Introduction
Adult T-cell leukemia (ATL) is an aggressive
malignancy of CD4+ T cells caused by the retrovirus,
human T-cell leukemia virus type 1 (HTLV-1) (1–3). ATL
is associated with poor prognosis mainly due to resistance to
conventional chemotherapies, hypercalcemia and frequent
opportunistic infections due to the associated severe
immunosuppression (4). In addition
to the classical structural genes required for retroviral
replication, the HTLV-1 genome encodes the viral transcriptional
activator Tax. The oncogenic potential of Tax has been extensively
studied. Especially, permanent activation of the nuclear factor-κB
(NF-κB) pathway by Tax is a key event in the process of
HTLV-1-induced T-cell immortalization and leukemogenesis (5,6). In
the cell nucleus, NF-κB binds promoter DNA elements to initiate or
enhance transcription of the genes involved in T-cell survival and
cell cycle progression (5,6). Understanding cell survival pathways
should enhance the design of new therapeutic approaches against
ATL.
The protein kinase C (PKC) family is subdivided into
three categories: conventional, novel and atypical PKCs (7). PKC-δ, a novel PKC, plays a crucial
role in the cellular response to genotoxic stress. It generally
functions as a pro-apoptotic protein during DNA damage-induced
apoptosis (8). In contrast, it can
act as an anti-apoptotic protein during receptor-initiated cell
death (8). PKC-δ promotes cell
survival through several well-known pro-survival pathways,
including NF-κB, Akt and ERK (8).
Thus, PKC-δ has numerous pleiotropic effects and has been
implicated in tumor suppression as well as survival of various
cancers (8).
The present study demonstrates the constitutive
activation of PKC-δ in HTLV-1-infected T-cell lines and freshly
isolated ATL cells, and that inhibition of PKC-δ induces cell cycle
arrest at the G1 phase and apoptosis. The results also
demonstrate that PKC-δ pathway plays an important role in
Tax-mediated NF-κB activation.
Materials and methods
Cells
The HTLV-1-infected MT-2 and -4, C5/MJ, SLB-1,
HUT-102, MT-1, TL-OmI and ED-40515(−) T-cell lines, and the
negative control uninfected human leukemia Jurkat, MOLT-4 and
CCRF-CEM T-cell lines, were grown in Roswell Park Memorial
Institute-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum (FBS) and antibiotics. JPX-9 cells are derivatives of
Jurkat with Tax gene, which is stably integrated under the
control of a metallothionein promoter (9). To induce Tax expression, JPX-9 cells
were cultured in the presence of 20 μM CdCl2. After
obtaining informed consent, blood samples were obtained from a
healthy volunteer, six patients with acute type ATL and a patient
with chronic type ATL. Peripheral blood mononuclear cells (PBMC)
were isolated from the heparinized blood samples by centrifugation
over a Ficoll-Paque layer (GE Healthcare Bio-Sciences AB, Uppsala,
Sweden). The diagnosis of ATL was based on clinical features,
hematological findings and the presence of anti-HTLV-1 antibodies
in the serum.
Reagents and antibodies
The selective PKC-δ inhibitor, rottlerin, and the
general PKC inhibitor, bisindolylmaleimide, were purchased from
Calbiochem (Darmstadt, Germany). Antibodies specific for PKC-δ,
phospho-PKC-δ (Tyr311), cleaved caspase-3, -8 and -9, cleaved
poly(ADP-ribose) polymerase (PARP), survivin, Bak,
Bcl-xL, phospho-Bcl-2 (Ser70), and phospho-IκBα (Ser32
and 36) were purchased from Cell Signaling Technology (Beverly, MA,
USA). Antibodies against c-IAP2, cyclin D2 and IκBα were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Antibodies against XIAP and phospho-retinoblastoma protein (pRb)
(Ser780) were obtained from Medical & Biological Laboratories,
Co., Ltd. (Aichi, Japan). Antibodies against Bax, Bcl-2, c-FLIP,
CDK4 and 6, c-Myc and actin were purchased from Neomarkers, Inc.
(Fremont, CA, USA). An antibody against active form-specific Bax
was purchased from BD Transduction Laboratories (San Jose, CA,
USA). Mouse monoclonal antibody to Tax, Lt-4, was described
previously (10).
Immunoblotting analysis
To investigate cell proteins by western blot
analysis, cells were lysed in a lysis buffer containing 62.5 mM
Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 10% glycerol, 6%
2-mercaptoethanol and 0.01% bromophenol blue. Lysates were
centrifuged and supernatants were collected. Total protein
concentration for each sample was determined by the Bradford assay
(Bio-Rad, Hercules, CA, USA). Total extracts (20 μg/lane) were
subjected to sodium dodecyl sulfate-polyacrylamide gels and western
blot analysis was performed on polyvinylidene difluoride membranes.
Protein expression was analyzed by immunoblotting using the
specific antibodies. The bands were visualized with the enhanced
chemiluminescence system (Amersham Biosciences Corp, Piscataway,
NJ, USA).
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde for 20 min
at 37°C. Fixed cells were washed twice with phosphate-buffered
saline (PBS) containing 7% of FBS and permeabilized with PBS
containing 0.1% Triton X-100 for 10 min at room temperature. In the
next step, the cells were washed once with PBS containing 7% of FBS
and resuspended in PBS/7% FBS containing antibody against PKC-δ or
phospho-PKC-δ (Tyr311) for 20 min at room temperature. Then, the
cells were washed twice with PBS/7% FBS and resuspended in PBS/7%
FBS containing Alexa Fluor 488-labeled goat anti-rabbit IgG
(Invitrogen Life Technologies, Carlsbad, CA, USA) for 20 min at
room temperature. The nuclei were stained with Hoechst 33342 (Wako
Pure Chemical Industries, Ltd., Osaka, Japan). Finally, the cells
were washed twice with PBS and examined under a Leica DMI6000
microscope (Leica Microsystems, Wetzlar, Germany). Mounted
coverslips were imaged through a 63x oil immersion lens (NA1.4) on
a Leica TCS confocal system.
