Introduction
Metastasis, a highly coordinated step-wise process
that includes detachment from the primary tumor, local proteolytic
degradation of extracellular matrices (ECMs), extravasation leading
to invasion into the new tissue, and formation of metastatic foci,
is a hallmark of malignant tumors and major cause of death in
cancer patients (1–3). Degradation of ECMs and surrounding
basement membranes is promoted by the action of proteinases, such
as matrix metalloproteinases (MMPs), plasminogen activators (PAs),
serine proteinases and cathepsins (4,5).
MMPs, particularly MMP-2 and MMP-9, are elevated in malignant
cancer cells and are closely related to poor prognosis and relapse
in cancer patients. In addition, MMP-9 is critical for tumor
angiogenesis, because it enhances the availability of vascular
endothelial cell growth factor (VEGF) in malignant tumors (6,7).
Angiogenesis, the formation of new blood vessels
within the primary tumor or metastatic foci, supplies oxygen,
nutrients and growth factors for tumor growth, as well as provides
a path for cancer cell migration and infiltration (8). Thus, tumors with abundant vasculature
exhibit enhanced metastatic potential. During angiogenesis,
secretion of angiogenic factors from cancer cells, such as VEGF,
basic fibroblast growth factor (bFGF), and platelet-derived growth
factor (PDGF), is one of the initial events (9–11).
Therefore, inhibition of metastasis and angiogenesis via
suppression of MMP activity and angiogenic factor secretion is
currently considered a promising strategy in cancer management.
Anisi stellati fructus (ASF), commonly known
as star anise (Illicium verum Hook. f), is an important
traditional Chinese medicine used in the treatment of vomiting,
stomach ache, inflammation, insomnia and rheumatic pain (12). In addition, ASF essential oil is
used as a flavoring in confectionery, tobacco and liquor. In
addition, ASF is the major source of the chemical compound shikimic
acid, a primary ingredient in the pharmaceutical synthesis of
anti-influenza drug Tamiflu (13).
In recent studies, it has been demonstrated that ASF crude
extracts, as well as its active compounds, exhibit a wide range of
pharmacological actions, including anti-microbial, anti-fungal,
anti-oxidant, insecticidal, analgesic and sedative activity
(12). In gas chromatography (GC)
and gas chromato-graphy-mass spectrometry (GC-MS) analyses,
trans-anethole was determined to be a major component in ASF, which
possesses muscle relaxant, anti-fungal and anti-cholinesterase
activities (14,15). Methanol extracts from fruits and
the stem of I. verum moderately inhibited tube-like
formation in HUVECs with approximately 50% inhibition at 10 μg/ml,
suggesting its anti-angiogenic and anti-cancer activities (16).
In the present study, we examined the effect of ASF
on the metastatic and angiogenic potentials of malignant cancer
cells in an in vitro and in vivo system. In addition,
we elucidate the detailed underlying mechanism. Finally, we also
investigated whether oral administration of ASF inhibits pulmonary
metastasis of murine melanoma B16F10 cells.
Materials and methods
Cells and mice
Human fibrosarcoma HT1080 cells (KCLB no. 10121) and
human prostate adenocarcinoma PC-3 cells (KCLB no. 21435) were
purchased in 2012 from Korean Cell Line Bank (Seoul, Korea), where
they were characterized by DNA fingerprinting. Cells were cultured
in RPMI-1640 (Lonza, Walkersville, MD, USA) supplemented with 10%
(v/v) heat-inactivated fetal bovine serum (FBS; Gibco/Invitrogen,
Carlsbad, CA, USA) and 100 U/ml penicillin/100 μg/ml streptomycin
(Gibco/Invitrogen) at 37°C in a humidified 5% CO2
incubator and routinely screened for mycoplasma contamination.
Human umbilical vein endothelial cells (HUVECs) purchased from
Innopharmascreen (Asan, Korea) in October, 2012 were maintained in
Endothelial Growth Medium-2 (EGM-2; PromoCell, Heidelberg, Germany)
and used at passages 3 to 8 in the experiments. Murine melanoma
B16F10 cells (KCLB no. 80008), which are metastatic to the lungs of
C57BL/6J mice, were obtained in 2012 from Korean Cell Line Bank and
cultured in complete Dulbecco’s modified Eagle’s medium (DMEM;
Lonza). Specific pathogen-free female C57BL/6J mice and athymic
nude mice were purchased from Taconic Farms Inc. (Samtako Bio
Korea, Osan, Korea) and Nara Biotech (Seoul, Korea), respectively,
and maintained in our animal facility for 1 week before use. Mice
were housed in a barrier facility with 12-h light-dark cycles at
24±1°C and 55±5% humidity under specific pathogen-free conditions.
All animal experimental procedures were approved by Korea Institute
of Oriental Medicine Care and Use Committee with a reference number
of #13-67 and #13-94, and performed according to the guidelines
established by the Korea Institute of Oriental Medicine Care and
Use Committee.
Reagents and antibodies
Type A gelatin from porcine skin, casein from bovine
milk, type I collagen from calf skin,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT),
phorbol 12-myristate 13-acetate (PMA), mitomycin C, and curcumin
were purchased from Sigma Chemical Co. (St. L ouis, MO, USA).
Growth factor-reduced Matrigel Basement Membrane Matrix was
obtained from BD Biosciences (Bedford, MA, USA), and recombinant
mouse basic fibroblast growth factor (rMu bFGF) and recombinant
murine vascular endothelial growth factor 165 (rMu
VEGF165) were purchased from PromoKine (Heidelberg,
Germany). SB203580, PD98059 and SP600125 were obtained from
Calbiochem (San Diego, CA, USA). Antibodies against IκBα, pIκBα,
c-jun, p-c-jun, c-fos, p-c-fos, NF-κB p65, p38, p-p38, ERK, p-ERK,
JNK, p-JNK and tubulin were obtained from Cell Signaling Technology
(Danvers, MA, USA).
