Introduction
Cisplatin-based combination chemotherapy (i.e.,
M-VAC or G/C) in a mainstay treatment for metastatic bladder cancer
showing a 50–70% response rate and a 15–20% improvement in
survival. However, most patients have a recurrence within the first
year and in the recurrent cases, additional cisplatin combination
chemotherapy provides limited benefit (1,2).
Thus, over the past three decades there have been numerous trials
to overcome cisplatin resistance in bladder cancer.
Advances in the understanding of tumor biology have
established the critical role of targeted therapy as first- or
second-line treatment options for various malignant diseases
including genitourinary tumors. In addition, a growing body of data
suggests that the combination of targeted agents is a promising
strategy to enhance the antitumor effect of conventional
chemotherapies (3–5). PI3K (phosphatidylinositol
3-kinase)/Akt (protein kinase B)/mTOR (mammalian target of
rapamycin) signaling axis is a major survival pathway and its
abnormal activation is frequently involved in the development and
progression of various tumors including invasive bladder cancers
(6–9). Moreover, several studies have
observed a synergistic antitumor effect between PI3K or mTOR
inhibitors and conventional chemotherapy agents such as cisplatin
in chemo-naïve or resistant cancers like melanoma, ovarian, and
nasopharyngeal cancer (10–12).
These findings provide a rationale for targeting PI3K/Akt/mTOR
survival pathway for the treatment of patients with advanced
bladder cancer, especially after failure of first-line chemotherapy
such as cisplatin-based regimens. Based on these ideas, a few
studies have assessed and demonstrated the antitumor effect of mTOR
inhibitors for bladder cancers in pre-clinical or clinical settings
(13–15). However, no study has tested in
detail the synergistic effect between cisplatin and mTOR inhibitors
in human bladder cancers, especially for cisplatin-resistant tumors
(16,17).
We report that the PI3K/mTOR dual inhibitor NVP-
BEZ235, an orally bioavailable imidazoquinoline derivative
synergistically potentiates the antitumor effect of cisplatin in
bladder cancer cells.
Materials and methods
Cell lines and chemicals
Bladder cancer cell lines (J82, SW1710, T24, HTB5,
HTB9, UMUC14 and 253J) were maintained in MEM, DMEM, and RPMI-1640
supplemented with 10% fetal bovine serum (Mediatech, Herndon, VA,
USA) and 100 U/ml penicillin/100 mg/l streptomycin (Gibco BRL,
Grand Island, NY, USA) with 5% CO2 at 37°C. UMUC14 was
donated by Professor E.S. Lee (Seoul National University, Seoul,
Korea) and all other cells lines obtained from ATCC (American Type
Culture Collection, Manassas, VA, USA) or KCLB (Korean Cell Line
Bank, Seoul, Korea). The cisplatin-resistant T24R2 cell line was
established by serial desensitization of T24 cells. NVP-BEZ235 was
kindly provided by Norvatis Pharmaceuticals Inc. (Basel,
Switzerland). Everolimus and temsirolimus were purchased from LC
laboratories (Woburn, MA, USA). Cisplatin was obtained from Pfizer
(Pfizer Korea Ltd., Seoul, Korea). NVP-BEZ235 compound was
dissolved in 100% DMSO at 85°C to prepare 10 mM stock solution and
kept at 4°C before use.
Cytotoxicity assay
Cells were treated with cisplatin (0.039–40.0
μg/ml), NVP-BEZ235 (0.19 nM-20.0 μM), temsirolimus (0.313–10.0 μM)
or, everolimus (0.313–10.0 μM) and the antitumor effect was
determined by CCK-8 assay (Cell counting kit-8; Dojindo Molecular
Technologies, Gaithersburg, MD, USA) according to the
manufacturer’s instructions.
Determination of synergism
The synergy between drugs was determined by
combination index (CI) in which CI values <1.0, >1.0, and 1.0
indicated synergism, antagonism, and additivity, respectively
(18).
Data were also analyzed using the MacSynergy II
software program at 95% confidence limits. The degree of
interaction is expressed for data represented as percentages in
which 0–25, 25–50, 50–100 and >100 μM/ml2% calculated
values in either a positive (synergy) or negative (antagonism)
direction are defined as insignificant, minor, moderate and strong
interaction respectively (19–21).
Clonogenic assay
T24R2 cells (2×102) were plated in a
6-well culture plate and treated with NVP-BEZ235 (0.5 μM) and/or
cisplatin (0.5 μg/ml) for 48 h with 5% CO2 at 37°C.
After washing with PBS, cells were maintained for another 10 days
and visualized by 0.4% crystal violet staining.
Flow cytometric analysis of cell cycle
and apoptosis
T24R2 cells were treated with NVP-BEZ235 (0.5 μM)
and/or cisplatin (0.5 μg/ml) for 48 h, fixed in 70% ethanol, and
stained with a propidium iodide solution [970 μl PBS and 40 μl of 1
mg/ml propidium iodide (Sigma)] and 3 μl of RNase A (Sigma).
Alteration in the cell cycle was determined by FACSCalibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA).
