Introduction
Lung cancer has been and remains one of the most
common malignancy in the world with an estimated 1.6 million new
cases per annum (1). Small-cell
lung cancer (SCLC), which constitutes between 10 and 15% of all
lung neoplasm and is different from non-small-cell lung cancer
(NSCLC) types in that it has neuroendocrine features, grows more
rapidly, spreads earlier and has a lower cure rate (1,2).
Furthermore, SCLC metastasizes rapidly to distant sites within the
body and is most often discovered after it has spread extensively
(1,3).
Whilst SCLC is initially sensitive to chemotherapy
and radiotherapy, relapse is almost inevitable and the efficacy of
treatment beyond first-line therapy diminishes as it becomes
increasingly resistant to treatment (1–3).
Although continuous efforts to improve clinical outcomes in
patients with SCLC by progressing clinical trials with novel
promising drugs are ongoing, platinum (such as cisplatin)-etoposide
doublets still represent the gold standard of a first-line therapy
(1). Furthermore, the 5-year
survival rates have not improved significantly over the last 40
years and have currently plateaued, although advance in diagnosis
and treatment have resulted in improved survival of other
solid-tumor malignancies (1).
Therefore, there is an obvious need to develop new therapeutic
strategies for SCLC.
Pyrrolidine dithiocarbamate (PDTC), a thiol compound
derived from dithiocarbamates, was initially described as an
antioxidant agent and reported to induce cytotoxicity in some tumor
cell lines (4,5). On the other hand, PDTC has been
described to act as a pro-oxidant agent (6–9). In
fact, its pro-oxidant activity has been demonstrated to induce
growth inhibition and cell death in rat cortical astrocytes
(6), a human pancreatic
adenocarcinoma cell line, PaGa44 (10) and promyelocytic leukemic cell line,
HL-60 (11), respectively. It
should be noted that PDTC has been demonstrated to sensitize human
cervical cancer line and metastatic renal cell carcinoma to
cisplatin (12,13). As mentioned above, cisplatin-based
regimens have become and remain the first-line treatment for SCLC
from the 1980s (14,15). Therefore, we hypothesize that PDTC
may be a promising candidate of adjunct therapeutic reagent for
SCLC. However, the effect of PDTC in SCLC cell lines, alone or in
combination with cisplatin, has not yet been investigated.
Increased intracellular Cu content has been
associated with PDTC-mediated cytotoxicity due to its ionophore
properties (6,9,11).
Furthermore, the presence of CuCl2 has been demonstrated
to potentiate the cytotoxic effects of PDTC in rat cortical
astrocytes and HL-60 cells (6,11).
Intracellular Cu homeostasis is well known to be maintained by Cu
transporters including copper-transporting P-type adenosine
triphosphatase (ATP7A) responsible for Cu efflux, and copper
transporter 1 (CTR1) responsible for Cu uptake (16). Moreover, ATP7A has been reportedly
associated with platinum drug resistance in various types of solid
tumors (16–18). It is especially noteworthy that
ATP7A plays a critical role in cisplatin-resistance of NSCLC, and
is a negative prognostic factor of NSCLC patients treated with
platinum-based chemotherapy (19–21).
However, to our knowledge, little is known about the expression of
Cu transporters in SCLC cell lines as well as the impact of PDTC on
their expression.
In the present study, we investigated first the
cytocidal effects of PDTC on SCLC cell lines in view of growth
inhibition, cell cycle arrest and apoptosis induction. We further
evaluated whether oxidative stress is involved in the cytocidal
effects by determining alterations in intracellular reactive oxygen
species (ROS) level along with expression levels of
oxidative-related genes. At the same time, the effect
N-acetyl-l-
cysteine (NAC), a free radical scavenger, on PDTC-mediated
cytotoxicity was also evaluated. We further investigated the
cytocidal effect of PDTC in combination with CuCl2 in
the presence or absence of bathocuproine disulfonate (BCPS), a
non-permeable copper-specific chelator, in order to verify whether
Cu is involved in the cytocidal effect. Importantly, we
investigated for the first time the effect of PDTC on the
expression levels of copper transporters such as ATP7A and CTR1,
and further evaluated the effects of cisplatin in combination with
non-toxic dose of PDTC on SCLC cell lines.
Materials and methods
Materials
PDTC, RPMI-1640 medium, CuCl2, NAC, and
ISOGEN (Wako Pure Chemical Industry, Osaka, Japan); fetal bovine
serum (FBS) (Nichirei Biosciences, Tokyo, Japan); agarose X (Nippon
Gene, Tokyo, Japan); dichlorofluorescin diacetate (DCFH-DA)
(Invitrogen, Carlsbad, CA, USA); propidium iodide (PI),
ribonuclease A (RNaseA),
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)
carbony]-2H-tetrazolium hydroxide (XTT), BCPS and cisplatin
(Sigma-Aldrich, St. Louis, MO, USA) were purchased from indicated
suppliers.
Cell culture and treatment
Human SCLC cell lines (NCI-H196 and NCI-H889) and
normal human embryonal lung fibroblast MRC-5 cells were obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). SCLC cell lines and MRC-5 cells were cultured in RPMI-1640
medium and Eagle’s minimum essential medium (MEM), respectively,
supplemented with 10% heat-inactivated FBS, 100 U/ml of penicillin
and 100 μg/ml of streptomycin at 37°C in a humidified atmosphere
(5% CO2 in air). Cells were treated with indicated
concentrations of PDTC for the indicated time-points. In order to
investigate whether oxidative stress is involved in PDTC-induced
cytotoxicity, cells were treated with 0.3 or 1 μM PDTC in the
presence or absence of 1 or 2 mM NAC for 24 h. Moreover, in order
to clarify whether copper ions are implicated in the PDTC-induced
cytotoxicity, cells were treated with CuCl2 (10 μM) and
BCPS (50 μM) (a non-permeable copper-specific chelator), alone or
in combination, for 3 h prior to treatment with 0.1 and 0.3 μM PDTC
in the presence or absence of these reagents. Cells were also
treated with 5 μM cisplatin in the presence or absence of 0.1 μM
PDTC for 24 h to investigate whether PDTC sensitize SCLC cell lines
to cisplatin. Cell viability was measured by the XTT assay as
described previously (22).