Preparation of water extract from
ASF
Dried ASF was purchased from Korea Medicine Herbs
Association (Yeongcheon, Korea) and deposited in the herbal bank of
Korea Institute of Oriental Medicine (KIOM; Daejeon, Korea) after
verifying by Professor Ki Hwan Bae of the College of Pharmacy,
Chungnam National University (Daejeon, Korea). To prepare aqueous
extract of ASF, dried ASF (50 g) were placed in 1 liter of
distilled water, and then heat-extracted at 115°C for 3 h in a
Cosmos-600 Extractor (Gyeonseo Co., Inchon, Korea). Aqueous extract
of ASF was filtered using standard testing sieves (150 μm, Retsch,
Haan, Germany), and then concentrated by lyophilization.
Freeze-dried ASF powder (50 mg) dissolved in 1 ml of DW was kept at
−20°C prior to use after filtration through a 0.22-μm disk
filter.
Safety assessment after oral
administration of ASF
Five-week-old female C57BL/6J mice (n=3 per group)
were daily administered vehicle (saline) or 50 mg/kg ASF. During
15-day experimental period, gross appearance and behavior were
carefully monitored and body weight was daily measured. After
sacrifice, organs including heart, liver, lung, spleen and kidneys
were weighed. Hematological and serological parameters were
measured using ADVIA 2120i hematology system (Siemens Healthcare
Diagnostics, Tarrytown, NY, USA) and XL 200 (Erba Diagnostics,
Mannheim, Germany), respectively.
Cell viability assay
To examine cytotoxicity of ASF, cells
(5×103 cells/well/96-well plate) were treated with
various concentrations of ASF (10 to 1,000 μg/ml) for 48 h, and
then MTT assay was performed as described previously (17). Relative cell viability was
represented as percentage compared with that of untreated control
cells.
Anchorage-independent colony forming
activity
After HT1080 cells (5×104) were suspended
in 2 ml RPMI medium with specified concentrations of ASF, 0.3% agar
and 10% FBS, they were applied to the bottom agar pre-solidified
with 3 ml RPMI medium containing 0.6% agar and 10% FBS. During
incubation for 2 weeks, colony formation was daily observed under a
phase-contrast microscope and photographed.
Wound healing assay
In vitro wound healing assay was performed as
described previously (17). In
brief, HT1080 cells with 90% confluence were pre-incubated with
mitomycin C (25 μg/ml) for 30 min and scratched by scraping across
the cell monolayer. After washing with PBS to eliminate floating
cell debris, cells were permitted to migrate for 36 h in the
presence of ASF and wound healing was observed under a
phase-contrast microscope and photographed.
Transwell migration and invasion
assays
Using a Transwell chamber with polycarbonate
membrane (10 mm diameter, 8 μm pore size), in vitro
migration and invasion assays were performed. Transwell chamber
coated with 20 μl 1:8 mixture of Matrigel: RPMI as the intervening
barrier was used for invasion assay. Cells were pre-incubated with
indicated concentrations of ASF for 12 h. After filling the lower
chamber with 600 μl 10% FBS/RPMI, cells (1×105)
suspended in 100 μl serum-free RPMI were added to upper chamber,
and incubated for 12 or 24 h for migration and invasion,
respectively. For migration of HUVECs, experimental conditions are
specified in the Figures. After removal of the cells remaining in
the upper surface of the chamber, migrated and invaded cells were
stained with 0.2% crystal violet/20% methanol (w/v) solution.
Zymography
The secretions of MMP-2 and MMP-9 in the culture
supernatants were measured by gelatin zymography. In brief, cells
pre-incubated with ASF in serum-free medium for 12 h were
stimulated with 5 nM PMA for an additional 24 h. Collected
serum-free conditioned medium (CM) were electrophoresed on an 8%
SDS-PAGE containing 0.1% gelatin and gels were washed with washing
buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 2.5% Triton X-100) and
incubated in reaction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl,
10 mM CaCl2, 0.02% NaN3, 1 μM
ZnCl2) at 37°C. Gels were stained with Coomassie
Brilliant Blue R-250 staining solution (Bio-Rad Laboratories,
Hercules, CA, USA) and destained with 10% isopropanol/10% acetic
acid (v/v) solution. The secretion of uPA was examined by
casein-plasminogen zymography. Conditioned medium were
electrophoresed on a 10% SDS-PAGE containing casein (1 mg/ml) and
human plasminogen (15 μg/ml), and gels were washed, incubated and
stained as described earlier. For the detection of collagenase
activity, conditioned medium were electrophoresed on a 10% SDS-PAGE
containing collagen (4 mg/ml). MMP-2, MMP-9 and uPA were detected
as clear bands against blue background at the size of 72/64, 92 and
55/52 kDa, respectively.
Reverse transcription and polymerase
chain reaction (RT-PCR)
Total RNA was isolated using PureHelix™ RNA
extraction solution and 3 μg RNA was reverse transcribed to cDNA
using HelixCript™ 1st Strand cDNA Synthesis kit (NanoHelix Co.,
Daejeon, Korea) according to the manufacturer’s instructions. The
cDNA aliquots were analyzed by semi-quantitative PCR using specific
primers (Table I) and PCR products
were electrophoresed on 1% agarose gels and stained with EcoDye™
(Solgent Co., Daejeon, Korea). Band intensity was quantitatively
analyzed using ImageJ software (National Institute of Health, USA)
for each gene.
 | Table IPrimers used for PCR. |
Table I
Primers used for PCR.
