1. Insulin resistance and
hyperinsulinemia
Insulin is a peptide hormone produced by pancreatic
β islet cells, it stimulates the transport of glucose, amino acids,
and potassium from circulating blood serum into cells. Binding of
the insulin molecule to the insulin receptor (IR) on muscle and
liver cells stimulates the induction of glycogen synthesis, fatty
acid esterification, lipolysis inhibition, protein catabolism, and
gluconeogenesis. IRs are present in two isoforms, IR A and B. As a
result of glucose and amino acid transport into cells, as well as
regulation of intracellular signaling pathways, insulin
significantly affects both the cellular transcriptome and proteome
(1). Moreover, high serum insulin
concentration inhibits autophagocytosis, proteasome activity, and
apoptosis (2). Therefore,
physiologic insulin concentrations are mainly associated with
metabolic effects and higher concentrations stimulate
anti-apoptotic and mitogenic effects (3) (Fig.
1).
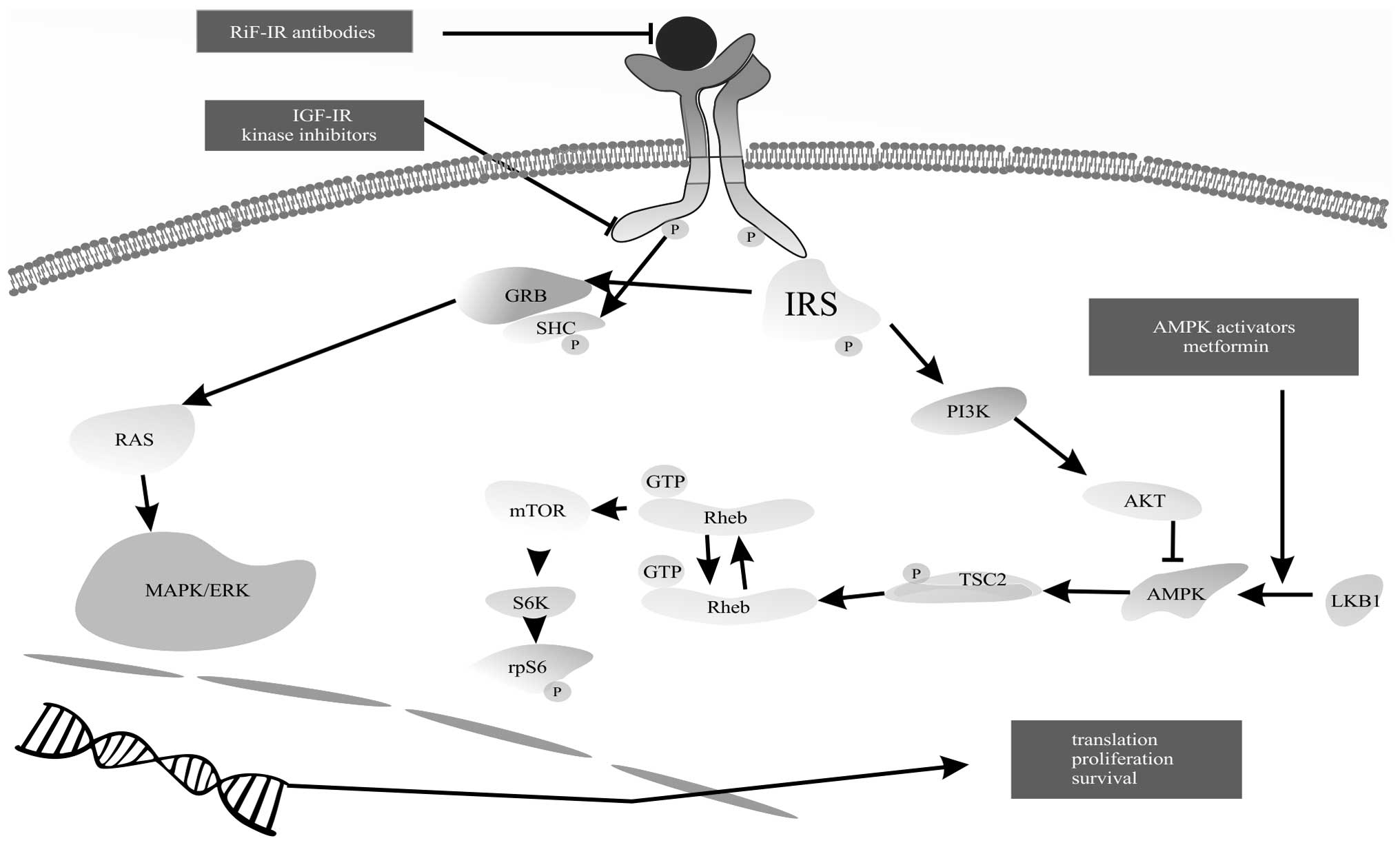
When insulin binds to its receptor, two pathways
become activated: mitogen-activated protein kinase (MAPK) and
phosphoinositide 3-kinase (PI3K) pathway (4,5).
Activation of MAPK results in transmission of mitogenic signals to
the nucleus. Activation of the PI3K pathway leads to protein kinase
B (PKB) activation and conversion to its active form (Akt/PKB).
