Introduction
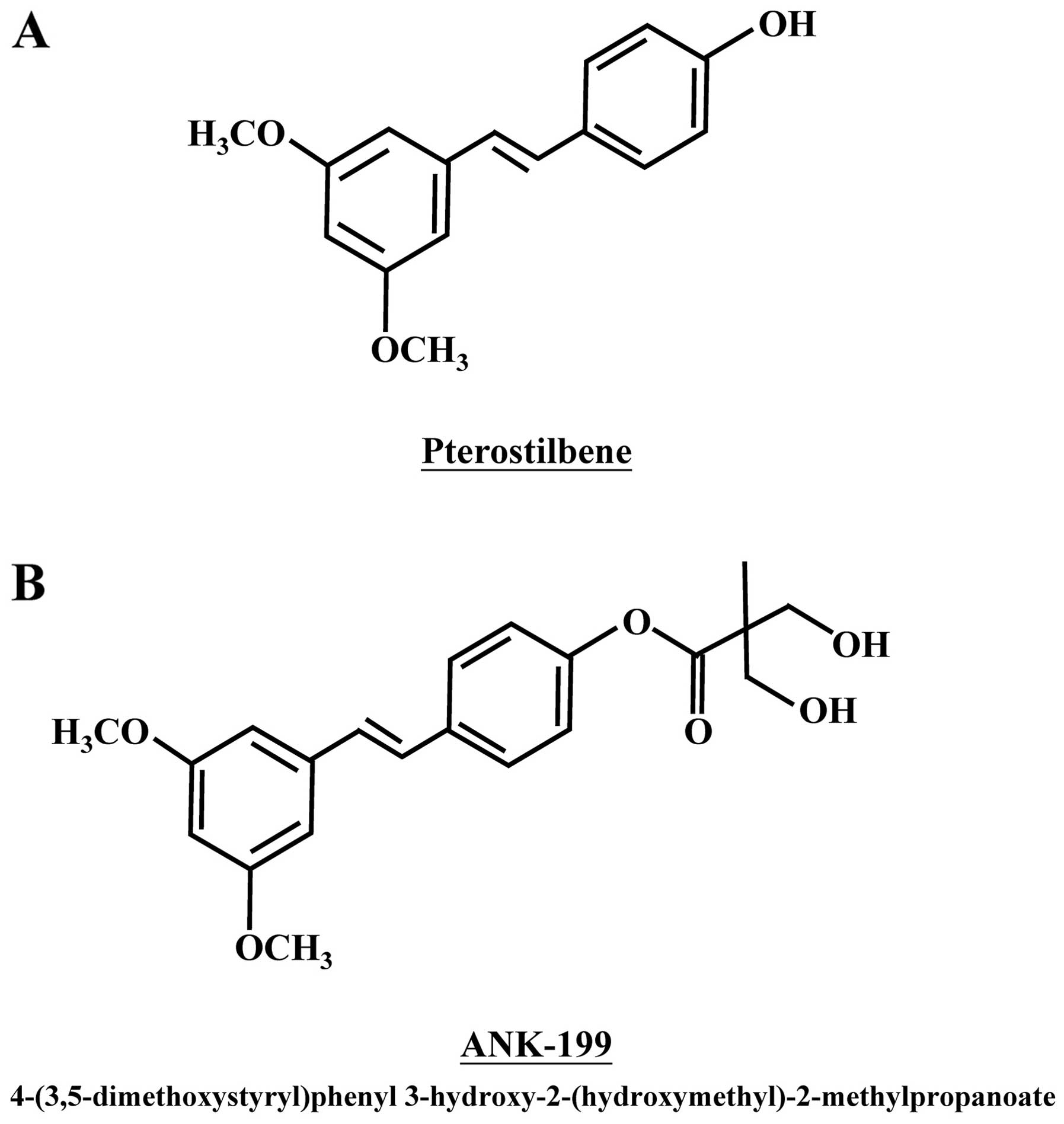
Pterostilbene, a natural stilbenoid compound of
phenolic phytoalexin analogue, is found in narra tree, grape and
blueberries (Fig. 1A) (1–4). It
possesses many different pharmacological and biologic activities,
such as anticancer activity with low intrinsic toxicity (4–6),
anti-inflammatory properties (7–9),
anti-oxidative effect (2),
regulation of neutrophil function (10,11)
and protection against free radical-mediated oxidative damage
(12–14). The anticancer activity of
pterostilbene has drawn the most attention among of them so far
(1,4–6). As
reported in previous studies, pro-apoptosis (4,15,16),
pro-autophagy (17–19), telomerase inhibition (20), DNA damage (12,13,15),
anti-angiogenesis (21),
anti-metastasis (4,21) and immuno-stimulatory effects
(10,11) are possible mechanisms responsible
for its anticancer activity.
Pterostilbene is able to induce apoptosis in many
different cancer cell lines, such as pancreatic cancer cells
(22,23), breast cancer MCF-7 cells (20,24,25),
docetaxel-induced multiple drug resistance (MDR) lung cancer cells
(26), osteosarcoma cells
(27), prostate cancer PC-3 and
LNCaP cells (28,29), leukemia K562 cells (30,31),
MDR and BCR-ABL-expressing leukemia cells (30,31),
colon cancer cells (32–34), hepatocellular carcinoma cells
(35,36) and gastric carcinoma cells (7,15).
On the other hand, it is also reported that autophagic death can be
triggered by pterostilbene in leukemia HL60 (17) and MOLT4 cells (37), lung cancer cells (18,32,38),
colon cancer HT29 cells (32),
breast cancer MCF-7 cells (39),
bladder cancer cells (17,40) and vascular endothelial cells
(41). In addition, pterostilbene
is capable of inhibiting tumorigenesis and metastasis with minor
toxicity in vivo (4,22,38).
It is safe in doses up to 250 mg/day in human clinical trial, and
deserves further investigation as a potential anticancer agent
(42). A novel pterostilbene
derivative, ANK-199, was therefore designed and synthesized by our
group (Fig. 1B).
Chewing the mixtures of betel leaf and areca nut is
a popular custom in many South and Southeast Asia countries. It is
a high risk factor for oral cavity carcinoma (43,44),
and is the 4th most common cause of cancer death in Taiwanese males
(45). Natural product with high
anticancer activity and low toxicity, like pterostilbene, appears
to be an ideal candidate to prevent or treat oral cancer, as it can
directly contact with human oral mucosa without intravenous
administration or surgery (22).
The anti-oral cancer activity of pterostilbene derivative, ANK-199,
was first investigated in this study. Both normal human oral cell
lines and cisplatin-resistant CAR human oral cancer cell lines were
used. Here, we report the cytotoxic effect and anticancer mechanism
of ANK-199 in human oral cancer CAR cells.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide (DMSO), 3-methyladenine (3-MA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), monodansyl cadaverine (MDC), cisplatin, β-actin antibody,
and Tween-20 were obtained from Sigma-Aldrich Corp. (St. Louis, MO,
USA). Fetal bovine serum (FBS), L-glutamine,
penicillin/streptomycin, Dulbecco’s modified Eagle’s medium (DMEM),
acridine orange (AO), and trypsin-EDTA were purchased from Life
Technologies (Carlsbad, CA, USA). The primary antibodies
(anti-Atg5, anti-Atg7, anti-Atg12, anti-Atg14, anti-Atg16L1,
anti-beclin 1, anti-PI3K class III, anti-LC3-II, and anti-Rubicon)
were obtained from Cell Signaling Technology (Danvers, MA, USA),
and the horseradish peroxidase (HRP)-conjugated secondary
antibodies against rabbit or mouse immunoglobulin for western blot
analysis were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). ANK-199 [4-(3,5-dimethoxystyryl)phenyl
3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate] was synthesized by
Dr Sheng-Chu Kuo.