| Target gene | Sequence |
|---|
| hMMP-1 | F:
5′-GCAAGTTGAAAAGCGGAGAAAT-3′
R: 5′-AAGATTTCCTCCAGGTCCATC-3′ |
| hMMP-2 | F:
5′-CCCCAAAACGGACAAAGAGTT-3′
R: 5′-AAAGGCATCATCCACTGTCTC-3′ |
| hMMP-3 | F:
5′-CACCAGCATGAACCTTGTTCAG-3′
R: 5′-CAGCATCTTTTGGCAAATCTGG-3′ |
| hMMP-9 | F:
5′-TCTTCCCTGGAGACTGAGAA-3′
R: 5′-GGCAAGTCTTCCGAGTAGTTT-3′ |
| hMMP-13 | F:
5′-GGCAAACTTGACGATAACACC-3′
R: 5′-GCCCATCAAATGGGTAGAAGT-3′ |
| hMT1-MMP | F:
5′-CATGCAGAAGTTTTACGGCTTG-3′
R: 5′-ACGGATGTAGGCATAGGCA-3′ |
| hTIMP-1 | F:
5′-CACCAGAAGTCAACCAGACCA-3′
R: 5′-GGGGATGGATAAACAGGGAAAC-3′ |
| hTIMP-2 | F:
5′-AGGGCAGCACTGTGAAATAGA-3′
R: 5′-TCTTGGACAAGCAGCTTTAGG-3′ |
| huPA | F:
5′-AGGGCAGCACTGTGAAATAGA-3′
R: 5′-TCTTGGACAAGCAGCTTTAGG-3′ |
| hPAI | F:
5′-GCACAATCCCCCATCCTACG-3′
R: 5′-GGCTCTCTCCACCTCTGAAA-3′ |
| GAPDH | F:
5′-CCACCCATGGCAAATTCCATGGCA-3′
R: 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ |
Western blot analysis
Whole cell lysates were extracted using M-PER
Mammalian Protein Extraction Reagent (Thermo Scientific, Rockford,
IL, USA), and protein concentrations were determined by
Bicinchoninic Acid (BCA) kit (Sigma). After immunoblot analysis,
proteins were detected using a PowerOpti-ECL Western blotting
detection reagent (Animal Genetics, Inc. Korea) and an ImageQuant
LAS 4000 mini (GE Healthcare, Piscataway, NJ, USA), and band
intensity was analyzed using ImageJ software.
Matrigel plug assay
For tumor-induced angiogenesis, mixture containing
PC-3 cells (3×106/100 μl serum-free medium), heparin (50
U/ml), and Matrigel (500 μl) with or without ASF was subcutaneously
injected into the abdomen of athymic nude mice. At days 10–14 after
implantation, Matrigel plugs were carefully removed and hemoglobin
content of the gel plug was measured using a Drabkin’s reagent kit
(Sigma) as described previously (18). The concentration of hemoglobin was
calculated based on the set of hemoglobin standard (Sigma).
HUVEC tube formation assay
Using an ECMatrix assay kit (Millipore, Temecula,
CA, USA), HUVEC tube formation assay was performed according to the
manufacturer’s instructions. In brief, 10 μl chilled ECMatrix was
transferred to each well of a pre-cooled 1 μ-Slide Angiogenesis
ibiTreat chamber (ibidi GmbH, Germany) and allowed to solidify for
at least 1 h at 37°C. A mixture of HUVECs (5×103 cells),
bFGF (1 μg/ml), VEGF165 (100 ng/ml), and/or ASF in
endothelial cell basal medium-2 (EBM-2; PromoCell, Heidelberg,
Germany), or HUVECs in ASF-treated CM was seeded onto the surface
of polymerized ECMatrix, and incubated for 4 to 24 h at 37°C. For
the collection of CM, cells were treated with ASF for 24 h in
complete media conditions, washed twice with 0.5% serum media, and
then incubated for another 24 h in 0.5% serum media. Cellular
network-like structures were examined under phase-contrast inverted
light microscopy and photographed. Tube formation was quantified by
counting tube number and represented as the average of five fields
of view in each well.
ELISA for VEGF-α, bFGF and PDGF
Cells were treated with indicated concentrations of
ASF for 48 h. The levels of VEGF165, bFGF and PDGF in
culture media were determined with the Human ELISA Development kit
(Peprotech, Rocky Hill, NJ, USA) according to the manufacturer’s
instructions.
In vivo pulmonary metastasis assay
After injection of B16F10 cells (3×105
cells/200 μl PBS) via the tail veins, C57BL/6J mice were randomly
divided into 2 groups (n=4 for each group). Administration of
vehicle (saline) or 50 mg/kg ASF (day 0), for 17 days, mice
received daily oral administration and were then sacrificed. Lungs
were fixed in Bouin’s solution (Sigma) and black colonies were
counted macroscopically.
Statistical analysis
The values are presented as means ± standard
deviation (SD). Statistical significance of the difference between
groups was analyzed by Two-way analysis of variance (ANOVA) and
Student’s t-test with SigmaPlot 8.0 software. P-value <0.05 was
considered significantly different.
Results
ASF significantly suppresses in vivo
pulmonary metastasis of B16F10 cells without side effects
To examine the anti-metastatic effect of ASF in
vivo, we first examined whether repeated administration of ASF
(50 mg/kg) is non-toxic systemically. Over 15 days, corresponding
to the in vivo experimental conditions, ASF intake did not
influence the body weight of mice (Table II) or cause abnormal behavior or
death. Organ weights were not significantly different between
control and ASF-treated mice (Table
III). In serological analyses, the aspartate
aminotransferase/alanine aminotransferase (AST/ALT) and blood urea
nitrogen/creatinine (BUN/CRE) ratios were not significantly
affected by ASF intake, indicating that ASF did not cause hepatic
or renal damage, respectively (Table
IV). In hematological analyses, the number of red blood cells
(RBCs) and the hemoglobin (Hb) level, an indicator of RBC balance
and anemia, were not altered by ASF intake. In addition, the number
of white blood cells (WBCs) and other hematologic parameters were
also similar to those of control mice (Table V). Next, we investigated the
inhibitory effect of ASF on in vivo tumor metastasis by
comparing the ability of B16F10 cells to colonize the lungs of
C57BL/6J mice after tail vein injection. As shown in Fig. 1, B16F10 cells in control mice
metastasized to the lungs and formed a considerable number of black
colonies (1273.5±157.1). In ASF-administered mice, the number of
black colony in lungs were significantly decreased (402.6±160.6) to
approximately 30% of control mice. These data indicate that ASF
intake effectively inhibited pulmonary metastasis of intravenously
injected B16F10 cells with no systemic side effects during
treatment.