Further activation of the mammalian target of rapamycin (mTOR)
pathway enhances protein and fatty acid synthesis and inhibits
apoptosis (Fig. 2). Proteins
homologous to insulin, insulin-like growth factors I and II (IGF-I
and -II), regulate cellular growth and differentiation. This is
accomplished by signal transduction pathways through their
respective receptors, IGF-IR and -IIR, and also by interactions
with insulin-like growth factor-binding proteins (IGFBPs), IGFBP-1
through 6 (6,7). IGF-I binding to IGF-IR results in
increased cellular proliferation and apoptosis inhibition. IGF-II
has a similar effect but its function is mainly limited to the
fetal period, and plays a major role in the development of major
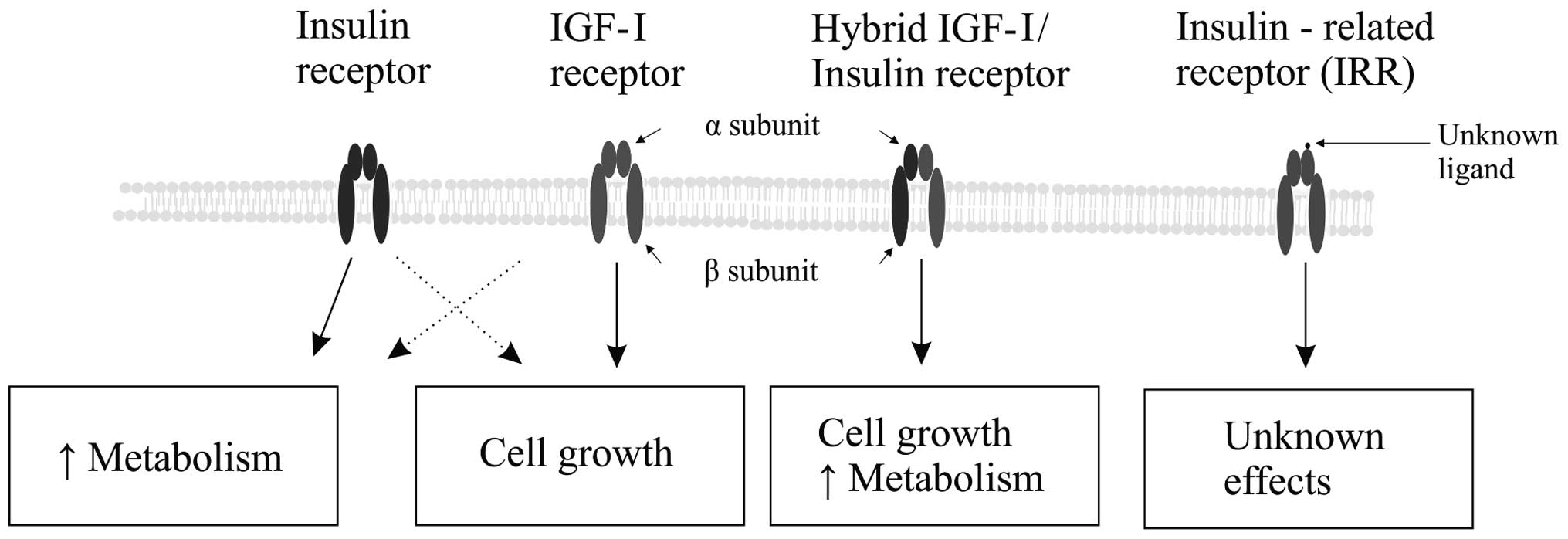
organs. IR and IGF-IR belong to a molecular class of proteins
called tyrosine kinase receptors. Intracellular pathways activated
through IGF-IR by IGF-I are similar to those pathways activated by
insulin binding its receptor (8).
In high concentrations, insulin activates its own receptor, IR, as
well as IGF-IR, stimulating cellular growth and proliferation. A
similar cross-receptor activation phenomenon was reported with high
IGF-I concentrations (Fig. 3)
(9). High post-prandial or
exogenously-administrated insulin levels lead to the inhibition of
IGFBP-1 synthesis and, therefore, result in increased free IGF-I
concentrations (10).
Additionally, the activation of IGF-IR and IR appears to be
affected by the function of another class of regulatory proteins
called tyrosine phosphatases. Specifically, protein tyrosine
phosphatase 1B (PTP1B) acts directly on IR, resulting in decreased
cellular insulin sensitivity. Furthermore, IR inhibitors appear to
increase insulin sensitivity, especially in tumor cells (11).
In vitro and biochemical studies have
identified three critical aspects of the insulin signal
transduction pathway: IR and IGF-IR binding with their substrates,
multiple isoforms of PI3K and Akt (Akt1-3), and multiple isoforms
of atypical protein kinase C (PKC), including PKCλ and ζ. Processes
associated with the regulation of these aspects impact downstream
metabolic steps, cell growth, and cellular viability, and possible
carcinogenesis (Fig. 3) (12).
A subset of regulatory proteins affecting cytokine
signal pathways also influence the insulin transduction pathway.
Examples of these proteins include SOCS1 and 3 proteins, IGF-R
binding protein (Grb10), and plasma cell membrane glycoprotein-1
(PC-1/ENPP1) particles (13–18).
This subset of proteins reduces the functionality of IR by blocking
the interaction of protein kinases, as well as modifying
receptor-dependent activation. Moreover, SOCS protein
concentrations are significantly increased in obesity, promoting
insulin resistance. Furthermore, suppressors of cytokine signaling
proteins (SOCS proteins) regulate the janus kinase (JAK)/signal
transducer and activator of transcription (STAT) pathway; this
pathway plays a significant role in the development of many cancer
types, especially hematologic malignancies (Fig. 4).
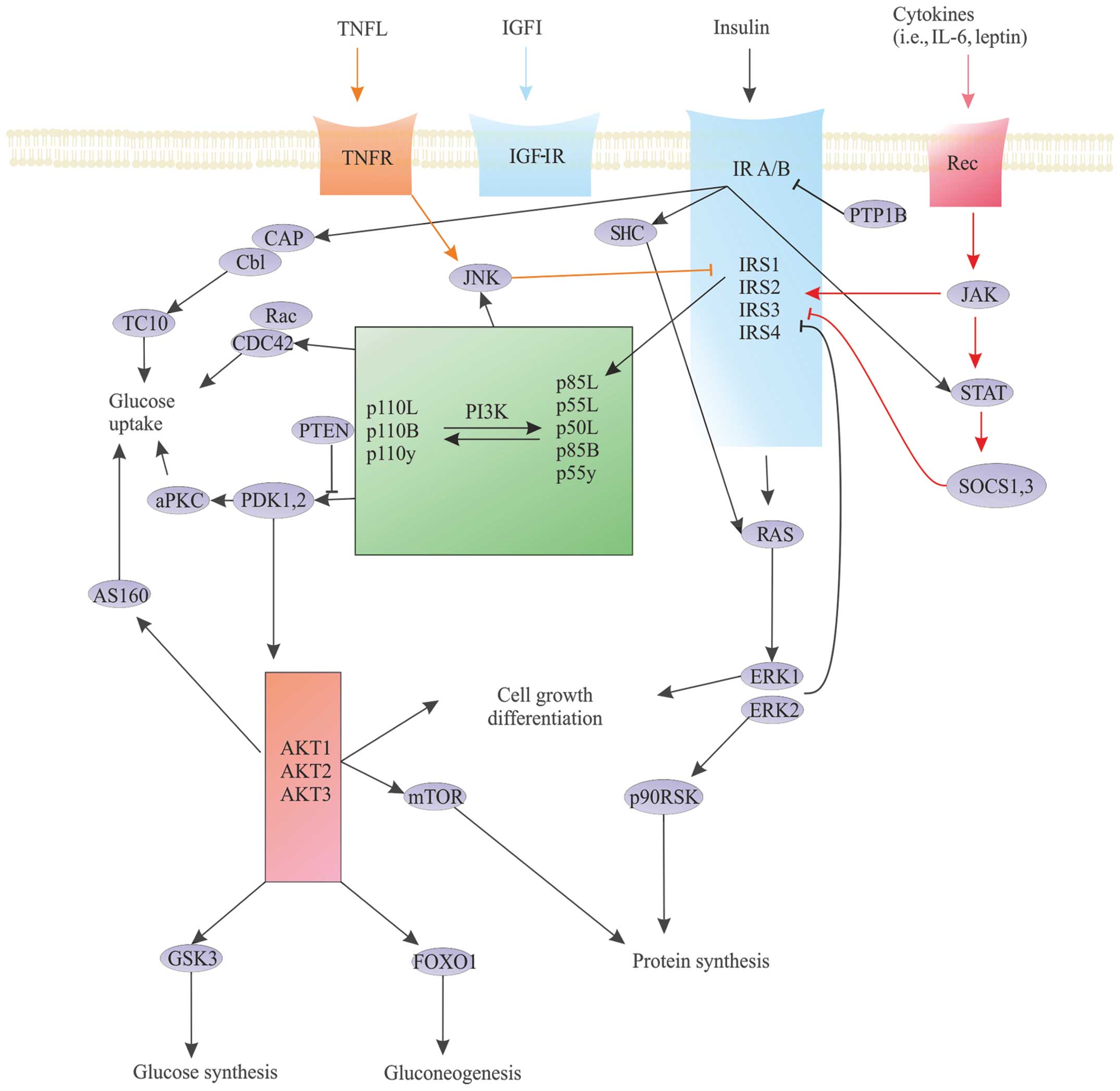 | Figure 4Crucial insulin cell signaling
pathway nodes. The concept of critical nodes in the insulin
signaling pathways (dark blue arrows) and the very similar network
of IGF-IR-dependent signalling (light blue arrows). Cytokines [TNF,
interleukin (IL)-6, leptin] interfere with insulin-dependent
pathway (orange and red arrows). The three main nodes in the signal
transduction pathway of insulin to its receptor (blue box) paired
with the insulin receptor substrate (IRS) proteins (light blue
box), phosphoinositide 3-kinase (PI3K) (green), and Akt (pink box).