IC50 value of PDTC was ~0.3 μM calculated from the cell
proliferation inhibition curve of NCI-H196 cells after 24-h
treatment.
DNA fragmentation analysis
DNA fragmentation analysis was carried out according
to a method described previously (23). Briefly, DNA samples [~20 μg DNA/20
μl TE buffer (10 mM Tris-HCl, pH 7.8, 1 mM EDTA)] and a Tracklt™
100 bp DNA Ladder (Invitrogen) as a DNA size marker were
electrophoresed, respectively, on a 2% agarose X gel, and
visualized by ethidium bromide staining, followed by viewing under
UV Light Printgraph (ATTO Corp., Tokyo, Japan).
Cell cycle analysis
After treatment with the IC50 value of
PDTC at 0.3 μM for 24 h, cell cycle analysis was performed using a
FACSCanto flow cytometer (Becton-Dickinson, CA, USA) according to
methods reported previously with modifications (24,25).
A total of 10,000 events were acquired and Diva software and ModFit
LT™ Ver.3.0 (Verity Software House, ME, USA) were used to calculate
the number of cells at each sub-G1,
G0/G1, S and G2/M phase
fraction.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA isolation and complementary DNA were
prepared according to methods described previously with
modifications (23). Total RNA was
extracted from cells using an RNA extraction kit, ISOGEN.
Complementary DNA was synthesized from 1 μg of RNA using 100 pmol
random primer and 50 U M-MLV RT in a total volume of 20 μl,
according to the manufacturer’s instructions. PCR was performed
according to the methods previously described (23) using a Takara Thermal Cycler MP
(Takara Shuzo Co., Osaka, Japan). DNA sequences of PCR primers and
optimal conditions for PCR are shown in Table I. PCR primers were purchased from
Sigma-Aldrich (Hokkaido, Japan). PCR products and 100 bp DNA Ladder
were electrophoresed on a 2% UltraPure™ agarose gel (Invitrogen),
respectively, and visualized by ethidium bromide staining, followed
by viewing under UV Light Printgraph. The relative expression
levels of target gene/β-actin gene were calculated as the ratios
against those at 0-time using ImageJ 1.46 m (Wayne Rasband,
USA).
 | Table IRT-PCR analysis. |
Table I
RT-PCR analysis.
| A, PCR primers and
optimal numbers of PCR cycle |
|---|
|
|---|
| Target mRNA | | DNA sequence of PCR
primer | Optimal cycles |
|---|
| Cox-2 | Sense |
5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ | 36 |
| Antisense |
5′-AGATCATCTCTGCCTGAGTATCTT-3′ | |
| HO-1 | Sense |
5′-CCAGCAACAAAGTGCAAGATTC-3′ | 27 |
| Antisense |
5′-CTGCAGGAACTGAGGATGCTG-3′ | |
| Mn-SOD | Sense |
5′-GCACTAGCAGCATGTTGAGCCG-3′ | 27 |
| Antisense |
5′-CAGTTACATTCTCCCAGTTGATTAC-3′ | |
| Cu/Zn-SOD | Sense |
5′-GCCTAGCGAGTTATGGCGACGA-3′ | 27 |
| Antisense |
5′-GGCCTCAGACTACATCCAAGG-3′ | |
| Catalase | Sense |
5′-CAGATGGACATCGCCACATG-3′ | 27 |
| Antisense |
5′-AAGACCAGTTTACCAACTGGG-3′ | |
| GPx1 | Sense |
5′-TGGCTTCTTGGACAATTGCG-3′ | 27 |
| Antisense |
5′-CCACCAGGAACTTCTCAAAG-3′ | |
| β-actin | Sense |
5′-CCTTCCTGGGCATGGAGTCCTG-3′ | 24 |
| Antisense |
5′-GGAGCAATGATCTTGATCTTC-3′ | |
|
| B, Conditions for
PCR |
|
| Denaturation
reaction | Annealing
reaction | Extension
reaction |
|
|
|
|
| Target mRNA | Temperature
(°C) | Time (sec) | Temperature
(°C) | Time (sec) | Temperature
(°C) | Time (sec) |
|
| Cox-2 | 94 | 30 | 58 | 30 | 72 | 30 |
| HO-1 | 94 | 60 | 65 | 60 | 72 | 60 |
| Mn-SOD | 94 | 60 | 62 | 60 | 72 | 60 |
| Cu/Zn-SOD | 94 | 60 | 65 | 60 | 72 | 120 |
| Catalase | 94 | 60 | 55 | 120 | 72 | 180 |
| GPx1 | 94 | 60 | 55 | 120 | 72 | 180 |
| β-actin | 94 | 45 | 60 | 45 | 72 | 120 |
Measurement of intracellular ROS
levels
Intracellular ROS levels were analyzed using DCFH-DA
as a ROS-reactive fluorescence probe as described previously
(24,26). In brief, after treatment with 0.3
μM PDTC for 3 or 12 h, cells (1×106 cells) were
suspended in 1 ml of PBS with 5 mM DCFH-DA and incubated for 20 min
at 37°C. Next, cells were washed with PBS twice, and resuspended in
500 μl of 2 μg/ml PI/PBS. The mean fluorescence intensity (MFI) of
DCF green fluorescence from 3×104 cells were acquired
for flow cytometry analysis using a FACSCanto flow cytometer and
Diva software.
Statistical analysis
Data were analyzed using Student’s t-test and
p<0.05 was considered as statistically significant.
Results
Cytocidal effects of PDTC on SCLC cell
lines
After the treatment with various concentrations of
PDTC (0.1, 1 and 10 μM) for 24 h, a significant decrease in cell
viability was observed in a dose-dependent manner in NCI-H196 cells
with IC50 value of 0.3 μM (Fig. 1A). On the other hand, although PDTC
also exhibited dose-dependent cytotoxic activity against NCI-H889,
the IC50 value of PDTC could not be calculated from its
proliferation inhibition curve since >50% of cells survived even
when treated with the highest concentration of PDTC (10 μM)
(Fig. 1A), indicating NCI-H196 is
more sensitive to the cytotoxicity of PDTC compared to NCI-H889.