 | Table IIMeans of body weights of mice
administered with ASF or saline. |
Table II
Means of body weights of mice
administered with ASF or saline.
| Body weight
(g) |
|---|
|
|
|---|
| Treatment | Day 0 | Day 5 | Day 10 | Day 15 |
|---|
| Control | 15.99±0.30 | 17.12±0.20 | 18.16±0.48 | 18.75±0.94 |
| 50 mg/kg | 16.15±0.29 | 17.14±0.13 | 18.19±0.09 | 18.94±0.26 |
 | Table IIIOrgan weights of mice administered
with ASF or saline. |
Table III
Organ weights of mice administered
with ASF or saline.
| Weight of organs
(g) |
|---|
|
|
|---|
| Treatment | Liver | Heart | Lung | Spleen | Kidney (L) | Kidney (R) |
|---|
| Control | 0.96±0.02 | 0.10±0.01 | 0.16±0.02 | 0.08±0.01 | 0.13±0.02 | 0.13±0.01 |
| 50 mg/kg | 0.91±0.05 | 0.10±0.01 | 0.16±0.01 | 0.07±0.01 | 0.12±0.01 | 0.12±0.01 |
 | Table IVChemical analysis of sera obtained
from mice administered with ASF or saline. |
Table IV
Chemical analysis of sera obtained
from mice administered with ASF or saline.
| Treatment | GOT (IU/l) | GPT (IU/l) | BUN (mg/dl) | CRE (mg/dl) |
|---|
| Control | 44.7±5.0 | 24.3±0.6 | 15.6±2.7 | 0.4±0.1 |
| 50 mg/kg | 38.7±4.6 | 20.0±2.1 | 15.7±1.3 | 0.4±0.0 |
 | Table VHematological analysis of blood
obtained from mice administered ASF or saline. |
Table V
Hematological analysis of blood
obtained from mice administered ASF or saline.
| Parameter | Control | 50 mg/kg |
|---|
| WBCP
(×103 cells/μl) | 2.5±0.17 | 2.3±0.39 |
| WBCB
(×103 cells/μl) | 2.6±0.25 | 2.3±0.29 |
| RBC
(×106 cells/μl) | 9.2±0.75 | 9.2±0.24 |
| Means HGB
(g/dl) | 13.5±1.18 | 13.6±0.45 |
| HCT (%) | 49.1±4.37 | 49.4±1.45 |
| MCV (fl) | 53.1±0.92 | 53.5±0.31 |
| MCH (pg) | 14.6±0.26 | 14.8±0.15 |
| MCHC (g/dl) | 27.5±0.10 | 27.6±0.23 |
| PLT (×104
cells/μl) | 102.5±11.12 | 97.8±1.91 |
| % NEUT | 6.5±1.62 | 6.4±1.60 |
| % LYM | 87.9±3.30 | 86.4±6.52 |
| % MONO | 0.5±0.01 | 0.5±0.15 |
Non-cytotoxic concentrations of ASF
suppress the metastatic potential of HT1080 cells
Prior to evaluation of the in vitro
anti-metastatic potential of ASF, we first determined its
cytotoxicity in HT1080 cells in the absence or presence of serum
using MTT assays. At concentrations up to 250 μg/ml, ASF had no
cytotoxic effects; however, 500 μg/ml ASF exhibited a ~10% decrease
in cell viability (Fig. 2A).
Therefore, we treated cells with 25, 50 or 100 μg/ml ASF in all
subsequent experiments. Metastatic cancer cells possess the ability
to form colonies in semi-solid agar, which is termed
anchorage-independent growth. As shown in Fig. 2B, untreated control HT1080 cells
formed sizable colonies from a single cell, whereas at
concentrations of 25–100 μg/ml, ASF remarkably suppressed
anchorage-independent growth by approximately 30–63%. In wound
healing assays, control HT1080 cells rapidly migrated across the
wound region, leading to approximately 28, 50 and 70% healing at
12, 24 and 36 h, respectively. ASF treatments of 25, 50 and 100
μg/ml dose-dependently suppressed wound migration to approximately
45, 40 and 23% healing, respectively, at 36 h (Fig. 2C). Using the Transwell culture
system, serum-induced migration and invasion were significantly
reduced in a dose-dependent manner in ASF-treated HT1080 cells by
approximately 20–70 and 30–90%, respectively, compared with
untreated control cells (Fig.
2D).
