Introduction
Ovarian cancer is the leading cause of gynecologic
cancer deaths in the United States (US) (1). While ovarian cancer has a typically
good response to first-line combination chemotherapy after initial
cytoreductive surgery, the prognosis of patients with advanced
malignant ovarian cancer remains poor because of acquired
chemotherapy resistance (2).
Despite recent advances made in chemotherapies of ovarian cancer,
the overall survival of patients has not improved significantly
because a considerable number of patients harbor ovarian cancer
refractory to these therapies and the majority of the initially
responsive tumors become resistant to treatments (3). Thus, the development of novel
targeted therapy that retains activity against
chemotherapy-resistant ovarian cancer is an unmet and urgent
medical need.
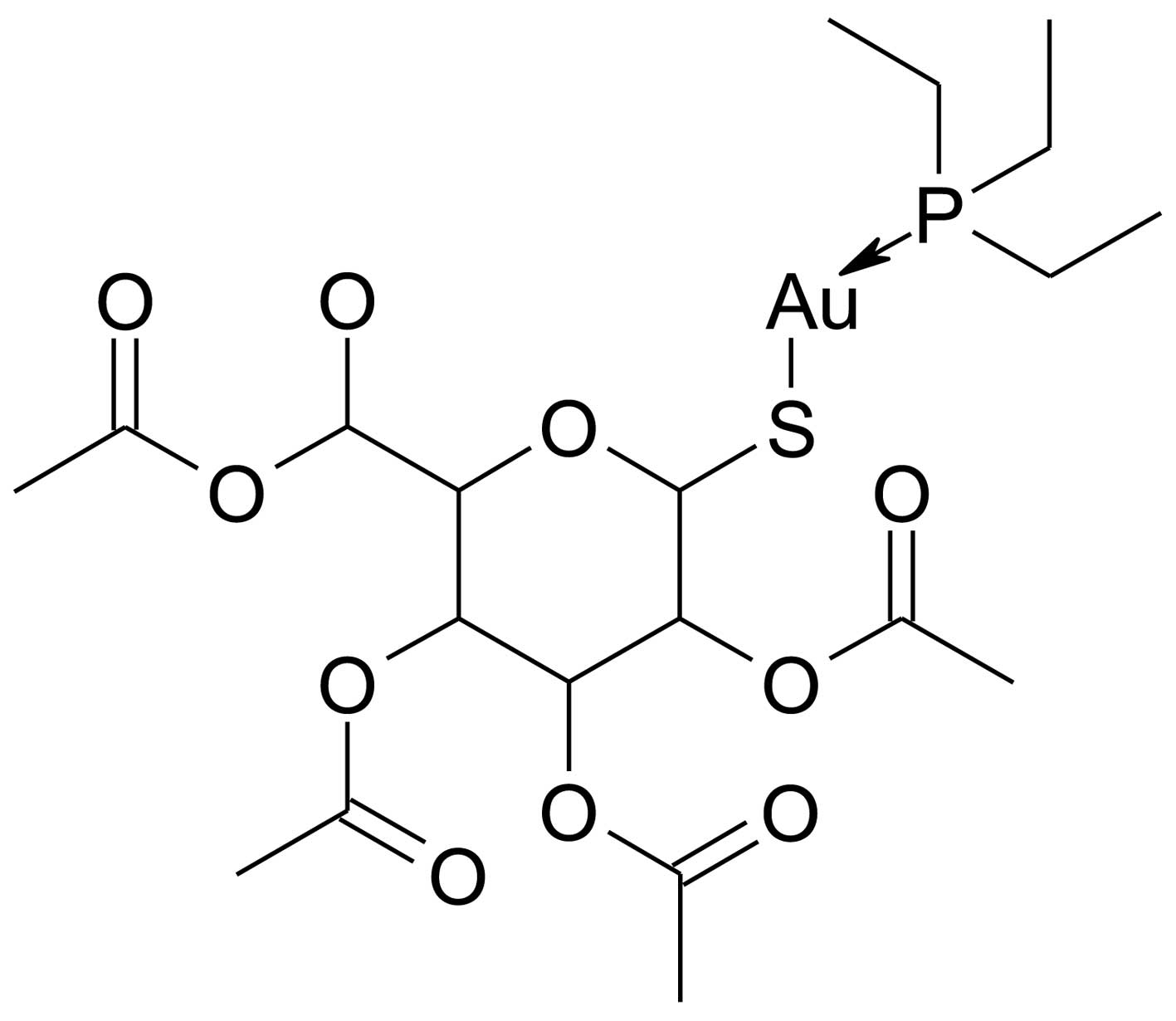
Auranofin
[2,3,4,6-tetra-o-acetyl-1-thio-β-D-glucopyranosato-S-(triethyl-phosphine)
gold] is a thiol-reactive gold (I)-containing compound (Fig. 1) that reduces the effects of the
inflammatory process in the body, and has been utilized to treat
rheumatoid arthritis by reducing pain and swelling (4,5).
Auranofin can inhibit IgE- and non-IgE-mediated histamine release
from human basophils and suppress the de novo synthesis of
sulfidopeptide leukotriene C4 (LTC4), which is induced by anti-IgE
from basophils and mast cells (6).
In addition, auranofin is a potent inhibitor of mitochondrial
thioredoxin reductase in vitro and in vivo, since
auranofin is able to block its active site (7). In addition, auranofin has been shown
to inhibit the activation of the IKK/NF-κB signaling pathway and
thereby downregulate the gene expression of pro-inflammatory
cytokines such as IL-1β and TNF-α (8–10).
