Introduction
Cancer is one of the leading causes of death. Breast
cancer is the most common cancer diagnosed in North American and
Western European women (1,2), and Asian populations generally have
the lowest risk, but rates in this population have been steadily
increasing. Particularly, in Korea, the incidence of breast cancer
has increased 4-fold from 1996 to 2010, showing the highest growth
rate of breast cancer among OECD countries (3). Majority of primary breast cancers
(70–80%) are estrogen receptor (ER)-positive (+), and
ER+ breast cancers generally have a better prognosis and
are responsive to antiestrogen therapy. In contrast, ER-independent
(ER−) breast cancers including refractory cancer to
antiestrogen therapy are more aggressive, possess high metastatic
potential (4,5). Most of these patients eventually die
of metastatic disease. Increasing evidence shows that breast cancer
cells undergo an epithelial-to-mesenchymal transition (EMT) to
metastasize, and this process is frequently observed in the most
aggressive subtype, estrogen receptor-negative
(ER−)/progesterone receptor (PR)-negative
(PR−)/human epithelial growth factor receptor 2-negative
(HER2−) triple-negative breast cancer (TNBC) (6).
Numerous materials isolated from plants are being
investigated for their therapeutic application against cancer
metastasis. Among compounds of known structure, flavonoids deserve
special attention because they are present in practically all
dietary plants, fruits and root. The flavonoids, including morin
(7,8), are non-toxic (9,10)
and display a variety of biological actions including
anti-carcinogenic (11–13). Mulberry trees are widely cultivated
in East Asia and the white mulberry, Morus alba L. is a rich
source of many bioactive phytochemicals. Five phenolic
constituents, including maclurin, rutin, isoquercitrin, resveratrol
and morin, have been identified in ethanolic extract of mulberry
twigs to account for its potential oxidation capability; among
them, maclurin and morin have been shown to be superior to the
others (14).
Morin (3,5,7,2′,4′-pentahydroxyflavone) is a kind of
flavonoid found in figs and other Moraceae which are used as herbal
medicines. It has certain biological activities, including
anti-oxidant properties (15,16)
and anti-inflammatory effects (17,18).
Morin also acts as an anti-mutagen (19,20)
and has an anti-promotion activity in a liver carcinogenesis model
(21). Most of all, the favorable
safety profile of this natural compound (8) makes it a potential candidate worthy
of further investigations. Such beneficial effects of morin could
be expected to work in in vitro and in vivo cancer
model. However, the effect of morin on cancer growth and metastasis
is not well known. Therefore, in the present study, we aimed to
investigate the effect of morin on the cancer growth and invasion
in highly metastatic human breast cancer cells MDA-MB-231. As
mentioned above, most lethal and aggressive subtype of breast
cancer is ER−/PR−/HER2− TNBC and
MDA-MB-231 is a well-known TNBC. Thus, MDA-MB-231 was used in this
study.
Materials and methods
Materials
Morin (Fig. 1A) was
obtained from Aging Tissue Bank (Pusan, Korea). Antibodies against
N-cadherin, phospho-Akt, Akt, phospho-GSK3β and GSK3β were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-biphenyl tetrazolium bromide
(MTT), 4′,6-diamidino-2-phenyindole, dilactate (DAPI) and
anti-β-actin antibody were obtained from Sigma-Aldrich Co. (St.
Louis, MO, USA). Recombinant human tumor necrosis factor-α (TNF-α)
was obtained from R&D Systems (Minneapolis, MN, USA). BD
Matrigel™ basement membrane matrix is supplied by BD Biosciences
(San Diego, CA, USA).
Cell culture
The human breast cancer cell MDA-MB-231 was grown in
RPMI-1640 supplemented with 10% FBS, 100 IU/ml penicillin and 10
μg/ml streptomycin. The human umbilical endothelial cell line EA.hy
926 was grown in DMEM supplemented with 10% FBS, 100 IU/ml
penicillin and 10 μg/ml streptomycin. All cells were incubated in a
humidified 5% CO2 incubator.
Cell proliferation assay
Cells were seeded at 104 cells per well
in 24-well plates. After treatments, 50 μl of 5 mg/ml MTT solution
was added to each well and incubated for 3 h. The supernatants were
aspirated and the formazan crystals were dissolved with 200 μl of 4
N HCl-isopropanol in each well. The optical density of the colored
product was measured at 570 nm, as suggested by the manufacturer,
using an Infinite 200 microplate reader (Tecan Austria GmbH,
Grödig, Austria).
Colony formation assay
Cells were seeded in 6-well plates at 1,000
cells/well. After serum starvation for 16 h, the cells were treated
with morin at the indicated doses in a 37°C cell culture incubator.
After 24 h, culture medium was discarded and changed with media
every 2–3 days. After 1–2 weeks, cells were fixed and stained using
crystal violet and photographed. The experiments were performed in
triplicate.