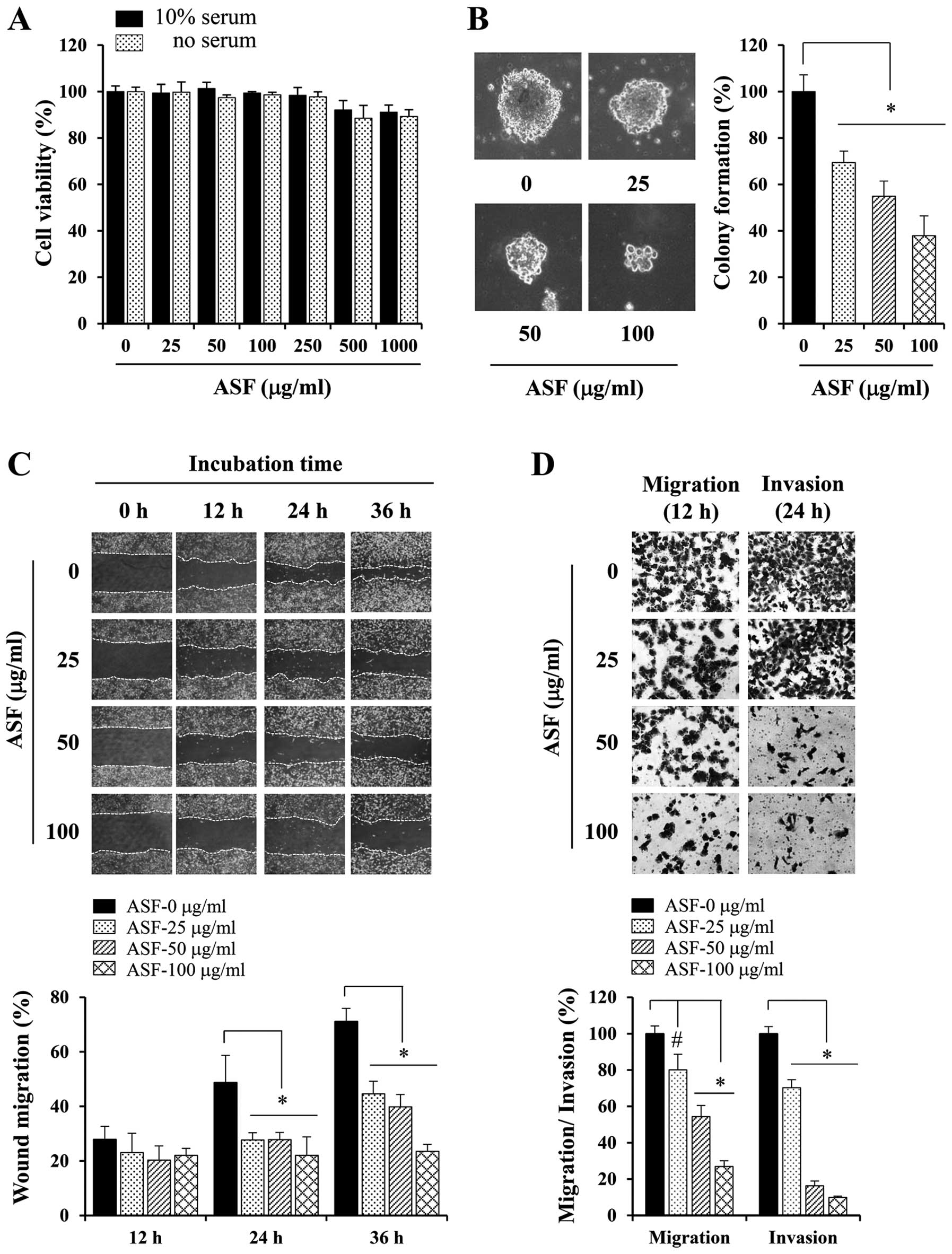 | Figure 2ASF treatment suppresses the
metastatic potential of human fibrosarcoma HT1080 cells. (A) Cells
(5×103/well) were seeded in 96-well culture plates,
treated with 25 to 1,000 μg/ml ASF in the presence or absence of
serum for 48 h, and cell viability was determined using MTT assays.
(B) Anchorage-independent colony formation in soft agar was
measured after incubation for 14 days with non-cytotoxic
concentrations of ASF (25, 50 and 100 μg/ml). The diameters of 10
representative colonies were measured and expressed as the mean ±
SD. (C) Confluent cell monolayers were pre-treated with mitomycin C
(25 μg/ml) for 30 min, and injury lines were then created. After
washing detached cell debris completely, cells were incubated with
ASF for 36 h, and migration was measured using phase-contrast
microscopy after 12, 24 and 36 h. Based on the width of injury at 0
h, relative wound migration was calculated and expressed as the
mean ± SD of four selected fields. (D) Cells pre-treated with ASF
were monitored for Transwell migration and Matrigel invasion. After
incubation, cells that migrated and invaded the lower surface of
the Transwell membrane were stained and observed using
phase-contrast microscopy. Relative migration and invasion were
quantified using ImageJ software, and data are expressed as the
means ± SD of five random fields from each well.
*p<0.01 vs. untreated control. |
ASF reduces MMP expression and suppresses
proteolytic activity in HT1080 cells
In order to elucidate the anti-metastatic effects of
ASF, we first examined the transcriptional levels of various MMPs
using semi-quantitative RT-PCR. Under resting conditions, HT1080
cells expressed substantial amounts of MMPs, including MMP-1, -2,
-3, -9, -13, MT1-MMP and uPA. PMA stimulation significantly
increased MMP-1, -3, -9, -13, MT1-MMP and uPA levels but decreased
PAI-1. MMP-2, TIMP-1 and TIMP-2 levels were essentially unchanged
by PMA stimulation. However, as shown in Fig. 3A, ASF treatment efficiently
inhibited the PMA-induced increase in MMP-9, -13, MT1-MMP and uPA
levels and decreased PAI-1 levels dose-dependently. Next, to
examine whether ASF influences proteolytic activity of HT1080
cells, we performed zymography using gelatin, collagen type I, and
casein-plasminogen as substrates (Fig.
3B). In gelatin zymography, HT1080 cells constitutively
secreted MMP-2 and MMP-9. PMA stimulation partially converted
proMMP-2 to active MMP-2 and increased MMP-9 activity. ASF
treatment dose-dependently suppressed MMP-9 activity in response to
PMA stimulation, but it did not prevent PMA-induced conversion to
active MMP-2. Collagenase activity in ASF-treated cells was also
lower compared with control cells. Furthermore, in
casein-plasminogen zymography, uPA activity after PMA-stimulation
was remarkably reduced by ASF treatment in a dose-dependent manner.
These results indicate that ASF suppresses the metastatic potential
of HT1080 cells via downregulation of proteolytic activities. In
U937 human leukemic monocyte lymphoma cells and PC-3 human prostate
cancer cells, ASF significantly decreased MMP-9 gelatinolytic
activity (Fig. 3C), further
supporting the inhibitory effect of ASF on proteolytic
degradation.
ASF suppresses PMA-induced NF-κB and AP-1
activation as well as p38 phosphorylation
It has been reported that the transcription factors
NF-κB and AP-1 are involved in regulating MMP expression and
invasion in various cancer cell types (19–21).
To examine whether the anti-metastatic effect of ASF is linked to
the suppression of NF-κB and AP-1 activities, we examined the
levels of p-IκBα, IκBα, p-c-jun, and p-c-fos in response to PMA
stimulation using western blot analyses in control cells and
ASF-treated cells. As shown in Fig.