Our previous study shows that IKK-β activation phosphorylates the
serine-644 residue in the FOXO3 protein and subsequently triggers
faster protein degradation of FOXO3 via the proteasome-mediated
ubiquitination (Ub) mechanism, which results in the stimulation of
human breast cancer cell proliferation in vitro and the
development of breast tumor in vivo (11,12).
These findings suggest that IKK-β may be a potential target for
anticancer therapy through FOXO3 activation. Interestingly, when we
screened for small molecules that can promote the activation of
FOXO3 in ovarian cancer cells, we identified auranofin as a
candidate FOXO3-activating small molecule from the US Food and Drug
Administration (FDA)-approved compound libraries. Since auranofin
is also a candidate inhibitor targeting IKK-β, it is a promising
small molecule candidate for development as anticancer
therapeutics.
FOXO3 is a member of the human Forkhead-box (FOX)
gene family that is known to have the distinct Forkhead DNA binding
domain (13). As a transcriptional
factor, FOXO3 is known to regulate various cellular processes such
as cell cycle (14,15), cellular apoptosis (16–19),
DNA damage repair (20,21), stress responses (15,22,23),
metabolism (24), aging (25) and tumor suppression in mammalian
cells (12,18,26).
The results from gene knockout exhibit key functions of FOXO family
members in tumor suppression (27)
and in preventing the decline of the hematopoietic stem cell pool
(28). The FOXO3 protein in cancer
cells can be regulated by various protein modification mechanisms
such as phosphorylation, acetylation and ubiquitination (25,29–31).
It has been shown that several active protein kinases (such as Akt,
IKK-β and MAPK) display the ability to phosphorylate the specific
serine/threonine residues on the FOXO3 protein and induce the
translocation of FOXO3 from the nucleus to the cytoplasm. This
nuclear exclusion and translocation of FOXO3 into the cytoplasm
inhibits FOXO3-dependent transcription and results in the
proteasome-mediated Ub and protein degradation of FOXO3 (11,12,18,25,31).
This inhibition of function of FOXO3 leads to tumor development and
progression, suggesting that FOXO3 is a crucial tumor suppressor.
Importantly, several clinical studies on the relationship between
the FOXO3 protein nuclear localization or expression level and
cancer patient survival rates have revealed that FOXO3 is a good
prognostic biomarker for cancer survival (12,32–34),
which suggests that the regulation of FOXO3 activation in cancer
cells may be a promising strategy for developing anticancer
therapeutic drugs.
Sequential activation of caspases (the cysteinyl
aspartate-specific proteases) plays an important role in the
execution phase of cellular apoptosis (35). In general, caspases exist as
inactive pro-enzymes that undergo proteolytic processing at the
conserved aspartic residues to produce two fragments that form a
functional dimer as an active protease. It has been shown that
cleavage of poly(ADP-ribose) polymerase 1 (PARP1) by caspase-3, a
crucial protease in regulating apoptosis, at the pro-apoptotic
cleavage site of PARP1 produces the p85-kDa proteolytic fragment,
which has been suggested as a biomarker associated with apoptosis
(36,37).
In the present study, we used human ovarian
carcinoma SKOV3 cells, which are p53-null, as the cell model system
to investigate the cytotoxic activity and the anticancer mechanisms
of auranofin. Cell-based assays and biochemical analyses were
employed to elucidate the molecular mechanism underlying the
anticancer activity of auranofin in SKOV3 cells. Based on our
findings, we propose that FOXO family members may upregulate the
pro-apoptotic genes and downregulate certain cell survival genes
that contribute to the cellular apoptosis response to auranofin in
a p53-independent manner. The important biological and pathological
significance of this new mechanism of auranofin in cancer therapy
is discussed below.
Materials and methods
Chemical reagents and antibodies
Auranofin, dimethylsulfoxide (DMSO), glycerol,
glycine, sodium chloride, thiazolyl blue tetrazolium bromide,
Trizma base and Tween-20 were purchased from Sigma (St. Louis, MO,
USA). Mouse anti-IκB kinase β (1:2,000 dilution), mouse anti-IκBα
(1:1,000 dilution), rabbit anti-p-IκBα (1:1,000 dilution), mouse
anti-PARP1 (1:1,000 dilution) and rabbit anti-FOXO3 (1:1,000
dilution) antibodies were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Rabbit anti-cleaved caspase-3 (1:1,000
dilution), rabbit anti-Bax (1:1,000 dilution), rabbit anti-Bim
(1:1,000 dilution) and rabbit anti-Bcl-2 (1:1,000 dilution)
antibodies were purchased from Cell Signaling Technology (Danvers,
MA, USA). Mouse anti-β-actin antibody (1:3,000 dilution) was
purchased from Sigma. Goat anti-mouse and goat anti-rabbit
horseradish peroxidase-conjugated IgG were obtained from Jackson
ImmunoResearch (West Grove, PA, USA). ECL Western Blotting
Detection reagents were obtained from Genedepot (Barker, TX,
USA).
Cells, cell culture and siRNA
transfection
Human ovarian carcinoma SKOV3 cells (from ATCC) were
maintained in DMEM/F12 media supplemented with 10% fetal bovine
serum, 3% L-glutamine and 1% streptomycin/penicillin at 37°C in a
humidified incubator containing 5% CO2 in air.
FOXO3-siRNA and control-siRNA were obtained from Santa Cruz
Biotechnology. Cells were transfected with FOXO3-siRNA or
control-siRNA by using DharmaFECT 1 transfection reagent (Thermo
Scientific, Rockford, IL, USA), according to the manufacturer’s
instructions and as described previously (19).