Induction of apoptosis by combination treatment was
also assessed using Annexin V-FITC apoptosis detection kit (BP
Pharmingen, San Jose, CA, USA) according to the manufacturer’s
instructions. Triplicate study results (≥5,000–10,000 counts per
each study) were used for the quantitative analysis.
Hoechst 33342 nuclear stating
T24R2 cells were exposed to NVP-BEZ235 (0.5 μM)
and/or cisplatin (0.5 μg/ml) for 48 h and fixed with 4%
paraformaldehyde before staining with 0.5 ml of Hoechst 33342 (10
μg/ml; Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C in the
dark.
Analysis of apoptosis-, cell cycle- and
survival-related protein expression
After 48 h of treatment with NVP-BEZ235 (0.5 μM)
and/or cisplatin (0.5 μg/ml), protein was extracted from T24R2
cells using RIPA buffer, fractionated by SDS-PAGE, transferred to
PVDF membrane, and incubated with the corresponding primary
antibodies [cyclin A, cyclin B1, cyclin D1, CDC2C, p-CDC2C (Tyr15),
CDC25C, p-CDC25C (Ser216), pRb (Ser807/811), cleaved cleaved
caspase (-3, -8 and -9), cleaved PARP, cIAP1, cIAP2, XIAP,
survivin, p-IKKα (Ser176/180), IKKα, p-IκBα (ser32), IκBα, NF-κB,
p-Akt (ser473), Akt, p-PI3K (Tyr199/458), PI3K, p-mTOR (Ser2448),
mTOR, p-p70S6K (Thr389), p70S6K, p-GSK-3β (Ser9), GSK-3β, p-4E-BP1
(Thr37/46), 4E-BP1, p-MEK1/2 (Ser217/221), MEK1/2, p-ERK1/2
(Thr202/Tyr204), and ERK1/2; Cell Signaling Technology, Danvers,
MA, USA; Bcl-2, Bax and Bad; Santa Cruz Biotechnology, Santa Cruz,
CA, USA] and secondary antibodies before signal detection with an
enhanced chemiluminescence Western blot substrate kit (Pierce,
Rockford, IL, USA).
Statistical analysis
All statistical analyses were carried out using the
SPSS 14.0K software (SPSS Inc., Chicago, IL, USA). Unless indicated
otherwise, the data sets consist of at least three biological
replicates, and the data are expressed as the mean ± SD.
Statistical significance was determined by a two-sample t-test, and
null hypotheses of no difference were rejected if p-values were
<0.05.
Results
Antitumor effect of NVP-BEZ235 in bladder
cancer cells
After 72 h of exposure, T24R2 showed virtually no
response to cisplatin treatment up to 5.0 μg/ml concentration,
while the proliferations of all other bladder cancer cell lines
tested were nearly completely inhibited by cisplatin in the same
dose ranges (Fig. 1A). NVP-BEZ235
exerted a dose-dependent but varying antitumor effect on bladder
cancer cells. However, T24R2 required a significantly higher amount
of NVP-BEZ235 for the proliferation suppression to a similar level
as other bladder cancer cell lines including T24 (Fig. 1B).
Up to 48 h, NVP-BEZ235 exerted dose- and
time-dependent antitumor effect in both T24 and T24R2 cells.
However, when exposed for >72 h, both cells at least partly
recovered from NVP-BEZ235-induced suppression and regained their
proliferation to the similar level as 24- (T24R2) and 48-h (T24)
treatment, respectively (Fig.
2).
At equimolar concentrations NVP-BEZ235 exerted
relatively more potent antitumor effect compared with mTOR
inhibitors (temsirolimus, everolimus) against bladder and breast
cancer cells (Fig. 3).
Synergistic antitumor effect of
NVP-BEZ235 and cisplatin in bladder cancer cells
To test synergistic effect T24R2 cells were exposed
to increasing doses of NVP-BEZ235 alone or in a 1:1 fixed ratio
combination with cisplatin and antitumor effect was assessed by
CCK-8 assay (Fig. 4A). Correlation
coefficient values (r) ranged from 0.926 to 0.988 indicating data
conforming to median effect principle (Table I). The IC50s (Dm) of
NVP-BEZ235 was 37.47 μM, cisplatin was 23.89 μg/ml, and the
combination 6.63 μM (Table I). The
isobole and DRI (dose reduction index) analysis showed a CI<1
over a wide range of fractions affected (fa, 0.1–0.99) (Fig. 4B and Table II).