4A, IκBα phosphorylation and degradation were markedly
increased by PMA stimulation in control cells. However, in
ASF-treated cells, the extent of IκBα phosphorylation and
degradation was insignificant. Phosphorylation of c-jun and c-fos
was also increased by PMA stimulation in control cells, whereas
c-fos phosphorylation was almost completely blocked by ASF
treatment. In addition, ASF-mediated suppression of phosphorylation
of both IκBα and c-fos was dose-dependent (Fig. 4B). In control cells, the p65
subunit translocated from the cytosol to the nucleus upon PMA
stimulation. In ASF-treated cells, however, p65 nuclear
translocation was significantly inhibited in a dose-dependent
manner (Fig. 4C). It has been
reported that activation of MAPKs, including p38, ERK and JNK,
plays a pivotal role in increasing MMP-9 activity and expression
(22). As shown in Fig. 4D, phosphorylation of p38, ERK and
JNK was rapidly increased by PMA stimulation in control cells. In
ASF-treated cells, PMA-induced p38 phosphorylation was almost
completely blocked, but ERK and JNK phosphorylation was only
partially inhibited. To confirm the involvement of these signal
transduction pathways in PMA-stimulated MMP-9 expression and
ASF-mediated inhibition, we used gelatin zymography to analyze the
effects of specific inhibitors on PMA-induced MMP-9 activity in
HT1080 cells. As reported previously, we found that PMA-induced
MMP-9 activity was almost completely inhibited by the p38 inhibitor
(SB203580) and was significantly inhibited by inhibitors of ERK
(PD98059), JNK (SP600125), and AP-1 (curcumin) (22). In addition, PMA-induced MMP-9
activity was significantly reduced by transient expression of
dominant negative IκB kinase, dnIKKα or dnIKKβ (data not shown)
(18). These results indicate that
specific inhibition of p38, ERK, JNK, NF-κB and AP-1 contributes to
suppression of PMA-induced MMP-9 activity.
 | Figure 4ASF treatment blocks PMA-induced
NF-κB and AP-1 activation, as well as p38 phosphorylation. (A)
Control and ASF (100 μg/ml)-treated HT1080 cells (12 h) were
stimulated with 5 nM PMA for 15, 30 or 60 min, and cell lysates
were evaluated for IκBα phosphorylation and degradation and
c-jun/c-fos phosphorylation using western blots. (B) Cells
pre-treated with ASF (25, 50 or 100 μg/ml) for 12 h were stimulated
with PMA for 30 min and then subjected to western blot analysis.
(C) To evaluate nuclear translocation of the NF-κB p65 subunit
after PMA stimulation, cell lysates were separated into cytosolic
and nuclear compartments. (D) Cell lysates prepared as described in
panel A were subjected to western blot analysis to evaluate
phosphorylation of p38, ERK and JNK. After normalization to tubulin
or TBP levels, the relative ratios of pIκBα/IκBα, nuclear
p65/cytosolic p65, and phosphorylated protein/total protein were
calculated. Data were derived from a single analysis,
representative of two independent experiments. |
ASF inhibits in vivo tumor angiogenesis
through suppression of pro-angiogenic factors in tumors
Angiogenesis, regulated by angiogenic factors
produced by tumor cells, is critical for growth, invasion and
metastasis of solid tumors (8). To
examine the effect of ASF on tumor-induced angiogenesis in
vivo, we performed Matrigel plug assays in athymic nude mice.
As reported previously, PC-3 cells remarkably induced formation of
new blood vessels in the Matrigel plug, and the plugs exhibited a
bright red color (Fig. 5A)
(23). In the presence of ASF,
Matrigel plugs were light red/yellow in color, indicating
inhibition of angiogenesis. The hemoglobin content in the plug with
the PC-3 cells was 15.6 mg/g. ASF treatment significantly decreased
hemoglobin levels by approximately 50%, suggesting that ASF can
inhibit tumor-induced angiogenesis in vivo. The levels of
pro-angiogenic factors, including VEGF-α, bFGF and PDGF, in
conditioned media (CM) from ASF-untreated and -treated PC-3 cells
were determined using ELISA analyses. We found that ASF treatment
significantly decreased the production of angiogenic factors
(Fig. 5B). In addition, we
observed that CM from control PC-3 cells strongly induced tube
formation in HUVECs. However, in HUVECs plated with CM from
ASF-treated PC-3, tube formation was considerably diminished in
terms of tubular network and tube number (Fig. 5C). During neovascularization,
cancer cells attract endothelial cells by secretion of
pro-angiogenic factors. To examine whether ASF could inhibit
endothelial cell migration toward PC-3 cells, we used Transwell
migration assays and seeded HUVECs in the upper chamber and
ASF-treated PC-3 cells in the lower chamber. As shown in Fig. 5D, PC-3 cells in the lower chambers
induced migration of HUVECs. ASF treatment of PC-3 cells reduced
HUVEC migration toward PC-3 cells in a dose-dependent manner,
indicating that ASF suppresses tumor cell-derived chemotactic
motility of endothelial cells. In ASF-treated HT1080 cells, we also
observed strong inhibitory effects on tube formation (Fig. 5E) and HUVEC migration (Fig. 5F). To determine the effects of ASF
on endothelial cells, HUVECs in EGM-2 media were treated for 24 h
with 25, 50 and 100 μg/ml ASF. Cell viability was analyzed using
MTT assays. As shown in Fig. 6A,
ASF had no effect on HUVEC proliferation. The degree of EGM-2-,
bFGF- and VEGF-α-stimulated tube formation in HUVECs was similar
between control and ASF-treated HUVECs (Fig. 6B). In addition, EGM-2-mediated
migration of HUVECs was not inhibited by ASF treatment (Fig. 6C). These data suggest that ASF
exerts its anti-angiogenic influence by targeting cancer cells but
not endothelial cells.