JAK, janus kinase; STAT, signal transducer and activator of
transcription; SOCS proteins, suppressors of cytokine signaling
proteins; PTP1B, protein tyrosine phosphatase 1B; ERK and JNK,
mitogen-activated kinases; p90RSK, ribosomal protein S6 kinase
subtype; CAP/Cbl/TC10, signaling pathway; CDC42, cell division
control protein 42 homolog; Rac, G protein (Roc family); PTEN,
phosphatase and tensin homolog - the protein encoded by the tumor
suppressor gene PTEN; PDK, threonine kinase; aPKC, activated
protein kinase C; AS160, substrate for the kinase Akt; GSK3,
glycogen synthase kinase 3; FOXO1, transcription factor involved in
gluconeogenesis and glycogenolysis. |
Prior studies demonstrated that tumor cells express
increased IR levels. Neoplastic cells may demonstrate >50%
higher IR expression rates, especially the IR A isoform. This
receptor is the fetal variant, activated primarily by IGF-II, and
is most frequently observed in breast, lung and thyroid cancers.
Moreover, tumor cells also overexpress IGF-I, IGF-IR, and hybrid
receptors (19–25). These overexpressed receptors may
promote neoplastic growth. IGF-II expression, through the influence
on IGF-IR overactivation, may cause growth-promoting conditions as
well (26–28). On the other hand, IGF-IIR can bind
with IGF-I as well as IGF-II, which sequestrates these signaling
molecules from pathologic receptors, promoting inhibitory effects
(29–31). Dysregulation of molecular
mechanisms involving insulin and IGF may be associated with p53
(TP53), the well-studied tumor suppressor protein p53 is known to
reduce the expression of both IR and IGF-IR in its normal state and
overexpression of these receptors in its mutated state (32).
Several in vitro and in vivo studies,
as well as epidemiological analyses, have shown that high levels of
insulin and insulin-like growth factors influence cancer
development and progression (33–35).
Multiple prospective studies demonstrated that the risk of
malignancy development and cancer-related deaths is associated with
increased serum levels of endogenous insulin, increased levels of
IGF-I, and decreased levels of IGFBP-3 (36).
Metabolic pathways activated by PI3K are responsible
for a wide range of cellular functions, including fatty acid
oxidation inhibition and increased glucose consumption by tumor
cells. Blockade of signaling pathways related to PI3K, such as
class I inhibitors of PI3K, mTOR, DNA-PK, PLK-1, CK2, ATM and
PIM-1, may become a therapeutic strategy for treatment of
malignancies (37–39).
2. Abnormal glucose metabolism and
colorectal cancer
The European Prospective Investigation into Cancer
and Nutrition (EPIC) trial demonstrated that hyperinsulinemia, as
reflected by increased plasma C-peptide concentration, is
associated with a significantly higher risk of developing
colorectal cancer, especially rectal cancer (40). The relative risk of cancer in the
patient population with the highest quintile of serum C peptide was
~56% higher compared to the patient quintile with the lowest
C-peptide concentration levels.
Similar conclusions were revealed in the Nurses’
Health Study (41). In this study
population of surveyed nurses, high C-peptide level was associated
with an increased risk of colon cancer by 76%. Moreover, a high
ratio of IGF-I to IGFBP-3 increased the risk of cancer by as much
as 285%. Interestingly, high IGFBP-1 levels appeared to
significantly reduce the risk of neoplasia.
In a prospective cohort study of 14,000 New York
females assessing colon cancer risk, it was found that it increases
in C-peptide concentration was associated with increased cancer
incidence (42). Additionally, it
was reported that subjects in the two highest quintiles of IGFBP-1
and -2 serum levels had a decreased colon cancer risk by 52 and
62%, respectively. However, high levels of IGFBP-3 appeared to be
associated with an increased cancer risk of up to 246%.
A similar association between colon cancer and
elevated C-peptide concentrations was identified in the Physicians’
Health Study, a prospective, case-control study (43). One aspect of this study assessed
risk factors for malignancy [including age, smoking, alcohol
consumption, body mass index (BMI), and physical activity] and
cancer diagnosis. Risk factors were highest in subjects in the
highest C-peptide concentration quintile.
Other studies assessing patients with colorectal
cancer have also demonstrated an association between colon cancer
diagnosis with elevated levels of insulin, C peptide and IFG-I as
well as reduced IGFBP-1 concentrations (44,45).
High levels of C peptide and low levels of IGFBP-1 were also
associated with increased risk of death in patients undergoing
surgical treatment for colorectal cancer, including radical
colectomy, hemicolectomy as well as high and low rectal amputations
(46). This association may be
partly explained by a prior diagnosis of diabetes mellitus (DM) and
its associated multi-organ system complications, concomitant
diagnosis of heart failure, the physiologic stress of surgery, and
postoperative surgical complications.
3. Abnormal glucose metabolism and
pancreatic cancer
Among the various gastrointestinal malignancies,
pancreatic cancer is strongly correlated with higher C-peptide
concentrations. Michaud et al, in a prospective cohort study
of 197 pancreatic cancer patients, determined that the risk of
pancreatic cancer diagnosis was increased by 424% in the highest
C-peptide concentration quintile (47). This association was not observed
with fasting concentrations of insulin or C peptide. These
observations may suggest that prandial insulin levels may play a
role in the development of pancreatic cancer.
