independent statistically
significant relationships with dropout. Independent significant
association with dropout was observed for subject’s age P= 0.001,
smoking duration P=0.006, systolic blood pressure P=0.03 and prior
diagnosis of malignant disease P= 0.01. There were high
probabilities of dropout over time for older subjects, smokers with
long duration of smoking history and those with prior diagnosis of
malignant disease.
 | Table II.Distribution of subject’s baseline
characteristics by overall (end of study) follow-up outcome. |
Table II.
Distribution of subject’s baseline
characteristics by overall (end of study) follow-up outcome.
| Characteristics | FU outcome status
| All subjects | P-value |
|---|
| Dropout | Active | Deceased |
|---|
| Age (years) | | | | | |
| <50 | 28 (24.6) | 82 (71.9) | 4 (3.5) | 114 (7.7) | |
| 50–59 | 161 (30.3) | 349 (65.7) | 21 (4.0) | 531 (35.7) | <0.01 |
| 60–69 | 179 (29.4) | 380 (62.5) | 49 (8.1) | 608 (40.9) | |
| ≥70 | 92 (39.5) | 113 (48.5) | 28 (12.0) | 233 (15.7) | |
| Mean ± SD | 62.2±7.7 | 60.5±7.5 | 64.8±7.4 | 61.3±7.7 | |
| Gender | | | | | |
| Male | 188 (28.3) | 415 (62.5) | 61 (9.2) | 664 (44.7) | 0.002 |
| Female | 272 (33.1) | 509 (61.9) | 41 (5.0) | 822 (55.3) | |
| Smoking status | | | | | |
| Current
smokers | 301 (33.2) | 554 (61.1) | 52 (5.7) | 907 (61.0) | 0.014 |
| Ex-smokers | 159 (27.5) | 370 (63.9) | 50 (8.6) | 579 (39.0) | |
| Smoking duration
(years) | | | | | |
| <40 | 125 (26.7) | 318 (68.0) | 25 (5.3) | 468 (31.5) | 0.021 |
| 40–60 | 312 (32.5) | 575 (59.9) | 73 (7.6) | 960 (64.7) | |
| ≥60 | 23 (40.4) | 30 (52.6) | 4 (7.0) | 57 (3.8) | |
| Pneumonia
diagnosis | | | | | |
| Yes | 86 (31.4) | 170 (62.0) | 18 (6.6) | 274 (18.4) | 0.97 |
| No | 374 (30.9) | 754 (62.2) | 84 (6.9) | 1,212 (81.6) | |
| Malignant
disease | | | | | |
| Yes | 43 (22.8) | 122 (64.6) | 24 (12.7) | 189 (12.7) | <0.01 |
| No | 417 (32.2) | 802 (61.8) | 78 (6.0) | 1,297 (87.3) | |
| Family history | | | | | |
| No history | 181 (33.9) | 310 (58.1) | 43 (8.1) | 534 (35.9) | 0.11 |
| Early onset | 151 (30.6) | 310 (62.9) | 32 (6.5) | 493 (33.2) | |
| Late onset | 128 (27.9) | 304 (66.2) | 27 (5.9) | 459 (30.9) | |
| Systolic BP
(mmHg) | | | | | |
| Normal
(<120) | 101 (27.3) | 246 (66.5) | 23 (6.2) | 370 (25.1) | |
| Pre-hypertension
(120–139) | 154 (28.1) | 359 (65.5) | 35 (6.4) | 548 (37.2) | 0.02 |
| High BP (≥140) | 198 (35.6) | 319 (57.3) | 40 (7.2) | 557 (37.8) | |
| Diastolic BP
(mmHg) | | | | | |
| Normal
(<80) | 202 (30.1) | 416 (62.0) | 53 (7.9) | 671 (45.5) | |
| Pre-hypertension
(80–89) | 133 (30.5) | 277 (63.5) | 26 (6.0) | 436 (29.6) | 0.48 |
| High BP (≥90) | 118 (32.1) | 231 (62.8) | 19 (5.2) | 368 (25.0) | |
Table III presents
the results of the association between dropout during the follow-up
period and all the patient’s characteristics. Elderly (aged ≥70),
hazard ratio (HR) 1.92 (95% CI 1.07–3.45), female gender 1.35
(1.09–1.66), current smoking 1.26 (1.02–1.57), prior diagnosis of
malignant disease 0.54 (0.36–0.79), home visits 0.67 (0.48–0.94)
and systolic blood pressure 1.46 (1.10–1.94) were significantly
associated with dropout rate in the multivariable model. The
separate analysis of the two dropout outcomes, withdrawn and LTFU,
showed a different pattern of associations with patient’s
covariates. There was a statistically significant increase in
subject’s withdrawal hazard rate with age; elderly participants
aged ≥70 years were approximately three times more likely to
withdraw from the follow-up compared to those aged <50 years.