Moreover, no apparent cytotoxic activity was observed in normal
human embryonal lung fibroblast MRC-5 when treated with the same
range of PDTC concentrations even for 48 h (Fig. 1B). No DNA fragmentation was
observed in either SCLC cell line, indicating no involvement of
apoptosis induction in the PDTC-mediated cytotoxicity (Fig. 1C).
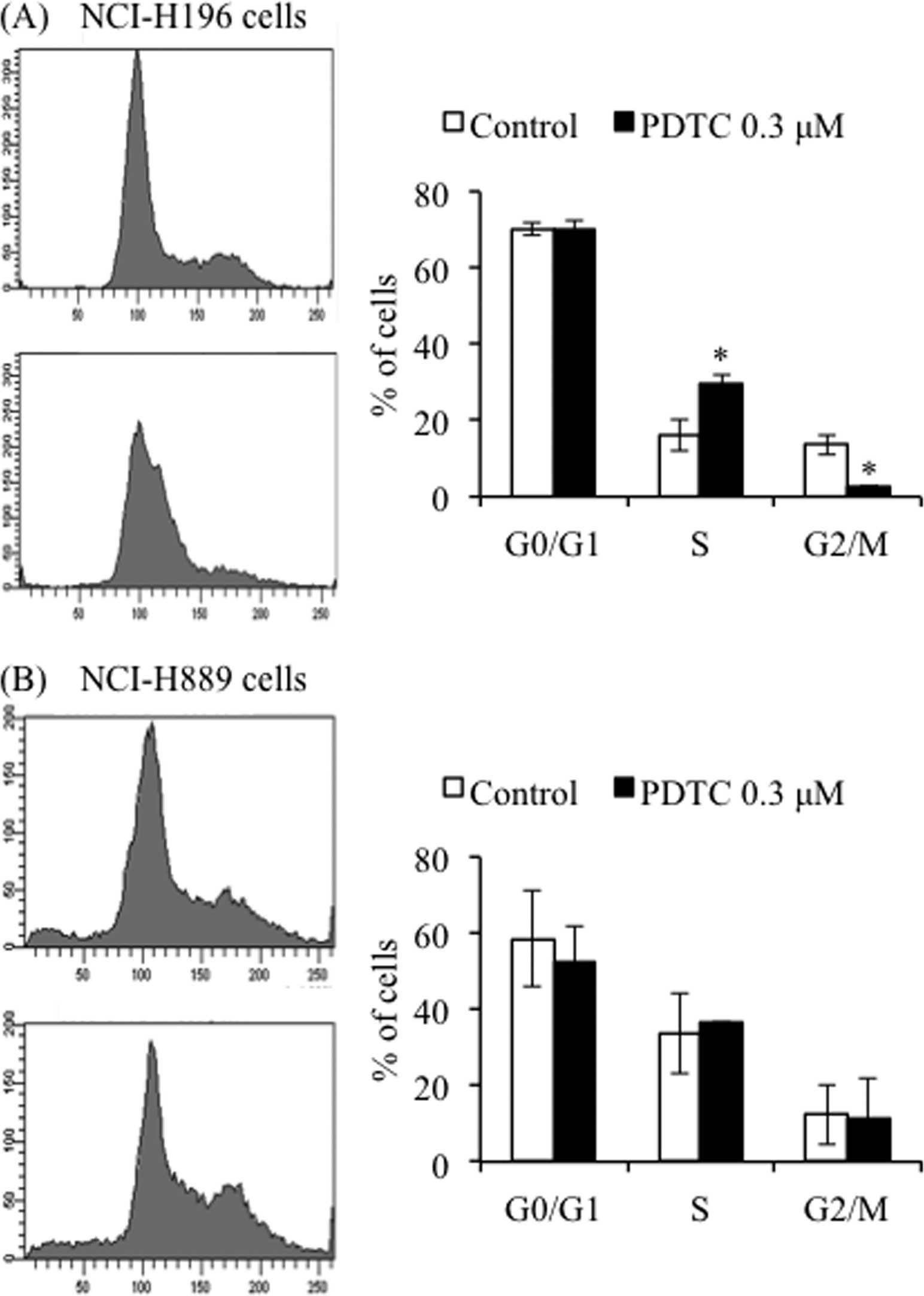
Effects of PDTC on cell cycle of SCLC
cell lines
S phase arrest along with a significant decrease in
the number of cells in G2/M phase was observed in
NCI-H196 cells after treatment with the IC50 value of
PDTC at 0.3 μM for 24 h (Fig. 2A).
However, no apparent alterations of cell cycle were observed in
NCI-H889 cells under the same treatment conditions (Fig. 2B). Furthermore, in agreement with
the results presented in Fig. 1C,
no significant accumulation of cells in sub-G1 phase was
observed in the treated cells (Fig.
2).