4. Abnormal glucose metabolism and liver
cancer
The Paris Prospective Study assessed 6,200
non-diabetic French men and identified an association between
increased insulin levels and death from liver cancer (48). The study evaluated both fasting
insulin levels and insulin levels after 120 min following
initiation of the standardized oral glucose tolerance test (OGTT).
This study found that both elevated fasting insulin levels and
elevated insulin levels 120 min following initiation of the OGTT
were significantly associated with an increased risk of dying from
liver cancer. The risk of fatal liver cancer was increased by 272
and 341% in the two highest quintiles for fasting insulin levels
and insulin levels 120 min after initiation of the OGTT,
respectively.
5. Abnormal glucose metabolism and breast
cancer
Recently published studies evaluating the
association between derangements in glucose metabolism and
subsequent development of breast cancer have demonstrated
contradictory findings. In the prospective Nurses’ Health Study II,
~75% of the female subjects were premenopausal (49). This study found that high
concentrations of C peptide were not associated with increased risk
of breast cancer. A similar case-control Japanese study, evaluating
only postmenopausal women, found that increased C-peptide levels
were associated with an increased risk of breast cancer, but only
in women with a BMI >28 kg/m2 (50). A meta-analysis assessing 20
studies, from 1966 to 2007, demonstrated that DM [primarily type 2
diabetes mellitus (DM2)] increased the risk of breast cancer
development by ~20% (51). Further
studies will be required to determine how DM specifically promotes
oncogenesis in breast cancer, in both the pre- and postmenopausal
females. Specific questions that need to be addressed include the
role of insulin transduction pathways and insulin’s effects on
cellular proliferation, anti-apoptosis, and sex hormone
metabolism.
6. Abnormal glucose metabolism and
endometrial cancer
Results from the aforementioned EPIC study, a
prospective, multi-institutional study of the European population,
demonstrated that the risk of endometrial cancer increases with
higher concentrations of serum C peptide (52). When BMI is taken into account, the
increased risk of endometrial cancer is as high as 56%. A
case-control study conducted in the United States, Sweden, and
Italy, by Lukanova et al, demonstrated similar findings with
regard to the relationship between higher C-peptide levels and
increased risk of endometrial cancer (53,54).
After adjusting for BMI, the odds ratio assessing the risk of
developing endometrial cancer in the highest quintile of serum
C-peptide concentration was 4.40. Lukanova et al also
assessed the risk of developing ovarian cancer and found no
significant correlation between the risk of ovarian cancer
diagnosis and elevated C-peptide concentrations. Furthermore, the
authors demonstrated that increased concentrations of IGFBP-1 and
-2 were not associated with a reduced risk of endometrial cancer
development after adjustment for confounders (53).
7. Abnormal glucose metabolism and prostate
cancer
Multiple studies have assessed the association
between DM and the subsequent development of prostate cancer. A
meta-analysis, by Kasper and Giovannucci, showed that the risk of
prostate cancer was ~16% lower in males with DM compared to
non-diabetic males (55). One
theory to explain this phenomenon is decreased levels of
circulating androgens. Decreased androgen levels are associated
with insulin resistance states, as in DM2. Reduced levels of
endogenous and exogenous insulin may have a protective role in the
development of prostate cancer in poorly controlled diabetic males.
Conversely, a prospective, multi-institutional study with a
multiracial population, conducted in the United States and Canada
by Borugian et al, did not identify any association between
serum C-peptide concentrations and increased risk of developing
prostate cancer (56).
Additionally, Roddam et al performed a metanalysis of twelve
prospective studies showing little association between prostate
cancer risk and IGF-I levels (57).
Ma et al, in a prospective Swedish study,
evaluated the impact of many factors associated with impaired
glucose metabolism, including BMI, concentrations of C peptide,
leptin, glycated hemoglobin (HbA1c), fasting glucose, OGTT test
results, and an index of tissue homeostasis model assessment of
insulin resistance (HOMA-IR) on the risk of prostate cancer
development (58). They observed
that the relative risk of death from prostate cancer increased by
56% in patients who were overweight. Moreover, the increased risk
of cancer-related death in obese patients was >2.6-fold.
Additionally, men with C-peptide concentrations in the highest
quartile versus the lowest quartile had a higher risk of prostate
cancer mortality (HR 2.38). The relative risk of death from
prostate cancer in patients with a BMI >25 kg/m2 and
C-peptide concentration in the upper quintiles was 4-fold higher
than in patients with normal BMI and C-peptide concentrations in
the lower quintiles. The study suggested a relationship between
impaired glucose metabolism and increased risk of aggressive forms
of prostate cancer development; however, these findings did not
reach statistical significance. According to Ma et al
(57), these results may suggest
that in diabetic males, an important factor affecting the initial
phase of prostate cancer development is reduced androgen levels
whereas in later stages of tumor development, insulin mitogenic
effects may play a dominant role; however, further studies are
warranted to explore this theory.
8. Abnormal glucose metabolism and lung
cancer
Lung cancer has the highest malignancy incidence and
mortality rates in developed countries. Given its high global
prevalence, many molecular studies on lung cancer tumor cell lines
and animal models exist. Additionally, multiple studies have
assessed possible associations between abnormal glucose metabolism
conditions and the subsequent development of lung cancer. Numerous
clinical studies demonstrated no statistically significant
association between serum abnormalities reflective of abnormal
glucose metabolism and subsequent development of lung cancer
(59,60). London et al, in a
prospective study of Chinese men, found no relationship between
high concentrations of IFG-I and increased risk of lung cancer
(61). Moreover, they noted a
decreased risk of lung cancer with higher IGFBP-3 concentrations.
On the other hand, a nested case-control study based on the
β-Carotene and Retinol Efficacy Trial (CARET), an American study
assessing lung cancer chemoprevention in smokers and individuals
exposed to passive smoking, found that increased IGF-I
concentrations were associated with a reduced risk of developing
lung cancer, while higher levels of IGFBP-3 were associated with an
increased risk of developing lung cancer (62).
9. DM, obesity, and the immune system
Diabetic patients have dysfunctional innate and
adaptive immune systems, resulting in increased susceptibility to
infections and subsequent development of immune system disorders
(1,63,64).
Immune system dysfunction may ultimately lead to increased risk of
cancer development. The cell types most actively involved in the
destruction of malignant cells are natural killer (NK) cells and
natural killer T cells (NKT cells).
NK cells are a type of peripheral blood lymphocyte
and account for ~10–19% of peripheral lymphocytes. NK cells are
responsible for the phenomenon of ‘natural cytotoxicity’ and are
involved in the early stages of non-specific immune surveillance
and response to abnormal foreign and host cells. They are likely
derived from a common lymphopoietic progenitor cell line and are
subjected to selection mechanisms, as evidenced by the observed
phenomenon of hybrid resistance. NK cells belong to a group of
immune cells called ‘K cells’ that are involved in
antibody-dependent cytotoxicity. Other K cells include,
macrophages, monocytes, certain T lymphocytes, neutrophils,
eosinophils, and thrombocytes. NK cellular membrane surfaces
contain characteristic protein markers, including CD16, 56 and 57.