MTT cell viability assay
A 200 μl aliquot of cells (1×103 cells in
media) was added to each well of a 96-well plate and incubated for
18 h at 37°C in a humidified incubator containing 5% CO2
in air. After incubation, each dose (0, 50, 100, 200 and 400 nM) of
auranofin was added into the wells for 72 h for the dose-dependent
response assay and 100 nM of auranofin was added into the wells for
0, 24, 72 and 120 h for the time-dependent response assay. Control
cultures were treated with DMSO. After incubation, a 20 μl MTT
solution (5 mg/ml in phosphate buffer) was added to each well and
the incubation continued for 4 h, after which time the solution was
carefully removed. The blue crystalline precipitate was dissolved
in DMSO 200 μl. The visible absorbance at 560 nm of each well was
quantified using a microplate reader.
Cell counting assay
SKOV3 cells (1×104) were seeded in 6-cm
dishes and incubated at 37°C in a humidified incubator containing
5% CO2 in air incubator for 18 h. After incubation,
cells were treated with DMSO as control vehicle and the indicated
concentration of auranofin (100 nM) for 0, 24, 72 and 120 h. Each
day, cell numbers were measured by using a hemocytometer.
Colony formation assay
SKOV3 cells (0.5×103) were seeded in 6-cm
dishes and incubated at 37°C in a humidified incubator containing
5% CO2 in air incubator for 18 h. After incubation,
cells were treated with DMSO as control vehicle and the indicated
concentration of auranofin (100 nM) for 7 days. The colonies were
washed twice with PBS, fixed with 3.7% paraformaldehyde and stained
with 1% crystal violet solution in distilled water.
Western blot analysis
Western blot analysis was performed as described
previously (11,12,19).
Briefly, cells were washed with PBS and lysed in lysis buffer (50
mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.1% SDS, pH
8.0) with protease and phosphatase inhibitors. Cell lysates were
centrifuged (10,000 × g, 4°C, 10 min) and the supernatants were
separated on 6 or 10% SDS-PAGE gels and blotted onto nitrocellulose
membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes
were blocked in 3% non-fat dry milk for 1 h at room temperature and
probed with appropriate antibodies. Membranes were then probed with
HRP-tagged anti-mouse or anti-rabbit IgG antibodies diluted
1:5,000–1:15,000 in 3% non-fat dry milk for 1 h at room
temperature. Chemiluminescence was detected using enhanced ECL.
Cytoplasmic and nuclear protein
fractionation
Cells from each condition were trypsinized,
centrifuged, washed, re-suspended in a cytoplasmic fractional
buffer (10 mM HEPES, pH 8.0, 50 mM NaCl, 500 mM sucrose, 1 mM EDTA,
0.5 mM spermidine, 0.15 mM spermine, 0.2% Triton X-100, 1 mM DTT, 2
μM PMSF and 0.15 U/ml aprotinin) and incubated at 4°C for 30 min on
a rotator. The cell suspension was centrifuged at 10,000 rpm for 30
min at 4°C and the supernatant was collected for cytoplasmic
fraction. The nuclear pellet was washed twice with the washing
buffer (10 mM HEPES pH 8.0, 50 mM NaCl, 25% glycerol, 0.1 mM EDTA,
0.5 mM spermidine and 0.15 mM spermine). The remaining pellet was
re-suspended with a nuclear fractional buffer (10 mM HEPES pH 8,
350 mM NaCl, 25% glycerol, 0.1 mM EDTA, 0.5 mM spermidine and 0.15
mM spermine) and incubated at 4°C for 30 min on a rotator. The
nuclear suspension was centrifuged at 13,000 rpm for 30 min at 4°C,
the supernatant was collected for nuclear fraction. Protein in each
fraction was quantified by the Bradford protein determination
reagent (Bio-Rad Laboratories), using BSA as a standard.
DNA fragmentation assay
SKOV3 cells (2×107 per sample) were
trypsinized, lysed in the lysis buffer (10 mM Tris-HCl, 10 mM EDTA,
0.1% Triton-X 100, 0.1% SDS and pH 7.5) and incubated on ice for 30
min. The lysates were digested with RNase I followed by digestion
with proteinase K. The DNA was extracted by phenol-chloroform (1:1,
v/v), precipitated with 2 volumes of EtOH plus 10% NaAc (3 M, pH
5.2) and then dissolved in distilled water. Equal amounts of the
extracted DNA (2 μg/lane) and size markers (1-kb ladder) were
subjected to electrophoresis on 2% agarose gels, which were stained
with ethidium bromide and photographed.
Statistical analysis
Results are expressed as arithmetic mean ± SEM (the
standard error of the mean). To compare the statistical meaning
between the groups, two-sided unpaired Student’s t-test was used.
All experiments were repeated three times and the representative
data are shown. Statistical analyses were performed using SPSS
software (version 19.0, SPSS Inc., Chicago, IL, USA). Mean
differences with P-values <0.05 were considered statistically
significant.