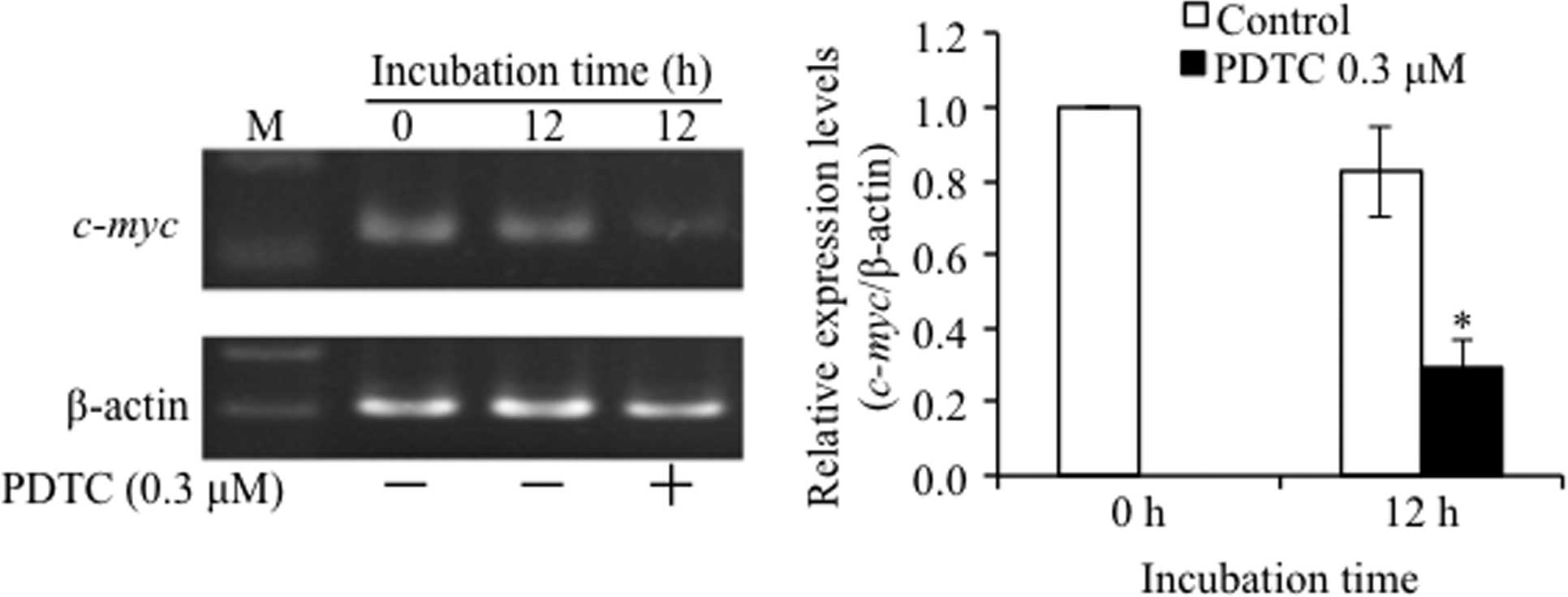
Suppression of c-myc expression in
PDTC-treated NCI-H196 cells
A large body of physiological evidence has shown
that either upregulation or downregulation of c-myc activity
has profound consequences on cell cycle progression (27,28).
Therefore, the alteration of c-myc gene expression was
evaluated in NCI-H196 cells after treatment with 0.3 μM of PDTC for
12 h. As shown in Fig. 3, the
expression level of c-myc gene was significantly suppressed
in PDTC-treated cells when compared to that in untreated cells.
Furthermore, similar phenomena were not observed in NCI-H889 cells
(data not shown).
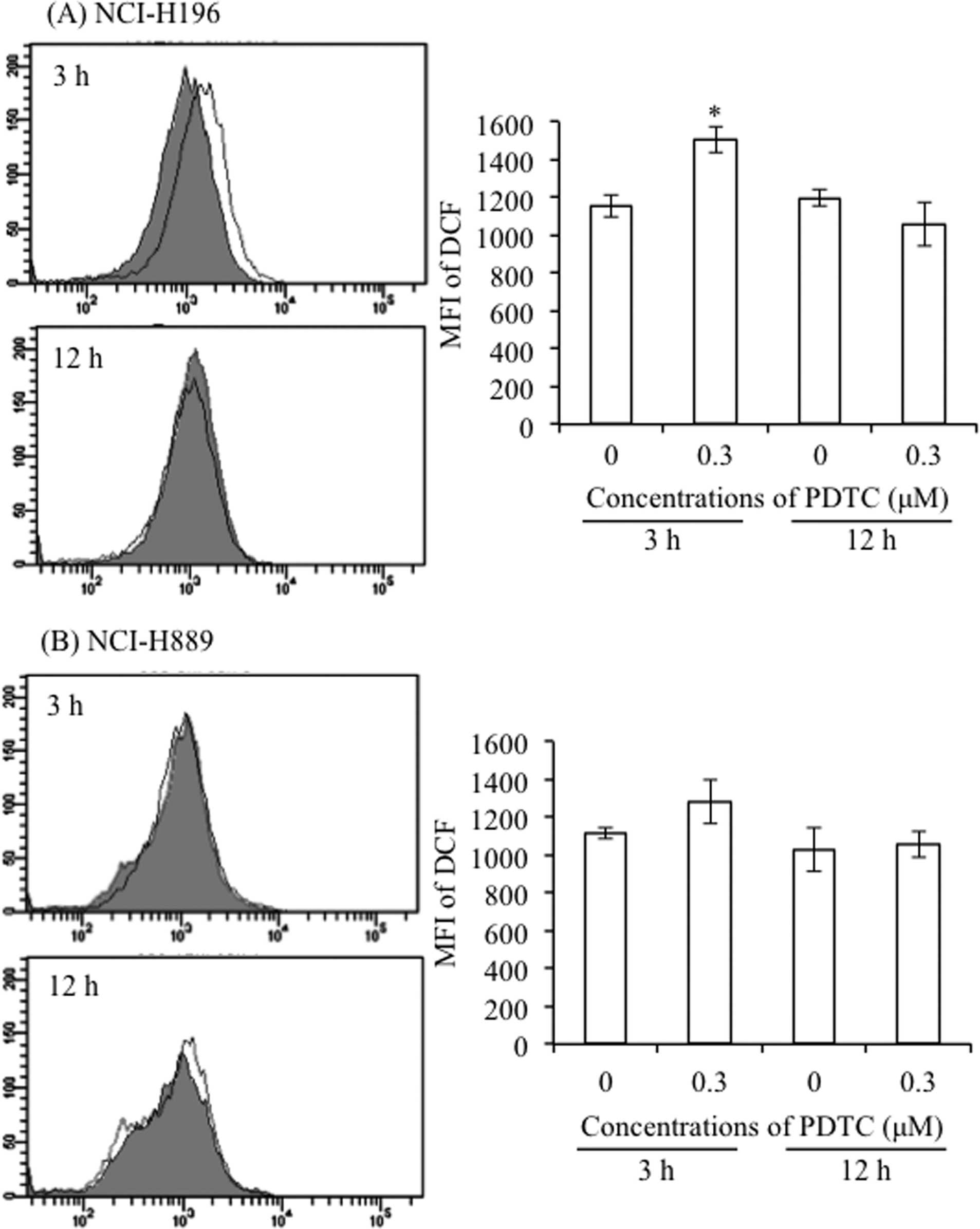
Alteration of intracellular ROS levels in
PDTC-treated SCLC cell lines
Since the pro-oxidant activity of PDTC has been
described to induce growth inhibition and cell death in different
types of cancer cells (10,11),
intracellular ROS levels were evaluated in both SCLC cell lines
after treatment with 0.3 μM of PDTC for 3 and 12 h. FACS analysis
using DCFH-DA as a ROS-reactive fluorescence probe showed that a
transient increase in the intracellular ROS levels was observed in
NCI-H196 cells at 3-h post-treatment, followed by restoration to
the control levels at 12-h post-treatment (Fig. 4A). On the other hand, a slight, but
non-significant, increase in the accumulation of intracellular ROS
was observed in NCI-H889 cells after treatment with PDTC for 3 h
(Fig. 4B).