They lack CD3. NK cells are distinguished by their ability to
spontaneously kill targeted foreign and abnormal host cells without
prior immunization. NK cells identify their targets by evaluating
the targeted cell’s concentration of surface cell markers. A
reduced concentration or complete loss of cell surface molecules,
such as class I major histocompatibility complex (MHC) proteins,
activates the NK cell’s cytotoxic mechanism and subsequent
destruction of the targeted cell. Reduction or complete lack of
class I MHC proteins is characteristic of viruses and cancer cells.
The class I MHC status of cells is verified through various
receptors, including immunoglobulin-like receptors (KIR, NCR, ILT,
LAIR, lectin receptors), and the CD94/NKG2 family of receptors.
Potent activators of NK cells are interleukin (IL)-2 and -4,
interferon (INF)-α and -γ. NK cell inhibitors include prostaglandin
E2 and transforming growth factor-β (TGF-β).
Individual differences in NK cell activity levels
depend on multiple genetic and environmental factors. Prior studies
demonstrated increased NK cellular activity in physically active
individuals, especially athletes (1,65–67).
Reduced activity was observed in individuals with high-fat diets.
Additionally, it was observed that chronic stress and depression
result in abnormal NK cell activity (68,69).
NK cells were first described in the 1970s when
mechanisms of lymphocyte-specific tumor cell cytotoxicity were
being investigated (70–72). Their antitumor mechanisms were
confirmed in subsequent animal studies (73,74).
Prior studies have shown that high NK cell activity is associated
with a lower risk of cancer development (75). Moreover, the activity of these
cells in oncologic patients is dependent on the disease phase and
progression. NK activity in patients with malignant tumors without
distant metastases is generally similar or slightly reduced
compared to healthy individuals. On the other hand, NK activity is
significantly reduced in patients with metastatic disease (76).
NKT cells are the subject of many studies evaluating
the role of the immune system in autoimmune diseases and malignancy
(77–80). They are aptly named ‘natural killer
T cells’ due to the cells possessing surface T-cell markers
(CD3-TCR complex), surface NK cell markers (CD56, 161 and 94), and
marker CD57. Studies suggest that NKT cells mature in the thymus,
like B and T lymphocytes. A particularly large number of NKT cells
are located in the liver, accounting for up to 40% of total cells
in the organ. Conversely, NKT cells make up a very small percentage
of peripheral blood lymphocytes, accounting for ~1–5% of all
peripheral blood T cells (81).
Despite their low number, NKT cells play an important role in
immunoregulation. Due to their cytotoxic abilities, NKT cells are
likely involved in the elimination of certain pathogens, including
viruses, intracellular bacteria, and protozoa, as well as abnormal
host cells, such as tumor cells. The NKT cell receptor has low
molecular variability and is able to recognize abnormal surface
class I MHC molecules presented by dysfunctional host cells, such
as host cells infected by viruses or intracellular bacteria or
malignantly-transformed host cells. NKT cells usually lack CD4 and
8 surface markers and, when activated, secrete large concentrations
of IL-4 and IFN-γ (82–86). NKT cells, similar to T cell
lymphocytes, may also be subject to immune polarization, an
observed phenomenon when immune cells differentiate into distinct
effector cell types in response to different cytokines.
Prior studies have shown that obesity and
prediabetes are associated with a reduction in both NK and NKT cell
activity levels (87). Lynch et
al evaluated NK cell populations in three different patient
groups: obese individuals with metabolic syndrome, obese
individuals without metabolic syndrome, and non-overweight
individuals without metabolic syndrome (serving as the control
group) (88). The mean age and BMI
in both obese groups were comparable. They observed that in obese
subjects, peripheral blood NK cell and cytotoxic lymphocyte numbers
were significantly lower when compared to the control group.
Moreover, regardless of age or actual BMI value, obese subjects
without metabolic syndrome had significantly higher percentages of
NK and cytotoxic lymphocytes than obese subjects with metabolic
syndrome (NK cells 11.7 vs. 6.5%, respectively, p=0.0001; CD8 cells
13.4% vs. 9.3%, respectively, p=0.04). Additionally, NK cell
activity was also significantly higher in obese individuals without
metabolic syndrome versus obese individuals with metabolic
syndrome. Further studies are warranted to determine whether
metabolic disorders play a causal role in the reduction of NK cell
number and function or whether a better functioning immune system
protects obese individuals from metabolic disorders and
complications.
In some organs, such as the liver as discussed
above, the percentage of NKT cells is greater than in peripheral
blood. Examination of human peritoneal samples confirmed such
findings (89). Analysis revealed
up to 15% of NKT cells in the peritoneum depending on the NKT cell
subtypes (10% iNKT, 15% with CD1d expression) compared to ~1% in
peripheral blood. Compared to healthy controls with normal BMI, the
peritoneal iNKT cell number was significantly lower in morbidly
obese subjects (p=0.005) and in subjects with colorectal cancer
(p=0.004) (89).
Numerous studies suggest that obesity, metabolic
disorders, and cancer are accompanied by adverse changes in NK and
NKT cell populations, which are manifested primarily by a decrease
in cell number and activity. The mechanisms responsible for these
adverse immunologic changes are not yet understood.
10. Conclusions
The most important molecular mechanisms underlying
the development and progression of cancer in patients with DM
include oxidative stresses, generation of reactive oxygen species
and nitric oxide with subsequent damage to cell membranes and DNA,
overproduction of lactate byproducts, and pathological
overexpression of certain enzymes. Additionally, derangements in
the insulin-receptor signal transduction pathways and an impaired
immune system may contribute to oncogenesis. Obesity and metabolic
disorders contribute to a chronic inflammatory state, dysfunctional
humoral and cellular immune responses, and decreased number and
activity levels of NK and NKT cell populations. Additionally,
studies have demonstrated an association between abnormal glucose
metabolism and increased incidence of multiple malignancies,
including colorectal, pancreatic, liver, breast, endometrial and
prostate cancers.
Acknowledgements
This study was supported by the National Science
Centre (NCN) (Krakow, Poland) grant no. UMO-
2012/05/D/NZ5/01844.