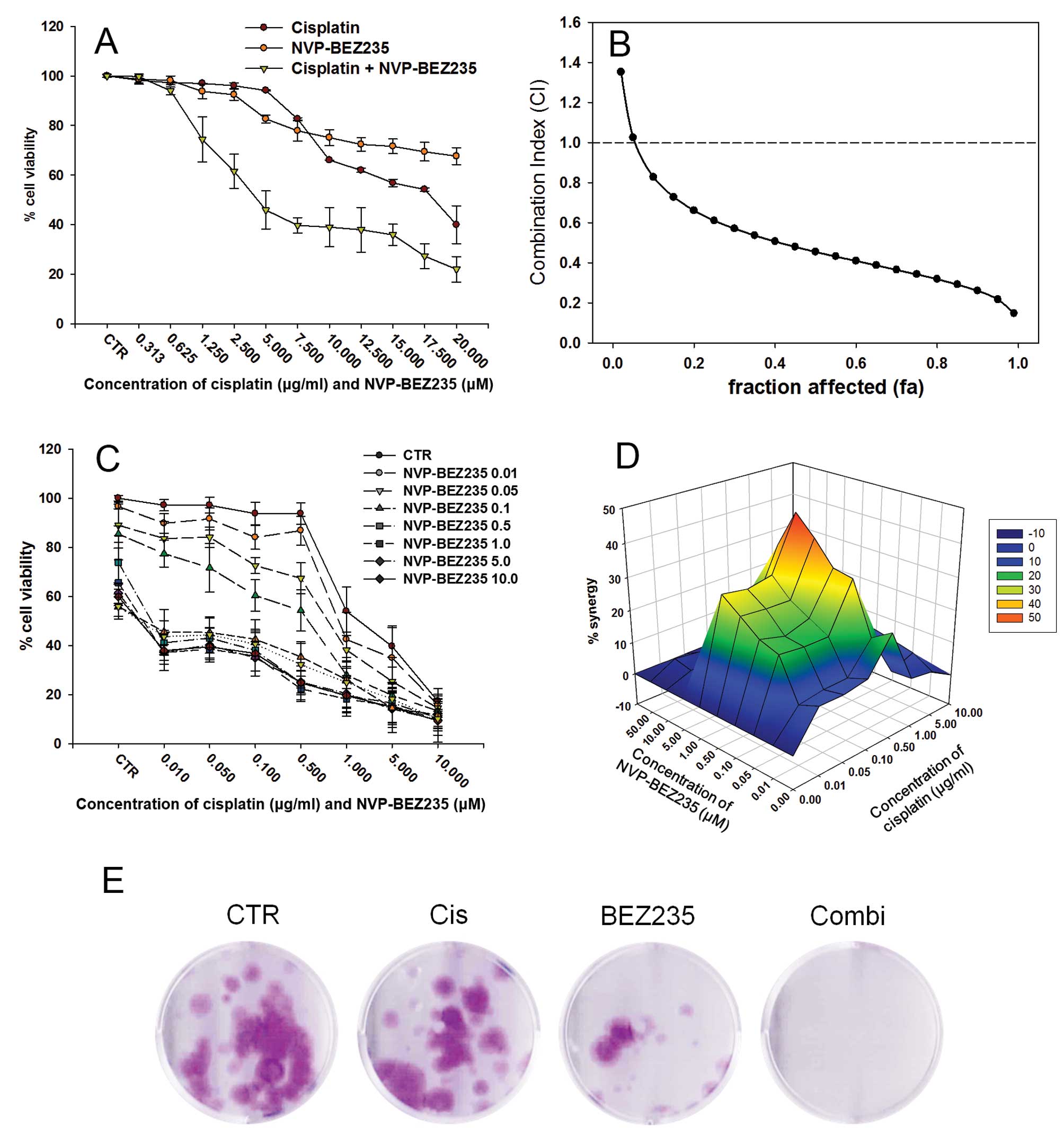 | Figure 4Synergistic antitumor effect between
NVP-BEZ235 and cisplatin in cisplatin-resistant bladder cancer
cells 72 h after treatment. Dose-response study (A) of 1:1 fixed
ratio combination of NVP-BEZ235 (0.313–20 μM) and cisplatin
(0.313–20 μg/ml) against T24R2 cells and (B) fa-CI plot in which fa
and CI indicate fraction affected, and combination index,
respectively. CI<1, CI=1, and CI>1 denote synergistic,
additive, and antagonistic interaction, respectively. Each data
point represents the mean ± SD of triplicate experiments. For more
detailed analysis of synergism, four independent combination
experiments of NVP-BEZ235 (0.01–10.0 μM) and cisplatin (0.01–10
μg/ml) were performed and the results were three-dimensionally
reconstructed by MacSynergy II data analysis program where peak,
depression, and horizontal plane indicate the interactions were
synergistic, antagonistic, and additive, respectively (C and D).
(E) Clonogenic assay of T24R2 cells exposed to NVP-BEZ235 (0.5 μM)
alone or in combination with cisplatin (0.5 μg/ml). CTR, Cis,
BEZ235 and Combi stand for untreated control, cisplatin single
treatment, NVP-BEZ235 single treatment and combination treatment
respectively. |
 | Table IDose-effect relationship parameters
of cisplatin, NVP-BEZ235 and combination treatment of T24R2 cells
in vitro. |
Table I
Dose-effect relationship parameters
of cisplatin, NVP-BEZ235 and combination treatment of T24R2 cells
in vitro.
| Compound | ma | Dmb | rc |
|---|
| Cisplatin | 1.076 | 23.895 | 0.941 |
| NVP-BEZ235 | 9.895 | 37.476 | 0.988 |
| Cisplatin +
NVP-BVEZ235 | 1.353 | 6.629 | 0.926 |
 | Table IIDose reduction index (DRI) for T24R2
cells. |
Table II
Dose reduction index (DRI) for T24R2
cells.
| faa | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 |
|---|
| Cisplatin | 2.4 | 2.8 | 3.1 | 3.3 | 3.6 | 3.9 | 4.2 | 4.7 | 5.5 |
| NVP-BEZ235 | 2.5 | 3.4 | 4.1 | 4.9 | 5.7 | 6.6 | 7.8 | 9.5 | 13.0 |
For a more detailed evaluation of synergy, four
independent combination experiments of NVP-BEZ235 (0.01–10.0 μM)
and cisplatin (0.01–10 μg/ml) were performed to generate synergy
plot using MacSynergy II data analysis program (Fig. 4C). There was strong synergy between
NVP-BEZ235 and cisplatin in T24R2 cells (synergy volume of 388.25
μM/ml2%) with minimal antagonism (-2.41
μM/ml2%) (Fig. 4D).