Cell culture
The human oral cancer cell line CAL 27 was obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). CAR, a cisplatin-resistant cell line, was established by
clonal selection of CAL 27 using 10 cycles of 1 passage treatment
with 10–80 μM of cisplatin followed by a recovery period of another
passage. CAR cells were cultivated in DMEM supplemented with 10%
FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, 2 mM L-glutamine
and 80 μM cisplatin. Human normal gingival fibroblasts cells (HGF)
and human normal oral keratinocyte cells (OK) were kindly provided
by Dr Tzong-Ming Shieh (Department of Dental Hygiene, China Medical
University). HGF and OK cells were cultivated in DMEM as previously
described for our study (45).
Cell viability and morphological
examination
CAR cells (1×104 cells) in a 96-well
plate were incubated with 0, 25, 50, 75 and 100 μM of ANK-199 for
24, 48 and 72 h. For incubation with the autophagy inhibitor, cells
were pretreated with 3-MA (10 mM) for 1 h, followed by treatment
with or without ANK-199 (50 and 75 μM) for 48 h. After washing the
cells, DMEM containing MTT (0.5 mg/ml) of was added to detect
viability as previously described (6). The cell viability was expressed as %
of the control. Cell morphological examination of autophagic
vacuoles was determined utilizing a phase-contrast microscope
(46,47).
Observation of autophagic vacuoles by MDC
and acidic vesicular organelles (AVO) with AO staining
CAR cells were seeded on sterile coverslips in
tissue culture plates with a density of 5×104 cells/per
coverslip. After 0, 50, 75 μM of ANK-199 treatment for 24 h, cells
were stained with either 1 μg/ml AO or 0.1 mM MDC at 37°C for 10
min. The occurrece of autophagic vacuoles and AVO were immediately
observed under fluorescence microscopy (Nikon, Melville, NY, USA)
(46–48).
Autophagy assay by LC3B-GFP imaging and
nuclear stain
The induction of autophagy was detected with the
Premo™ Autophagy Sensor (LC3B-GFP) BacMam 2.0 kit (Molecular
Probes/Life Technologies). CAR cells were seeded on sterile
coverslips in tissue culture plates with a density of
1×104 cells/per coverslip. After CAR cells were
transfected with LC3B-GFP in accordance with the manufacturer’s
protocol, cells were treated with 0, 50 and 75 μM of ANK-199 for 24
h. Cells were then fixed on ice with 4% paraformaldehyde, and the
slides were mounted and analyzed by a fluorescence microscope.
After treatments, cells were stained with
4′,6-diamidino-2-phenylindole (DAPI, Molecular Probes/Life
Technologies) and photographed using a fluorescence microscope
(46,47,49).
Western blot analysis
CAR cells (1×107 cells/75-T flask) were
treated with ANK-199 (50 and 75 μM) for 48 h. At the end of
incubation, the total proteins were prepared, and the protein
concentration was measured by using a BCA assay kit (Pierce
Chemical, Rockford, IL, USA). Equal amounts of cell lysates were
run on 10% SDS-polyacrylamide gel electrophoresis and further
employed by immunoblotting as described by Lin et al
(46).
Real-time PCR analysis
CAR cells at a density of 5×106 in T75
flasks were incubated with or without 50 and 75 μM of ANK-199 for
24 h. Cells were collected, and total RNA was extracted by the
Qiagen RNeasy mini kit (Qiagen Inc., Valencia, CA, USA). Each RNA
sample was individually reverse-transcribed using the High Capacity
cDNA Reverse Transcription kits (Applied Biosystems, Foster City,
CA, USA). Quantitative PCR was assessed for amplifications with 2X
SYBR-Green PCR Master mix (Applied Biosystems), as well as forward
and reverse primers for Atg7, Atg12, beclin 1
and LC3-II gene. (Human ATG7-F-CAGCAGTGACGATCGGATGA; human
ATG7-R-GACGGGAAGGACATTATCAAACC; human
ATG12-F-TGTGGCCTCAGAACAGTTGTTTA; human
ATG12-R-CGCCTGAGACTTGCAGTAATGT; human
BECN1-F-GGATGGTGTCTCTCGCAGATTC; human BECN1-R-GGTGCCGCCATCAGATG;
human LC3-II-F-CCGACCGCTGTAAGGAGGTA; human
LC3-II-R-AGGACGGGCAGCTGCTT) Applied Biosystems 7300 Real-Time PCR
System was run in triplicate, and each value was expressed in the
comparative threshold cycles (CT) method for the housekeeping gene
GAPDH.
cDNA microarray analysis
CAR cells (5×106 per T75 flask) were
incubated with or without 75 μM of ANK-199 for 24 h. Cells were
scraped and collected by centrifugation. The total RNA was
subsequently isolated as stated above, and the purity was assessed
at 260 and 280 nm using a Nanodrop (ND-1000; Labtech
International). Each sample (300 ng) was amplified and labeled
using the GeneChip WT Sense Target Labeling and Control Reagents
(900652) for Expression Analysis. Hybridization was performed
against the Affymetrix GeneChip Human Gene 1.0 ST array. The arrays
were hybridized for 17 h at 45°C and 60 rpm. Arrays were
subsequently washed (Affymetrix Fluidics Station 450), stained with
streptavidin-phycoerythrin (GeneChip Hybridization, Wash, and Stain
Kit, 900720), and scanned on an Affymetrix GeneChip Scanner 3000.
Resulting data were analyzed by using Expression Console software
(Affymetrix) with default RMA parameters. Genes regulated by
ANK-199 were determined with a 1.5-fold change. For detection of
significantly over-represented GO biological processes, the DAVID
functional annotation clustering tool (http://david.abcc.ncifcrf.gov) was used (DAVID
Bioinformatics Resources 6.7). Enrichment was determined at DAVID
calculated Benjamini value <0.05. Significance of overexpression
of individual genes was determined (50).
Statistical analysis
All the statistical results were expressed as the
mean ± SEM of triplicate samples. Statistical analyses of data were
done using one-way ANOVA followed by Student’s t-test, and
*p<0.05 and ***p<0.001 were considered
significant.
Results
ANK-199 exhibits cytotoxicity and
inhibits viable CAR cells
CAR cells were treated with different concentrations
of ANK-199 for 24, 48 and 72 h. ANK-199 concentration- and
time-dependently decreased cell viability of CAR cells (Fig. 2A). The half maximal inhibitory
concentration (IC50) for a 24, 48 and 72-h treatment of
ANK-199 in CAR cells were 106.21±3.21, 73.25±4.20 and 32.58±2.39
μM, respectively. To investigate whether the cell death was
mediated through apoptosis by ANK-199, cells were treated with 50
and 75 μM ANK-199 for 48 h. The appearance of DNA fragmentation was
not observed (data not shown), suggesting that ANK-199 was unable
to induce apoptosis in CAR cells. As shown in Fig. 2B, ANK-199 was able to induce the
formation of autophagic vacuoles in CAR cells in a time-dependent
manner in the presence of 50 μM ANK-199 for 24, 48 and 72 h. This
result implies that autophagic cell death plays a pivotal role in
ANK-199-induced cell death. However, no viability impact and
morphological trait change was observed in ANK-199-treated HGF and
OK cells, suggesting that ANK-199 has an extremely low toxicity in
normal oral cell lines. In accordance with this observation, the
IC50 value of HGF and OK cells is greater than 100 μM
(Fig. 3A and B). In short,
ANK-199-induced cell death of CAR cells is mediated through
autophagic death, rather than apoptosis.