Western blot analysis
Cells were washed in ice-cold PBS and lysed in
PRO-PREP protein extraction solution (iNtRON Biotechnology, Seoul,
Korea). The samples were centrifuged at 13,000 rpm, for 15 min at
4°C. An aliquot of the whole cell lysate was subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene difluoride membrane. Membranes were
blocked with 5% non-fat milk in Tris-buffered saline (TBS)
containing 0.05% Tween-20 for 2 h at room temperature and incubated
with primary antibodies at 1:1,000 in TBS containing 0.05% Tween-20
and 3% bovine serum albumin overnight at 4°C. The membranes were
then incubated with horseradish peroxidase-conjugated anti-rabbit
IgG (1:5,000) antibody for 1 h at room temperature. After washing,
the membranes were developed using the ECL reagent (Bionote,
Gyeonggi-do, Korea).
Matrigel invasion assay
The upper chamber of 24-well cell culture inserts
(8-μm pore size, Falcon, Franklin Lakes, NJ, USA) were washed with
a serum-free medium, coated with 100 μl of Matrigel (1 mg/ml) and
dried for 30 min at 37°C. MDA-MB-231 cells treated with morin were
collected; 2×105 cells were loaded to the upper chambers
filled with serum-free media, and 500 μl of RPMI media containing
10% FBS was added to the lower chambers. The invasion chambers were
incubated for 24 h in a 37°C cell culture incubator. The
non-invasive cells that remained on the upper surface of the insert
membranes were removed by scrubbing. The cells on the lower insert
membranes were stained with DAPI, and cells were counted under a
fluorescence microscope. Each sample was measured in triplicate,
and each experiment was repeated three times.
Gelatin zymography
Gelatin zymography was performed as described by Jin
et al (22). Briefly,
conditioned media were concentrated using a protein concentrator
(Thermo Pierce, Rockford, IL, USA) and subjected to electrophoresis
on 8% PAGE gels containing 1 mg/ml gelatin. Gels were washed twice
with 2.5% Triton X-100, stained with 0.2% Coomassie Brilliant Blue
and destained (50% methanol and 10% acetic acid). Gelatinolytic
activity was detected as clear bands in the background of blue
staining.
Animal experiments
Athymic nude mice were divided into 2 groups (5
mice/group) and received morin at the dose of 10 and 50 mg/kg
(daily, i.p.), respectively for 7 days. Mice were injected
subcutaneously with MDA-MB-231 (5×106 cells/100 μl of
serum-free RPMI). Tumors were allowed to grow until they reached 4
mm. At this point, mice were divided into 2 groups (7 mice/group):
control and morin-treated mice. The mice were administered a daily
i.p. injection of 10 mg/kg (non-toxic dose) morin for 45 days. The
mice were sacrificed at day 45, and the tumors were extracted. Body
weights and tumor volumes were measured every 3 days, starting at 7
days after injection. The experimental protocol was approved by the
Institutional Animal Care and Use Committee at Gyeongsang National
University.
Statistical evaluations
Scanning densitometry was performed using Image
Master® VDS (Pharmacia Biotech Inc., San Francisco, CA,
USA). The treatment groups were compared using one-way analysis of
variance and the post hoc test by Scheffe. P<0.05 was
considered statistically significant. All data were expressed as
the mean ± standard error (SEM).
Results
Morin inhibits the colony forming ability
of the human breast cancer MDA-MB-231 cells without cytotoxicity to
human endothelial cells
First, we examined the cell viability of MDA-MB-231
cells and normal endothelial cells in response to morin. When
MDA-MB-231 cells and endothelial cells were treated with indicated
doses of morin (10, 50, 100 and 200 μM) for 24 h, the results
revealed that morin did not affect cell viability at the doses
<100 μM both in MDA-MB-231 cells and endothelial cells. High
concentrations (100 and 200 μM) of morin reduced cell viability of
MDA-MB-231 a little but rather increased cell viability in
endothelial cells (Fig. 1B). Thus,
we investigated the effect of morin on the ability of MDA-MB-231
cells to form colonies. Interestingly, morin effectively inhibited
colony formation of MDA-MB-231 cells in a dose-dependent manner
(Fig. 1C).