Results
Auranofin inhibits cell survival or
growth of SKOV3 cells
To examine the potential anticancer activity of
auranofin against ovarian cancer cells, we treated human ovarian
carcinoma SKOV3 cells with auranofin and measured the survival
and/or growth rate of SKOV3 cells using the MTT, cell counting and
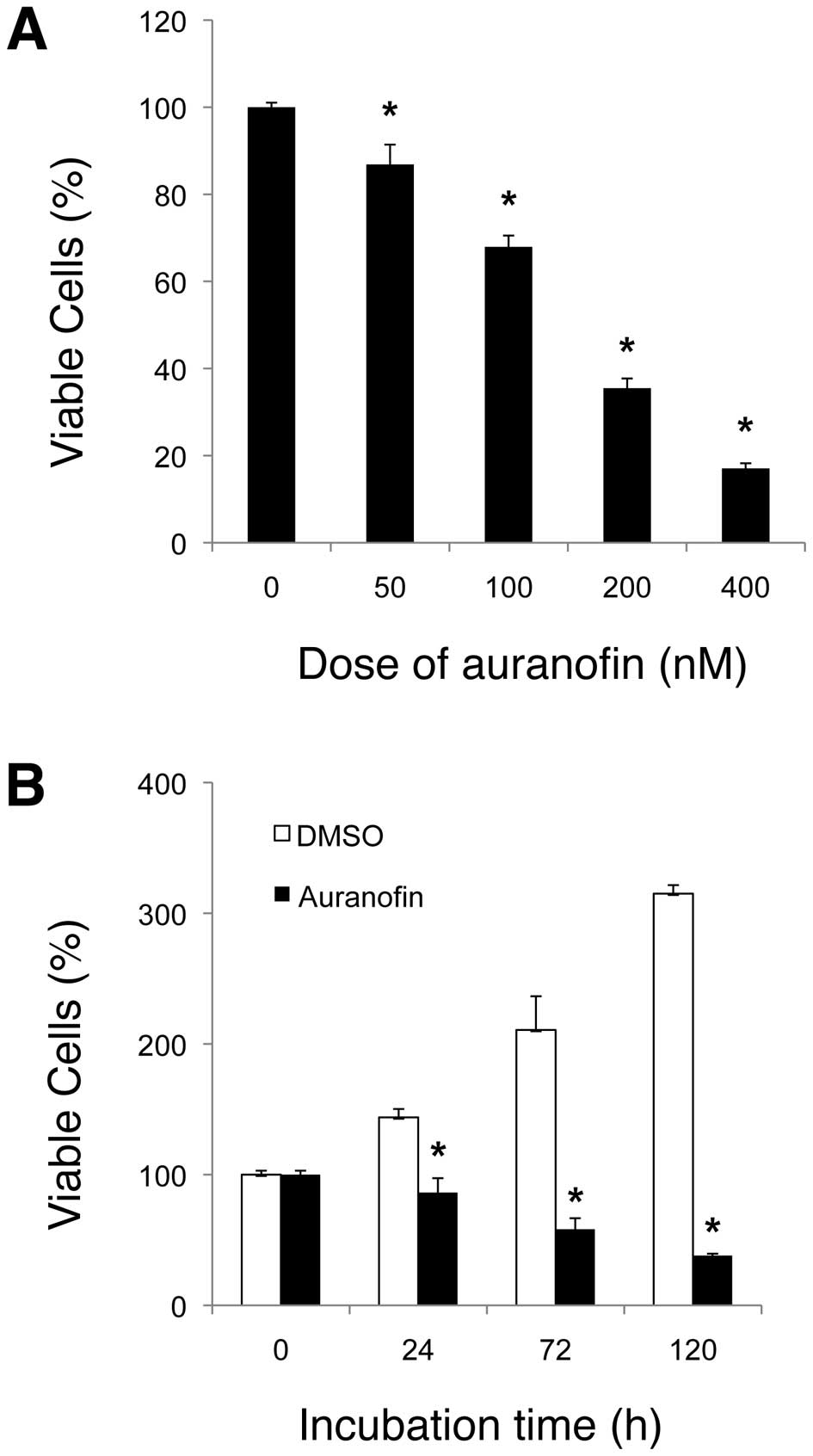
colony formation assays. We showed that auranofin had an inhibitory
effect on SKOV3 cell survival/growth in a dose- and time-dependent
manner. After 72 h incubation, the dose-dependent assay data
indicated that the IC50 value of auranofin on SKOV3 was
~150 nM (Fig. 2A), which is
thought to be relatively low. The time-dependent assay results also
demonstrated the anti-survival/ proliferation activity of auranofin
(Fig. 2B), which was confirmed by
cell counting assay using the same treatment condition of auranofin
against SKOV3 cells (Fig. 3).
After 120 h incubation, the cell number in SKOV3 cells treated with
auranofin (100 nM) was significantly lower (~13 times) than that of
the control (DMSO) treatment. Furthermore, the clonogenic assay
results showed that auranofin treatment significantly suppressed
the colony-forming ability of SKOV3 cells (Fig. 4). Taken together, these results
support that auranofin displays a potent inhibitory effect on cell
survival/proliferation of ovarian carcinoma SKOV3 cells.
Auranofin induces cellular apoptosis in
SKOV3 cells
To examine the effect of auranofin on apoptosis in
SKOV3 cells, we performed western blot analyses and DNA
fragmentation assays. We found that the auranofin treatment (100 nM
for 48 h) increased the cleavage of PARP1 and caspase-3. Auranofin
also upregulated the expression of Bax and Bcl-2 interacting
mediator of cell death (Bim) in SKOV3 cells as compared with the
DMSO control treatment, whereas the auranofin treatment decreased
the Bcl-2 expression level under the same condition (Fig. 5A). These results suggest that
auranofin may exhibit its apoptotic effect through the
caspase-3-mediated mechanism in SKOV3 cells, the upregulation of
the mitochondrial proapoptotic Bax and Bim proteins and the
downregulation of the anti-apoptotic Bcl2 protein expression. Also,
when compared with the DMSO control, treatment of SKOV3 cells with
auranofin (100 nM) for 48 h resulted in an increase in the amount
of DNA fragmentation, a typical marker of apoptosis caused by the
cleaved (active) caspase-3 (Fig.
5B). Collectively, our results show that auranofin treatment
can induce cellular apoptosis in SKOV3 cells.