Cell viability and proliferation
assays
Cell viability and proliferation were assessed by
measuring mitochondrial-dependent conversion of water-soluble
tetrazolium (WST)-8 (Nacalai Tesque, Inc., Kyoto, Japan) to a
colored formazan product. Cell lines or PBMC were seeded in 96-well
plates and then treated with rottlerin or bisindolylmaleimide for
48 or 24 h, respectively. This was followed by the addition of a
solution containing WST-8 to the cells for 4–6 h at 37°C and the
viable growing cells were estimated by monitoring the adsorption of
the product at 450 nm. All experiments were performed in
triplicates.
Analysis of cell death
Apoptosis was assessed by the APO2.7 assay. Cells
were seeded in culture plates then treated with rottlerin (20 μM)
for 48 h, followed by analysis by flow cytometry after staining
with phycoerythrin-conjugated APO2.7 antibody (Beckman Coulter,
Marseille, France), which specifically detects 7A6, a 38-kDa
mitochondrial membrane antigen expressed during apoptosis (11). In addition, apoptosis was also
assessed by monitoring cleavage of caspase-3, -8 and -9, as well as
PARP by western blot analysis.
In vitro measurement of caspase
activity
Caspase activity was measured using Colorimetric
Caspase Assay kits (Medical & Biological Laboratories, Co.,
Ltd.). Briefly, cell extracts were recovered using the cell lysis
buffer supplied with the kit and assessed for caspase-3, -8 and -9
activities using colorimetric probes. The assay kits are based on
detection of chromophore ϱ-nitroanilide after cleavage from
caspase-specific labeled substrates. Colorimetric readings were
performed in an automated microplate reader.
Cell cycle analysis
Cells were harvested and the CycleTEST Plus DNA
Reagent kit (Becton-Dickinson Immunocytometry Systems, San Jose,
CA, USA) was used for analysis of changes in the cell cycle. Cell
suspensions were analyzed by flow cytometry. The MultiCycle
software calculated the percentage of cells in each cell cycle
phase.
Immunohistochemical analysis
Lymph node biopsies were obtained from patients with
ATL. Phospho-PKC-δ immunohistochemistry was performed using an
anti- phospho-PKC-δ (Tyr311) antibody after pre-treatment of the
deparafinized tissue sections with ready-to-use proteinase K (Dako,
Carpinteria, CA, USA). The sections were counterstained with methyl
green, hydrated in ethanol, cleaned in xylene, and mounted. The
stained cells were examined under a light microscope (Axioskop 2
Plus) with an Achroplan 40x/0.65 lens (both from Zeiss, Jena,
Germany). Images were acquired with an AxioCam MRc camera and
AxioVision 4.7 software (Zeiss). A signed consent form was obtained
from each tissue donor.
Plasmids and transfection
The PKC-δ constructs of pGFP-PKC-δ (δWT), in which
the GFP tag was placed on the C-terminus of the kinase,
pGFP-PKC-δK376R (δKN), pGFP-PKC-δD327A (δCM),
pGFP-NLS PKC-δ (NLS-δWT), pGFP-NLS PKC-δK 376R (NLS-δK
N) and pGFP-NLS PKC-δD327A (NLS-δCM) were previously
described (12,13). These mouse wild-type PKC-δ and its
mutants were cloned into pEGFP-N1 (Clontech Laboratories, Palo
Alto, CA, USA). The K376→R mutation in kinase-negative residues in
the ATP-binding site and the protein has been shown to function as
an isoform-specific dominant inhibitory kinase (14). The D327→A mutation in the caspase
cleavage site resulted in loss of caspase cleavage (12). To drive nuclear import, an SV40
nuclear localization signal (NLS) was fused to the N-terminus of
PKC-δ (NLS-δWT) (13). A reporter
plasmid, expressing luciferase through a minimal promoter linked to
five copies of the typical NF-κB responsive element from the
interleukin-2 receptor α chain gene (κB-LUC), was used to
measure the NF-κB transcriptional competence (15). Plasmid expressing the HTLV-1 Tax
through β-actin promoter has been described (16). 293T cells were transfected by the
calcium phosphate DNA co-precipitation method. Each transfection
included the phRL-TK plasmid (Promega Corp., Madison, WI, USA) as
an internal control for variation in the transfection efficiency.
Total DNA was completed to 2,042 ng in all samples with an empty
plasmid. The luciferase activity was determined by the
Dual-Luciferase Reporter system (Promega Corp.) using the protocol
supplied by the manufacturer.
Preparation of nuclear extracts and
EMSA
Nuclear proteins were extracted and transcription
factors bound to specific DNA sequences were examined by
electrophoretic mobility shift assay (EMSA), as described
previously (17). The top strand
sequences of the oligonucleotide probes were as follows: for a
typical NF-κB element from the interleukin-2 receptor α
chain gene, 5′-GATCCGGCAGGGGAATCTCCCTCTC-3′; and for the
consensus sequence of the octamer binding motif,
5′-GATCTGTCGAATGCAAATCACTAGAA-3′. The latter was used to identify
specific binding of the transcription factor Oct-1, which regulates
the transcription of a number of so-called housekeeping genes. The
above underlined sequences represent the NF-κB and Oct-1 binding
sites, respectively.
Statistical analysis
Data were expressed as mean ± SD, and statistical
differences in parameters between groups were examined using the
Student’s t test. P<0.05 denoted the presence of a statistically
significant difference.