ANK-199 induces autophagic cell death in
CAR cells
To further confirm the formation of autophagosome
vesicles in ANK-199-treated CAR cells, the autophagic cell death
caused by ANK-199 was monitored by using MDC staining, a popular
fluorescent marker that preferentially accumulates in autophagic
vacuoles (46,48). After cells were treated with 50 and
75 μM of ANK-199 for 48 h, autophagic vacuoles were easily observed
under fluorescence microscopy (Fig.
4A). The intensity of MDC staining was directly proportional to
the concentration of ANK-199. The ANK-199-triggered autophagic cell
death was also examined by using AO staining. In Fig. 4B, AO staining of ANK-199-treated
CAR cells clearly showed the presence of AVOs within the cytoplasm
compared to control. Microtubule-associated protein 1 light-chain 3
(LC3) is an autophagic membrane marker for the detection of early
autophagosome formation (46,49).
The LC3 distribution in ANK-199-treated CAR cells was also
investigated. A more punctate pattern of LC3B-GFP was observed in
ANK-199 treated cells (Fig. 4C).
The occurrence of DNA condensation was also investigated in the
presence of 50 and 75 μM ANK-199 for 24 h. No significant change
was observed in ANK-199-treated CAR cells under microscope, which
means that ANK-199-induced cell death triggered by apoptosis is
quite unlikely (Fig. 4D). Again,
all of the above results support that ANK-199-induced cell death in
CAR cells is mediated through the induction of autophagic death
instead of apoptosis.
ANK-199 upregulates the
autophagy-associated protein levels in CAR cells
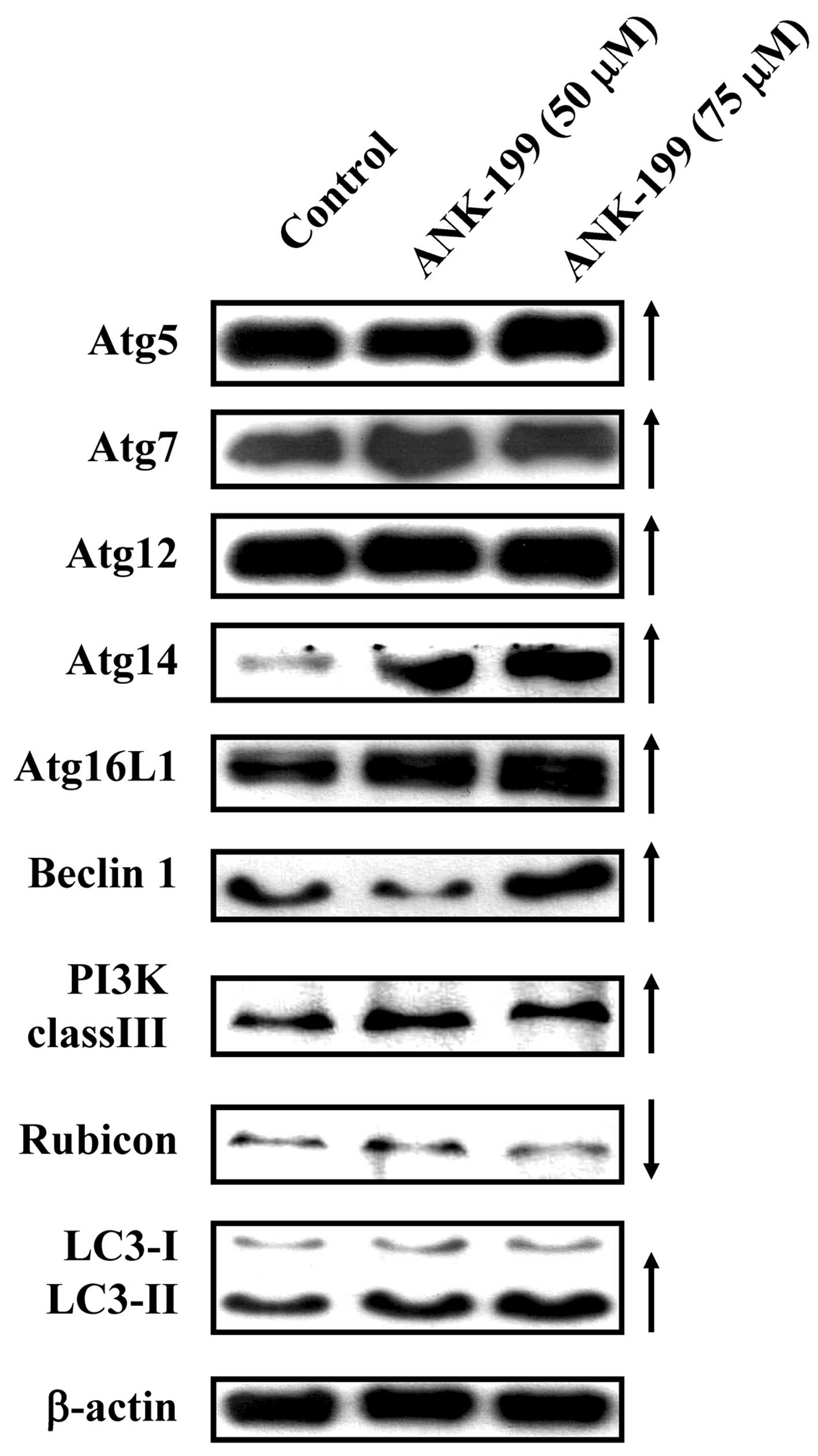
The protein level of autophagy marker proteins, like
Atg complex (Atg5, Atg7, Atg12, Atg14 and Atg16L1), beclin 1, PI3K
class III, rubicon and LC3, was also investigated in
ANK-199-treated CAR cells. As shown in Fig. 5, ANK-199 at 50 and 75 μM increased
the protein levels of Atg5, Atg7, Atg12, Atg14 and Atg16L1, beclin
1, PI3K class III and LC3, but decreased the protein level of
rubicon in CAR cells. Our results imply that ANK-199 induced
autophagic cell death in CAR cells through interfering with the
kinase class III/beclin 1/Atg-associated signal pathway.