Abbreviations:
|
PI3K
|
phosphoinositide 3-kinase
|
|
PTEN
|
phosphatase and tensin homolog
|
|
mTOR
|
mammalian target of rapamycin
|
|
NK
|
natural killer
|
|
NKT cell
|
natural killer T cell
|
|
IR
|
insulin receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PKB
|
protein kinase B
|
|
IGF-I and -II
|
insulin-like growth factors I and
II
|
|
IGFBP
|
insulin-like growth factor-binding
protein
|
|
PTP1B
|
protein tyrosine phosphatase 1B
|
|
PKC
|
protein kinase C
|
|
SOCS proteins
|
suppressors of cytokine signaling
proteins
|
|
PC-1/ENPP1
|
plasma cell membrane
glycoprotein-1
|
|
JAK
|
janus kinase
|
|
STAT
|
signal transducer and activator of
transcription
|
|
DM
|
diabetes mellitus
|
|
OGTT
|
oral glucose tolerance test
|
|
HbA1c
|
glycated hemoglobin
|
|
HOMA-IR
|
homeostasis model assessment of
insulin resistance
|
|
MHC
|
major histocompatibility complex
|
|
IL
|
interleukin
|
|
INF
|
interferon
|
|
TGF-β
|
transforming growth factor-β
|
|
IRS
|
insulin receptor substrate
|
|
GRB protein
|
growth factor receptor-bound
protein
|
|
SHC
|
adapter protein
|
|
RAS
|
RAS protein
|
|
TSC1/2
|
hamartin/tuberin
|
|
LKB1 gene
|
liver kinase B1 gene
|
|
Rheb
|
GTP binding protein
|
|
rpS6
|
ribosomal protein S6
|
|
ERK and JNK
|
mitogen-activated kinases
|
|
p90RSK
|
ribosomal protein S6 kinase
subtype
|
|
CAP/Cbl/TC10
|
signaling pathway
|
|
CDC42
|
cell division control protein 42
homolog
|
|
Rac
|
G protein (Roc family)
|
|
PDK
|
threonine kinase
|
|
aPKC
|
activated protein kinase C
|
|
AS160
|
substrate for the kinase Akt
|
|
GSK3
|
glycogen synthase kinase 3
|
|
FOXO1
|
transcription factor involved in
gluconeogenesis and glycogenolysis
|
References
|
1
|
Piatkiewicz P and Czech A: Glucose
metabolism disorders and the risk of cancer. Arch Immunol Ther Exp
(Warsz). 59:215–230. 2011. View Article : Google Scholar
|
|
2
|
Bergamini E, Cavallini G, Donati A and
Gori Z: The role of autophagy in aging: its essential part in the
anti-aging mechanism of caloric restriction. Ann NY Acad Sci.
1114:69–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Meyts P: The structural basis of
insulin and insulin-like growth factor-I receptor binding and
negative co-operativity, and its relevance to mitogenic versus
metabolic signalling. Diabetologia. 37(Suppl 2): S135–S148. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jensen M and De Meyts P: Molecular
mechanisms of differential intracellular signaling from the insulin
receptor. Vitam Horm. 80:51–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hermann C, Assmus B, Urbich C, Zeiher AM
and Dimmeler S: Insulin-mediated stimulation of protein kinase Akt:
A potent survival signaling cascade for endothelial cells.
Arterioscler Thromb Vasc Biol. 20:402–409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwa V, Oh Y and Rosenfeld RG: The
insulin-like growth factor-binding protein (IGFBP) superfamily.
Endocr Rev. 20:761–787. 1999.PubMed/NCBI
|
|
7
|
Holly J and Perks C: The role of
insulin-like growth factor binding proteins. Neuroendocrinology.
83:154–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: biological actions.
Endocr Rev. 16:3–34. 1995.PubMed/NCBI
|
|
9
|
Werner H, Weinstein D and Bentov I:
Similarities and differences between insulin and IGF-I: structures,
receptors, and signalling pathways. Arch Physiol Biochem.
114:17–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee PD, Giudice LC, Conover CA and Powell
DR: Insulin-like growth factor binding protein-1: recent findings
and new directions. Proc Soc Exp Biol Med. 216:319–357. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gum RJ, Gaede LL, Koterski SL, et al:
Reduction of protein tyrosine phosphatase 1B increases
insulin-dependent signaling in ob/ob mice. Diabetes. 52:21–28.
2003. View Article : Google Scholar
|
|
12
|
Taniguchi CM, Emanuelli B and Kahn CR:
Critical nodes in signalling pathways: insights into insulin
action. Nat Rev Mol Cell Biol. 7:85–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ueki K, Kondo T and Kahn CR: Suppressor of
cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance
through inhibition of tyrosine phosphorylation of insulin receptor
substrate proteins by discrete mechanisms. Mol Cell Biol.
24:5434–5446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueki K, Kondo T, Tseng YH and Kahn CR:
Central role of suppressors of cytokine signaling proteins in
hepatic steatosis, insulin resistance, and the metabolic syndrome
in the mouse. Proc Natl Acad Sci USA. 101:10422–10427. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Emanuelli B, Peraldi P, Filloux C,
Sawka-Verhelle D, Hilton D and Van Obberghen E: SOCS-3 is an
insulin-induced negative regulator of insulin signaling. J Biol
Chem. 275:15985–15991. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Emanuelli B, Peraldi P, Filloux C, et al:
SOCS-3 inhibits insulin signaling and is up-regulated in response
to tumor necrosis factor-alpha in the adipose tissue of obese mice.
J Biol Chem. 276:47944–47949. 2001.PubMed/NCBI
|
|
17
|
Wick KR, Werner ED, Langlais P, et al:
Grb10 inhibits insulin- stimulated insulin receptor substrate
(IRS)-phosphatidylinositol 3-kinase/Akt signaling pathway by
disrupting the association of IRS-1/IRS-2 with the insulin
receptor. J Biol Chem. 278:8460–8467. 2003. View Article : Google Scholar
|
|
18
|
Dong H, Maddux BA, Altomonte J, et al:
Increased hepatic levels of the insulin receptor inhibitor,
PC-1/NPP1, induce insulin resistance and glucose intolerance.
Diabetes. 54:367–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kellerer M, von Eye Corleta H, Mühlhöfer
A, et al: Insulin- and insulin-like growth-factor-I receptor
tyrosine-kinase activities in human renal carcinoma. Int J Cancer.
62:501–507. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Corleta HE, Capp E and Corleta OC: Insulin
receptor tyrosine kinase activity in colon carcinoma. Braz J Med
Biol Res. 29:1593–1597. 1996.PubMed/NCBI
|
|
21
|
Werner H and Le Roith D: New concepts in
regulation and function of the insulin-like growth factors:
implications for understanding normal growth and neoplasia. Cell
Mol Life Sci. 57:932–942. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yi HK, Hwang PH, Yang DH, Kang CW and Lee
DY: Expression of the insulin-like growth factors (IGFs) and the
IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J
Cancer. 37:2257–2263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hudelist G, Wagner T, Rosner M, et al:
Intratumoral IGF-I protein expression is selectively upregulated in
breast cancer patients with BRCA1/2 mutations. Endocr Relat Cancer.
14:1053–1062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim WY, Jin Q, Oh SH, et al: Elevated
epithelial insulin-like growth factor expression is a risk factor
for lung cancer development. Cancer Res. 69:7439–7448. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Werner H and Bruchim I: The insulin-like
growth factor-I receptor as an oncogene. Arch Physiol Biochem.