Results
Constitutive phosphorylation of PKC-δ in
HTLV-1-infected T-cell lines
In contrast to serine/threonine phosphorylation
sites, tyrosine phosphorylation is a relatively specific regulatory
mechanism for PKC-δ and not a common regulatory mechanism for the
entire family of PKC enzymes. Phosphorylation of Tyr311 in the
hinge region of PKC-δ has been reported to initiate a series of
phosphorylation reactions on other tyrosines that play important
roles in the regulation of its activity (18). We initially examined the
phosphorylation of PKC-δ at Tyr311 in HTLV-1-infected and
-uninfected T-cell lines. HTLV-1-transformed T-cell lines,
including MT-2 and -4, C5/MJ, SLB-1 and HUT-102, constitutively
expressed Tax mRNA. In contrast, ATL-derived T-cell lines,
including MT-1, TL-OmI and ED-40515(−), did not express Tax
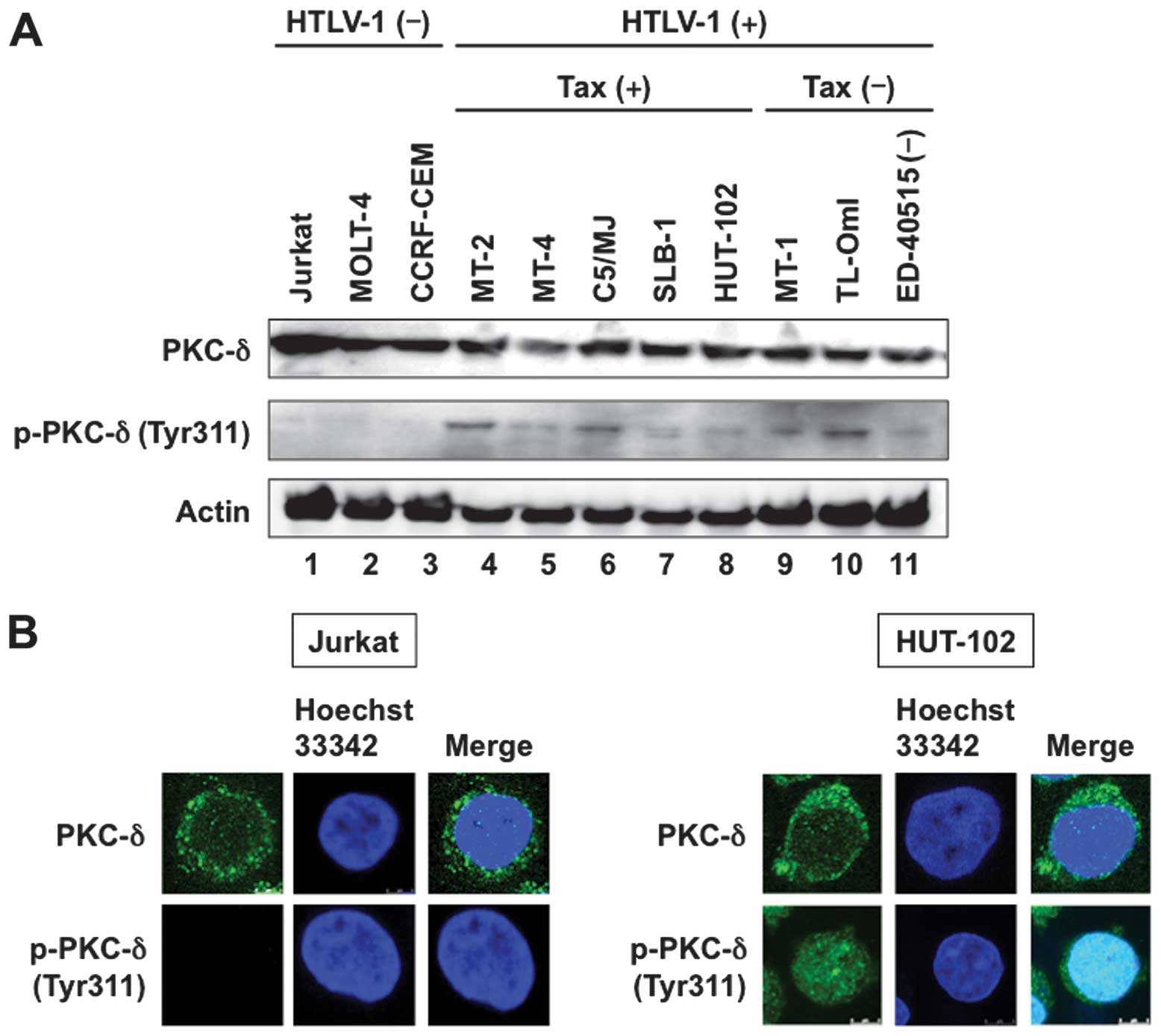
(19). Western blot analysis
showed ubiquitous expression of PKC-δ in all human T-cell lines
(Fig. 1A, top panel). All eight
HTLV-1-infected T-cell lines constitutively expressed the
phosphorylated form of PKC-δ, although the phosphorylated level
varied widely. On the other hand, the phosphorylated form of PKC-δ
was not detected in three uninfected T-cell lines (Fig. 1A, middle panel).
In the next step, immunofluorescent staining was
used to map the site(s) of PKC-δ. In uninfected Jurkat cells, PKC-δ
was localized primarily in the cytoplasm and the phosphorylated
form of PKC-δ could not be detected (Fig. 1B, left panels). PKC-δ was also
localized primarily in the cytoplasm in HTLV-1-infected HUT-102
cells. However, the nuclear phosphorylated form of PKC-δ was
detected in HUT-102 cells (Fig.
1B, right panels).
Tax induces PKC-δ phosphorylation
Next, we analyzed the role of Tax in the regulation
of PKC-δ phosphorylation. For this purpose, JPX-9 cells transfected
with the Tax gene, regulated by the inducible
metallothionein promoter, were used for Tax-function analysis, as
described previously (9).