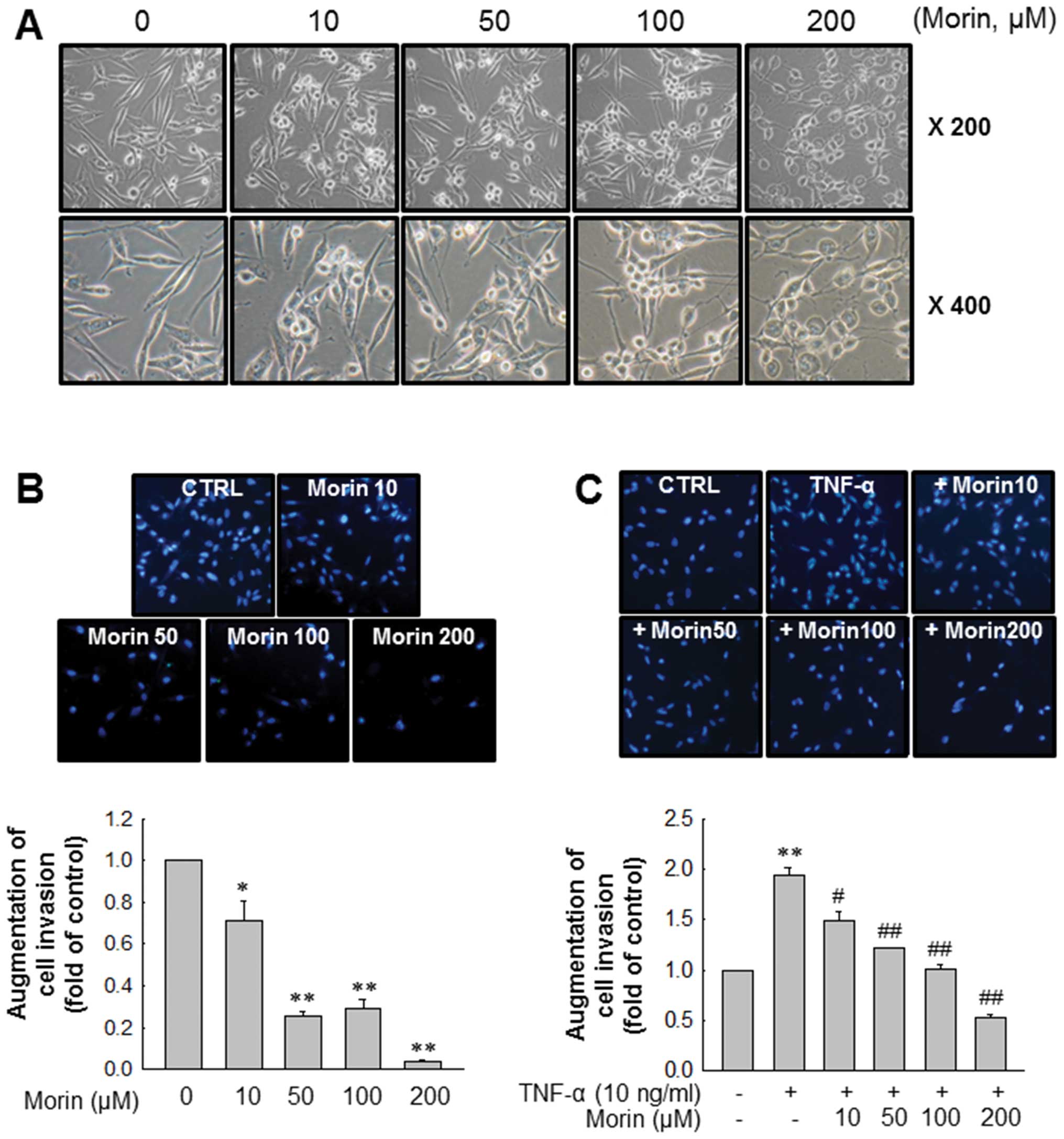
Morin affects cell morphology and
inhibits the invasion of MDA-MB-231 breast cancer cells
Next, we observed changes of MDA-MB-231 cells in
morphology after morin treatment. As shown in Fig. 2A, morin induced morphologic changes
of MDA-MB-231 cells from mesenchymal form to epithelial form in a
dose-dependent manner. We then determined the effects of morin on
MDA-MB-231 cell invasion because cancer cell invasion is the first
step for cancer metastasis. Matrigel invasion assays revealed that
morin significantly inhibited cell invasion in a dose-dependent
manner (Fig. 2B). Moreover, morin
also effectively inhibited TNF-α-induced MDA-MB-231 cell invasion
in a similar manner (Fig. 2C).
Morin decreases matrix
metalloproteinase-9 (MMP-9) secretion and the mesenchymal marker
N-cadherin expression involved in cancer metastasis
Proteolytic digestion of the extracellular matrix
(ECM) by secreted MMPs is one of major steps for cancer invasion
(23,24). MMP-2, -3 and -9 are also biomarkers
for EMT (25). In particular,
MMP-9 expression is associated with pathological processes,
including inflammation, atherosclerosis and tumor-cell invasion and
metastasis (26–28). Thus, we examined the effect of
morin on the activity of the secreted MMP-9 from MDA-MB-231 cells
in the presence of TNF-α or not by gelatin zymographic analysis.
Morin dose-dependently suppressed the gelatinolytic activities of
secreted MMP-9 in MDA-MB-231 cells. MMP-9 secretion augmented by
TNF-α was also significantly inhibited by a low dose of morin (10
μM) (Fig. 3A). In addition, we
also assessed the changes in EMT biomarkers to confirm that morin
has inhibitory effects on EMT. Fig.