ANK-199 stimulates the
autophagy-associated mRNA levels in CAR cells
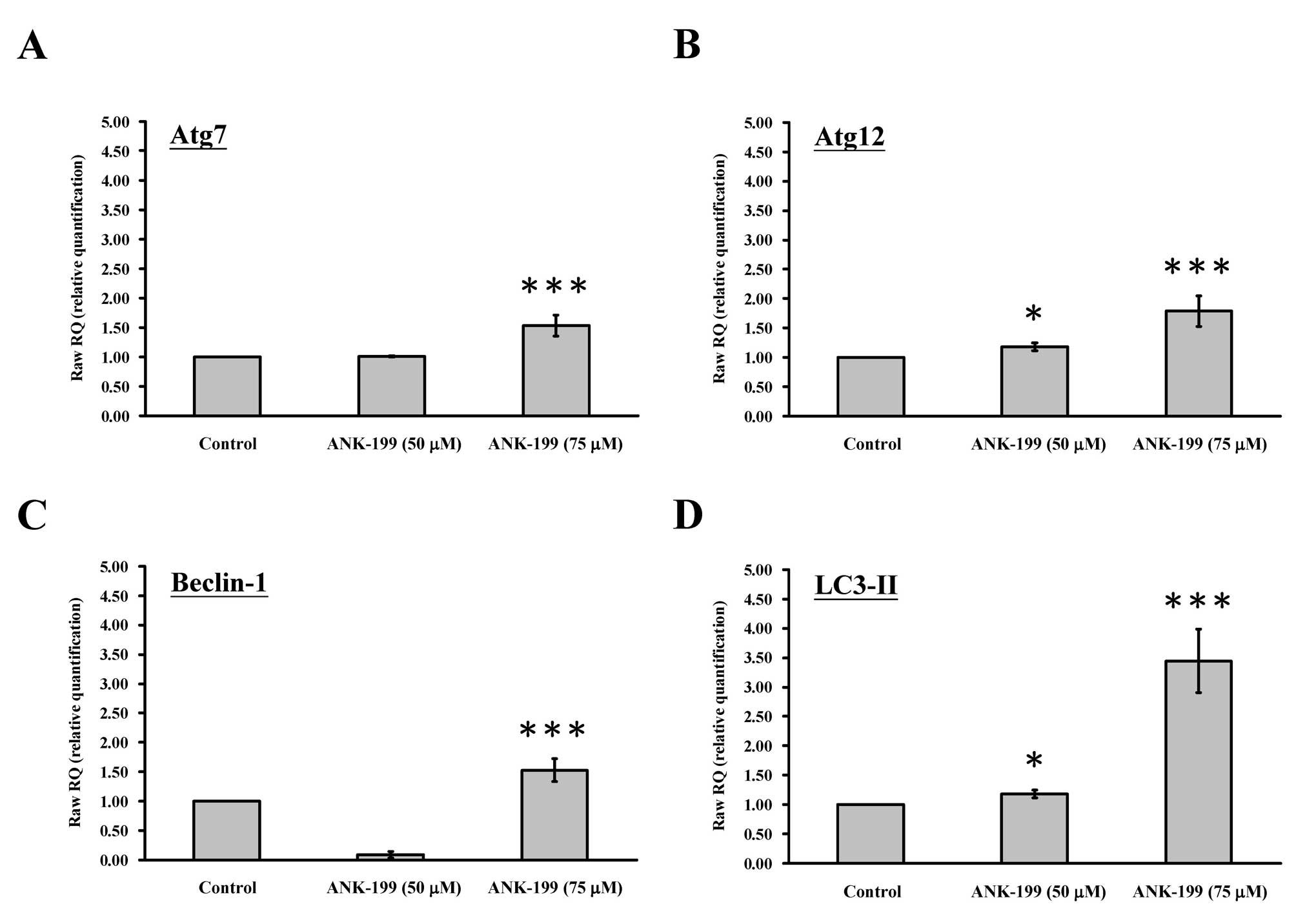
The mRNA level of autophagy-associated gene was also
investigated in ANK-199-treated CAR cells. As shown in Fig. 6, ANK-199 is able to enhance the
expression level of Atg7 gene (Fig. 6A), Atg12 gene (Fig. 6B), beclin 1 gene (Fig. 6C) and LC3-II gene (Fig. 6D) in CAR cells.
Protection effect of 3-MA against
autophagy in ANK-199- treated CAR cells
3-MA, an inhibitor of PI3K kinase class III, has
been shown to potently inhibit autophagy-dependent protein
degradation and suppress the formation of autophagosomes. CAR cells
were pretreated with 3-MA and then exposed to 50 or 75 μM of
ANK-199. The formation of autophagic vacuoles and cell viability
were then monitored under phase contrast microscopy. Our results
showed that 3-MA can inhibit the formation of autophagic vacuoles
(Fig. 7A) and enhance the
viability of ANK-199-treated CAR cells (Fig. 7B), suggesting that ANK-199-induced
autophagy in CAR cells is mediated through interference with the
PI3K kinase class III.
Microarray analysis
The cDNA microarray experiments were carried out to
examine the gene expression in ANK-199-treated CAR cells. The
transcripts of 26 genes were upregulated, while these of 96 genes
were downregulated in ANK-199-treated CAR cells (Table I). The important biological
processes and Gene to Go Molecular Function test regulated by
ANK-199 are listed in Table II
and Table III. The formation of
autophagosomes and autophagolysosome was observed during the course
of ANK-199 induced autophagic cell death. In a good agreement with
above results, membrane formation or reorganization is closely
associated with following biological processes: cellular component
biogenesis, actin cytoskeleton organization, regulation of actin
filament-based process, regulation of cytoskeleton organization,
regulation of actin polymerization or depolymerization, regulation
of actin filament length.
 | Table IThe genes with more than 1.5-fold
changes in mRNA levels in CAR cells after ANK-199 (50 μM) 24-h
treatment identified by DNA microarray. |
Table I
The genes with more than 1.5-fold
changes in mRNA levels in CAR cells after ANK-199 (50 μM) 24-h
treatment identified by DNA microarray.
| Accession | Gene | FC |
|---|
| XR_042379 | LOC401875:
hypothetical LOC401875 | 7.34 |
| NM_198581 | ZC3H6: zinc finger
CCCH-type containing 6 | 5.68 |
| NM_004755 | RPS6KA5: ribosomal
protein S6 kinase, 90 kDa, polypeptide 5 | 3.27 |
| NM_006472 | TXNIP: thioredoxin
interacting protein | 3.00 |
| NM_001506 | GPR32: G
protein-coupled receptor 32 | 2.15 |
| NM_018387 | STRBP: spermatid
perinuclear RNA binding protein | 2.04 |
| BC007928 | C21orf119:
chromosome 21 open reading frame 119 | 1.93 |
| BC108718 | LOC389765: similar
to KIF27C | 1.88 |
| NM_153335 | LYK5: protein
kinase LYK5 | 1.86 |
|
ENST00000021776 | CCT8L1: chaperonin
containing TCP1, subunit 8 (theta)-like 1 | 1.81 |
| NM_018434 | RNF130: ring finger
protein 130 | 1.75 |
| NM_181684 | KRTAP12-2: keratin
associated protein 12-2 | 1.72 |
| NM_201266 | NRP2: neuropilin
2 | 1.71 |
| NM_181351 | NCAM1: neural cell
adhesion molecule 1 | 1.63 |
| NM_004064 | CDKN1B:
cyclin-dependent kinase inhibitor 1B (p27, Kip1) | 1.63 |
| NM_007051 | FAF1: Fas (TNFRSF6)
associated factor 1 | 1.62 |
| NM_001039752 | SLC22A10: solute
carrier family 22, member 10 | 1.62 |
| NM_198993 | STAC2: SH3 and
cysteine rich domain 2 | 1.62 |
| NM_018257 | PCMTD2:
protein-L-isoaspartate (D-aspartate) O-methyltransferase domain
containing 2 | 1.62 |
| NM_000901 | NR3C2: nuclear
receptor subfamily 3, group C, member 2 | 1.62 |
| BC093665 | FAM92B: family with
sequence similarity 92, member B | 1.56 |
| NM_016609 | SLC22A17: solute
carrier family 22, member 17 | 1.55 |
| NM_181605 | KRTAP6-3: keratin
associated protein 6-3 | 1.55 |
| XM_938903 | LOC649839: similar
to large subunit ribosomal protein L36a | 1.53 |
| BC021739 | LOC554201:
hypothetical LOC554201 | 1.53 |
|
ENST00000329244 | LOC100132169:
similar to hCG1742852 | 1.50 |
| NM_021109 | TMSB4X: thymosin
beta 4, X-linked | −1.50 |
| NM_019896 | POLE4: polymerase
(DNA-directed), epsilon 4 (p12 subunit) | −1.51 |
| NM_015475 | FAM98A: family with
sequence similarity 98, member A | −1.51 |
| NM_006136 | CAPZA2: capping
protein (actin filament) muscle Z-line, alpha 2 | −1.51 |
| NM_001128619 | LUZP6: leucine
zipper protein 6 | −1.51 |
| NM_014248 | RBX1: ring-box
1 | −1.52 |
| NM_024755 | SLTM: SAFB-like,
transcription modulator | −1.53 |
| NM_003168 | SUPT4H1: suppressor
of Ty 4 homolog 1 (S. cerevisiae) | −1.53 |
| NM_017892 | PRPF40A: PRP40
pre-mRNA processing factor 40 homolog A (S. cerevisiae) | −1.53 |
| NM_004776 | B4GALT5:
UDP-Gal:betaGlcNAc beta 1,4-galactosyltransferase, polypeptide
5 | −1.53 |
| NM_002090 | CXCL3: chemokine
(C-X-C motif) ligand 3 | −1.53 |
| NM_001005333 | MAGED1: melanoma
antigen family D, 1 | −1.53 |
| NM_000937 | POLR2A: polymerase
(RNA) II (DNA directed) polypeptide A, 220 kDa | −1.54 |
| NM_001349 | DARS: aspartyl-tRNA
synthetase | −1.55 |
| NM_003348 | UBE2N:
ubiquitin-conjugating enzyme E2N (UBC13 homolog, yeast) | −1.56 |
| NM_001614 | ACTG1: actin, gamma
1 | −1.56 |
| NM_053024 | PFN2: profilin
2 | −1.56 |
| NM_003010 | MAP2K4:
mitogen-activated protein kinase kinase 4 | −1.57 |
| NM_001099771 | A26C1B:
ANKRD26-like family C, member 1B | −1.57 |
| NM_006000 | TUBA4A: tubulin,
alpha 4a | −1.58 |
| NM_133494 | NEK7: NIMA (never
in mitosis gene a)-related kinase 7 | −1.59 |
| NM_173647 | RNF149: ring finger
protein 149 | −1.59 |
| NM_182917 | EIF4G1: eukaryotic
translation initiation factor 4 gamma, 1 | −1.59 |