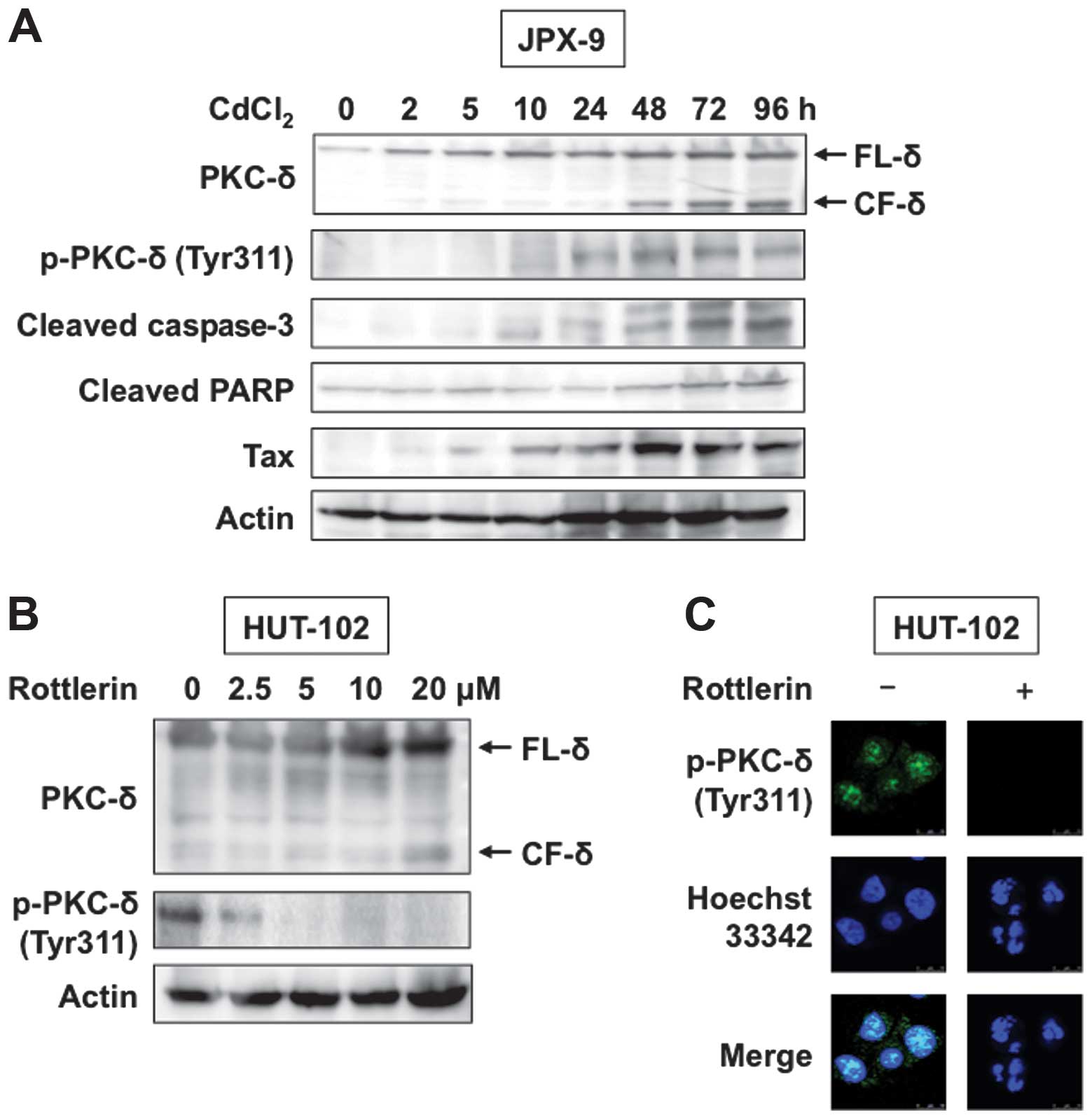
Treatment of JPX-9 cells with CdCl2 induced significant
expression of Tax protein (Fig.
2A, panel 5). Phosphorylation of PKC-δ was also induced in
CdCl2-treated JPX-9 cells (Fig. 2A, second panel). Various apoptotic
agents can cleave PKC-δ from caspases, resulting in the production
of a 40-kDa catalytic fragment (CF-δ) (12). Tax is reported to cause apoptosis
of JPX-9 cells (20), although it
is believed to play a significant role in transforming host cells.
To characterize and confirm that death of JPX-9 cells was due to
apoptosis, we used western blot analysis to investigate cleavage of
caspase-3 and its specific substrate PARP. Consistent with
apoptotic death, we observed processing of caspase-3 and PARP in
JPX-9 cells treated with CdCl2 (Fig. 2A, panels 3 and 4). Treatment with
CdCl2 induced Tax-mediated apoptosis, resulting in
caspase cleavage of PKC-δ (Fig.
2A, top panel).
Inhibition of PKC-δ by rottlerin
decreases cell viability and induces apoptosis of HTLV-1-infected T
cells
We next analyzed the effects of rottlerin, a PKC-δ
inhibitor, on HTLV-1-infected HUT-102 cells. Western blot analysis
and immunofluorescent staining showed that rottlerin reduced
tyro-sine phosphorylation of PKC-δ (Fig. 2B and C). Furthermore, rottlerin
induced PKC-δ cleavage as demonstrated by western blot analysis, as
well as chromatin condensation and nuclear fragmentation, as
demonstrated using Hoechst 33342 staining (Fig. 2B and C). In the next step, we
compared the effects of PKC-δ-specific inhibitor rottlerin and
general PKC inhibitor bisindolylmaleimide on the viability of
HTLV-1-infected T-cell lines. Rottlerin inhibited the viability of
HTLV-1-infected T-cell lines, MT-2 and -4, C5/MJ, SLB-1, HUT-102,
MT-1 and ED-40515(−) in a dose-dependent manner. In contrast to
HTLV-1-infected T-cell lines, uninfected T-cell line CCRF-CEM was
less susceptible to rottlerin (Fig.