3B showed that morin inhibited mesenchymal markers N-cadherin,
but did not influence the expressions of either vimentin or
E-cadherin of MDA-MB-231 cells (data not shown). These results
suggest that morin might suppress the invasion of highly metastatic
MDA-MB-231 breast cancer cells through regulating the EMT
process.
Morin inhibits the invasion of MDA-MB-231
breast cancer cells through inhibiting Akt pathway but not GSK3β
pathway
Concerning the upstream signals that affect
N-cadherin, β-catenin induces N-cadherin expression, and β-catenin
is negatively regulated by GSK-3β. GSK-3β is regulated by
intracellular signaling pathways including PI3K/Akt. In other
words, activation of PI3K/Akt results in the phosphorylation of
GSK-3β (inactivation of GSK-3β), which in turn increases β-catenin
protein levels. Thus, we investigated whether morin modulates Akt
and GSK-3β pathways in MDA-MB-231. Morin reduced phosphorylated Akt
level in MDA-MB-231 cells but failed to inhibit GSK-3β
phosphorylation. In addition, TNF-α (10 ng/ml) enhanced Akt
phosphorylation in MDA-MB-231, which was also significantly
inhibited by morin. Morin did not reduce the TNF-α-mediated
phosphorylation of GSK-3β (Fig. 4A and
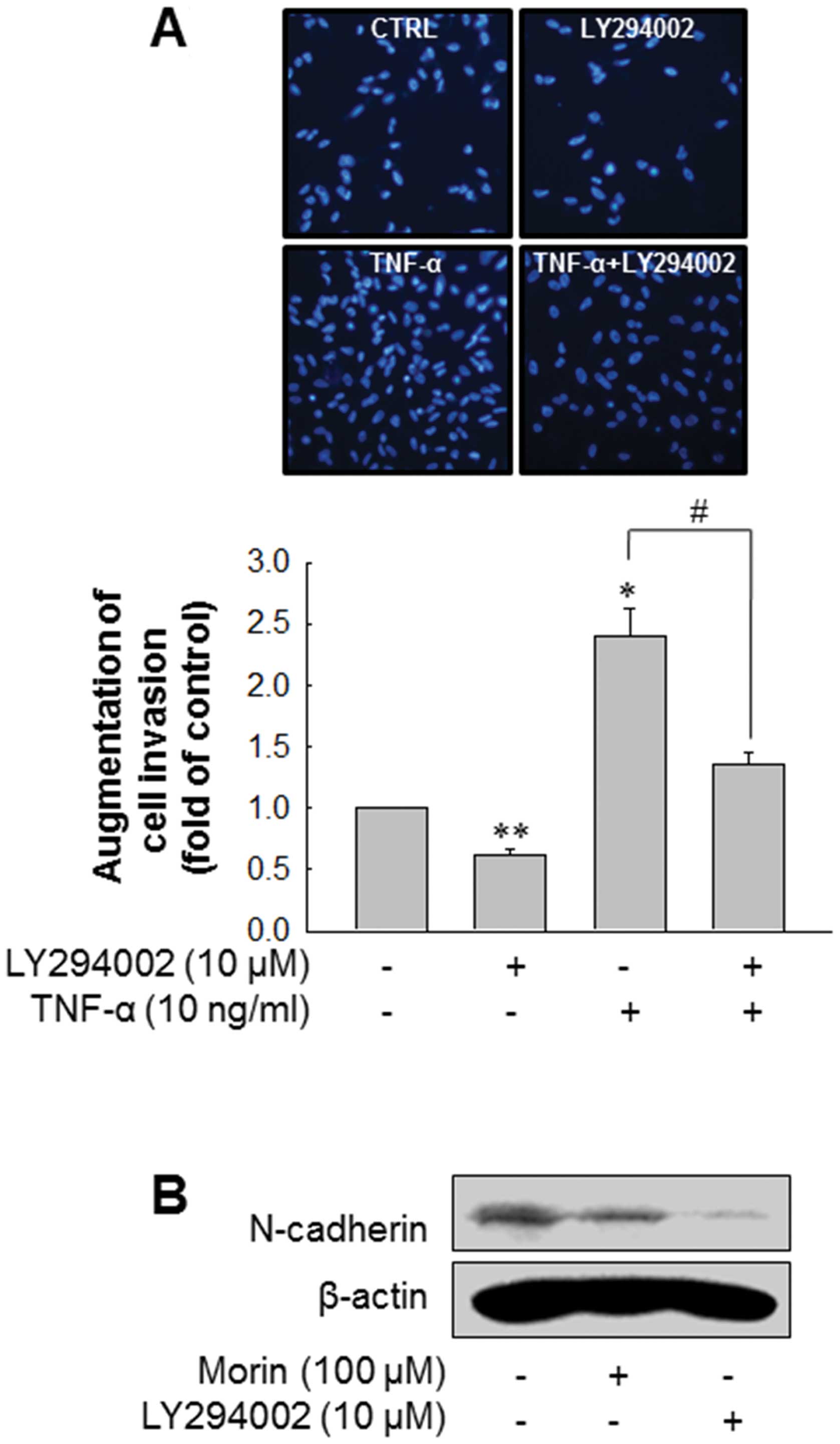
B). Then, we confirmed whether inhibition of Akt pathway could
block the invasion of MDA-MB-231. As expected, inhibition of Akt
pathway using LY294002 significantly decreased the invasion of
MDA-MB-231 as well as TNF-α-enhanced invasion of MDA-MB-231
(Fig. 5A). In addition, LY294002
downregulated N-cadherin expression level (Fig. 5B). These results suggest that morin
suppresses the invasion of MDA-MB-231 breast cancer cell through
inhibiting Akt pathway and N-cadherin expression.