| NM_002599 | PDE2A:
phosphodiesterase 2A, cGMP-stimulated | −1.62 |
| NM_005066 | SFPQ: splicing
factor proline/glutamine-rich (polypyrimidine tract binding protein
associated) | −1.64 |
| NM_001039479 | KIAA0317:
KIAA0317 | −1.64 |
| NM_001127649 | PEX26: peroxisomal
biogenesis factor 26 | −1.64 |
| NM_015153 | PHF3: PHD finger
protein 3 | −1.64 |
| NM_007189 | ABCF2: ATP-binding
cassette, sub-family F (GCN20), member 2 | −1.64 |
| NM_007126 | VCP:
valosin-containing protein | −1.64 |
| NM_012234 | RYBP: RING1 and YY1
binding protein | −1.65 |
| NR_004845 | LOC644936:
cytoplasmic beta-actin pseudogene | −1.65 |
| NM_014795 | ZEB2: zinc finger
E-box binding homeobox 2 | −1.66 |
| NM_005998 | CCT3: chaperonin
containing TCP1, subunit 3 (gamma) | −1.67 |
| NM_001099692 | EIF5AL1: eukaryotic
translation initiation factor 5A-like 1 | −1.67 |
| NM_138689 | PPP1R14B: protein
phosphatase 1, regulatory (inhibitor) subunit 14B | −1.67 |
| NM_015665 | AAAS: achalasia,
adrenocortical insufficiency, alacrimia (Allgrove, triple-A) | −1.67 |
| NM_001099692 | EIF5AL1: eukaryotic
translation initiation factor 5A-like 1 | −1.68 |
| NM_001099692 | EIF5AL1: eukaryotic
translation initiation factor 5A-like 1 | −1.68 |
| NM_002154 | HSPA4: heat shock
70 kDa protein 4 | −1.68 |
| NM_013451 | FER1L3: fer-1-like
3, myoferlin (C. elegans) | −1.70 |
| NM_000303 | PMM2:
phosphomannomutase 2 | −1.71 |
| NM_002795 | PSMB3: proteasome
(prosome, macropain) subunit, beta type, 3 | −1.71 |
| NM_001363 | DKC1: dyskeratosis
congenita 1, dyskerin | −1.71 |
| NM_001102 | ACTN1: actinin,
alpha 1 | −1.73 |
| NM_004299 | ABCB7: ATP-binding
cassette, sub-family B (MDR/TAP), member 7 | −1.73 |
| NM_005857 | ZMPSTE24: zinc
metallopeptidase (STE24 homolog, S. cerevisiae) | −1.74 |
| NM_152265 | BTF3L4: basic
transcription factor 3-like 4 | −1.74 |
| NM_020409 | MRPL47:
mitochondrial ribosomal protein L47 | −1.74 |
| NM_006148 | LASP1: LIM and SH3
protein 1 | −1.76 |
| BC065192 | C2orf12: chromosome
2 open reading frame 12 | −1.76 |
| NM_001414 | EIF2B1: eukaryotic
translation initiation factor 2B, subunit 1 alpha, 26 kDa | −1.78 |
| NM_003222 | TFAP2C:
transcription factor AP-2 gamma (activating enhancer binding
protein 2 gamma) | −1.78 |
| NM_007350 | PHLDA1: pleckstrin
homology-like domain, family A, member 1 | −1.79 |
|
ENST00000242577 | DYNLL1: dynein,
light chain, LC8-type 1 | −1.79 |
| NM_032830 | CIRH1A: cirrhosis,
autosomal recessive 1A (cirhin) | −1.79 |
| NM_152265 | BTF3L4: basic
transcription factor 3-like 4 | −1.80 |
| NM_176816 | CCDC125:
coiled-coil domain containing 125 | −1.80 |
| NM_001039690 | CTF8: chromosome
transmission fidelity factor 8 homolog (S. cerevisiae) | −1.83 |
| NM_001127257 | SLC39A10: solute
carrier family 39 (zinc transporter), member 10 | −1.84 |
| XM_001716411 | LOC128322:
hypothetical LOC128322 | −1.86 |
| NM_002370 | MAGOH: mago-nashi
homolog, proliferation-associated (Drosophila) | −1.90 |
| NM_138578 | BCL2L1: BCL2-like
1 | −1.91 |
| NM_003580 | NSMAF: neutral
sphingomyelinase (N-SMase) activation associated factor | −1.92 |
| NM_015922 | NSDHL: NAD(P)
dependent steroid dehydrogenase-like | −1.92 |
| NM_001797 | CDH11: cadherin 11,
type 2, OB-cadherin (osteoblast) | −1.93 |
| NM_021242 | MID1IP1: MID1
interacting protein 1 [gastrulation specific G12 homolog
(zebrafish)] | −1.93 |
| NM_005968 | HNRNPM:
heterogeneous nuclear ribonucleoprotein M | −1.95 |
| NM_012338 | TSPAN12:
tetraspanin 12 | −1.97 |
| NM_014953 | DIS3: DIS3 mitotic
control homolog (S. cerevisiae) | −2.02 |
| NM_003130 | SRI: sorcin | −2.11 |
| NM_018993 | RIN2: Ras and Rab
interactor 2 | −2.12 |
| NM_004093 | EFNB2:
ephrin-B2 | −2.13 |
| NM_032256 | TMEM117:
transmembrane protein 117 | −2.14 |
| NM_005415 | SLC20A1: solute
carrier family 20 (phosphate transporter), member 1 | −2.15 |
| NM_017872 | THG1L:
tRNA-histidine guanylyltransferase 1-like (S. cerevisiae) | −2.20 |
| NM_014624 | S100A6: S100
calcium binding protein A6 | −2.24 |
| NM_153618 | SEMA6D: sema
domain, transmembrane domain (TM), and cytoplasmic domain,
(semaphorin) 6D | −2.27 |
| NM_014604 | TAX1BP3: Tax1
(human T-cell leukemia virus type I) binding protein 3 | −2.27 |
| NM_033505 | SELI: selenoprotein
I | −2.30 |
| NM_003666 | BLZF1: basic
leucine zipper nuclear factor 1 | −2.35 |
| NM_002714 | PPP1R10: protein
phosphatase 1, regulatory (inhibitor) subunit 10 | −2.50 |
| NM_002714 | PPP1R10: protein
phosphatase 1, regulatory (inhibitor) subunit 10 | −2.50 |
| NM_002714 | PPP1R10: protein
phosphatase 1, regulatory (inhibitor) subunit 10 | −2.50 |
| NR_003003 | SCARNA17: small
Cajal body-specific RNA 17 | −2.51 |
| NR_002738 | SNORD57: small
nucleolar RNA, C/D box 57 | −2.57 |
| NM_006080 | SEMA3A: sema
domain, immunoglobulin domain (Ig), short basic domain, secreted,
(semaphorin) 3A | −2.57 |
| NM_009587 | LGALS9: lectin,
galactoside-binding, soluble, 9 | −2.65 |
| NM_003234 | TFRC: transferrin
receptor (p90, CD71) | −2.85 |
| NM_138966 | NETO1: neuropilin
(NRP) and tolloid (TLL)-like 1 | −2.97 |
| NM_001098272 | HMGCS1:
3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 (soluble) | −2.97 |
| NM_006350 | FST:
follistatin | −4.21 |
| NM_024090 | ELOVL6: ELOVL
family member 6, elongation of long chain fatty acids(FEN1/Elo2,
SUR4/Elo3-like, yeast) | −4.46 |
| NM_005328 | HAS2: hyaluronan
synthase 2 | −5.00 |
| NM_001753 | CAV1: caveolin 1,
caveolae protein, 22 kDa | −5.04 |
| NM_033439 | IL33: interleukin
33 | −5.96 |
 | Table IIGene to GO Biological Process test
for over-representation (ANK-199 to control). |
Table II
Gene to GO Biological Process test
for over-representation (ANK-199 to control).