The hazards of withdrawal were also higher for female compared to
male and current smokers against ex-smokers, but no significant
association was seen with smoking duration. Participants with prior
diagnosis of malignant disease had low follow-up withdrawal
hazards. The withdrawal hazard was lower 0.54 (0.35–0.84) for those
on home visits compared to those not on home visit list and
participants who were hypertensive (high systolic BP) were more
likely to withdraw from the study compared to normal subjects HR
1.70 (1.19–2.43). However, none of the patient characteristics were
associated with LTFU and none of the dropout outcomes was
significantly associated with predicted lung cancer risk, although
the median predicted risk was slightly higher among the dropouts
(5.30±5.09; median, 4.16) compared to those that remain active
(4.72±4.81; median, 3.43).
 | Table III.Hazard ratio from multivariable Cox
proportional model for predictors of subject’s participation in
annual follow-up. |
Table III.
Hazard ratio from multivariable Cox
proportional model for predictors of subject’s participation in
annual follow-up.
|
Characteristics | Withdrawn
| Lost to follow-up
| Dropout (withdrawn
+ LTFU)
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Current status | | | | | | |
| Deceased | 1.45
(0.76–2.75) | 0.26 | 0.33
(0.05–2.40) | 0.27 | 1.10
(0.60–2.03) | 0.75 |
| Age | | | | | | |
| <50 | 1.00 | | 1.00 | | 1.00 | |
| 50–59 | 2.01
(1.03–3.93) | 0.02 | 1.02
(0.58–1.80) | 0.75 | 1.38
(0.90–2.11) | 0.10 |
| 60–69 | 2.00
(0.99–4.05) | | 0.89
(0.46–1.72) | | 1.34
(0.85–2.12) | |
| ≥70 | 3.35
(1.48–7.58) | | 0.64
(0.23–1.77) | | 1.92
(1.07–3.45) | |
| Gender | | | | | | |
| Female | 1.61
(1.24–2.09) | 0.002 | 1.00
(0.71–1.41) | 0.99 | 1.35
(1.09–1.66) | 0.005 |
| Smoking status | | | | | | |
| Ex-smokers | 1.00 | | 1.00 | | 1.00 | |
| Current
smoker | 1.21
(0.92–1.59) | 0.16 | 1.37
(0.93–2.02) | 0.11 | 1.26
(1.02–1.57) | 0.04 |
| Smoking
duration | | | | | | |
| <40 | 1.00 | | 1.00 | | 1.00 | |
| 40–60 | 1.06
(0.73–1.55) | 0.43 | 0.76
(0.48–1.22) | 0.50 | 0.96
(0.72–1.29) | 0.47 |
| ≥60 | 0.75
(0.37–1.51) | | 0.64
(0.21–1.92) | | 0.72
(0.40–1.29) | |
| Pneumonia | | | | | | |
| Yes | 0.82
(0.57–1.17) | 0.27 | 1.00
(0.64–1.57) | 0.99 | 0.91
(0.69–1.21) | 0.43 |
| Malignant
disease | | | | | | |
| Yes | 0.46
(0.28–0.76) | 0.002 | 0.71
(0.38–1.32) | 0.28 | 0.54
(0.36–0.79) | 0.002 |
| Family history | | | | | | |
| No history | 1.00 | | 1.00 | | 1.00 | |
| Early onset
(<60 years) | 0.70
(0.50–0.99) | | 0.91
(0.59–1.40) | 0.26 | 0.78
(0.60–1.02) | 0.06 |
| Late onset (≥60
years) | 0.77
(0.58–1.03) | 0.08 | 0.72
(0.47–1.08) | | 0.76
(0.60–0.97) | |
| Home visit | | | | | | |
| Yes | 0.54
(0.35–0.84) | 0.005 | 1.04
(0.60–1.79) | 0.90 | 0.67
(0.48–0.94) | 0.02 |
| Diastolic blood
pressure (mmHg) | | | | | | |