Clonogenic assay also showed synergistic interaction between
NVP-BEZ235 and cisplatin for T24R2 cells (Fig. 4E).
NVP-BEZ235 and cisplatin combination
induces S phase cell cycle arrest and apoptosis
Neither NVP-BEZ235 (0.5 μM) nor cisplatin (0.5
μg/ml) single-agent treatment caused any significant changes in the
cell cycle distribution, while the concomitant treatment resulted
in a marked increase of cells in the sub-G1 (3.6±0.6%) and S
(46.9±4.3%) phase compared with the untreated control (0.8±0.3% for
sub-G1, 5.16±1.1% for S phase), NVP-BEZ235 (0.84±0.3% for sub-G1,
12.6±2.1% for S phase), or cisplatin (0.61±0.3% for sub-G1,
5.49±0.1.3% for S) single-treated cells (Fig. 5).
Flow cytometric analysis after Annexin V-FITC/PI
double stating showed significantly increased population of Annexin
V-positive and PI-negative cells (in late apoptotic stage) in
concomitant treatment group (25.7±3.6%) compared with untreated
control (1.7±0.7%) and cisplatin (4.6±1.3%) or NVP-BEZ235
(4.4±3.6%) single treatment groups (p<0.05, Fig. 6).
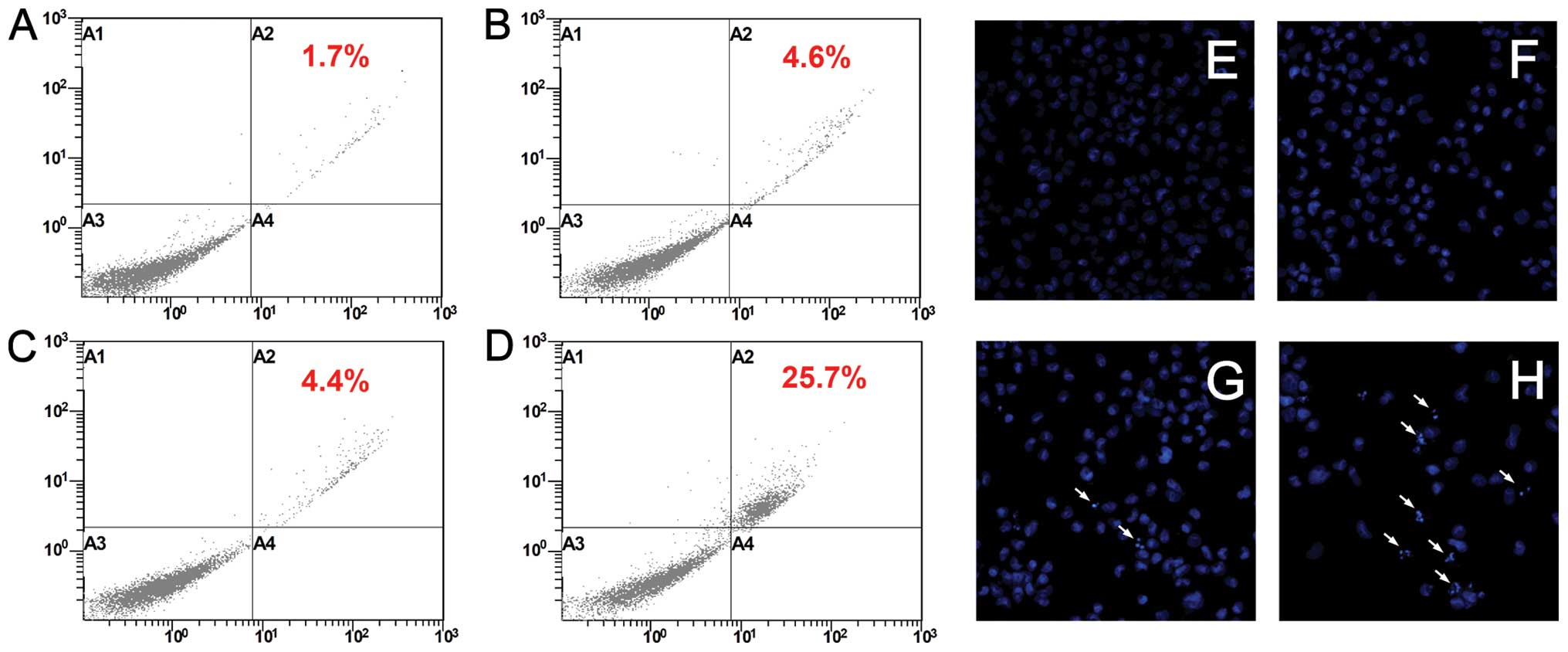 | Figure 6T24R2 cells were treated with
cisplatin [(B and F)/0.5 μg/ml], NVP-BEZ235 [(C and G)/0.5 μM)]
alone or in combination (D and H) for 72 h and induction of
apoptosis was assessed by Annexin V-FITC/PI flow cytometry (left
panel) in which A3, Annexin V−/PI−,
representing viable cells; A4, Annexin
V+/PI−, representing early apoptotic cells;
A2, Annexin V+/PI+ cells, representing late
apoptotic cells; and A1, Annexin V−/PI+
cells, representing genuine necrotic response, respectively.