| Term | Count | % | p-value |
|---|
| GO:0044087 -
regulation of cellular component biogenesis | 9 | 6.382979 | 8.59E-06 |
| GO:0030036 - actin
cytoskeleton organization | 10 | 7.092199 | 3.75E-05 |
| GO:0032956 -
regulation of actin cytoskeleton organization | 7 | 4.964539 | 4.43E-05 |
| GO:0043254 -
regulation of protein complex assembly | 7 | 4.964539 | 4.72E-05 |
| GO:0032970 -
regulation of actin filament-based process | 7 | 4.964539 | 5.34E-05 |
| GO:0051493 -
regulation of cytoskeleton organization | 8 | 5.673759 | 5.75E-05 |
| GO:0030029 - actin
filament-based process | 10 | 7.092199 | 6.17E-05 |
| GO:0007010 -
cytoskeleton organization | 13 | 9.219858 | 7.13E-05 |
| GO:0008064 -
regulation of actin polymerization or depolymerization | 6 | 4.255319 | 7.77E-05 |
| GO:0030832 -
regulation of actin filament length | 6 | 4.255319 | 9.07E-05 |
 | Table IIIGene to GO Molecular Function test
for over-representation (ANK-199 to control). |
Table III
Gene to GO Molecular Function test
for over-representation (ANK-199 to control).
| Term | Count | % | p-value |
|---|
| GO:0003723 - RNA
binding | 21 | 14.89362 | 1.54E-07 |
| GO:0000166 -
nucleotide binding | 33 | 23.40426 | 6.28E-05 |
| GO:0008092 -
cytoskeletal protein binding | 11 | 7.801418 | 0.00346 |
| GO:0032553 -
ribonucleotide binding | 24 | 17.02128 | 0.005183 |
| GO:0032555 - purine
ribonucleotide binding | 24 | 17.02128 | 0.005183 |
| GO:0017076 - purine
nucleotide binding | 24 | 17.02128 | 0.008794 |
| GO:0003779 - actin
binding | 8 | 5.673759 | 0.009366 |
| GO:0019900 - kinase
binding | 6 | 4.255319 | 0.009636 |
| GO:0047485 -
protein N-terminus binding | 4 | 2.836879 | 0.016454 |
| GO:0003924 - GTPase
activity | 6 | 4.255319 | 0.018492 |
Discussion
Apoptosis, autophagy and necrosis are three major
routes that lead to cell death (51). Both apoptosis and autophagy belong
to the form of cell programmed death, but necrosis does not
(51–54). Autophagic death can promote cell
survival or cell death when cells experience stress, such as
damage, nutrient starvation, aging and pathogen infection (55–57).
Indeed, induction of autophagic death for cancer cells is thought
to be one of the best strategies in chemotherapy (58–60).
Not only many autophagy-related proteins (Atgs) are involved in
this process, but also a specific morphological and biochemical
modification can be observed (56,60,61).
Autophagy is first triggered by membrane nucleation, which is
mediated by phosphatidylinositol 3-kinase (PI3K) class III, beclin
1 (the mammalian ortholog of yeast ATG6), rubicon and Atg14
(62–64). The cytoplasm and phagophore of
various organelles are then sequestered by a membrane to form an
autophagosomes. Atg16L1-Atg12-Atg7-Atg5 complex and
microtubule-associated protein 1 light chain 3 type II (LC3-II)
(membrane-bound form) are absolutely required for autophagosome
formation (55,65–67).
The autophagosome fuses with the lysosome then forming
autophagolysosome, eventually resulting in the degradation of the
captured proteins or organelles by lysosomal enzymes (55,68,69).
Once cells undergo autophagic cell death, an autophagosomal marker
LC3-II increases from the conversion of LC3-I (70,71).
Our results demonstrated that the ANK-199 can induce
the formation of autophagic vesicle (Fig. 4A) and acidic vesicular organelles
(Fig. 4B). It also simultaneously
enhances the protein level of autophagic proteins, Atg complex,
beclin 1, PI3K class III and LC3-II (Fig. 5), and mRNA expression of autophagic
genes Atg7, Atg12, beclin 1 and LC3-II
(Fig. 6). More importantly, 3-MA,
a specific inhibitor of PI3K kinase class III, can inhibit the
autophagic vesicle formation induced by ANK-199 (Fig. 7). All of the above results support
that ANK-199-induced cell death in CAR cells is mediated through
the induction of autophagic death. This molecular signaling pathway
induced by ANK-199 is summarized in Fig. 8.
However, ANK-199 treatment duration for CAR cells is
72 h. We cannot completely rule out the possibility that apoptotic
cell death or other signaling pathways can be induced by ANK-199
for longer treatment time. A series of pterostilbene derivatives
have been synthesized as less toxic anticancer candidates (30,40,72,73).
ANK-199 is less toxic than pterostilbene (unpublished data). More
importantly, ANK-199 has much less cytotoxicity in the normal oral
cells (HGF and OK) than that in CAR cells (Fig. 3). This is a novel finding regarding
that ANK-199 represents a promising candidate as an anti-oral
cancer drug with low toxicity to normal cells. Results presented in
this study show that ANK-199 may become a novel therapeutic reagent
for the treatment of oral cancer in the near future (patent
pending).
Acknowledgements
This study was supported by AnnCare Bio-Tech Center
Inc. and a research grant from the Ministry of Health and Welfare,
Executive Yuan, Taiwan (DOH101-TD-C-111-005) awarded to S.-C.K.