| Pre-hypertension
(120–139) | 0.78
(0.58–1.06) | 0.27 | 1.33
(0.89–1.98) | 0.37 | 0.95
(0.75–1.20) | 0.87 |
| High BP
(≥140) | 0.87
(0.63–1.21) | | 1.11
(0.68–1.79) | | 0.94
(0.71–1.23) | |
| Systolic blood
pressure (mmHg) | | | | | | |
| Pre-hypertension
(120–139) | 1.20
(0.86–1.67) | 0.006 | 0.88
(0.57–1.38) | 0.64 | 1.08
(0.83–1.41) | 0.01 |
| High BP
(≥140) | 1.70
(1.19–2.43) | | 1.06
(0.65–1.72) | | 1.46
(1.10–1.94) | |
| Predicted LLP risk
(%) | 1.04
(0.99–1.10) | 0.08 | 1.03
(0.96–1.10) | 0.43 | 1.03
(0.99–1.08) | 0.10 |
Table IV shows the
distribution of retrospective 5-year absolute risk predicted by the
LLP risk model. The majority of participants (61.8%) selected as
‘high-risk’ had 5-year absolute risk below the threshold of 5.0%
currently used in an on-going primary care implementation programme
(PCIP) early detection study and in the United Kingdom Lung
Screening (UKLS) trial. There was an increase in the proportion of
lung cancer cases with predicted risk; participants with 5-year
risk above 10% had the highest proportion of lung cancer occurrence
(6%) and no lung cancer was identified during the follow-up period
among individuals with very low predicted 5-year absolute risk that
were selected as part of the ‘high-risk’ group.
 | Table IV.Distribution of predicted 5-year risk
of lung cancer by disease status. |
Table IV.
Distribution of predicted 5-year risk
of lung cancer by disease status.
| Predicted risk
(%) | Subject’s cohort
status
| All subjects
(n=1,485) |
|---|
| Malignancy (n=46,
3.1%) | Disease-free
(n=1,439, 96.9%) |
|---|
| <1 | 0 (0.0) | 267 (100.0) | 267 (18.0) |
| 1.0–2.49 | 2 (0.8) | 246 (99.2) | 248 (16.7) |
| 2.5–4.90 | 12 (3.0) | 390 (97.0) | 402 (27.1) |
| 5.0–9.90 | 22 (5.5) | 376 (94.5) | 398 (26.8) |
| ≥10 | 10 (5.9) | 160 (94.1) | 170 (11.5) |
| Summary
measures | | | |
| Mean ± SD | 8.72±6.12 | 4.93±4.91 | 5.05±4.98 |
| Median | 6.65 | 3.7 | 3.81 |
| Minimum | 1.47 | 0.13 | 0.13 |
| Maximum | 31.57 | 51.32 | 51.32 |
Discussion
This study examined risk factors that are associated
with dropout of individuals at high-risk of developing lung cancer
in the Liverpool Lung Project (LLP) ‘high-risk’ follow-up study.
Female gender, current smoking, prior diagnosis of malignant
disease, home visitation and systolic blood pressure were
significantly associated with dropout rate.
The high number of deaths in the first year of
follow-up as reported in Table I
is due to the fact that follow-up period in this group included all
follow-up from the first recruitment of the study participants in
1996. The overall attrition (dropout) rate in the LLP ‘high-risk’
follow-up of 31% (withdrawal, 20%; LTFU, 11%) was considerably
modest and similar to rates reported in other longitudinal studies
involving regular recalls of patients, which ranges between 14 and
33% (5,7,15).