Discussion
The anise-scented star-shaped fruit ASF has long
been used in traditional Chinese herbal medicine and the food
industry in Asian countries. ASF dispels cold and relieves pain,
and it is also used in teas to treat nervousness and insomnia
(12). ASF contains essential
oils, sesquiterpenes, prenylated C6-C3 compounds, lignans and
flavonoids. In particular, essential oil from ASF includes
trans-anethole (70–94%), estragole, limonene, pinene,
β-phellandrene and α-terpineol (24). To extract the essential oil from
ASF, various techniques can be used, including hydrodistillation
(HD), steam distillation (SD), solvent extraction (SE),
supercritical fluid CO2 extraction (SFE),
hydrodistillation-headspace solvent microextraction (HD-HSME) and
microwave-assisted extraction (MAE). In addition, it is important
to select appropriate solvents for extraction to improve yield and
the effect of the medicine. Recent studies have shown that ethanol
extract of ASF exerts substantial anti-bacterial activity against
67 drug-resistant isolates (25).
Additionally, methanol extract and decoction of ASF also exhibit
anti-bacterial activity against Eikenella corrodens
(26). Furthermore, ASF has been
reported to possess antifungal, anti-oxidant, insecticidal,
analgesic, sedative and convulsive activities (14,27–29).
In a study by Nam et al, tube-like formation in HUVECs was
considerably inhibited by the methanol extract from the fruits and
stem of I. verum, suggesting its anticancer activity
(16). However, the effects of ASF
decoction on tumor growth or the metastatic potential of malignant
tumor cells, as well as the underlying mechanism, have not yet been
established.
In this study, we demonstrated that water extract of
ASF alleviates the metastatic and angiogenic potential of malignant
HT1080 cells via suppression of proteolytic activities and
reduction of pro-angiogenic factors. In addition, we observed that
oral administration of 50 mg/kg ASF decreases pulmonary metastatic
colonies of B16F10 melanoma in vivo with no systemic
toxicity, suggesting that ASF can be used as a safe remedy for
cancer treatment. In other studies, oral administration of ethyl
acetate extract of ASF at a high dose (500 mg/kg) caused
convulsions and lethal toxicity in mice, and veranisatins were
identified as the active components (28,29).
Veranisatins are also known as analgesic and sedative compounds,
indicating that the medicinal and toxic effects depend on the
dose.
Highly malignant tumors, which are relatively
resistant to current anticancer agents and related to high
recurrence and mortality, can efficiently degrade surrounding
basement membranes and stromal ECMs via MMP activation (7). The tumors then invade surrounding
tissues and enter the bloodstream to metastasize to distant sites.
Among MMPs, MMP-9 expression and activity have been closely linked
to vascular invasion and aggressiveness (5,30).
Our results showed that ASF treatment significantly decreased MMP-9
expression, secretion, and proteolytic activity for gelatin, type I
collagen, and casein-plasminogen as substrates, suggesting that ASF
can be used to treat malignant metastatic cancers. We also observed
that ASF inhibited the production of pro-angiogenic factors in
cancer cells, thereby suppressing in vitro tumor-induced
migration and tube formation of endothelial cells. Furthermore,
using Matrigel plug assays, we found that ASF-treated cancer cells
showed lower vascularization in plugs than control cells,
supporting the in vivo anti-angiogenic activity of ASF.
Because endothelial cells in the tumor microenvironment play a
critical role in cancer growth and progression, ASF could be
considered to control both cancer cells and endothelial cells.
In response to various agonists, including PMA,
MMP-9 activation is induced by MAPKs and the subsequent activation
of the transcription factors NF-κB and AP-1, which are involved in
cancer cell adhesion, invasion and angiogenesis (4,31).
In this study, ASF almost completed blocked PMA-induced p38
phosphorylation and dramatically decreased PMA-induced NF-κB and
AP-1 activation. As reported in earlier studies, pre-treatment with
specific inhibitors significantly decreased PMA-induced MMP-9
activity in our HT1080 cell system (22). These results indicate that blockade
of p38, NF-κB and AP-1 activation by ASF contributes to suppression
of the metastatic potential via downregulation of MMP-9
activity.
Anethole, a main ASF phytoconstituent, was reported
to possess muscle relaxant and anti-cholinesterase activities
(15). In addition, anethole
exhibited chemopreventive activities in its suppression of the
incidence and multiplicity of both invasive and non-invasive
carcinomas (32). Anethole also
inhibited cell migration and invasion in human fibrosarcoma cells
by inhibiting MMP-2/-9 and Akt/MAPK/NF-κB signaling pathways
(33). These results suggest that
anethole derived from ASF may suppress metastasis and angiogenesis
in cancer cells.
Collectively, our results demonstrate that ASF
exerts anti-metastatic and anti-angiogenic effects on highly
malignant cancer cells via downregulation of proteolytic activities
and pro-angiogenic factors. Furthermore, we found that repeated
oral administration of ASF (50 mg/kg) significantly reduced the
number of metastatic colonies in mice with no adverse effects.
These results collectively suggest that ASF may be a safe herbal
product used to control metastatic cancer.
Acknowledgements
This study was supported by the Grant K14050 awarded
to Korea Institute of Oriental Medicine (KIOM) from Ministry of
Education, Science and Technology (MEST), Republic of Korea.
References
|
1
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel LR, Camacho DF, Shiozawa Y, Pienta
KJ and Taichman RS: Mechanisms of cancer cell metastasis to the
bone: a multistep process. Future Oncol. 7:1285–1297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
4
|
Westermarck J and Kahari VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
5
|
Gondi CS, Lakka SS, Dinh DH, Olivero WC,
Gujrati M and Rao JS: Downregulation of uPA, uPAR and MMP-9 using
small, interfering, hairpin RNA (siRNA) inhibits glioma cell
invasion, angiogenesis and tumor growth. Neuron Glia Biol.
1:165–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lockhart AC, Braun RD, Yu D, et al:
Reduction of wound angiogenesis in patients treated with
BMS-275291, a broad spectrum matrix metalloproteinase inhibitor.
Clin Cancer Res. 9:586–593. 2003.
|
|
7
|
Zheng H, Takahashi H, Murai Y, et al:
Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth,
invasion, metastasis and angiogenesis of gastric carcinoma.