3A, left panel). While bisindolylmaleimide reduced cell
viability of HTLV-1-infected T-cell lines, its effect was less
pronounced than that of rottlerin. Furthermore, there was no
difference in the susceptibility of HTLV-1-infected and -uninfected
T-cell lines to bisindolylmaleimide (Fig. 3A, right panel).
We also investigated the effects of rottlerin and
bisindolylmaleimide on peripheral ATL cells freshly isolated from
seven patients with ATL. Rottlerin reduced ATL cell survival but
not that of PBMC obtained from a healthy donor (Fig. 3B, left panel). Bisindolylmaleimide
also reduced cell viability though its effect was milder than that
of rottlerin (Fig. 3B, right
panel).
Since rottlerin reduced the viability of
HTLV-1-infected T cells, we next assessed the effects of rottlerin
on apoptosis. Inhibition of PKC-δ by 20 μM rottlerin increased the
number of apoptotic cells as measured by APO2.7 staining (Fig. 4A). Interestingly, HTLV-1-infected
T-cell lines were more susceptible to rottlerin than uninfected
CCRF-CEM cells (Fig. 4A). Next, we
studied the role of caspases in this process by determining
cleavage of endogenous caspases. Western blot analysis demonstrated
increased levels of activated cleaved forms of caspase-3, -8 and
-9, as well as PARP, and that such increases were rottlerin
dose-dependent (Fig. 4B).
Immunoblotting allowed us to examine the processing of caspases,
but did not indicate whether the cleavage products were
enzymatically active. Therefore, we used colorimetric assays to
determine caspase-3, -8 and -9 activities based on cleavage of
caspase-specific-labeled substrates. As shown in Fig. 4C, rottlerin activated caspase-3, -8
and -9 in HUT-102 cells. These results confirmed that caspase
activation mediates rottlerin-induced apoptosis of HTLV-1-infected
T-cell lines.
 | Figure 4Rottlerin-induced apoptosis involves
activation of caspase-3, -8 and -9. (A) Flow cytometric analysis of
APO2.7 reactive cells. Human T-cell lines were treated with or
without 20 μM of rottlerin for 48 h. The cells were analyzed by
flow cytometry after staining with phycoerythrin-conjugated APO2.7
antibody. Data are mean ± SD percentages of apoptotic cells for
both untreated (open bars) and rottlerin-treated (solid bars) cells
(n=3). (B) Immunoblot analysis of cleaved caspase-3, -8 and -9, as
well as poly(ADP-ribose) polymerase (PARP). HUT-102 cells were
treated with the indicated concentrations of rottlerin for 48 h.
Samples of 20 μg of whole cell lysates were examined by
immunoblotting. The immunoblot of actin served as a control. (C)
Rottlerin-induced apoptosis is caspase-dependent, based on
treatment of HUT-102 cells with or without 20 μM of rottlerin.
After 48 h, cell lysates were prepared and incubated with the
labeled caspase substrates, and caspase activity was measured using
an automated microplate reader. Caspase activity is expressed
relative to untreated cells, which was assigned an arbitrary value
of 1. Data are mean ± SD (n=3). (D) Immunoblot analysis of cleaved
caspase-3, -8 and -9, as well as PARP. Peripheral blood mononuclear
cells (PBMC) from a patient with adult T-cell leukemia (ATL) were
treated with or without 10 μM of rottlerin for 24 h. Samples of 20
μg of whole cell lysates were examined by immunoblotting. (E)
Immunohistochemical staining of phosphorylated protein kinase C
(PKC)-δ in ATL lymph nodes. Tissue sections from ATL lymph nodes
were stained with anti-phospho PKC-δ antibody and counterstained
with methyl green. Low-power images of representative lymph nodes
from patients with ATL (original magnification, ×400). The inset
represents higher magnification of the small boxed region (original
magnification, ×1,200). |
As shown in Fig.
4D, rottlerin inhibited PKC-δ in freshly isolated primary ATL
cells, and resulted in cleavage of caspase-3, -8 and -9, as well as
PARP in these cells. Furthermore, immunohistochemical staining
showed nuclear staining for phosphorylated PKC-δ (Tyr311) in ATL
cells found in lymph nodes (Fig.
4E).
Rottlerin induces cell cycle block in
HTLV-1-infected T cells
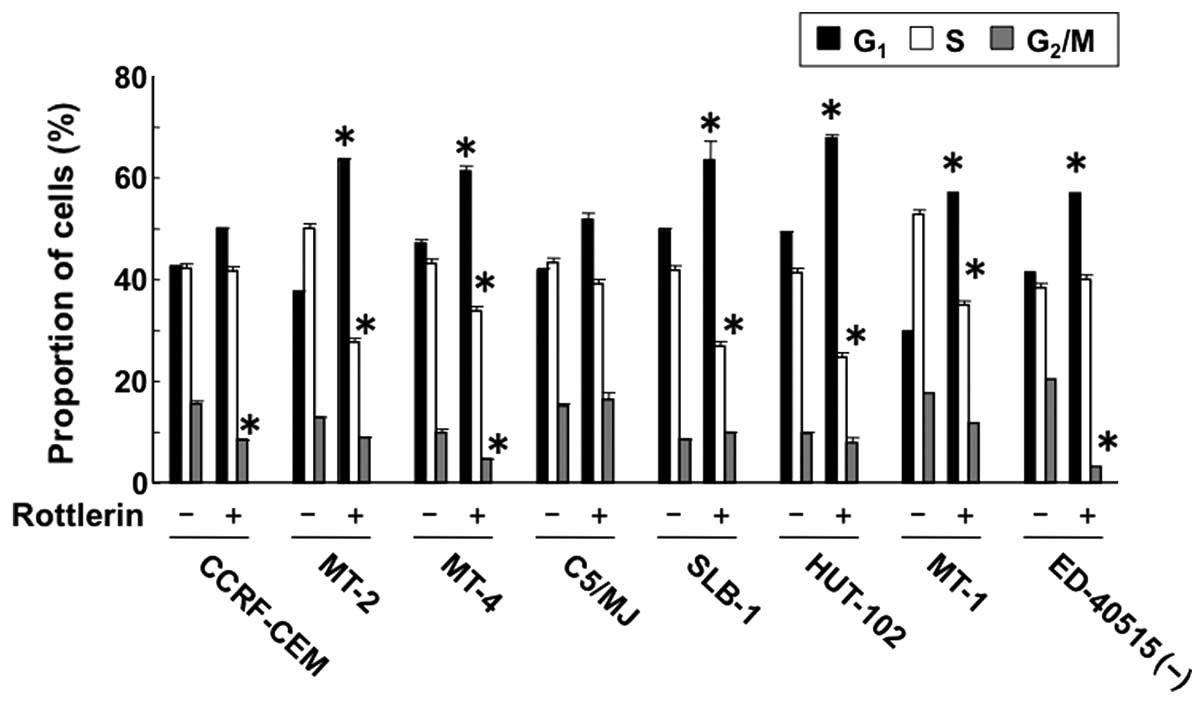
Cell cycle analysis showed that rottlerin
significantly increased the population of HTLV-1-infected T cells
at the G1 phase of the cell cycle, and at the same time
it decreased the population of cells at the S phase. However,
rottlerin had no effect on the percentage of CCRF-CEM cells in the
G1 and S phases, compared to the control (Fig. 5). These results suggest that PKC-δ
accelerates the cell cycle in HTLV-1-infected T cells, i.e.,
inhibition of PKC-δ causes cell cycle arrest at the G1
phase.