Hoechst 33342 nuclear stating (x200, right panel). (A and E)
untreated controls. Arrows indicate apoptotic bodies formed in
T24R2 cells. |
Hoechst 33342 nuclear staining showed more frequency
chromatin fragmentation and condensation in T24R2 cells exposed to
concomitant treatment compared with untreated control and also
cisplatin or NVP-BEZ235 single agent-treated groups (Fig. 6).
Expression of apoptosis, cell cycle and
survival regulators
The combined treatment of T24R2 cells with
NVP-BEZ235 and cisplatin markedly enhanced the cleavage of
caspase-3, -8, and -9 accompanied by increased PARP cleavage
(Fig. 7). A colorimetric assay
also exhibited increased caspase-3, -8, and -9 activity in
concomitant treatment group (169.4±3.8, 149.7±1.2 and 148.7±2.9% of
untreated controls, respectively, Fig.
7). Combination treatment suppressed the expression or
activation of anti-apoptotic (cIAP2, XIAP and surviving) and cell
cycle regulatory protein (cyclin A, cyclin B, pCDC2C, CDC2C,
pCDC25C, CDC25C, and pRb expression, Fig. 7). The phosphorylations of cell
survival-regulatory proteins (PI3K, Akt, mTOR, 4E-BP1, GSK-3β and
p70S6k) were efficiently suppressed by combination treatment
(Fig. 8). Both the NVP-BEZ235
single and the combination treatment with cisplatin increased the
phosphorylation of MEK1/2 and ERK1/2 in T24R2 cells (Fig. 8).
 | Figure 7Apoptosis and cell cycle regulatory
protein expression in T24R2 cells. T24R2 cells were exposed to
NVP-BEZ235 (0.5 μM) and/or cisplatin (0.5 μg/ml) for 72 h and
apoptosis- [cleaved caspase (-3, -8 and -9), PARP, cIAP1, cIAP2,
XIAP, survivin, BAD, Bcl-2, Bax, p-IKKα, IKKa, p-IκBα, IκBα and
NF-κB] and cell cycle-related (cyclin A, cyclin B1, cyclin D1,
pCDC2, CDC2, pCDC25C, CDC25C and pRb) protein expression was
analyzed by western blot analysis. Caspase activity was also
quantitatively analyzed using colorimetric caspase activity assay
kit. Asterisks indicate significant difference between each
treatment group and untreated control. Double asterisk denotes
significant difference between NVP-BEZ235 single and
cisplatin/NVP-NEZ235 combined treatment groups. |
 | Figure 8Cell survival-related protein
expression in T24R2 cells. T24R2 cells were treated with NVP-BEZ235
(0.5 μM) alone or in combination with cisplatin (0.5 μg/ml) for 72
h and expression of cell survival-related proteins (p-Akt, Akt,
p-PI3K, PI3K, p-mTOR, mTOR, p-4E-BP1, 4E-BP1, p-GSK-3β, GSK-3β,
p-p70S6K, p70S6K, p-MEK1/2, MEK1/2, p-ERK1/2 and ERK1/2) was
analyzed by western blot analysis. |
Discussion
The sensitivity of cancer cells to chemotherapeutic
drug-induced apoptosis depends on the balance between pro-apoptotic
and anti-apoptotic signals. Therefore, targeting of anti-apoptotic
signals that promote cell survival is proposed as a promising
strategy to enhance the efficacy of conventional chemotherapeutic
agents. One signaling pathway that has recently drawn much
attention for this purpose is the PI3K/AKT/mTOR axis and abnormal
activation of this pathway has been reported to play an important
role in progression, metastasis, and also chemoresistance in a
variety of tumors including bladder cancer (22–26).
These findings ignited enthusiasm for targeting mTOR signaling as
an anticancer modality and led to the development and clinical
application of mTOR inhibitors such as rapamycin, temsirolimus, and
everolimus. Although these 1st generation rapalogs have shown
promise, due to their allosteric inhibition of mTORC1, but not
mTORC2, they usually permit mTORC2-mediated Akt phosphorylation,
causing paradoxical activation of survival axis signaling (27–29).
Thus, the concomitant dual inhibition of PI3K/mTOR or Akt/mTOR may
be a solution to these feedback loops and provide a superior
strategy for overcoming development of resistance of cancer cells
to targeted therapy.
In the present study we demonstrated dose-dependent
antitumor effect of PI3K/mTOR dual inhibitor NVP-BEZ235 in bladder,
prostate and kidney cancer cell lines. In bladder cancer cells,
NVP-BEZ235 showed relatively more potent antitumor effect than mTOR
inhibitor such as temsirolimus and everolimus. In addition,
NVP-BEZ235 prevented the negative feedback activation of Akt
usually observed after treatment with 1st generation rapalogues.