Anticancer Res. 26:3579–3583. 2006.PubMed/NCBI
|
|
8
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bicknell R: Vascular targeting and the
inhibition of angiogenesis. Ann Oncol Suppl. 4:45–50. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Appelmann I, Liersch R, Kessler T, Mesters
RM and Berdel WE: Angiogenesis inhibition in cancer therapy:
platelet-derived growth factor (PDGF) and vascular endothelial
growth factor (VEGF) and their receptors: biological functions and
role in malignancy. Recent Results Cancer Res. 180:51–81. 2010.
View Article : Google Scholar
|
|
11
|
Poveshchenko AF and Konenkov VI:
Mechanisms and factors of angiogenesis. Usp Fiziol Nauk. 41:68–89.
2010.(In Russian).
|
|
12
|
Wang GW, Hu WT, Huang BK and Qin LP:
Illicium verum: a review on its botany, traditional use,
chemistry and pharmacology. J Ethnopharmacol. 136:10–20. 2011.
View Article : Google Scholar
|
|
13
|
Ghosh S, Chisti Y and Banerjee UC:
Production of shikimic acid. Biotechnol Adv. 30:1425–1431. 2012.
View Article : Google Scholar
|
|
14
|
Huang Y, Zhao J, Zhou L, et al: Antifungal
activity of the essential oil of Illicium verum fruit and
its main component trans-anethole. Molecules. 15:7558–7569.
2010.PubMed/NCBI
|
|
15
|
Bhadra S, Mukherjee PK, Kumar NS and
Bandyopadhyay A: Anticholinesterase activity of standardized
extract of Illicium verum Hook. f. fruits. Fitoterapia.
82:342–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nam NH, Kim HM, Bae KH and Ahn BZ:
Inhibitory effects of Vietnamese medicinal plants on tube-like
formation of human umbilical venous cells. Phytother Res.
17:107–111. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim A, Im M, Yim NH, Jung YP and Ma JY:
Aqueous extract of Bambusae Caulis in Taeniam inhibits
PMA-induced tumor cell invasion and pulmonary metastasis:
suppression of NF-kappaB activation through ROS signaling. PLoS
One. 8:e780612013.
|
|
18
|
Kim A, Kim MJ, Yang Y, Kim JW, Yeom YI and
Lim JS: Suppression of NF-kappaB activity by NDRG2 expression
attenuates the invasive potential of highly malignant tumor cells.
Carcinogenesis. 30:927–936. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho HJ, Kang JH, Kwak JY, et al:
Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9
gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent
mechanisms. Carcinogenesis. 28:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato T, Koike L, Miyata Y, et al:
Inhibition of activator protein-1 binding activity and
phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy
flavonoid, results in augmentation of tissue inhibitor of
metalloproteinases-1 production and suppression of production of
matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080
cells. Cancer Res. 62:1025–1029. 2002.
|
|
21
|
Choi JH, Han EH, Hwang YP, et al:
Suppression of PMA-induced tumor cell invasion and metastasis by
aqueous extract isolated from Prunella vulgaris via the
inhibition of NF-kappaB-dependent MMP-9 expression. Food Chem
Toxicol. 48:564–571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW
and Jeong HG: Suppression of PMA-induced tumor cell invasion by
dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and
NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol.
79:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang J, Zhou Q, Liu LZ, et al: Apigenin
inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF
expression. Carcinogenesis. 28:858–864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Wang L, Li T, et al: Rapid
analysis of the essential oils from dried Illicium verum
Hook. f and Zingiber officinale Rosc by improved
solvent-free microwave extraction with three types of
microwave-absorption medium. Anal Bioanal Chem. 386:1863–1868.
2006.PubMed/NCBI
|
|
25
|
Yang JF, Yang CH, Chang HW, et al:
Chemical composition and antibacterial activities of Illicium
verum against antibiotic-resistant pathogens. J Med Food.
13:1254–1262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iauk L, Lo Bue AM, Milazzo I, Rapisarda A
and Blandino G: Antibacterial activity of medicinal plant extracts
against periodontopathic bacteria. Phytother Res. 17:599–604. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo DJ, Cheng HL, Chan SW and Yu PH:
Antioxidative activities and the total phenolic contents of tonic
Chinese medicinal herbs. Inflammopharmacology. 16:201–207. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakamura T, Okuyama E and Yamazaki M:
Neurotropic components from star anise (Illicium verum Hook.
fil.). Chem Pharm Bull. 44:1908–1914. 1996. View Article : Google Scholar
|
|
29
|
Okuyama E, Nakamura T and Yamazaki M:
Convulsants from star anise (Illicium verum Hook. F.). Chem
Pharm Bull. 41:1670–1671. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mira E, Lacalle RA, Buesa JM, et al:
Secreted MMP9 promotes angiogenesis more efficiently than
constitutive active MMP9 bound to the tumor cell surface. J Cell
Sci. 117:1847–1857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SO, Jeong YJ, Yu MH, et al: Wogonin
suppresses TNF-alpha-induced MMP-9 expression by blocking the
NF-kappaB activation via MAPK signaling pathways in human aortic
smooth muscle cells. Biochem Biophys Res Commun. 351:118–125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lubet RA, Steele VE, Eto I, Juliana MM,
Kelloff GJ and Grubbs CJ: Chemopreventive efficacy of anethole
trithione, N-acetyl-L-cysteine, miconazole and
phenethylisothiocyanate in the DMBA-induced rat mammary cancer
model. I J Cancer. 72:95–101. 1997.PubMed/NCBI
|
|
33
|
Choo EJ, Rhee YH, Jeong SJ, et al:
Anethole exerts antimetatstaic activity via inhibition of matrix
metalloproteinase 2/9 and AKT/mitogen-activated kinase/nuclear
factor kappa B signaling pathways. Biol Pharm Bull. 34:41–46. 2011.
View Article : Google Scholar
|




