Inhibition of PKC-δ results in
downregulation of anti-apoptotic proteins and activation of
pro-apoptotic protein
High levels of anti-apoptotic proteins are commonly
found in HTLV-1-infected T cells, suggesting their potential
involvement in the pathophysiology of the disease. Therefore, we
studied the expression of anti- and pro-apoptotic proteins in
HTLV-1-infected T cells cultured with rottlerin. As shown in
Fig. 6 (left panels), HUT-102
cells expressed high levels of survivin, c-IAP2, XIAP,
Bcl-xL, Bcl-2 and c-FLIP. Incubation of these cells with
rottlerin induced downregulation of survivin, XIAP,
Bcl-xL and c-FLIP. Although rottlerin had no effect on
the level of Bcl-2, it significantly reduced phosphorylation of
Bcl-2 at Ser70 when used at concentration of 2.5 μM and completely
abolished the phosphorylation at 20 μM. Bcl-2 phosphorylation at
Ser70 is necessary for its full and potent anti-apoptosis function
(21).
The pro-apoptotic Bcl-2 family proteins, such as Bax
and Bak, act on the mitochondria and counterbalance Bcl-2. The
protein expression of Bax and Bak remained stable regardless of
PKC-δ inhibition. Bax-mediated cell death occurs via
well-controlled steps, including a conformational change that
facilitates the dimerization and translocation of Bax to the
mitochondrial outer membrane (22,23).
For this reason, we also tested the involvement of Bax activation
in apoptosis. Conformational changes in Bax can be evaluated using
conformation-specific anti-Bax antibodies (24). In the present study, immunoblot
analysis using an antibody specific for the active form of Bax
(clone 3) demonstrated that rottlerin changed Bax conformation in a
dose-dependent manner.
Inhibition of PKC-δ alters the expression
of proteins involved in cell cycle regulation
In order to determine whether the rottlerin-induced
cell cycle block is accompanied by changes in proteins involved in
cell cycle regulation, we studied the expression of cyclin D2, CDK4
and 6 proteins in HUT-102 cells. Fig.
6 (right panels) shows that rottlerin decreased cyclin D2, CDK4
and 6 protein levels compared to the control. The product of the
proto-oncogene c-Myc is a potent activator of cell proliferation.
Cyclin D2 and CDK4 genes are direct targets of c-Myc
(25). CDK4 and 6 are responsible
for phosphorylation of pRB, the product of the retinoblastoma tumor
suppressor gene (25). Rottlerin
significantly reduced c-Myc and the hyperphosphorylated pRb
form.
PKC-δ kinase activity is required for
Tax-induced NF-κB activation
NF-κB is a target of Tax, and its activation
progresses cell cycle and prevents apoptosis through regulation of
expression of pro-proliferation and anti-apoptosis genes in
HTLV-1-infected T cells (5,6). To
determine the involvement of PKC-δ in Tax-induced NF-κB activation,
293T cells were co-transfected with a luciferase reporter plasmid
containing five copies of the NF-κB motif (κB-LUC) and expression
plasmids for wild-type PKC-δ and its mutants in the presence or
absence of Tax expression plasmid. Tax induced κB-LUC activity
(Fig. 7A), and the induction was
significantly increased by wild-type PKC-δ (δWT), but markedly
inhibited by the kinase-dead PKC-δ (δKN). To determine whether
nuclear retention of PKC-δ is necessary for Tax-mediated NF-κB
activation, 293T cells were co-transfected with NLS-δWT. Fusion of
the SV40 NLS to PKC-δ (NLS-δWT) resulted in a much higher κB-LUC
activity. In contrast, the kinase-negative construct of NLS-δWT
(NLS-δKN) significantly inhibited Tax-mediated κB-LUC activity. As
expected, mutation at the caspase cleavage site (δCM and NLS-δCM)
did not affect κB-LUC activity by δWT and NLS-δWT. These results
highlight the importance of active nuclear PKC-δ in Tax-mediated
NF-κB activation.