Auranofin downregulates the expression of
IKK-β and induces the nuclear translocation of FOXO3 protein in
SKOV3 cells
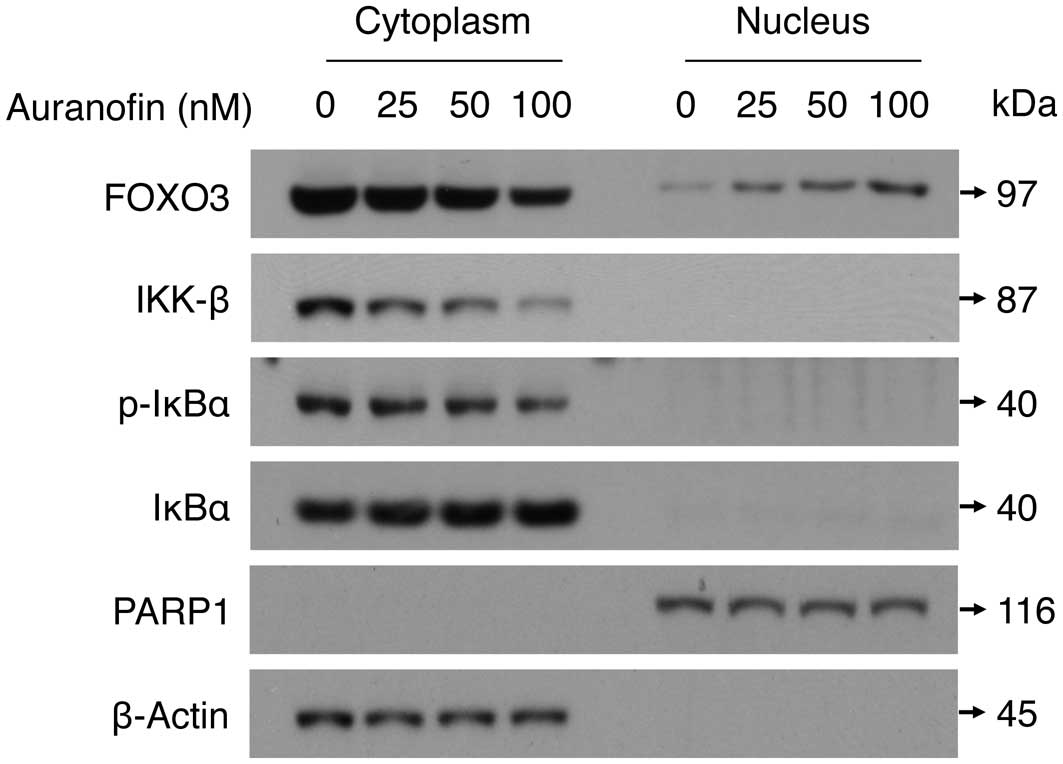
To elucidate the anticancer mechanism of auranofin
treatment against SKOV3 cells, we carried out western blot analyses
with cytoplasmic and nuclear extracts that had been fractionated
from SKOV3 cells previously treated with auranofin. We showed that
auranofin treatment (0, 25, 50 and 100 nM for 48 h) decreased the
expression level of IKK-β protein in the cytoplasm in a
dose-dependent manner. These data were confirmed by a parallel
decrease of the phosphorylation level of IκBα, which is a
well-known substrate of IKK-β, while the total amount of IκBα
protein was not affected by auranofin treatment (Fig. 6). At the same time, the level of
the cytoplasmic FOXO3 protein was decreased by auranofin treatment,
while the level of the nuclear FOXO3 protein was significantly
increased in a dose-dependent manner (Fig. 6). These results suggest that
auranofin may display its anticancer effect through downregulation
of IKK-β, which then triggers the translocation of the FOXO3
protein from the cytoplasm into the nucleus of ovarian cancer
cells.
Auranofin promotes apoptosis in SKOV3
cells in a FOXO3-dependent manner
To examine the role of FOXO3 in auranofin-induced
apoptosis in SKOV3 cells, we silenced FOXO3 expression in SKOV3
cells and analyzed the protein status of PARP1, caspase-3, Bax,
Bim, Bcl-2 and FOXO3 using western blot assays. Interestingly,
knockdown of FOXO3 expression in SKOV3 cells significantly
attenuated the caspase-3-mediated cleavage of PARP1 and caspase-3
proteins and reduced the expression levels of Bax and Bim, extra
large (EL) isoform, when these cells were treated with auranofin
(Fig. 7). In contrast, silencing
FOXO3 decreases the repressive effect of auranofin on Bcl-2
expression. Collectively, these results suggest that FOXO3 may play
an essential role in promoting the caspase-3-mediated apoptosis
after auranofin treatment in SKOV3 cells.
Discussion
FOXO3 has received great attention as a potential
prognostic biomarker for overall survival in patients with cancer
because several clinical studies with primary tumor specimens have
indicated that FOXO3′s nuclear exclusion or downregulation
correlates significantly with poor prognosis and survival in breast
and ovarian carcinomas (12,32–34).