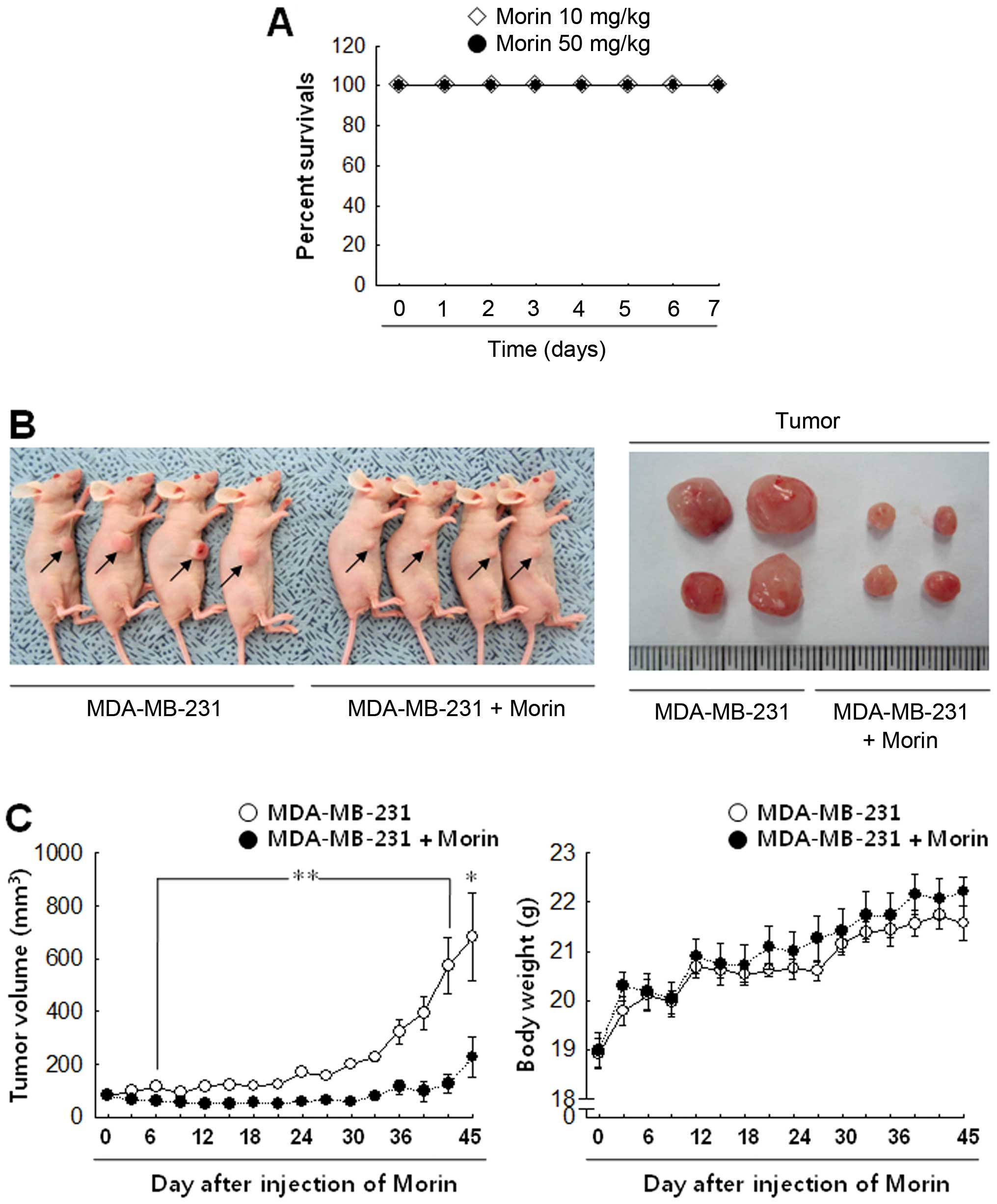
Morin reduces breast cancer cell growth
in xenograft mouse model in vivo
To confirm the in vivo effect of morin in
tumor progression, morin was injected into tumor-bearing mice for
45 days. Before that, to determine whether morin has a toxic effect
in vivo, the mice received i.p. a daily injection of 10 or
50 mg/kg morin for 7 days (n=7/group). Both 10 and 50 mg/kg
concentrations of morin caused no lethality in mice (Fig. 6A), however 50 mg/kg made the mice
somewhat nervous. Thus, we used 10 mg/kg of morin for further
experiments. Control animals developed significant tumor growth
during the 45-day follow-up period, as shown by the tumor volume in
Fig. 6B and C. In contrast,
animals that received 10 mg/kg morin daily showed that tumor growth
was significantly inhibited (Fig. 6B
and C). Body weight does not show any significant differences
between the groups.