NF-κB activation in HTLV-1-infected T
cells is mediated by PKC-δ
Overexpression of a dominant negative form of PKC-δ
resulted in significant attenuation of Tax-mediated NF-κB
activation. To further delineate the involvement of PKC-δ in NF-κB
activation in HTLV-1-infected T cells, HUT-102 cells were treated
with rottlerin. Western blot analysis detected phosphorylation and
degradation of IκBα in control HUT-102 cells (Fig. 7B). Rottlerin inhibited the
phosphorylation and degradation of IκBα. EMSA studies showed that
rottlerin inhibited NF-κB binding activity (Fig. 7C). Under the same experimental
conditions, rottlerin did not affect Oct-1 binding activity,
suggesting the specificity of rottlerin inhibition. Taken together,
the above results stress the role of PKC-δ in NF-κB activation in
HTLV-1-infected T cells.
Discussion
HTLV-1-infected T cells exhibit constitutive
activation of various signaling pathways (26). Such activation plays an important
role in apoptosis in ATL. One of the important features of
HTLV-1-infected T cells is the high level of NF-κB activity
(5,6).
In the present study, we demonstrated constitutive
phosphorylation of PKC-δ in HTLV-1-infected T cells and that
specific inhibition of this kinase with rottlerin increased
apoptosis and induced cell cycle arrest at the G1 phase
in HTLV-1-infected T cells. In contrast, normal PBMC from a healthy
donor were resistant to rottlerin-induced cell death. We also
demonstrated that shutting down the constitutive activity of PKC-δ
resulted in a strong apoptotic response in HTLV-1-infected T cells,
primarily by affecting the expression and activity of proteins
acting on both the cell membrane and mitochondria. Activation of
initiator caspases (caspase-8 and -9) led to the proteolytic
activation of downstream effector caspase-3. Two independent
pathways, death receptors and the mitochondria, lie upstream of
caspase activation. Downregulation of c-FLIP by rottlerin may
result in activation of caspase-8, because c-FLIP has
anti-apoptotic properties by blocking the activity of FLICE.
Anti-apoptotic Bcl-2 family proteins, such as Bcl-2 and
Bcl-xL, disrupt the mitochondrial apoptotic machinery,
while the pro-apoptotic member Bax is central to cell apoptosis.
Downregulation of Bcl-xL and phosphorylated Bcl-2
proteins, and activation of Bax may result in the activation of
caspase-9. Survivin and XIAP, members of the inhibitor of apoptosis
(IAP) family, can directly bind to and suppress several caspases
(27). In addition to changes in
Bcl-2 family proteins, any decrease in IAP family protein levels
may result in the activation of caspase-3 and -9.
The results also showed that rottlerin increased the
percentage of HTLV-1-infected T cells in the G1 phase of
the cell cycle. The growth arrest evoked by rottlerin was caused by
marked decrease in the amount of cyclin D2, CDK4 and 6, as well as
c-Myc, which directly regulate the G1 to S phase
progression of the cell cycle.
The expression of genes that suppress apoptosis
(e.g., survivin, XIAP, Bcl-xL and c-FLIP) or mediate
cell proliferation (e.g., cyclin D2, CDK4 and 6, as well as c-Myc)
is regulated by NF-κB in HTLV-1-infected T cells (28–34).
NF-κB is normally present in the cytoplasm by inhibitory proteins
such as IκBα. Induction of NF-κB is associated with phosphorylation
and release of IκBα from NF-κB complexes followed by proteolytic
degradation. PKC-δ activates NF-κB signaling, and rottlerin can
prevent NF-κB activation (35–41).
In this regard, the present study showed that rottlerin interfered
with the NF-κB activation process by lowering the levels of
phospho-IκBα. These findings demonstrate the interfering action of
rottlerin in NF-κB signaling pathway, inhibition of which is fully
consistent with the decrease in the above NF-κB-regulated gene
products.
HTLV-1 Tax induces NF-κB activation (5,6). The
present study provides evidence for the importance of PKC-δ in the
NF-κB induction pathway. Overexpression of dominant negative PKC-δ
inhibits Tax induction of NF-κB. Furthermore, forced expression of
wild-type PKC-δ augmented Tax-induced NF-κB activation, and fusion
of the SV40 NLS to PKC-δ resulted in targeting the protein to the
nucleus and further increase in Tax-mediated NF-κB activation.
Endogenous PKC-δ is located primarily in the cytosol, but
phosphorylated PKC-δ is present within the nucleus of
HTLV-1-infected T cells and ATL cells invading lymph nodes. Our
studies clearly demonstrate that nuclear import of active PKC-δ is
required for Tax-mediated NF-κB activation. This conclusion is
supported by the findings of previous studies (42), which showed direct interaction of
Tax with PKC-δ and Tax-induced phosphorylation of PKC, and that
subsequent steps in the PKC cascade seem to have stimulated IκBα
phosphorylation.
Phosphorylation of PKC-δ was also observed in
ATL-derived T-cell lines that did not express Tax. Similarly,
ATL-derived T-cell lines as well as ATL leukemic cells sustain
NF-κB activity without Tax (43).
These findings suggest that phosphorylated PKC-δ may play an
important role in Tax-independent NF-κB activation.
In conclusion, we demonstrated in the present study
selective phosphorylation of PKC-δ in HTLV-1-infected T cells.
Importantly, we showed that Tax activates NF-κB and this action is
mediated through PKC-δ activation. Together with our previous
findings that inhibition of NF-κB can induce cell apoptosis
(44), our present study further
delineates potential upstream regulators of NF-κB, which may play a
role in the enhanced survival of HTLV-1-infected T cells. Based on
our results, we suggest that rottlerin inhibits the proliferation
and survival of HTLV-1-infected T cells through its inhibitory
effect on the NF-κB activation process, which diminishes the levels
of proliferative and anti-apoptotic NF-κB-regulated gene products.
These results could be useful in the design of new PKC-δ-targeting
therapies that can potentially improve the prognosis of patients
with ATL.