These findings also suggest that small molecules that can induce
nuclear localization (activation) of FOXO3 in cancer cells may
become promising antitumor chemotherapeutic drugs. The promotion of
nuclear localization of the FOXO transcription factors by
gold-containing compounds such as auranofin has not been reported
and the roles of FOXO transcription factors in auranofin-mediated
cellular apoptosis have not been determined. In this study, our
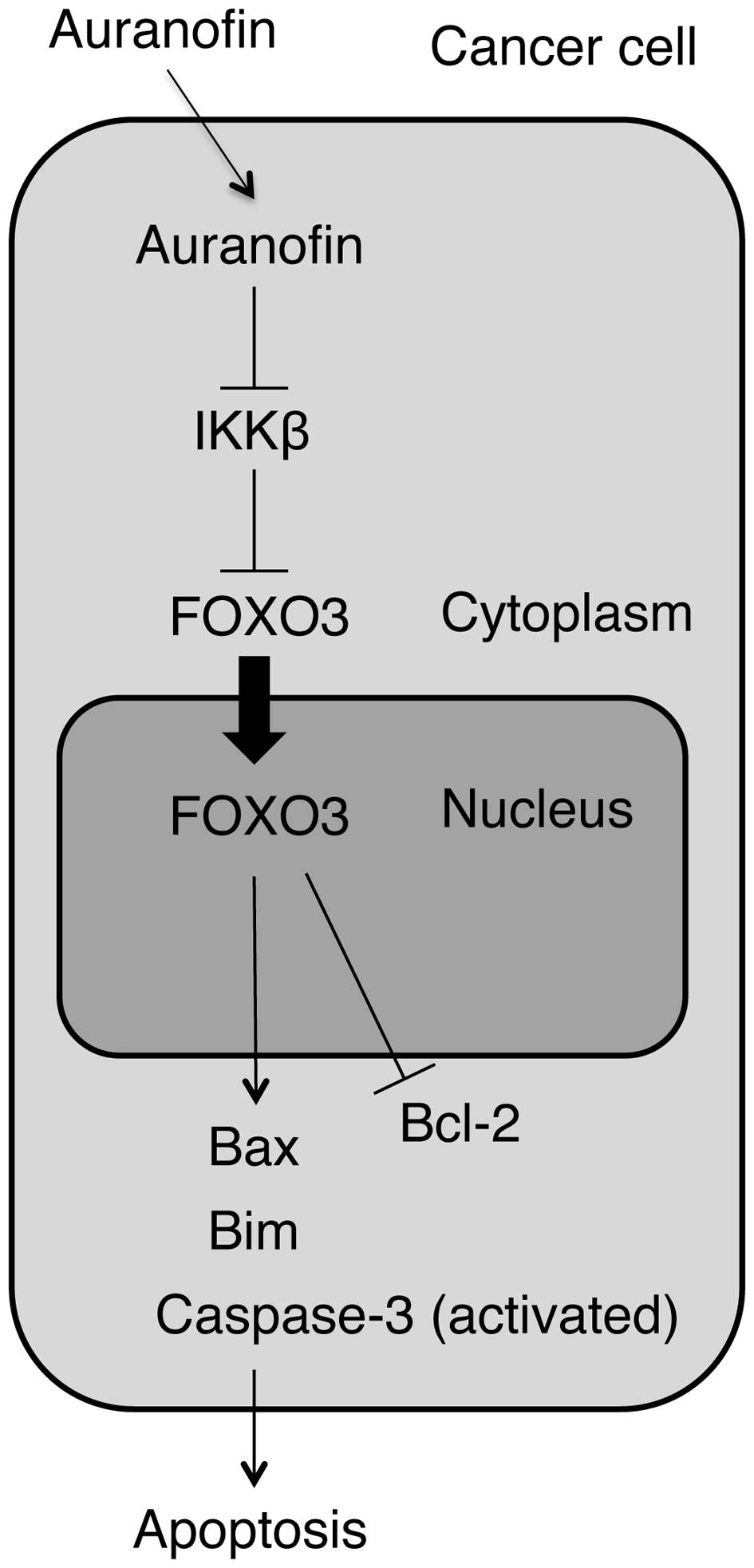
results provide the first evidence that auranofin promotes the
FOXO3 protein to translocate from the cytoplasm into the nucleus,
where it upregulates the expression of the target genes Bax and Bim
and downregulates the expression of Bcl-2 (an important gene
regulating cell survival) in ovarian cancer cells. Because this
phenomenon is discovered in a p53-null cell line SKOV3, it suggests
that activation of FOXO3, Bax and Bim by auranofin may be through a
mechanism independent of p53. It is known that the tumor suppressor
p53 plays a key role in genotoxic stress responses including repair
of DNA damage, cell cycle arrest and apoptosis, which are
complicated and mostly through a p53-dependent pathway (38–40).
Therefore, we propose a new mechanism by which FOXO3 induces
apoptosis in ovarian cancer cells in response to auranofin
treatment through upregulation of Bax and Bim and downregulation of
Bcl-2 in a p53-independent manner (Fig. 8).
We found that auranofin treatment increased the
cleavage of PARP1 and caspase-3 in SKOV3 cells, while the level of
parental caspase-3 protein appear to be not significantly affected
by auranofin treatment (Figs. 5A
and 7). These results suggest that
auranofin may exhibit caspase-3-mediated apoptotic effect through
the activation of caspase-3 protein instead of through the
upregulation of caspase-3 expression in SKOV3 cells. Interestingly,
silencing the expression of FOXO3 in SKOV3 cells significantly
reduced the caspase-3-mediated cleavage of PARP1 and caspase-3
proteins (Fig. 7). Currently,
there is no literature to our knowledge that demonstrates FOXO3 is
required for the caspase-3-mediated cleavage of PARP1 and caspase-3
proteins and for auranofin-induced apoptotic signaling in cancer
cells. However, it remains largely unknown how FOXO3 regulates the
activation of caspase-3 protein in cancer cells in response to
auranofin treatment. It is of interest to further elucidate the
molecular mechanisms governing the control of the FOXO3-mediated
activation of caspase-3 protein in promoting the apoptotic
signaling pathways in cancer cells.
As an anti-rheumatoid arthritis agent, auranofin has
been shown to inhibit the activation of NF-κB by blocking IKK
activity in the LPS-stimulated RAW 264.7 mouse macrophages
(8). In this study, our results
provide initial evidence of the anticancer effect of auranofin on
human ovarian cancer cells, in which auranofin treatment
downregulates the expression of IKK-β and reduces the level of
phospho-IκBα (p-IκBα) (Fig. 6).