Expression profiles of oxidative
stress-related mRNAs in PDTC-treated NCI-H196 cells
Since a transient increase in the intracellular ROS
levels was observed in PDTC-treated NCI-H196 cells, expression
profiles of oxidative stress-related gene mRNAs were evaluated in
the cells after treatment with 0.3 and 1 μM of PDTC for 1, 3, 6 and
12 h, respectively. It should be noted that the expression profiles
of these gene mRNAs in cells treated with 0.3 μM PDTC were very
similar to those in cells treated with 1 μM PDTC. As shown in
Fig. 5A and B, a significant
increase in the expression level of Cox-2, which is known to play a
central role in ROS production (29), was observed 6 h after treatment
with PDTC, and the increase continued up to 12 h. Furthermore, the
expression of HO-1, which is closely associated with oxidative
stress mediated cellular damage (30,31),
was significantly induced from as early as 3 h after the treatment
with PDTC. In comparison to untreated cells, the level of HO-1 gene
expression increased approximately 6- or 9-fold after 6- and 12-h
treatment, respectively. Moreover, a significant decrease in the
expression levels of Mn-SOD and Cu/Zn-SOD was observed 3 h after
treatment with PDTC, and continued up to 12 h. A slight but
significant downregulation in catalase expression was observed 1 h
after treatment with PDTC, followed by a substantial decrease at 3-
and 6-h post-treatment. Similar to the expression of other
antioxidant enzymes, the expression levels of GPx1 were also
significantly downregulated at 6- and 12-h post-treatment,
respectively. Moreover, PDTC-mediated alterations in expression
level of the above-mentioned oxidative-related genes were abolished
by the addition of 2 mM NAC, a free radical scavenger (Fig. 5C). However, similar phenomena were
not observed in NCI-H889 cells, except the behavior of HO-1 gene
expression (data not shown).
Blocking of PDTC-induced cytotoxicity by
NAC
To further verify whether oxidative stress is
responsible for the cytotoxicity of PDTC against SCLC cell lines,
we investigated the effects of NAC on the PDTC-induced cytotoxicity
in NCI-H196 cells. As shown in Fig.
6A, dose-dependent cytotoxic effects of PDTC on NCI-H196 cells
were reconfirmed by the reduction in cell viability. Furthermore,
the cells treated with 0.3 μM PDTC for 24 h displayed apparent
morphological changes such as shrinkage, spherical morphology, and
detachment of the cells (Fig. 6B).
In contrast, PDTC-induced reduction in cell viability and
morphological changes were almost completely abrogated by the
addition of 1 or 2 mM NAC (Fig.
6).
Inhibition of enhanced cytotoxicity of
PDTC in combination with CuCl2 by BCPS
As shown in Fig. 7,
no cytocidal effects was observed in either of the SCLC cell lines
after treatment with 0.1 μM PDTC alone for 24 h, consistent with
the results in Fig. 1. However, a
significant decrease in cell viability was observed in both cells
after treatment with 0.1 μM PDTC in combination with 10 μM
CuCl2. Furthermore, a much stronger cytocidal effect
against NCI-H196 cells was confirmed. Intriguingly, the addition of
50 μM BCPS, a non-permeable copper-specific chelator, completely
abrogated the enhanced cytotoxicity of PDTC in combination with
CuCl2. Moreover, after treatment 0.3 μM PDTC alone for
24 h, a significant decrease in the cell viability was observed in
NCI-H196, but not in NCI-H889, reconfirming that NCI-H196 is more
sensitive to the cytotoxicity of PDTC. Similarly, the addition of
50 μM BCPS completely abrogated 0.3 μM PDTC-mediated cytotoxicity
in NCI-H196 cells. When both cells were treated with 0.3 μM PDTC
combined with 10 μM CuCl2, the enhanced cytotoxicity was
significantly abrogated by the addition of 50 μM BCPS. Moreover, no
alteration in cell viability was observed in the cells when treated
with BCPS and CuCl2, either alone or in combination.
Downregulation of expression levels of
ATP7A in PDTC-treated NCI-H196 cells
It has been demonstrated that cellular oxidative
stress induced by PDTC may be attributed to increased intracellular
concentrations of Cu (9,32). Indeed, it has been clarified that
treatment with a relatively high concentration of PDTC (10–100 μM)
markedly increased an intracellular concentrations of Cu, and that
the combination of PDTC (0.1–1 μM) with Cu (1 μM) also
significantly increased the concentrations of intracellular Cu in
parallel with an enhanced cytotoxicity in HL-60 cells and rat
thymocytes, respectively (9,11).
Therefore, the expression levels of ATP7A responsible for Cu
efflux, and CTR1 responsible for Cu uptake were investigated in
PDTC-treated SCLC cell lines. As shown in Fig. 8, in comparison to control at 24-h
time-point, almost no alteration in the expression level of CTR1
was observed in the cells after treatment with 0.3 μM PDTC for 24
h, although there was a trend showing an increase in its expression
level. On the other hand, the expression level of ATP7A
significantly decreased by >50% in NCI-H196 cells, whereas
displayed a slight, but not significant, increase in NCI-H889
cells. Interestingly, the expression level of two Cu transporters
appeared to be affected by medium change, since notable differences
in their expression levels were observed in control groups between
0- and 24-h time-points.
Enhancement of cytotoxicity in NCI-H196
cells treated with combination of cisplatin and PDTC
A correlation between the expression level of Cu
transporters and the degree of the acquired resistance to platinum
drug, known as a first line treatment for SCLC patients, suggested
that Cu transporters play critical roles in regulation of
sensitivity to platinum drugs (16,19–21).
Since the results presented in Fig.