115:58–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vella V, Pandini G, Sciacca L, et al: A
novel autocrine loop involving IGF-II and the insulin receptor
isoform-A stimulates growth of thyroid cancer. J Clin Endocrinol
Metab. 87:245–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boulle N, Logié A, Gicquel C, Perin L and
Le Bouc Y: Increased levels of insulin-like growth factor II
(IGF-II) and IGF-binding protein-2 are associated with malignancy
in sporadic adreno-cortical tumors. J Clin Endocrinol Metab.
83:1713–1720. 1998.PubMed/NCBI
|
|
28
|
Moorehead RA, Sanchez OH, Baldwin RM and
Khokha R: Transgenic overexpression of IGF-II induces spontaneous
lung tumors: a model for human lung adenocarcinoma. Oncogene.
22:853–857. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JS, Weiss J, Martin JL and Scott CD:
Increased expression of the mannose 6-phosphate/insulin-like growth
factor-II receptor in breast cancer cells alters tumorigenic
properties in vitro and in vivo. Int J Cancer. 107:564–570. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hebert E: Mannose-6-phosphate/insulin-like
growth factor II receptor expression and tumor development. Biosci
Rep. 26:7–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
El-Shewy HM and Luttrell LM: Insulin-like
growth factor-2/mannose-6 phosphate receptors. Vitam Horm.
80:667–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Larsson O, Girnita A and Girnita L: Role
of insulin-like growth factor 1 receptor signalling in cancer. Br J
Cancer. 92:2097–2101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frasca F, Pandini G, Sciacca L, et al: The
role of insulin receptors and IGF-I receptors in cancer and other
diseases. Arch Physiol Biochem. 114:23–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Godsland IF: Insulin resistance and
hyperinsulinaemia in the development and progression of cancer.
Clin Sci (Lond). 118:315–332. 2009. View Article : Google Scholar
|
|
36
|
Renehan AG, Zwahlen M, Minder C, O’Dwyer
ST, Shalet SM and Egger M: Insulin-like growth factor (IGF)-I, IGF
binding protein-3, and cancer risk: systematic review and
meta-regression analysis. Lancet. 363:1346–1353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ihle NT and Powis G: Take your PIK:
phosphatidylinositol 3-kinase inhibitors race through the clinic
and toward cancer therapy. Mol Cancer Ther. 8:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robey RB and Hay N: Is Akt the ‘Warburg
kinase’? -Akt-energy metabolism interactions and oncogenesis. Semin
Cancer Biol. 19:25–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jenab M, Riboli E, Cleveland RJ, et al:
Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal
cancers in the European Prospective Investigation into Cancer and
Nutrition. Int J Cancer. 121:368–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei EK, Ma J, Pollak MN, et al: A
prospective study of C-peptide, insulin-like growth factor-I,
insulin-like growth factor binding protein-1, and the risk of
colorectal cancer in women. Cancer Epidemiol Biomarkers Prev.
14:850–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaaks R, Toniolo P, Akhmedkhanov A, et al:
Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding
proteins, and colorectal cancer risk in women. J Natl Cancer Inst.
92:1592–1600. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma J, Giovannucci E, Pollak M, et al: A
prospective study of plasma C-peptide and colorectal cancer risk in
men. J Natl Cancer Inst. 96:546–553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schoen RE, Tangen CM, Kuller LH, et al:
Increased blood glucose and insulin, body size, and incident
colorectal cancer. J Natl Cancer Inst. 91:1147–1154. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Le Marchand L, Wang H, Rinaldi S, et al:
Associations of plasma C-peptide and IGFBP-1 levels with risk of
colorectal adenoma in a multiethnic population. Cancer Epidemiol
Biomarkers Prev. 19:1471–1477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wolpin BM, Meyerhardt JA, Chan AT, et al:
Insulin, the insulin-like growth factor axis, and mortality in
patients with nonmetastatic colorectal cancer. J Clin Oncol.
27:176–185. 2009. View Article : Google Scholar :
|
|
47
|
Michaud DS, Wolpin B, Giovannucci E, et
al: Prediagnostic plasma C-peptide and pancreatic cancer risk in
men and women. Cancer Epidemiol Biomarkers Prev. 16:2101–2109.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Balkau B, Kahn HS, Courbon D, Eschwège E
and Ducimetière P; Paris Prospective Study. Hyperinsulinemia
predicts fatal liver cancer but is inversely associated with fatal
cancer at some other sites: the Paris Prospective Study. Diabetes
Care. 24:843–849. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eliassen AH, Tworoger SS, Mantzoros CS,
Pollak MN and Hankinson SE: Circulating insulin and c-peptide
levels and risk of breast cancer among predominately premenopausal
women. Cancer Epidemiol Biomarkers Prev. 16:161–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hirose K, Toyama T, Iwata H, Takezaki T,
Hamajima N and Tajima K: Insulin, insulin-like growth factor-I and
breast cancer risk in Japanese women. Asian Pac J Cancer Prev.
4:239–246. 2003.PubMed/NCBI
|
|
51
|
Larsson SC, Mantzoros CS and Wolk A:
Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J
Cancer. 121:856–862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cust AE, Allen NE, Rinaldi S, et al: Serum
levels of C-peptide, IGFBP-1 and IGFBP-2 and endometrial cancer
risk; results from the European prospective investigation into
cancer and nutrition. Int J Cancer. 120:2656–2664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lukanova A, Lundin E, Micheli A, et al:
Risk of ovarian cancer in relation to prediagnostic levels of
C-peptide, insulin-like growth factor binding proteins-1 and -2
(USA, Sweden, Italy). Cancer Causes Control. 14:285–292. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lukanova A, Zeleniuch-Jacquotte A, Lundin
E, et al: Prediagnostic levels of C-peptide, IGF-I, IGFBP -1, -2
and -3 and risk of endometrial cancer. Int J Cancer. 108:262–268.
2004. View Article : Google Scholar
|
|
55
|
Kasper JS and Giovannucci E: A
meta-analysis of diabetes mellitus and the risk of prostate cancer.
Cancer Epidemiol Biomarkers Prev. 15:2056–2062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Borugian MJ, Spinelli JJ, Sun Z, et al:
Prediagnostic C-peptide and risk of prostate cancer. Cancer
Epidemiol Biomarkers Prev. 16:2164–2165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Roddam AW, Allen NE, Appleby P, et al:
Insulin-like growth factors, their binding proteins, and prostate
cancer risk: analysis of individual patient data from 12
prospective studies. Ann Intern Med. 149:461–471. w83–w88. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ma J, Li H, Giovannucci E, et al:
Prediagnostic body-mass index, plasma C-peptide concentration, and
prostate cancer-specific mortality in men with prostate cancer: a
long-term survival analysis. Lancet Oncol. 9:1039–1047. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ahn J, Weinstein SJ, Snyder K, Pollak MN,
Virtamo J and Albanes D: No association between serum insulin-like
growth factor (IGF)-I, IGF-binding protein-3, and lung cancer risk.