This percentage decreases to 24% after correction for deceaced
subjects. The LTFU were typically subjects who have moved out of
the area and were therefore untraceable. The lack of significant
predictors for LTFU is a suggestion of non-selective participation
in terms of participants that were LTFU, and an indication that
LTFU in this study is a random process. This provides reassurance
that potential effects of systematic bias on risk effects may not
be present.
Among the patient characteristics examined, the
gender, smoking status, home visits and systolic blood pressure,
were statistically associated with dropout whereas subject’s age,
gender, malignant disease, home visits and systolic blood pressure
were significantly associated with withdrawal. None of the
aforementioned patient characteristics were associated with LTFU.
Apart from the gender effect on withdrawal rate, all other factors
identified as determinants of dropout indicate that withdrawal in
the LLP ‘high-risk’ follow-up study may be linked to ill health and
lack of mobility. Poor health condition is a major factor often
reported to influence participation in follow-up studies (4,5,7). In
this study, a substantial number of participants refused to
continue participation because they could not provide the required
specimen or were too old to travel to attend the LLP clinics. In
addition, our results suggest that females were more likely to drop
out of the follow-up than their male counterparts. This observation
is consistent with the result from other studies (6).
The comprehensive investigation of attrition as
reported herein is very important not only for gauging follow-up
intervals, but also for designing strategies to maximise compliance
and encouraging continued participation in future studies.
Interventions may include setting up criteria to exclude at onset
people with potential for health deterioration during the
follow-up. However, projecting future health conditions at baseline
would be difficult. The exclusion of individuals may also lead to
non-representation of the follow-up study with respect to the
general population distribution of the event under investigation.
Instead, an oversampling of those individuals at the initial phases
of the follow-up could be undertaken to ensure that sufficient
numbers remain at the end of the follow-up period to ensure that
the result is generalizable to the population it aims to depict.
One important constraint for oversampling of a particular group of
subjects is cost-effectiveness of such approach; one would need to
assess the relative cost of such an approach to its effectiveness.
Another useful strategy to encourage continued participation would
be to provide special assistance to individuals according to their
needs and preferences, such as making home visit provision for
individuals who are unable to attend either due to ill health or
old age; this however would inevitably be a more expensive
follow-up method. In the LLP follow-up study, withdrawal rate was
about 54% lower among those on home visit; this may be an
indication of the effectiveness and value for money for the
approach.
The strengths of this study include the
population-based design, the large sample size, the long follow-up
period and the use of ONS and the HES data minimises the chances of
missing information. In addition, detailed information concerning
the potential risk factors in the LLP was collected using
standardised questionnaires. However, the result of this study must
be considered in the light of a number of limitations. First, we
have examined dropout in relation to only a number of possible
baseline characteristics that may be influential determinants of
either withdrawal or LTFU. We do not know if dropout was related to
other factors that are latent (unmeasured) in our analysis.
Secondly, the design of the ‘high-risk’ follow-up
study estimated that the size of the planned cohort would enable
the selection of about 1,500 individuals from which 60–70% (90–105)
lung cancer cases would be expected to occur in 10 years of
follow-up (10). However, the
total number of lung cancer cases that occurred in this ‘high-risk’
follow-up cohort with a 3 years average follow-up period was only
47 (3.2%). This number of lung cancer occurrence may be a
reflection of selection of individuals with risk not sufficiently
high enough even though this reflects the best available lung
cancer model as at the time of selection, inclusion of low risk
individuals will mean long follow-up time before occurrence of the
event outcome.
The retrospective prediction of absolute risk of
lung cancer for the ‘high-risk’ using the LLP risk model showed
that only 38% of selected individuals would qualify to be in the
follow-up using the 5% risk threshold currently in use in our early
detection primary care implementation programme and UKLS. This
finding suggests a need for the modification of the ‘high-risk’
group in terms of making changes to selection criteria in order to
achieve its specific outcome events.