References
|
1
|
Estrela JM, Ortega A, Mena S, Rodriguez ML
and Asensi M: Pterostilbene: Biomedical applications. Crit Rev Clin
Lab Sci. 50:65–78. 2013. View Article : Google Scholar
|
|
2
|
McCormack D and McFadden D: A review of
pterostilbene antioxidant activity and disease modification. Oxid
Med Cell Longev. 2013:5754822013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cherniack EP: A berry thought-provoking
idea: the potential role of plant polyphenols in the treatment of
age-related cognitive disorders. Br J Nutr. 108:794–800. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCormack D and McFadden D: Pterostilbene
and cancer: current review. J Surg Res. 173:e53–e61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mikstacka R and Ignatowicz E:
Chemopreventive and chemotherapeutic effect of trans-resveratrol
and its analogues in cancer. Pol Merkur Lekarski. 28:496–500.
2010.(In Polish).
|
|
6
|
Rimando AM and Suh N:
Biological/chemopreventive activity of stilbenes and their effect
on colon cancer. Planta Med. 74:1635–1643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li N, Ma Z, Li M, Xing Y and Hou Y:
Natural potential therapeutic agents of neurodegenerative diseases
from the traditional herbal medicine Chinese Dragons Blood. J
Ethnopharmacol. 152:508–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paul S, Rimando AM, Lee HJ, Ji Y, Reddy BS
and Suh N: Anti-inflammatory action of pterostilbene is mediated
through the p38 mitogen-activated protein kinase pathway in colon
cancer cells. Cancer Prev Res (Phila). 2:650–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan MH, Chang YH, Tsai ML, et al:
Pterostilbene suppressed lipopolysaccharide-induced up-expression
of iNOS and COX-2 in murine macrophages. J Agric Food Chem.
56:7502–7509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perecko T, Drabikova K, Rackova L, et al:
Molecular targets of the natural antioxidant pterostilbene: effect
on protein kinase C, caspase-3 and apoptosis in human neutrophils
in vitro. Neuro Endocrinol Lett. 31(Suppl 2): 84–90.
2010.PubMed/NCBI
|
|
11
|
Perecko T, Jancinova V, Drabikova K, Nosal
R and Harmatha J: Structure-efficiency relationship in derivatives
of stilbene. Comparison of resveratrol, pinosylvin and
pterostilbene. Neuro Endocrinol Lett. 29:802–805. 2008.PubMed/NCBI
|
|
12
|
Rossi M, Caruso F, Antonioletti R, et al:
Scavenging of hydroxyl radical by resveratrol and related natural
stilbenes after hydrogen peroxide attack on DNA. Chem Biol
Interact. 206:175–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Acharya JD and Ghaskadbi SS: Protective
effect of pterostilbene against free radical mediated oxidative
damage. BMC Complement Altern Med. 13:2382013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rimando AM, Cuendet M, Desmarchelier C,
Mehta RG, Pezzuto JM and Duke SO: Cancer chemopreventive and
antioxidant activities of pterostilbene, a naturally occurring
analogue of resveratrol. J Agric Food Chem. 50:3453–3457. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan MH, Chang YH, Badmaev V, Nagabhushanam
K and Ho CT: Pterostilbene induces apoptosis and cell cycle arrest
in human gastric carcinoma cells. J Agric Food Chem. 55:7777–7785.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrer P, Asensi M, Priego S, et al:
Nitric oxide mediates natural polyphenol-induced Bcl-2
down-regulation and activation of cell death in metastatic B16
melanoma. J Biol Chem. 282:2880–2890. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siedlecka-Kroplewska K, Jozwik A,
Boguslawski W, et al: Pterostilbene induces accumulation of
autophagic vacuoles followed by cell death in HL60 human leukemia
cells. J Physiol Pharmacol. 64:545–556. 2013.PubMed/NCBI
|
|
18
|
Hsieh MJ, Lin CW, Yang SF, et al: A
combination of pterostilbene with autophagy inhibitors exerts
efficient apoptotic characteristics in both chemosensitive and
chemoresistant lung cancer cells. Toxicol Sci. 137:65–75. 2014.
View Article : Google Scholar
|
|
19
|
Kapoor S: Pterostilbene and its emerging
antineoplastic effects: a prospective treatment option for systemic
malignancies. Am J Surg. 205:4832013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tippani R, Prakhya LJ, Porika M, Sirisha
K, Abbagani S and Thammidala C: Pterostilbene as a potential novel
telomerase inhibitor: Molecular docking studies and its in vitro
evaluation. Curr Pharm Biotechnol. Jan 12–2014.(Epub ahead of
print).
|
|
21
|
Li K, Dias SJ, Rimando AM, et al:
Pterostilbene acts through metastasis-associated protein 1 to
inhibit tumor growth, progression and metastasis in prostate
cancer. PLoS One. 8:e575422013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCormack DE, Mannal P, McDonald D, Tighe
S, Hanson J and McFadden D: Genomic analysis of pterostilbene
predicts its antiproliferative effects against pancreatic cancer in
vitro and in vivo. J Gastrointest Surg. 16:1136–1143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mannal PW, Alosi JA, Schneider JG,
McDonald DE and McFadden DW: Pterostilbene inhibits pancreatic
cancer in vitro. J Gastrointest Surg. 14:873–879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moon D, McCormack D, McDonald D and
McFadden D: Pterostilbene induces mitochondrially derived apoptosis
in breast cancer cells in vitro. J Surg Res. 180:208–215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan MH, Lin YT, Lin CL, Wei CS, Ho CT and
Chen WJ: Suppression of heregulin-beta1/HER2-modulated invasive and
aggressive phenotype of breast carcinoma by pterostilbene via
inhibition of matrix metalloproteinase-9, p38 kinase cascade and
Akt activation. Evid Based Complement Alternat Med.
2011:5621872011.PubMed/NCBI
|
|
26
|
Yang Y, Yan X, Duan W, et al:
Pterostilbene exerts antitumor activity via the Notch1 signaling
pathway in human lung adenocarcinoma cells. PLoS One. 8:e626522013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Wang L, Wu Y, et al: Pterostilbene
exerts antitumor activity against human osteosarcoma cells by
inhibiting the JAK2/STAT3 signaling pathway. Toxicology.
304:120–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin VC, Tsai YC, Lin JN, et al: Activation
of AMPK by pterostilbene suppresses lipogenesis and cell-cycle
progression in p53 positive and negative human prostate cancer
cells. J Agric Food Chem. 60:6399–6407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chakraborty A, Gupta N, Ghosh K and Roy P:
In vitro evaluation of the cytotoxic, anti-proliferative and
anti-oxidant properties of pterostilbene isolated from
Pterocarpus marsupium. Toxicol In Vitro. 24:1215–1228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roslie H, Chan KM, Rajab NF, et al:
3,5-Dibenzyloxy-4′-hydroxystilbene induces early caspase-9
activation during apoptosis in human K562 chronic myelogenous
leukemia cells. J Toxicol Sci. 37:13–21. 2012.PubMed/NCBI
|
|
31
|
Tolomeo M, Grimaudo S, Di Cristina A, et
al: Pterostilbene and 3′-hydroxypterostilbene are effective
apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia
cells. Int J Biochem Cell Biol. 37:1709–1726. 2005.
|
|
32
|
Mena S, Rodriguez ML, Ponsoda X, Estrela
JM, Jaattela M and Ortega AL: Pterostilbene-induced tumor
cytotoxicity: a lysosomal membrane permeabilization-dependent
mechanism. PLoS One. 7:e445242012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harun Z and Ghazali AR: Potential
chemoprevention activity of pterostilbene by enhancing the
detoxifying enzymes in the HT-29 cell line. Asian Pac J Cancer
Prev. 13:6403–6407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nutakul W, Sobers HS, Qiu P, et al:
Inhibitory effects of resveratrol and pterostilbene on human colon
cancer cells: a side-by-side comparison. J Agric Food Chem.