However, relatively high dose of NVP-BEZ235 was required for the
growth inhibition of bladder cancer cells and even at the dose of
0.5 μM or higher, NVP-BEZ235 caused only partial inhibition of
bladder cancer cells. This is compatible with a previous report in
which NVP-BEZ235 showed only partial inhibition (~60%) of prostate
cancer cell growth at the concentration of 0.5 μM (30).
Also in bladder cancer cells, NVP-BEZ235 showed only
a transient effect resulting in partial loss of its antitumor
activity 3 days after initial treatment and this phenomenon was
more prominent in cisplatin-resistant T24R2 cells compared with
cisplatin-sensitive parental T24 cells. These findings are
compatible with previous studies in which mTOR inhibitors showed
only cytostatic and transient antitumor effect in many tumors
including bladder cancer cells (13,31).
Moreover, T24R2 cells showed significantly higher resistance to
NVP-BEZ235 compared with other bladder cancer cell lines including
cisplatin-sensitive T24. These findings suggest possible
cross-resistance between NVP-BEZ235 and cisplatin in human bladder
cancers, and thus limited antitumor effect of NVP-BEZ235 in the
patients with cisplatin-resistant bladder cancer, a potential
target population for these novel therapies.
Based on previous reports that the mTOR inhibitors
can exert synergism with various conventional chemotherapeutic
agents including cisplatin in chemo-naïve or resistant tumors, we
reasoned that the combination of NVP-BEZ235 and cisplatin might
enhance antitumor effect of both agents in cisplatin-resistant
bladder cancer cells (17,32,33).
To test this hypothesis, we performed a synergy test for the
NVP-BEZ235 and cisplatin and found a significant enhancement in the
antitumor effect compared with that of either agent as a single
treatment. The fa-CI plot showed that the two drugs exert
synergistic effect over a wide range of dose combinations in
cisplatin-resistant T24R2 cells. Dose-reduction index (DRI)
analysis demonstrated that when used in combination to treat T24R2
cells, the IC50 of cisplatin and NVP-BEZ235 can be
reduced by 3.6- and 5.6-fold, respectively, indicating strong
synergistic interaction between the two drugs. 3D synergy test
resulted in synergy volume 388.25 μM/ml2% with minimal
antagonism. According to previous guidelines such an extent of the
synergy volume indicates that this effect may be important in
vivo (19).
Flow cytometry showed that combination of NVP-BEZ235
and cisplatin causes a mild increase in sub-G1 fraction while
inducing marked increase in S phase population suggesting prominent
cytostatic effect of combined treatment rather than apoptogenic
effect. Western blot analysis demonstrated that combined treatment
caused a marked decrease in cyclin A, cyclin B1, cyclin D1, pCDC2C,
CDC2C, pCDC25C, CDC25C, and pRb in T24R2 cells, supporting the flow
cytometry data of cell cycle arrest. The combination treatment also
caused a significant decrease in the anti-apoptotic cIAP1, cIAP2,
XIAP, survivin, and Bcl-2 expression, while causing upregulation of
proapoptotic Bad, and Bax expression. Both western blot analysis
and colorimetric assay exhibited increased cleavage of caspase-3,
-8, and -9 in NVP-BEZ235 and cisplatin co-treated T24R2 cells
indicating induction of the caspase-dependent apoptotic pathway.
Flow cytometric analysis after Annexin V-FITC/PI double staining
and Hoechst 33342 nuclear stating also showed increased apoptosis
in concomitant treatment group.
The exposure of T24R2 cells to concomitant treatment
suppressed the phosphorylation of IκB kinase α (p-IKKα) and IκBα in
conjunction with an increase in cytoplasmic NF-κB and reciprocal
decrease of nucleic NF-κB levels, suggesting the suppression of
NF-κB signaling by NVP-BEZ235 and cisplatin co-treatment.
The exposure of T24R2 cells to NVP-BEZ235 alone or
in combination with cisplatin resulted in the suppression of PI3K
and mTOR phosphorylation, as well as its immediate target GSK-3β
and 4E-BP1, which was accompanied by slight increased expression or
activities of its downstream target BAD, caspase-3 and -9 and
accompanying suppression of Bcl-2, cyclin A, and D1. NVP-BEZ235
and/or cisplatin treatment suppressed Akt phosphorylation without
any paradoxical activation which was reported in 1st generation
mTOR inhibitors such as temsirolimus and everolimus. Akt promotes
cell cycle progression through the inhibition of GSK-3β and it
suppresses the expression of the Bcl-2 antagonist Bad, maintaining
cell survival (34). Thus
simultaneous suppression of PI3K and mTOR without activation of Akt
and its downstream target signaling also supports the potent
antiproliferative and proapoptotic activities of NVP-BEZ235 and
cisplatin combination treatment in cisplatin-resistant bladder
cancer cells.