Discussion
Cancer metastasis is responsible for most cancer
death rather than the primary tumors (29–31).
Cancer metastasis is a complex process involving the coordinated
responses among cancer cells, normal cells and ECM. Variable growth
factors, MMPs and cytokines including TNF-α stimulate cancer
metastasis (32–34). Considered that tumor metastasis is
the main cause of mortality of cancer patients, it is more
beneficial to develop drugs that are able to suppress the highly
metastatic cancer cells progression. Indeed, human breast cancer
cells MDA-MB-231 express putative aggressiveness markers and are
well known as an ER−/PR−/HER2−
TNBC. Therefore, in this study, we examined the anticancer effect
of morin using the highly metastatic human breast cancer cells
MDA-MB-231. Our results showed that morin significantly inhibited
the ability of MDA-MB-231 cells to form colony and invade. In
addition, morin suppressed the EMT process. Interestingly, morin
has no severe cytotoxicity to cancer cells or normal endothelial
cells; high concentrations (100 and 200 μM) of morin slightly
reduced cell viability of MDA-MB-231 but rather increased cell
viability in endothelial cells. These results suggest that morin
could be used in cancer patients without serious cytotoxicity.
Previous studies demonstrated that natural compounds could safely
induce anticancer effects.
A family of extracellular matrix-degrading enzymes,
the MMPs, has been implicated in inflammation and cancer (35). In particular, MMP-9 expression is
associated with pathological processes, including inflammation,
atherosclerosis and tumor cell invasion and metastasis (26–28).
Increased levels of serum and tissue expression of MMP-9 are
associated with a poor prognosis of breast cancer (36). Our results also showed that MMP-9
secretion was more prominent than MMP-2 in MDA-MB-231 (data not
shown), which was effectively inhibited by morin.
For the evaluation of EMT process, it is important
to assess the expression of E-cadherin an epithelial marker, but
E-cadherin expression level of MDA-MB-231 was too low to detect
(22). Instead, we showed that
morin downregulated N-cadherin expression in the MDA-MB-231. The
expression of N-cadherin (37,38)
is frequently upregulated in moderately-to-poorly invasive duct
carcinomas (IDCs) (39,40) and HER2-amplified tumors.
Accordingly, it is suggesting that N-cadherin might play a role in
cancer invasion and EMT in breast cancer. In this respect, morin
might downregulate the EMT process by suppressing N-cadherin
expression. Activation of PI3K/Akt causes an inactivation of
GSK-3β, which in turn increases β-catenin protein levels, and
finally results in the N-cadherin expression. Our results showed
that morin significantly decreased phosphorylation of Akt in
MDA-MB-231 cells as well as in TNF-α-stimulated MDA-MB-231 cells.
Inhibition of Akt pathway using LY294002, a PI3K/Akt inhibitor,
significantly reduced MDA-MB-231 invasion in the presence of TNF-α
or not, suggesting that morin suppresses EMT at least in part
through suppressing Akt pathway. However, morin did not affect
GSK-3β phosphorylation. Without GSK-3β phosphorylation, MMP
expression and activity can regulate N-cadherin function (41–43).
In addition, gene expression of MMPs can be regulated via the Akt
pathways (44,45). These findings support that morin
may suppress the EMT process by inhibiting MMP-9 activity followed
by N-cadherin expression through suppression of Akt pathway.
Furthermore, MMP-9 is also involved in EMT. Finally, we confirmed
the anticancer effect of morin using in vivo xenograft mouse
model in which animals were injected with MDA-MB-231 cells.
Taken together, our findings suggest that morin
exhibits an inhibitory effect on the cancer progression by
inhibiting EMT in the highly metastatic breast cancer MDA-MB-231
cells at least in part through inhibiting Akt activation. These
findings suggest that morin may serve as an effective therapeutic
strategy against metastatic breast cancer without side effects.
Acknowledgements
This study was supported by grants from the National
R&D Program for Cancer Control, Ministry of Health &
Welfare, Republic of Korea (0820050) and the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (2012R1A1A3003268). We thank the Aging
Tissue Bank (ATB) for providing research materials and
information.