8 showed that treatment with a relatively low concentration of
PDTC significantly downregulated the expression level of ATP7A in
NCI-H196 cells, an investigation into the effects of the
combination of cisplatin and PDTC on the cells were followed in the
study. After the treatment of NCI-H196 cells with various
concentrations of cisplatin alone for 24 h, the relative cell
viabilities were 85, 70 and 25% of control for 5, 10, and 100 μM
cisplatin, respectively. Moreover, a significant decrease in the
cell viability was only observed in NCI-H889 cells when treated
with 100 μM cisplatin (the value of cell viability, 51%).
Intriguingly, treatment with 5 μM cisplatin in combination with
non-toxic dose of 0.1 μM PDTC decreased the cell viability of
NCI-H196 by ~50%, showing a synergistic effect in augmenting
cytotoxicity of cisplatin (Fig.
9). On the other hand, a slight decrease in the cell viability
of NCI-H889 was observed (Fig.
9).
Discussion
In the present study, we demonstrated that PDTC
exhibited a much stronger dose-dependent cytotoxic activity against
NCI-H196 compared to NCI-H889 cells. Furthermore, S phase arrest
along with a significant decrease in the number of cells in
G2/M phase, but not DNA fragmentation, was observed in
NCI-H196 cells. These results suggest that PDTC induces
cytotoxicity in the SCLC cells via cell cycle arrest in the
S-phase, rather than apoptosis induction. Similar phenomena have
also been observed in PDTC-treated human pancreatic adenocarcinoma
cell line, PaCa44 (10).
Interestingly, it has been demonstrated that NCI-H889 is more
sensitive to the cytotoxicity of ABT-737, a small-molecule
inhibitor of Bcl-2, Bcl-XL, and Bcl-w (33), compared to NCI-H196, due to
NCI-H889 having much higher Bcl-2 gene copy number (34). Therefore, the differential
sensitivities of the two SCLC cell lines to PDTC may not be
attributed to the different amounts of Bcl-2 gene number.
Furthermore, consistent with previous findings showing that normal
primary cells including human bone marrow CD34+ cells
and fibroblasts are insensitive to the cytocidal effects of PDTC
(10,35), we demonstrated that no alteration
was observed in cell viability of the human MRC-5 embryonal lung
fibroblasts when treated with toxic doses of PDTC in SCLC cell
lines. These findings imply that normal cells are equipped with
mechanisms by which they respond differently to PDTC effects with
respect to tumor cells, providing further supportive evidence for
the use of PDTC in wide clinical practice.
We further demonstrated that suppression of
c-myc gene expression was observed in PDTC-treated NCI-H196
cells. It has been demonstrated that c-Myc is a critical protein in
controlling proliferation, differentiation and survival of cancer
cells (27,28,36).
It was demonstrated (28) that
c-Myc downregulation caused by resveratrol, a plant polyphenol
naturally occurring in grapes with chemopreventive properties
(37), resulted in cell cycle
arrest at S phase and apoptotic cell death in human medulloblastoma
cells. It was further clarified that transfection of c-myc
directed antisense oligonucleotides to the cells reduced
c-myc expression, and consequently inhibited cell growth
concomitant with cell cycle arrest in the S phase (28). Taken together, we suggested that
c-myc downregulation is a critical molecular event in
PDTC-mediated cell cycle arrest of NCI-H196 cells. In fact, a
previous report demonstrated that treatment of leukemic cell line
U-937 with 100 μM of PDTC markedly downregulated the expression
level of c-myc, associated with cell growth inhibition
(38). Furthermore, Donadelli
et al have demonstrated that PDTC induced
P21WAF1/CIP1 expression post-transcriptionally
via a ROS/ERK mediated mechanism in PaCa44 cells, and consequently
resulted in cell cycle arrest at S phase (10). It should be noted that c-myc
has been reported to be a negative regulator of
P21WAF1/CIP1 expression in lung cancer cells
(39). Therefore, further studies
may be needed to clarify the molecular details of correlation
between c-myc and P21WAF1/CIP1 in
PDTC-treated SCLC cell lines.
We also demonstrated that PDTC induced accumulation
of intracellular ROS in NCI-H196 cells, paralleled with an
alteration of oxidative stress-related gene expression, suggesting
that PDTC acts as a pro-oxidant agent in the cells, although the
antioxidant activity of PDTC has been reported to induce
cytotoxicity in some tumor cell lines, such as colorectal (4) and prostatic carcinoma cells (5). In line with these results, the
addition of NAC, a free radical scavenger, not only abolished
PDTC-triggered alterations of the above-mentioned oxidative
stress-related gene expression but also almost completely abrogated
PDTC-induced reduction in cell viability and morphological changes
associated with cell damage.
In agreement with a previous report showing that
combination treatment with CuCl2 and PDTC enhanced the
cytocidal effects against HL-60 cells (11), we also demonstrated that a
significant decrease in cell viability was observed in SCLC cell
lines after treatment with 0.1 μM PDTC in combination with 10 μM
CuCl2, either of which was a non-toxic dose.
Interestingly, BCPS, a non-permeable copper-specific chelator,
completely abrogated the enhanced cytotoxicity of PDTC in
combination with CuCl2. In this regard, BCPS has been
demonstrated to block the penetration of Cu into cells and prevent
Cu overload, and consequently protect HL-60 cells from the
cytotoxicity caused by PDTC/Cu complex (11). Furthermore, it has been
demonstrated that cellular oxidative stress induced by PDTC may be
attributed to an increased intracellular concentrations of Cu due
to ionophore activity of PDTC (6,9,32).
Taking these previous results and our observations into account, we
suggested that PDTC induced cytocidal effects against SCLC cell
lines by raising intracellular levels of Cu, known to be
responsible for ROS generation via Fenton reaction (32,40).
Intriguingly, after treatment with PDTC, the
expression level of ATP7A, a copper export protein responsible for
maintaining copper homeostasis (16,19–21),
significantly decreased in NCI-H196 cells, whereas almost no
alteration was observed in the expression level of CTR1 responsible
for copper uptake (16). Although
the detailed mechanisms underlying the downregulation of the
expression level of ATP7A are need to clarify, these results
provide a new rationale for the clinical use of PDTC since
expression of ATP7A has been reportedly associated with
platinum-drug resistance in various types of solid tumors (17–19).