Interestingly, we found that both NVP-BEZ235
monotherapy and combination treatment caused increased
phosphorylation of MEK1/2, and ERK1/2. This result is compatible
with recent reports in which NVP-BEZ235 upregulated ERK
phosphorylation in Waldenstrom macroglobulinemia cell line although
it appeared to be less significant compared with mTOR inhibitor or
PI3K inhibitor (35). It has been
reported that activation of MAPK/ERK pathway signaling is supposed
to be a resistance mechanism in mTOR inhibitor-based therapy and
MAPK/ERK inhibitors can improved of antitumor effect of NVP-BEZ235
and other PI3K inhibitors (16,36–39).
Thus these findings suggest that concomitant targeting of MAPK/ERK
signaling is a promising strategy to enhance antitumor effect of
NVP-BEZ235 in bladder cancer patients.
Although the present study has several limitations
such as in vitro nature of the design and the small number
of cell lines tested, it demonstrated synergistic interaction
between cisplatin and NVP-BEZ235 in cisplatin-resistant human
bladder cancer cells. While NVP-BEZ235 by itself showed only a
limited antitumor effect in bladder cancer cells, it
synergistically potentiated cisplatin-mediated apoptosis and cell
cycle arrest without any paradoxical activation of Akt in
cisplatin-resistant human bladder cancer cells. These findings
suggest that dual targeting of PI3K/mTOR combined with cisplatin
can be a promising strategy for the patients with
cisplatin-resistant bladder cancer. Also our data indicate possible
crosstalk between PI3K/Akt/mTOR and MAPK/ERK pathway, thus
suggesting the potential of concomitant targeting of MAPK/ERK
pathway to enhance antitumor effect of PI3K/mTOR dual inhibitors in
cisplatin-resistant bladder cancer. Further comprehensive molecular
studies should be performed to test the safety and in vivo
synergistic antitumor effect of NVP-EBZ235 and cisplatin
combination therapy for the clinical application in
cisplatin-resistant bladder cancer.
Acknowledgements
This study was supported by the Research Foundation
Grant funded by the Korean Urological Association (KUA-2009-002, to
Cheol Yong Yoon) and SNUBH Research fund (03-2013-013, to Sang Eun
Lee).
References
|
1
|
Fossa SD, Aass N, Ous S, et al: Survival
after curative treatment of muscle-invasive bladder cancer. Acta
Oncol. 35(Suppl 8): 59–65. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saxman SB, Propert KJ, Einhorn LH, et al:
Long-term follow-up of a phase III intergroup study of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: a
cooperative group study. J Clin Oncol. 15:2564–2569. 1997.
|
|
3
|
Hoda MA, Mohamed A, Ghanim B, et al:
Temsirolimus inhibits malignant pleural mesothelioma growth in
vitro and in vivo: synergism with chemotherapy. J Thorac Oncol.
6:852–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O’Reilly T, McSheehy PM, Wartmann M, et
al: Evaluation of the mTOR inhibitor, everolimus, in combination
with cytotoxic antitumor agents using human tumor models in vitro
and in vivo. Anticancer Drugs. 22:58–78. 2011.PubMed/NCBI
|
|
5
|
Chen P, Wang L, Liu B, et al:
EGFR-targeted therapies combined with chemotherapy for treating
advanced non-small-cell lung cancer: a meta-analysis. Eur J Clin
Pharmacol. 67:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pinto-Leite R, Botelho P, Ribeiro E, et
al: Effect of sirolimus on urinary bladder cancer T24 cell line. J
Exp Clin Cancer Res. 28:32009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Obata T, Khan Q, et al: The
phosphatidylinositol-3 kinase pathway regulates bladder cancer cell
invasion. BJU Int. 93:143–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puzio-Kuter AM, Castillo-Martin M, Kinkade
CW, et al: Inactivation of p53 and Pten promotes invasive bladder
cancer. Genes Dev. 23:675–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia JA and Danielpour D: Mammalian
target of rapamycin inhibition as a therapeutic strategy in the
management of urologic malignancies. Mol Cancer Ther. 7:1347–1354.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thallinger C, Poeppl W, Pratscher B, et
al: CCI-779 plus cisplatin is highly effective against human
melanoma in a SCID mouse xenotranplantation model. Pharmacology.
79:207–213. 2007. View Article : Google Scholar
|
|
11
|
Ma BB, Lui VW, Hui EP, et al: The activity
of mTOR inhibitor RAD001 (everolimus) in nasopharyngeal carcinoma
and cisplatin-resistant cell lines. Invest New Drugs. 28:413–420.
2010. View Article : Google Scholar
|
|
12
|
Yardley DA: Combining mTOR inhibitors with
chemotherapy and other targeted therapies in advanced breast
cancer: rationale, clinical experience, and future directions.
Breast Cancer. 7:7–22. 2013.
|
|
13
|
Chiong E, Lee IL, Dadbin A, et al: Effects
of mTOR inhibitor everolimus (RAD001) on bladder cancer cells. Clin
Cancer Res. 17:2863–2873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fechner G, Classen K, Schmidt D, et al:
Rapamycin inhibits in vitro growth and release of angiogenetic
factors in human bladder cancer. Urology. 73:665–669. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu C, Wangpaichitr M, Feun L, et al:
Overcoming cisplatin resistance by mTOR inhibitor in lung cancer.