Using the mutant forms of IKK-α and IKK-β subunits, Jeon et
al suggest a possible inhibitory mechanism to explain how
auranofin inhibited IKK activity in vitro (8). According to their report, a
substitution of cystine-179 of IKK-β with alanine (mutant
IKK-β-179A) made IKK-β resistant to inhibition by auranofin.
However, a similar protective effect was not observed with IKK-α
mutant. This result indicates that auranofin inhibited the two
subunits of IKK in a different mode and that the inhibition of IKK
activation induced by inflammatory signals in the auranofin-treated
cells may be via its interaction with cystine-179 of IKK-β. It will
be interesting to examine whether or not auranofin treatment
exhibits an inhibitory effect on mutant IKK-β-179A activity in
human cancer cells. Previously, our laboratory identified a novel
mechanism by which cancer cells can impair the tumor suppressive
function of FOXO3 protein by the oncogenic IKK-β-mediated
phosphorylation of the serine-644 residue in FOXO3 (FOXO3-pS644)
protein that leads to the translocation of FOXO3 from the nucleus
into the cytoplasm in cancer cells (11,12).
Subsequently, βTrCP1, an E3 Ub-ligase, interacts with the
FOXO3-pS644 protein and induces the Ub-mediated degradation of
FOXO3, resulting in the promotion of tumorigenesis and tumor growth
in vivo (11). This
IKK-β-mediated tumorigenic mechanism is consistent with and
supports our current finding that auranofin treatment can
downregulate the expression of IKK-β and promote FOXO3 nuclear
localization to regulate the expression of its downstream target
genes, resulting in the suppression of cell survival/growth and the
promotion of cellular apoptosis in ovarian cancer cells.
From the standpoint of cancer therapy, the initial
standard surgical management certainly plays an important and
essential role in ovarian cancer treatment. Most patients will have
appropriate surgical staging followed by optimal surgical
cytoreduction, with the goal being to remove all gross disease
before starting chemotherapy (2).
There are a number of important issues to be resolved in the
management of ovarian cancer that relates to surgery, including the
role of surgical interval cytoreduction, as well as the role of
second-look laparotomy (3). There
are basically three initial chemotherapy options considered the
standard of care at the present time for the treatment of ovarian
cancer. The first is the use of carboplatin and paclitaxel. The
second is a cisplatin and paclitaxel regimen. The third is the use
of a carboplatin and docetaxel regimen. Unfortunately, in the
second-line setting for the treatment of ovarian cancer, we have
far fewer encouraging data based upon randomized controlled trials
that give us definitive answers as to optimal management in the
malignancy. A considerable number of patients harbor ovarian tumor
refractory to these therapies and the majority of the initially
responsive tumors become resistant to treatments (41). Thus, the development of novel
targeted therapy that retains activity against
chemotherapy-resistant ovarian cancer is an unmet medical need.
Since auranofin is an FDA-approved small-molecule drug, it can be
expedited for future clinical trials as a promising anticancer
therapeutics and save time and money for the required pre-clinical
investigation and toxicity testing for new compounds (42).
Finally, further investigation of the signaling
mechanisms by which auranofin downregulates the expression of
antiapoptotic proteins IKK-β and Bcl-2 that in turn contributes to
tumor cell survival/growth may provide new cellular targets that
can be exploited therapeutically. On the other hand, further
elucidation of the molecular mechanisms underlying the
FOXO3-mdiated pro-apoptotic signaling pathways induced by auranofin
is necessary for understanding the molecular basis of auranofin
anticancer activity and its application as a new anticancer
therapy.
Acknowledgements
We thank the Freidenrich Center for Translational
Research for generously providing support. This study was supported
in part by R01 grant CA113859 (to M.C.T.H.) from the National
Cancer Institute, National Institutes of Health, the 2012
Developmental Cancer Research Award from Stanford Cancer Institute
(to M.C.T.H.), a grant 02-2013-051 from the Avon Foundation for
Women (to M.C.T.H.), and the 2012 Ann Schreiber Research Award from
the Ovarian Cancer Research Fund (to S.H.P.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Banerjee S and Kaye SB: New strategies in
the treatment of ovarian cancer: current clinical perspectives and
future potential. Clin Cancer Res. 19:961–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shigetomi H, Higashiura Y, Kajihara H and
Kobayashi H: Targeted molecular therapies for ovarian cancer: an
update and future perspectives. Oncol Rep. 28:395–408.
2012.PubMed/NCBI
|
|
4
|
Snyder RM, Mirabelli CK and Crooke SJ: The
cellular pharmacology of auranofin. Semin Arthritis Rheum.
17:71–80. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madeira JM, Gibson DL, Kean WF and
Klegeris A: The biological activity of auranofin: implications for
novel treatment of diseases. Inflammopharmacology. 20:297–306.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Columbo M, Galeone D, Guidi G,
Kagey-Sobotka A, Lichtenstein LM, Pettit GR and Marone G:
Modulation of mediator release from human basophils and pulmonary
mast cells and macrophages by auranofin. Biochem Pharmacol.
39:285–2891. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rigobello MP, Scutari G, Boscolo R and
Bindoli A: Induction of mitochondrial permeability transition by
auranofin, a gold(I)-phosphine derivative. Br J Pharmacol.