Cancer Epidemiol Biomarkers Prev. 15:2010–2012. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lukanova A, Toniolo P, Akhmedkhanov A, et
al: A prospective study of insulin-like growth factor-I,
IGF-binding proteins-1, -2 and -3 and lung cancer risk in women.
Int J Cancer. 92:888–892. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
London SJ, Yuan JM, Travlos GS, et al:
Insulin-like growth factor I, IGF-binding protein 3, and lung
cancer risk in a prospective study of men in China. J Natl Cancer
Inst. 94:749–754. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Spitz MR, Barnett MJ, Goodman GE,
Thornquist MD, Wu X and Pollak M: Serum insulin-like growth factor
(IGF) and IGF-binding protein levels and risk of lung cancer: a
case-control study nested in the beta-Carotene and Retinol Efficacy
Trial Cohort. Cancer Epidemiol Biomarkers Prev. 11:1413–1418.
2002.PubMed/NCBI
|
|
63
|
Joshi N, Caputo GM, Weitekamp MR and
Karchmer AW: Infections in patients with diabetes mellitus. N Engl
J Med. 341:1906–1912. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Muller LM, Gorter KJ, Hak E, et al:
Increased risk of common infections in patients with type 1 and
type 2 diabetes mellitus. Clin Infect Dis. 41:281–288. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Takahashi K, Iwase M, Yamashita K, et al:
The elevation of natural killer cell activity induced by laughter
in a crossover designed study. Int J Mol Med. 8:645–650.
2001.PubMed/NCBI
|
|
66
|
Li Q, Kobayashi M, Inagaki H, et al: A day
trip to a forest park increases human natural killer activity and
the expression of anti-cancer proteins in male subjects. J Biol
Regul Homeost Agents. 24:157–165. 2010.PubMed/NCBI
|
|
67
|
Hayashi T, Tsujii S, Iburi T, et al:
Laughter up-regulates the genes related to NK cell activity in
diabetes. Biomed Res. 28:281–285. 2007. View Article : Google Scholar
|
|
68
|
Maes M, Meltzer HY, Stevens W, Calabrese J
and Cosyns P: Natural killer cell activity in major depression:
relation to circulating natural killer cells, cellular indices of
the immune response, and depressive phenomenology. Prog
Neuropsychopharmacol Biol Psychiatry. 18:717–730. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Reiche EM, Nunes SO and Morimoto HK:
Stress, depression, the immune system, and cancer. Lancet Oncol.
5:617–625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kiessling R, Klein E, Pross H and Wigzell
H: ‘Natural’ killer cells in the mouse. II Cytotoxic cells with
specificity for mouse Moloney leukemia cells Characteristics of the
killer cell. Eur J Immunol. 5:117–121. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kiessling R, Petranyi G, Kärre K, Jondal
M, Tracey D and Wigzell H: Killer cells: a functional comparison
between natural, immune T-cell and antibody-dependent in vitro
systems. J Exp Med. 143:772–780. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Roder JC, Kiessling R, Biberfeld P and
Andersson B: Target-effector interaction in the natural killer (NK)
cell system. II The isolation of NK cells and studies on the
mechanism of killing. J Immunol. 121:2509–2517. 1978.PubMed/NCBI
|
|
73
|
Moretta L, Bottino C, Pende D, Vitale M,
Mingari MC and Moretta A: Human natural killer cells: molecular
mechanisms controlling NK cell activation and tumor cell lysis.
Immunol Lett. 100:7–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Waldhauer I and Steinle A: NK cells and
cancer immunosurveillance. Oncogene. 27:5932–5943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Furue H, Matsuo K, Kumimoto H, et al:
Decreased risk of colorectal cancer with the high natural killer
cell activity NKG2D genotype in Japanese. Carcinogenesis.
29:316–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Szkaradkiewicz A, Karpiński TM, Drews M,
Borejsza-Wysocki M, Majewski P and Andrzejewska E: Natural killer
cell cytotoxicity and immunosuppressive cytokines (IL-10,
TGF-beta1) in patients with gastric cancer. J Biomed Biotechnol.
2010:9015642010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hammond KJ, Pelikan SB, Crowe NY, et al:
NKT cells are phenotypically and functionally diverse. Eur J
Immunol. 29:3768–3781. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jerud ES, Bricard G and Porcelli SA:
CD1d-restricted natural killer t cells: roles in tumor
immunosurveillance and tolerance. Transfus Med Hemother. 33:18–36.
2006. View Article : Google Scholar
|
|
79
|
Montoya CJ, Pollard D, Martinson J, et al:
Characterization of human invariant natural killer T subsets in
health and disease using a novel invariant natural killer T
cell-clonotypic monoclonal antibody, 6B11. Immunology. 122:1–14.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Balato A, Unutmaz D and Gaspari AA:
Natural killer T cells: an unconventional T-cell subset with
diverse effector and regulatory functions. J Invest Dermatol.
129:1628–1642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chan AC, Serwecinska L, Cochrane A,
Harrison LC, Godfrey DI and Berzins SP: Immune characterization of
an individual with an exceptionally high natural killer T cell
frequency and her immediate family. Clin Exp Immunol. 156:238–245.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Smyth MJ, Thia KY, Street SE, et al:
Differential tumor surveillance by natural killer (NK) and NKT
cells. J Exp Med. 191:661–668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mercer JC, Ragin MJ and August A: Natural
killer T cells: rapid responders controlling immunity and disease.
Int J Biochem Cell Biol. 37:1337–1343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bendelac A, Savage PB and Teyton L: The
biology of NKT cells. Annu Rev Immunol. 25:297–336. 2007.
View Article : Google Scholar
|
|
85
|
Berzofsky JA and Terabe M: The contrasting
roles of NKT cells in tumor immunity. Curr Mol Med. 9:667–672.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Swann JB, Uldrich AP, van Dommelen S, et
al: Type I natural killer T cells suppress tumors caused by p53
loss in mice. Blood. 113:6382–6385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
O’Shea D, Cawood TJ, O’Farrelly C and
Lynch L: Natural killer cells in obesity: impaired function and
increased susceptibility to the effects of cigarette smoke. PLoS
One. 5:e86602010. View Article : Google Scholar :
|
|
88
|
Lynch LA, O’Connell JM, Kwasnik AK, Cawood
TJ, O’Farrelly C and O’Shea DB: Are natural killer cells protecting
the metabolically healthy obese patient? Obesity (Silver Spring).
17:601–605. 2009. View Article : Google Scholar
|
|
89
|
Lynch L, O’Shea D, Winter DC, Geoghegan J,
Doherty DG and O’Farrelly C: Invariant NKT cells and CD1d(+) cells
amass in human omentum and are depleted in patients with cancer and
obesity. Eur J Immunol. 39:1893–1901. 2009. View Article : Google Scholar : PubMed/NCBI
|