Abbreviations:
|
DAPI
|
4′,6-diamidino-2-phenyindole,
dilactate
|
|
ECM
|
extracellular matrix
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
ER
|
estrogen receptor
|
|
HER
|
human epithelial growth factor
receptor
|
|
MMP
|
metalloproteinase
|
|
MTT
|
3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide
|
|
PR
|
progesterone receptor
|
|
SEM
|
standard error
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
TBS
|
Tris-buffered saline
|
|
TNBC
|
triple-negative breast cancer
|
|
TNF
|
tumor necrosis factor
|
References
|
1
|
Hayes DF, Isaacs C and Stearns V:
Prognostic factors in breast cancer: current and new predictors of
metastasis. J Mammary Gland Biol Neoplasia. 6:375–392. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Korean Breast Cancer Society. Korean
breast cancer data of 1996. J Korean Surg Soc. 55:621–635.
1998.
|
|
4
|
Keen JC and Davidson NE: The biology of
breast carcinoma. Cancer. 97:825–833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anandappa SY, Sibson R, Platt-Higgins A,
Winstanley JH, Rudland PS and Barraclough R: Variant estrogen
receptor α mRNAs in human breast cancer specimens. Int J Cancer.
88:209–216. 2000.
|
|
6
|
Mostert B, Sleijfer S, Foekens JA and
Gratama JW: Circulating tumor cells (CTCs): detection methods and
their clinical relevance in breast cancer. Cancer Treat Rev.
35:463–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yugarani T, Tan BKH, The M and Das NP:
Effects of polyphenolic natural products on the lipid profiles of
rats fed high fat diets. Lipids. 27:181–186. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu TW, Zeng LH, Wu J and Fung KP: Morin: a
wood pigment that protects three types of human cells in the
cardiovascular system against oxyradical damage. Biochem Pharmacol.
47:1099–1103. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kleijnen J and Knipschild P: Ginkgo
biloba. Lancet. 340:1136–1139. 1992. View Article : Google Scholar
|
|
10
|
McGregor D: Diets, food components and
human cancer. Biotherapy. 11:189–200. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robak J and Gryglewski RJ: Flavonoids are
scavengers of superoxide anion. Biochem Pharmacol. 37:83–88.
1998.
|
|
12
|
Husain SR, Cillard J and Cillard P:
Hydroxyl radical scavenging activity of flavonoids. Phytochemistry.
26:2489–2492. 1987. View Article : Google Scholar
|
|
13
|
Stavric B: Role of chemopreventers in
human diet. Clin Biochem. 27:319–332. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang LW, Juang LJ, Wang BS, Wang MY, Tai
HM, Hung WJ, Chen YJ and Huang MH: Antioxidant and antityrosinase
activity of mulberry (Morus alba L.) twigs and root bark.
Food Chem Toxicol. 49:785–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramanathan L, Das NP and Li QT: Studies on
lipid oxidation in fish phospholipid liposomes. Biol Trace Elem
Res. 40:59–70. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanasaki Y, Ogawaa S and Fukui S: The
correlation between active oxygens scavenging and antioxidative
effects of flavonoids. Free Radic Biol Med. 16:845–850. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakadate T, Yamamoto S, Aizu E and Kato R:
Effects of flavonoids and antioxidants on
12-O-tetradecanoylphorbol-13-acetate-caused epidermal ornithine
decarboxylase induction and tumor promotion in relation to
lipoxygenase inhibition by these compounds. Gann. 75:214–222.
1984.
|
|
18
|
Baumann J, Bruchhausen FV and Wurm G:
Flavonoids and related compounds as inhibitors of arachidonic acid
peroxidation. Prostaglandins. 20:627–639. 1980. View Article : Google Scholar
|
|
19
|
Francis AR, Shetty TK and Bhattacharya RK:
Modulating effect of plant flavonoids on the mutagenicity of
N-methyl-N′-nitro-N-nitrosoguanidine. Carcinogenesis. 10:1953–1955.
1989.PubMed/NCBI
|
|
20
|
Huang MT, Wood AW, Newmark HL, Sayer JM,
Yagi H, Jerina DM and Conney AH: Inhibition of the mutagenicity of
bay-region diol-epoxides of polycyclic aromatic hydrocarbons by
phenolic plant flavonoids. Carcinogenesis. 4:1631–1637. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Denda A, Ura H, Tsujiuchi T, Tsutsumi M,
Eimoto H, Takashima Y, Kitazawa S, Kinugasa T and Konishi Y:
Possible involvement of arachidonic acid metabolism in
phenobarbital promotion of hepatocarcinogenesis. Carcinogenesis.