It is especially noteworthy that ATP7A overexpression plays an
important role in platinum-resistance of NSCLC, and was a negative
prognostic factor of NSCLC patients treated with platinum-based
chemotherapy (20). Furthermore, a
recent study demonstrated that microRNA-495 enhanced the
sensitivity of NSCLC cells to platinum-drug by modulation of ATP7A
expression (21). Although PDTC
has been demonstrated to sensitize different types of cancer cells
by inhibiting transcription activity of NF-κB (12,13),
the effects of PDTC on the expression of copper transporters still
remains unclear. Therefore, our current findings suggest that PDTC
can raise the intracellular level of Cu via downregulation of ATP7A
expression, result in the accumulation of intracellular ROS, and
consequently cause profound cytotoxicity in SCLC cell lines. Based
on the fact that the expression of ATP7A is closely related with
platinum-resistance in various types of solid tumors including lung
cancer (17,19–21,41),
we further hypothesized that PDTC could probably be able to
sensitize SCLC cells to cisplatin. As expected, combination of much
lower dose of cisplatin (5 μM) and non-toxic dose of PDTC (0.1 μM)
synergistically induced a significant cytotoxicity in NCI-H196
cells, the details of the study on the synergistic effects are
current underway.
In conclusion, PDTC-induced cytocidal effects
against SCLC cells were characterized by the S phase arrest
paralleled with suppression of c-myc expression.
Furthermore, the cytotoxicity triggered by PDTC and
CuCl2, alone or in combination, was significantly
abrogated by the addition of BCPS. Importantly, the downregulation
of ATP7A expression was observed for the first time in PDTC-treated
NCI-H196 cells. These results support the plausibility that
increased accumulation of intracellular Cu as a result of ATP7A
inhibition plays an important role in PDTC-mediated cytotoxicity in
SCLC cell lines, although its exact concentrations were not
evaluated in the current study. Given that ATP7A plays a critical
role in the resistance of platinum-drugs (such as cisplatin)
representing the first-line treatment of SCLC, PDTC could be a
promising candidate of adjunct therapeutic reagent for the patients
requiring treatment with platinum-based regimens.
Acknowledgements
This study was supported in part by grants from the
Ministry of Education, Culture, Sports, Science and Technology and
by the Promotion and Mutual Aid Corporation for Private Schools of
Japan.
References
|
1
|
Chan BA and Coward JI: Chemotherapy
advances in small-cell lung cancer. J Thorac Dis. 5:S565–S578.
2013.PubMed/NCBI
|
|
2
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755.
2011.
|
|
3
|
Jackman DM and Johnson BE: Small-cell lung
cancer. Lancet. 366:1385–1396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chinery R, Brockman JA, Peeler MO, Shyr Y,
Beauchamp RD and Coffey RJ: Antioxidants enhance the cytotoxicity
of chemotherapeutic agents in colorectal cancer: a p53-independent
induction of p21WAF1/CIP1 via C/EBPbeta. Nat Med.
3:1233–1241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herrmann JL, Beham AW, Sarkiss M, et al:
Bcl-2 suppresses apoptosis resulting from disruption of the
NF-kappa B survival pathway. Exp Cell Res. 237:101–109. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen SH, Liu SH, Liang YC, Lin JK and
Lin-Shiau SY: Death signaling pathway induced by pyrrolidine
dithiocarbamate-Cu(2+) complex in the cultured rat cortical
astrocytes. Glia. 31:249–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forman HJ, York JL and Fisher AB:
Mechanism for the potentiation of oxygen toxicity by disulfiram. J
Pharmacol Exp Ther. 212:452–455. 1980.PubMed/NCBI
|
|
8
|
Goldstein BD, Rozen MG, Quintavalla JC and
Amoruso MA: Decrease in mouse lung and liver glutathione peroxidase
activity and potentiation of the lethal effects of ozone and
paraquat by the superoxide dismutase inhibitor
diethyldithiocarbamate. Biochem Pharmacol. 28:27–30. 1979.
View Article : Google Scholar
|
|
9
|
Nobel CI, Kimland M, Lind B, Orrenius S
and Slater AF: Dithiocarbamates induce apoptosis in thymocytes by
raising the intracellular level of redox-active copper. J Biol
Chem. 270:26202–26208. 1995. View Article : Google Scholar
|
|
10
|
Donadelli M, Dalla Pozza E, Costanzo C, et
al: Increased stability of P21(WAF1/CIP1) mRNA is required for
ROS/ERK-dependent pancreatic adenocarcinoma cell growth inhibition
by pyrrolidine dithiocarbamate. Biochim Biophys Acta. 1763:917–926.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen SH, Lin JK, Liang YC, Pan MH, Liu SH
and Lin-Shiau SY: Involvement of activating transcription factors
JNK, NF-kappaB, and AP-1 in apoptosis induced by pyrrolidine
dithiocarbamate/Cu complex. Eur J Pharmacol. 594:9–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morais C, Gobe G, Johnson DW and Healy H:
Inhibition of nuclear factor kappa B transcription activity drives
a synergistic effect of pyrrolidine dithiocarbamate and cisplatin
for treatment of renal cell carcinoma. Apoptosis. 15:412–425. 2010.
View Article : Google Scholar
|
|
13
|
Venkatraman M, Anto RJ, Nair A, Varghese M
and Karunagaran D: Biological and chemical inhibitors of NF-kappaB
sensitize SiHa cells to cisplatin-induced apoptosis. Mol Carcinog.
44:51–59. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Evans WK, Shepherd FA, Feld R, Osoba D,
Dang P and Deboer G: VP-16 and cisplatin as first-line therapy for
small-cell lung cancer. J Clin Oncol. 3:1471–1477. 1985.PubMed/NCBI
|
|
15
|
Sundstrom S, Bremnes RM, Kaasa S, et al:
Cisplatin and etoposide regimen is superior to cyclophosphamide,
epirubicin, and vincristine regimen in small-cell lung cancer:
results from a randomized phase III trial with 5 years’ follow-up.
J Clin Oncol. 20:4665–4672. 2002.PubMed/NCBI
|
|
16
|
Safaei R: Role of copper transporters in
the uptake and efflux of platinum containing drugs. Cancer Lett.