136:1162–1168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeon KI, Jeong JY and Jue DM:
Thiol-reactive metal compounds inhibit NF-κB activation by blocking
IκB kinase. J Immunol. 164:5981–5989. 2000.PubMed/NCBI
|
|
9
|
Kim IS, Jin JY, Lee IH and Park SJ:
Auranofin induces apoptosis and when combined with retinoid acid
enhances differentiation of acute promyelocytic leukaemia cells in
vitro. Br J Pharmacol. 142:749–755. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SJ and Kim IS: The role of p38 MAPK
activation for auranofin-induced apoptosis of human promyelocytic
leukaemia HL-60 cells. Br J Pharmacol. 146:506–513. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai WB, Chung YM, Zou Y, Park SH, Xu Z,
Nakayama K, Lin SH and Hu MC: Inhibition of FOXO3 tumor suppressor
function by βTrCP1 through ubiquitin-mediated degradation in a
tumor mouse model. PLoS One. 5:e111712010.
|
|
12
|
Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang
F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R and Hung
MC: IkappaB kinase promotes tumorigenesis through inhibition of
forkhead FOXO3a. Cell. 117:225–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katoh M and Katoh M: Human FOX gene
family. Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
14
|
Alvarez B, Martinez AC, Burgering BM and
Carrera AC: Forkhead transcription factors contribute to execution
of the mitotic programme in mammals. Nature. 413:744–747. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Furukawa-Hibi Y, Kobayashi Y, Chen C and
Motoyama N: FOXO transcription factors in cell-cycle regulation and
the response to oxidative stress. Antioxid Redox Signal. 7:752–760.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sunters A, Fernández de Mattos S, Stahl M,
Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH,
Coombes RC and Lam EW: FOXO3 transcriptional regulation of Bim
controls apoptosis in paclitaxel-treated breast cancer cell lines.
J Biol Chem. 278:49795–49805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung YM, Park SH, Tsai WB, Wang SY, Ikeda
MA, Berek JS, Chen DJ and Hu MC: FOXO3 signalling links ATM to the
p53 apoptotic pathway following DNA damage. Nat Commun. 3:10002012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tran H, Brunet A, Grenier JM, Datta SR,
Fornace AJ Jr, DiStefano PS, Chiang LW and Greenberg ME: DNA repair
pathway stimulated by the forkhead transcription factor FOXO3a
through the Gadd45 protein. Science. 296:530–534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai WB, Chung YM, Takahashi Y, Xu Z and
Hu MC: Functional interaction between FOXO3 and ATM regulates DNA
damage response. Nat Cell Biol. 10:460–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J-Y, Xia W and Hu MC: Induction of
FOXO3a and Bim expression in response to ionizing radiation. Int J
Oncol. 29:643–648. 2006.PubMed/NCBI
|
|
23
|
Sunters A, Madureira PA, Pomeranz KM,
Aubert M, Brosens JJ, Cook SJ, Burgering BM, Coombes RC and Lam EW:
Paclitaxel-induced nuclear translocation of FOXO3 in breast cancer
cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res.
66:212–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nemoto S, Fergusson MM and Finkel T:
Nutrient availability regulates SIRT1 through a forkhead-dependent
pathway. Science. 306:2105–2108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seoane J, Le HV, Shen L, Anderson SA and
Massagué J: Integration of Smad and forkhead pathways in the
control of neuroepithelial and glioblastoma cell proliferation.
Cell. 117:211–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paik JH, Kollipara R, Chu G, Ji H, Xiao Y,
Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S,
Gilliland DG, Chin L, Wong WH, Castrillon DH and DePinho RA: FoxOs
are lineage-restricted redundant tumor suppressors and regulate
endothelial cell homeostasis. Cell. 128:309–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyamoto K, Araki KY, Naka K, Arai F,
Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen
C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M,
Motoyama N, Suda T and Hirao A: FOXO3 is essential for maintenance
of the hematopoietic stem cell pool. Cell Stem Cell. 1:101–112.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang H and Tindall DJ: FOXO factors: a
matter of life and death. Future Oncol. 2:83–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu MC and Hung MC: Role of IkappaB kinase
in tumorigenesis. Future Oncol. 1:67–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eijkelenboom A and Burgering BM: FOXOs:
signalling integrators for homeostasis maintenance. Nat Rev Mol
Cell Biol. 14:83–97. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu M, Zhao Y, Xu F, Wang Y, Xiang J and
Chen D: The expression and prognosis of FOXO3a and Skp2 in human
ovarian cancer. Med Oncol. 29:3409–3915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fei M, Zhao Y, Wang Y, Lu M, Cheng C,
Huang X, Zhang D, Lu J, He S and Shen A: Low expression of Foxo3a
is associated with poor prognostic in ovarian cancer patients.
Cancer Invest. 27:52–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Habashy HO, Rakha EA, Aleskandarany M,
Ahmed MA, Green AR, Ellis IO and Powe DG: FOXO3a nuclear
localization is associated with good prognosis in luminal-like
breast cancer. Breast Cancer Res Treat. 129:11–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumari SR, Mendoza-Alvarez H and
Alvarez-Gonzalez R: Functional interactions of p53 with
poly(ADP-ribose) polymerase (PARP) during apoptosis following DNA
damage: covalent poly(ADP-ribosyl)ation of p53 by exogenous PARP
and noncovalent binding of p53 to the M(r) 85,000 proteolytic
fragment. Cancer Res. 58:5075–5078. 1998.
|
|
38
|
Carvajal LA and Manfredi JJ: Another fork
in the road - life or death decisions by the tumour suppressor p53.
EMBO Rep. 14:414–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida K and Miki Y: The cell death
machinery governed by the p53 tumor suppressor in response to DNA
damage. Cancer Sci. 101:831–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Das S, Boswell SA, Aaronson SA and Lee SW:
P53 promoter selection: choosing between life and death. Cell
Cycle. 7:154–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fung-Kee-Fung M, Oliver T, Elit L, Oza A,
Hirte HW and Bryson P: Optimal chemotherapy treatment for women
with recurrent ovarian cancer. Curr Oncol. 14:195–208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chong CR and Sullivan DJ Jr: New uses for
old drugs. Nature. 448:645–646. 2007. View Article : Google Scholar : PubMed/NCBI
|