In conclusion, a modest dropout rate was recorded in
the LLP ‘high-risk cohort’ after an average of 3 years of follow-up
indicating feasibility of annual follow-up in this cohort of
subjects. Efforts to encourage continued participation should be
implemented. Given the aging nature of the cohort, arranging home
visits by the research nurse to elderly participants unable to
attend clinics may prove useful in reducing the study’s withdrawal
rate but may lead to extra cost. Finally, the use of the LLP risk
model will ensure that individuals at sufficiently high-risk of the
disease are the ones selected for follow-up, thereby maximising the
benefit-harm ratio of the study.
Acknowledgements
This study was supported by grants
from the Roy Castle Lung Cancer Foundation, the European
Community’s Seventh Framework Programme (FP7/2007-2013) under grant
agreement no. HEALTH-F2-2010-258677 (CURELUNG project) and grant
agreement no. 258868 (LCAOS project).
References
|
1.
|
Vandenbroucke JP: Observational research,
randomised trials, and two views of medical science. PLoS Med.
5:e672008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Rothman KJ and Greenland S: Cohort
studies. Rothman KJ, Greenland S and Lash TL: Modern Epidemiology.
3rd edition. Lippincott Williams & Wilkins; Philadelphia, PA:
pp. 100–110. 2008
|
|
3.
|
Pirie PL, Thomson SJ, Mann SL, et al:
Tracking and attrition in longitudinal school-based smoking
prevention research. Prev Med. 18:249–256. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Guey LT, Bromet EJ, Gluzman SF, Zakhozha V
and Paniotto V: Determinants of participation in a longitudinal
two-stage study of the health consequences of the Chornobyl nuclear
power plant accident. BMC Med Res Methodol. 8:272008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Oleske DM, Kwasny MM, Lavender SA and
Andersson GB: Participation in occupational health longitudinal
studies: predictors of missed visits and dropouts. Ann Epidemiol.
17:9–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Van Beijsterveldt CE, van Boxtel MP, Bosma
H, Houx PJ, Buntinx F and Jolles J: Predictors of attrition in a
longitudinal cognitive aging study: the Maastricht Aging Study
(MAAS). J Clin Epidemiol. 55:216–223. 2002.PubMed/NCBI
|
|
7.
|
Chatfield MD, Brayne CE and Matthews FE: A
systematic literature review of attrition between waves in
longitudinal studies in the elderly shows a consistent pattern of
dropout between differing studies. J Clin Epidemiol. 58:13–19.
2005. View Article : Google Scholar
|
|
8.
|
Phillips KA, Butow PN, Stewart AE, et al:
Predictors of participation in clinical and psychosocial follow-up
of the kConFab breast cancer family cohort. Fam Cancer. 4:105–113.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Field JK, Smith DL, Duffy S and Cassidy A:
The Liverpool Lung Project research protocol. Int J Oncol.
27:1633–1645. 2005.PubMed/NCBI
|
|
10.
|
Cassidy A, Myles JP, Liloglou T, Duffy SW
and Field JK: Defining high-risk individuals in a population-based
molecular-epidemiological study of lung cancer. Int J Oncol.
28:1295–1301. 2006.PubMed/NCBI
|
|
11.
|
Cassidy A, Myles JP, van Tongeren M, Page
RD, Liloglou T, Duffy SW and Field JK: The LLP risk model: an
individual risk prediction model for lung cancer. Br J Cancer.
98:270–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Spitz MR, Hong WK, Amos CI, et al: A risk
model for prediction of lung cancer. J Natl Cancer Inst.
99:715–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Singer JD and Willett JB: It’s about time:
Using Discrete-Time Survival Analysis to study duration and the
timing of events. J Educ Stat. 18:155–195. 1993.
|
|
14.
|
Willett JB and Singer JD: Investigating
onset, cessation, relapse, and recovery: why you should, and how
you can, use discrete-time survival analysis to examine event
occurrence. J Consult Clin Psychol. 61:952–965. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bildt C, Alfredsson L, Punnett L, Theobald
H, Torgen M and Wikman A: Effects of drop out in a longitudinal
study of musculoskeletal disorders. Occup Environ Med. 58:194–199.
2001. View Article : Google Scholar : PubMed/NCBI
|