234:34–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samimi G, Safaei R, Katano K, et al:
Increased expression of the copper efflux transporter ATP7A
mediates resistance to cisplatin, carboplatin, and oxaliplatin in
ovarian cancer cells. Clin Cancer Res. 10:4661–4669. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Safaei R, Katano K, Samimi G, et al:
Cross-resistance to cisplatin in cells with acquired resistance to
copper. Cancer Chemother Pharmacol. 53:239–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inoue Y, Matsumoto H, Yamada S, et al:
Association of ATP7A expression and in vitro sensitivity to
cisplatin in non-small cell lung cancer. Oncol Lett. 1:837–840.
2010.
|
|
20
|
Li ZH, Qiu MZ, Zeng ZL, et al:
Copper-transporting P-type adenosine triphosphatase (ATP7A) is
associated with platinum-resistance in non-small cell lung cancer
(NSCLC). J Transl Med. 10:212012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song L, Li Y, Li W, Wu S and Li Z: miR-495
enhances the sensitivity of non-small cell lung cancer cells to
platinum by modulation of copper-transporting P-type adenosine
triphosphatase A (ATP7A). J Cell Biochem. 115:1234–1242. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imai M, Kikuchi H, Denda T, Ohyama K,
Hirobe C and Toyoda H: Cytotoxic effects of flavonoids against a
human colon cancer derived cell line, COLO 201: a potential natural
anticancer substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar
|
|
23
|
Yuan B, Ohyama K, Bessho T and Toyoda H:
Contribution of inducible nitric oxide synthase and
cyclooxygenase-2 to apoptosis induction in smooth chorion
trophoblast cells of human fetal membrane tissues. Biochem Biophys
Res Commun. 341:822–827. 2006. View Article : Google Scholar
|
|
24
|
Kikuchi H, Yuan B, Yuhara E, Takagi N and
Toyoda H: Involvement of histone H3 phosphorylation through p38
MAPK pathway activation in casticin-induced cytocidal effects
against the human promyelocytic cell line HL-60. Int J Oncol.
43:2046–2056. 2013.PubMed/NCBI
|
|
25
|
Kon A, Yuan B, Hanazawa T, et al:
Contribution of membrane progesterone receptor alpha to the
induction of progesterone-mediated apoptosis associated with
mitochondrial membrane disruption and caspase cascade activation in
Jurkat cell lines. Oncol Rep. 30:1965–1970. 2013.
|
|
26
|
Hu XM, Yuan B, Tanaka S, et al:
Involvement of oxidative stress associated with glutathione
depletion and p38 mitogen-activated protein kinase activation in
arsenic disulfide-induced differentiation in HL-60 cells. Leuk
Lymphoma. 55:392–404. 2014. View Article : Google Scholar
|
|
27
|
Obaya AJ, Mateyak MK and Sedivy JM:
Mysterious liaisons: the relationship between c-Myc and the cell
cycle. Oncogene. 18:2934–2941. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang P, Li H, Wu ML, et al: c-Myc
downregulation: a critical molecular event in resveratrol-induced
cell cycle arrest and apoptosis of human medulloblastoma cells. J
Neurooncol. 80:123–131. 2006. View Article : Google Scholar
|
|
29
|
Payne CM, Bernstein C and Bernstein H:
Apoptosis overview emphasizing the role of oxidative stress, DNA
damage and signal-transduction pathways. Leuk Lymphoma. 19:43–93.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keyse SM and Tyrrell RM: Heme oxygenase is
the major 32-kDa stress protein induced in human skin fibroblasts
by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl
Acad Sci USA. 86:99–103. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Keyse SM and Tyrrell RM: Both near
ultraviolet radiation and the oxidizing agent hydrogen peroxide
induce a 32-kDa stress protein in normal human skin fibroblasts. J
Biol Chem. 262:14821–14825. 1987.
|
|
32
|
Furuta S, Ortiz F, Zhu Sun X, Wu HH, Mason
A and Momand J: Copper uptake is required for pyrrolidine
dithiocarbamate-mediated oxidation and protein level increase of
p53 in cells. Biochem J. 365:639–648. 2002.PubMed/NCBI
|
|
33
|
Oltersdorf T, Elmore SW, Shoemaker AR, et
al: An inhibitor of Bcl-2 family proteins induces regression of
solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olejniczak ET, Van Sant C, Anderson MG, et
al: Integrative genomic analysis of small-cell lung carcinoma
reveals correlates of sensitivity to bcl-2 antagonists and uncovers
novel chromosomal gains. Mol Cancer Res. 5:331–339. 2007.
View Article : Google Scholar
|
|
35
|
Malaguarnera L, Pilastro MR, DiMarco R, et
al: Cell death in human acute myelogenous leukemic cells induced by
pyrrolidinedithiocarbamate. Apoptosis. 8:539–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng YC, Lin H, Huang MJ, Chow JM, Lin S
and Liu HE: Downregulation of c-Myc is critical for valproic
acid-induced growth arrest and myeloid differentiation of acute
myeloid leukemia. Leuk Res. 31:1403–1411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aragones J, Lopez-Rodriguez C, Corbi A, et
al: Dithiocarbamates trigger differentiation and induction of CD11c
gene through AP-1 in the myeloid lineage. J Biol Chem.
271:10924–10931. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bae KM, Wang H, Jiang G, Chen MG, Lu L and
Xiao L: Protein kinase C epsilon is overexpressed in primary human
non-small cell lung cancers and functionally required for
proliferation of non-small cell lung cancer cells in a
p21/Cip1-dependent manner. Cancer Res. 67:6053–6063. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lloyd DR and Phillips DH: Oxidative DNA
damage mediated by copper(II), iron(II) and nickel(II) fenton
reactions: evidence for site-specific mechanisms in the formation
of double-strand breaks, 8-hydroxydeoxyguanosine and putative
intrastrand cross-links. Mutat Res. 424:23–36. 1999. View Article : Google Scholar
|
|
41
|
Zhang H, Wu JS and Peng F: Potent
anticancer activity of pyrrolidine dithiocarbamate-copper complex
against cisplatin-resistant neuroblastoma cells. Anticancer Drugs.
19:125–132. 2008. View Article : Google Scholar
|