Mol Cancer. 4:252005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manara MC, Nicoletti G, Zambelli D, et al:
NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer
Res. 16:530–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukherjee B, Tomimatsu N, Amancherla K, et
al: The dual PI3K/mTOR inhibitor NVP-BEZ235 is a potent inhibitor
of ATM- and DNA-PKCs-mediated DNA damage responses. Neoplasia.
14:34–43. 2012.PubMed/NCBI
|
|
18
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buckwold VE, Wei J, Wenzel-Mathers M, et
al: Synergistic in vitro interactions between alpha interferon and
ribavirin against bovine viral diarrhea virus and yellow fever
virus as surrogate models of hepatitis C virus replication.
Antimicrob Agents Chemother. 47:2293–2298. 2003. View Article : Google Scholar
|
|
20
|
Tarbet EB, Maekawa M, Furuta Y, et al:
Combinations of favipiravir and peramivir for the treatment of
pandemic influenza A/California/04/2009 (H1N1) virus infections in
mice. Antiviral Res. 94:103–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vilhelmova N, Jacquet R, Quideau S, et al:
Three-dimensional analysis of combination effect of ellagitannins
and acyclovir on herpes simplex virus types 1 and 2. Antiviral Res.
89:174–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee S, Choi EJ, Jin C, et al: Activation
of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification
contributes to cisplatin resistance in an ovarian cancer cell line.
Gynecol Oncol. 97:26–34. 2005. View Article : Google Scholar
|
|
23
|
Altomare DA, Wang HQ, Skele KL, et al: AKT
and mTOR phosphorylation is frequently detected in ovarian cancer
and can be targeted to disrupt ovarian tumor cell growth. Oncogene.
23:5853–5857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fahy BN, Schlieman MG, Virudachalam S, et
al: Inhibition of AKT abrogates chemotherapy-induced NF-kappaB
survival mechanisms: implications for therapy in pancreatic cancer.
J Am Coll Surg. 198:591–599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang HY, Zhang PN and Sun H: Aberration
of the PI3K/AKT/mTOR signaling in epithelial ovarian cancer and its
implication in cisplatin-based chemotherapy. Eur J Obstet Gynecol
Reprod Biol. 146:81–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao YK, Wu AT, Geethangili M, et al:
Identification of antrocin from Antrodia camphorata as a selective
and novel class of small molecule inhibitor of Akt/mTOR signaling
in metastatic breast cancer MDA-MB-231 cells. Chem Res Toxicol.
24:238–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wangpaichitr M, Wu C, You M, et al:
Inhibition of mTOR restores cisplatin sensitivity through
down-regulation of growth and anti-apoptotic proteins. Eur J
Pharmacol. 591:124–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mabuchi S, Kawase C, Altomare DA, et al:
mTOR is a promising therapeutic target both in cisplatin-sensitive
and cisplatin-resistant clear cell carcinoma of the ovary. Clin
Cancer Res. 15:5404–5413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yasumizu Y, Miyajima A, Kosaka T, et al:
Dual PI3K/mTOR inhibitor NVP-BEZ235 sensitizes docetaxel in
castration resistant prostate cancer. J Urol. 191:227–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mansure JJ, Nassim R, Chevalier S, et al:
Inhibition of mammalian target of rapamycin as a therapeutic
strategy in the management of bladder cancer. Cancer Biol Ther.
8:2339–2347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang F, Qian XJ, Qin W, et al: Dual
phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor
NVP-BEZ235 has a therapeutic potential and sensitizes cisplatin in
nasopharyngeal carcinoma. PLoS One. 8:e598792013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schult C, Dahlhaus M, Glass A, et al: The
dual kinase inhibitor NVP-BEZ235 in combination with cytotoxic
drugs exerts anti-proliferative activity towards acute
lymphoblastic leukemia cells. Anticancer Res. 32:463–474. 2012.
|
|
34
|
Bhatt AP, Bhende PM, Sin SH, et al: Dual
inhibition of PI3K and mTOR inhibits autocrine and paracrine
proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood.
115:4455–4463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roccaro AM, Sacco A, Husu EN, et al: Dual
targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in
Waldenstrom macroglobulinemia. Blood. 115:559–569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Winter JN, Fox TE, Kester M, et al:
Phosphatidic acid mediates activation of mTORC1 through the ERK
signaling pathway. Am J Physiol Cell Physiol. 299:C335–C344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zitzmann K, Ruden J, Brand S, et al:
Compensatory activation of Akt in response to mTOR and Raf
inhibitors - a rationale for dual-targeted therapy approaches in
neuroendocrine tumor disease. Cancer Lett. 295:100–109. 2010.
View Article : Google Scholar
|
|
38
|
Aziz SA, Jilaveanu LB, Zito C, et al:
Vertical targeting of the phosphatidylinositol-3 kinase pathway as
a strategy for treating melanoma. Clin Cancer Res. 16:6029–6039.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haagensen EJ, Kyle S, Beale GS, et al: The
synergistic interaction of MEK and PI3K inhibitors is modulated by
mTOR inhibition. Br J Cancer. 106:1386–1394. 2012. View Article : Google Scholar : PubMed/NCBI
|






