10:1929–1935. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin H, Lee WS, Yun JW, Jung JH, Lee SM,
Kim HJ, Choi YH, Kim GS, Jung JM, Ryu CH, Shin SC and Hong SC:
Flavonoids from Citrus unshiu Marc. inhibit cancer cell
adhesion to endothelial cells by selective inhibition of VCAM-1.
Oncol Rep. 30:2336–2342. 2013.
|
|
23
|
Vihinen P and Kahari VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
25
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lelongt B, Trugnan G, Murphy G and Ronco
PM: Matrix metalloproteinases MMP2 and MMP9 are produced in early
stages of kidney morphogenesis but only MMP9 is required for renal
organogenesis in vitro. J Cell Biol. 136:1363–1373. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarén P, Welgus HG and Kovanen PT:
TNF-alpha and IL-1beta selectively induce expression of 92-kDa
gelatinase by human macrophages. J Immunol. 157:4159–4165.
1996.PubMed/NCBI
|
|
28
|
Przybylowska K, Kluczna A, Zadrozny M,
Krawczyk T, Kulig A, Rykala J, Kolacinska A, Morawiec Z, Drzewoski
J and Blasiak J: Polymorphisms of the promoter regions of matrix
metalloproteinases genes MMP-1 and MMP-9 in breast cancer. Breast
Cancer Res Treat. 95:65–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steeg PS: Cancer: micromanagement of
metastasis. Nature. 449:671–673. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eccles SA and Welch DR: Metastasis: recent
discoveries and novel treatment strategies. Lancet. 369:1742–1757.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
White ES, Strom SR, Wys NL and Arenberg
DA: Non-small cell lung cancer cells induce monocytes to increase
expression of angiogenic activity. J Immunol. 166:7549–7555. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ueno T, Toi M, Saji H, Muta M, Bando H,
Kuroi K, Koike M, Inadera H and Matsushima K: Significance
macrophage chemoattractant protein-1 in macrophage recruitment,
angiogenesis, and survival in human breast cancer. Clin Cancer Res.
6:3282–3289. 2000.PubMed/NCBI
|
|
34
|
Naylor MS, Stamp GW, Davies BD and
Balkwill FR: Expression and activity of MMPS and their regulators
in ovarian cancer. Int J Cancer. 58:50–56. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD,
Yang F and Xu XC: Prognostic significance of MMP-9 and TIMP-1 serum
and tissue expression in breast cancer. Int J Cancer.
122:2050–2056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suyama K, Shapiro I, Guttman M and Hazan
RB: A signaling pathway leading to metastasis is controlled by
N-cadherin and the FGF receptor. Cancer Cell. 2:301–314. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hulit J, Suyama K, Chung S, Keren R,
Agiostratidou G, Shan W, Dong X, Williams TM, Lisanti MP, Knudsen K
and Hazan RB: N-cadherin signaling potentiates mammary tumor
metastasis via enhanced extracellular signal-regulated kinase
activation. Cancer Res. 67:3106–3116. 2007. View Article : Google Scholar
|
|
39
|
Nagi C, Guttman M, Jaffer S, Qiao R, Keren
R, Triana A, Li M, Godbold J, Bleiweiss IJ and Hazan RB: N-cadherin
expression in breast cancer: correlation with an aggressive
histologic variant--invasive micropapillary carcinoma. Breast
Cancer Res Treat. 94:225–235. 2005. View Article : Google Scholar
|
|
40
|
Walsh MM and Bleiweiss IJ: Invasive
micropapillary carcinoma of the breast: eighty cases of an
underrecognized entity. Hum Pathol. 32:583–589. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ho AT, Voura EB, Soloway PD, Watson KL and
Khokha R: MMP inhibitors augment fibroblast adhesion through
stabilization of focal adhesion contacts and up-regulation of
cadherin function. J Biol Chem. 276:40215–40224. 2001. View Article : Google Scholar
|
|
42
|
Covington MD, Burghardt RC and Parrish AR:
Ischemia-induced cleavage of cadherins in NRK cells requires
MT1-MMP (MMP-14). Am J Physiol Renal Physiol. 290:F43–F51. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pon YL, Auersperg N and Wong AS:
Gonadotropins regulate N-cadherin mediated human ovarian surface
epithelial cell survival at both posttranslational and
transcriptional levels through a cyclic AMP/protein kinase A
pathway. J Biol Chem. 280:15438–15448. 2005. View Article : Google Scholar
|
|
44
|
Yoon SO, Shin S, Lee HJ, Chun HK and Chung
AS: Isoginkgetin inhibits tumor cell invasion by regulating
phosphatidylinositol 3-kinase/Akt-dependent matrix
metalloproteinase-9 expression. Mol Cancer Ther. 5:2666–2675. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chung TW, Lee YC and Kim CH: Hepatitis B
viral HBx induces matrix metallo-proteinase-9 gene expression
through activation of ERK and PI-3K/AKT pathways: involvement of
invasive potential. FASEB J. 18:1123–1125. 2004.PubMed/NCBI
|