59:10964–10970. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang CS, Ho CT, Tu SH, et al: Long-term
ethanol exposure-induced hepatocellular carcinoma cell migration
and invasion through lysyl oxidase activation are attenuated by
combined treatment with pterostilbene and curcumin analogues. J
Agric Food Chem. 61:4326–4335. 2013. View Article : Google Scholar
|
|
36
|
Pan MH, Chiou YS, Chen WJ, Wang JM,
Badmaev V and Ho CT: Pterostilbene inhibited tumor invasion via
suppressing multiple signal transduction pathways in human
hepatocellular carcinoma cells. Carcinogenesis. 30:1234–1242. 2009.
View Article : Google Scholar
|
|
37
|
Siedlecka-Kroplewska K, Jozwik A,
Kaszubowska L, Kowalczyk A and Boguslawski W: Pterostilbene induces
cell cycle arrest and apoptosis in MOLT4 human leukemia cells.
Folia Histochem Cytobiol. 50:574–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen RJ, Tsai SJ, Ho CT, et al:
Chemopreventive effects of pterostilbene on urethane-induced lung
carcinogenesis in mice via the inhibition of EGFR-mediated pathways
and the induction of apoptosis and autophagy. J Agric Food Chem.
60:11533–11541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chakraborty A, Bodipati N, Demonacos MK,
Peddinti R, Ghosh K and Roy P: Long term induction by pterostilbene
results in autophagy and cellular differentiation in MCF-7 cells
via ROS dependent pathway. Mol Cell Endocrinol. 355:25–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen RJ, Ho CT and Wang YJ: Pterostilbene
induces autophagy and apoptosis in sensitive and chemoresistant
human bladder cancer cells. Mol Nutr Food Res. 54:1819–1832. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang L, Cui L, Zhou G, Jing H, Guo Y and
Sun W: Pterostilbene, a natural small-molecular compound, promotes
cytoprotective macroautophagy in vascular endothelial cells. J Nutr
Biochem. 24:903–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Riche DM, McEwen CL, Riche KD, et al:
Analysis of safety from a human clinical trial with pterostilbene.
J Toxicol. 2013:4635952013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shetty SR, Babu S, Kumari S, Prasad R,
Bhat S and Fazil KA: Salivary ascorbic acid levels in betel quid
chewers: A biochemical study. South Asian J Cancer. 2:142–144.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Arjungi KN: Areca nut: a review.
Arzneimittelforschung. 26:951–956. 1976.PubMed/NCBI
|
|
45
|
Chang PY, Peng SF, Lee CY, et al:
Curcumin-loaded nanoparticles induce apoptotic cell death through
regulation of the function of MDR1 and reactive oxygen species in
cisplatin-resistant CAR human oral cancer cells. Int J Oncol.
43:1141–1150. 2013.
|
|
46
|
Lin C, Tsai SC, Tseng MT, et al: AKT
serine/threonine protein kinase modulates baicalin-triggered
autophagy in human bladder cancer T24 cells. Int J Oncol.
42:993–1000. 2013.PubMed/NCBI
|
|
47
|
Tsai SC, Yang JS, Peng SF, et al: Bufalin
increases sensitivity to AKT/mTOR-induced autophagic cell death in
SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol.
41:1431–1442. 2012.PubMed/NCBI
|
|
48
|
Huang WW, Tsai SC, Peng SF, et al:
Kaempferol induces autophagy through AMPK and AKT signaling
molecules and causes G2/M arrest via downregulation of CDK1/cyclin
B in SK-HEP-1 human hepatic cancer cells. Int J Oncol.
42:2069–2077. 2013.PubMed/NCBI
|
|
49
|
Huang AC, Lien JC, Lin MW, et al:
Tetrandrine induces cell death in SAS human oral cancer cells
through caspase activation-dependent apoptosis and LC3-I and LC3-II
activation-dependent autophagy. Int J Oncol. 43:485–494. 2013.
|
|
50
|
Liu CY, Yang JS, Huang SM, et al: Smh-3
induces G(2)/M arrest and apoptosis through calcium-mediated
endoplasmic reticulum stress and mitochondrial signaling in human
hepatocellular carcinoma Hep3B cells. Oncol Rep. 29:751–762.
2013.
|
|
51
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
52
|
Jin S and White E: Role of autophagy in
cancer: management of metabolic stress. Autophagy. 3:28–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fink SL and Cookson BT: Apoptosis,
pyroptosis, and necrosis: mechanistic description of dead and dying
eukaryotic cells. Infect Immun. 73:1907–1916. 2005. View Article : Google Scholar
|
|
54
|
Lockshin RA and Zakeri Z: Apoptosis,
autophagy, and more. Int J Biochem Cell Biol. 36:2405–2419. 2004.
View Article : Google Scholar
|
|
55
|
Shimizu S, Yoshida T, Tsujioka M and
Arakawa S: Autophagic cell death and cancer. Int J Mol Sci.
15:3145–3153. 2014. View Article : Google Scholar
|
|
56
|
Mukhopadhyay S, Panda PK, Sinha N, Das DN
and Bhutia SK: Autophagy and apoptosis: where do they meet?
Apoptosis. 19:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ma Y, Galluzzi L, Zitvogel L and Kroemer
G: Autophagy and cellular immune responses. Immunity. 39:211–227.
2013. View Article : Google Scholar
|
|
58
|
Morselli E, Galluzzi L, Kepp O, et al:
Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta.
1793:1524–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li LC, Liu GD, Zhang XJ and Li YB:
Autophagy, a novel target for chemotherapeutic intervention of
thyroid cancer. Cancer Chemother Pharmacol. 73:439–449. 2014.
View Article : Google Scholar
|
|
60
|
Meschini S, Condello M, Lista P and
Arancia G: Autophagy: Molecular mechanisms and their implications
for anticancer therapies. Curr Cancer Drug Targets. 11:357–379.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu B, Bao JK, Yang JM and Cheng Y:
Targeting autophagic pathways for cancer drug discovery. Chin J
Cancer. 32:113–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ghavami S, Shojaei S, Yeganeh B, et al:
Autophagy and apoptosis dysfunction in neurodegenerative disorders.
Prog Neurobiol. 112:24–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fu LL, Cheng Y and Liu B: Beclin-1:
autophagic regulator and therapeutic target in cancer. Int J
Biochem Cell Biol. 45:921–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Parkes M: Evidence from genetics for a
role of autophagy and innate immunity in IBD pathogenesis. Dig Dis.
30:330–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Martinez-Lopez N and Singh R: ATGs:
Scaffolds for MAPK/ERK signaling. Autophagy. 10:535–537. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tam BT and Siu PM: Autophagic cellular
responses to physical exercise in skeletal muscle. Sports Med.
44:625–640. 2014. View Article : Google Scholar
|
|
68
|
Jiang P and Mizushima N: Autophagy and
human diseases. Cell Res. 24:69–79. 2014. View Article : Google Scholar
|
|
69
|
Pottier M, Masclaux-Daubresse C, Yoshimoto
K and Thomine S: Autophagy as a possible mechanism for
micronutrient remobilization from leaves to seeds. Front Plant Sci.
5:112014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Schmeisser H, Bekisz J and Zoon KC: New
function of type I IFN: Induction of autophagy. J Interferon
Cytokine Res. 34:71–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang W, Sviripa V, Kril LM, et al:
Fluorinated N,N-dialkylaminostilbenes for Wnt pathway inhibition
and colon cancer repression. J Med Chem. 54:1288–1297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fuendjiep V, Wandji J, Tillequin F, et al:
Chalconoid and stilbenoid glycosides from Guibourtia
tessmanii. Phytochemistry. 60:803–806. 2002. View Article : Google Scholar : PubMed/NCBI
|