Acknowledgements
The authors would like to thank Dr Sawako Nakachi
and Mr. Taro Uchida for the expert technical assistance. We also
thank Drs Mary E. Rayland, Kayoko Matsumoto and Yuetsu Tanaka for
providing expression vectors for PKC-δ and its mutants, expression
vector for Tax, and Tax antibody, as well as Dr Masataka Nakamura
for providing JPX-9, Dr Michiyuki Maeda for providing ED-40515(−),
and Fujisaki Cell Center, Hayashibara Biochemical Laboratories,
Inc. (Okayama, Japan) for providing C5/MJ, HUT-102 and MT-1.
References
|
1
|
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn
PA, Minna JD and Gallo RC: Detection and isolation of type C
retrovirus particles from fresh and cultured lymphocytes of a
patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA.
77:7415–7419. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hinuma Y, Nagata K, Hanaoka M, Nakai M,
Matsumoto T, Kinoshita KI, Shirakawa S and Miyoshi I: Adult T-cell
leukemia: antigen in an ATL cell line and detection of antibodies
to the antigen in human sera. Proc Natl Acad Sci USA. 78:6476–6480.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshida M, Miyoshi I and Hinuma Y:
Isolation and characterization of retrovirus from cell lines of
human adult T-cell leukemia and its implication in the disease.
Proc Natl Acad Sci USA. 79:2031–2035. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bazarbachi B, Ghez D, Lepelletier Y, Nasr
R, de Thé H, El-Sabban ME and Hermine O: New therapeutic approaches
for adult T-cell leukaemia. Lancet Oncol. 5:664–672. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kannian P and Green PL: Human T
lymphotropic virus type 1 (HTLV-1): molecular biology and
oncogenesis. Viruses. 2:2037–2077. 2010. View Article : Google Scholar
|
|
6
|
Cheng H, Ren T and Sun SC: New insight
into the oncogenic mechanism of the retroviral oncoprotein Tax.
Protein Cell. 3:581–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinberg SF: Structural basis of protein
kinase C isoform function. Physiol Rev. 88:1341–1378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basu A and Pal D: Two faces of protein
kinase Cδ: the contrasting roles of PKCδ in cell survival and cell
death. Sci World J. 10:2272–2284. 2010. View Article : Google Scholar
|
|
9
|
Ohtani K, Nakamura M, Saito S, Nagata K,
Sugamura K and Hinuma Y: Electroporation: application to human
lymphoid cell lines for stable introduction of a transactivator
gene of human T-cell leukemia virus type I. Nucleic Acids Res.
17:1589–1604. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka Y, Yoshida A, Takayama Y, Tsujimoto
H, Tsujimoto A, Hayami M and Tozawa H: Heterogeneity of antigen
molecules recognized by anti-tax1 monoclonal antibody Lt-4 in cell
lines bearing human T cell leukemia virus type I and related
retro-viruses. Jpn J Cancer Res. 81:225–231. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Ao Z, Seth A and Schlossman SF: A
mitochondrial membrane protein defined by a novel monoclonal
antibody is preferentially detected in apoptotic cells. J Immunol.
157:3980–3987. 1996.PubMed/NCBI
|
|
12
|
DeVries TA, Neville MC and Reyland ME:
Nuclear import of PKCδ is required for apoptosis: identification of
a novel nuclear import sequence. EMBO J. 21:6050–6060. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeVries-Seimon TA, Ohm AM, Humphries MJ
and Reyland ME: Induction of apoptosis is driven by nuclear
retention of protein kinase Cδ. J Biol Chem. 282:22307–22314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Yu JC, Shin DY and Pierce JH:
Characterization of a protein kinase C-δ (PKC-δ) ATP binding
mutant. An inactive enzyme that competitively inhibits wild type
PKC-δ enzymatic activity. J Biol Chem. 270:8311–8318. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki T, Hirai H, Murakami T and Yoshida
M: Tax protein of HTLV-1 destabilizes the complexes of NF-κB and
IκB-α and induces nuclear translocation of NF-κB for
transcriptional activation. Oncogene. 10:1199–1207. 1995.PubMed/NCBI
|
|
16
|
Matsumoto K, Shibata H, Fujisawa JI, Inoue
H, Hakura A, Tsukahara T and Fujii M: Human T-cell leukemia virus
type 1 Tax protein transforms rat fibroblasts via two distinct
pathways. J Virol. 71:4445–4451. 1997.PubMed/NCBI
|
|
17
|
Mori N and Prager D: Transactivation of
the interleukin-1α promoter by human T-cell leukemia virus type I
and type II Tax proteins. Blood. 87:3410–3417. 1996.PubMed/NCBI
|
|
18
|
Steinberg SF: Distinctive activation
mechanisms and functions for protein kinase Cδ. Biochem J.
384:449–459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimura R, Senba M, Cutler SJ, Ralph SJ,
Xiao G and Mori N: Human T cell leukemia virus type I Tax-induced
IκB-ζ modulates Tax-dependent and Tax-independent gene expression
in T cells. Neoplasia. 15:1110–1124. 2013.PubMed/NCBI
|
|
20
|
Chen X, Zachar V, Zdravkovic M, Guo M,
Ebbesen P and Liu X: Role of the Fas/Fas ligand pathway in
apoptotic cell death induced by the human T cell lymphotropic virus
type I Tax transactivator. J Gen Virol. 78:3277–3285.
1997.PubMed/NCBI
|
|
21
|
Ito T, Deng X, Carr B and May WS: Bcl-2
phosphorylation required for anti-apoptosis function. J Biol Chem.
272:11671–11673. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Autret A, Martin-Latil S, Mousson L,
Wirotius A, Petit F, Arnoult D, Colbère-Garapin F, Estaquier J and
Blondel B: Poliovirus induces Bax-dependent cell death mediated by
c-Jun NH2-terminal kinase. J Virol. 81:7504–7516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolter KG, Hsu YT, Smith CL, Nechushtan A,
Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar
|
|
24
|
Dewson G, Snowden RT, Almond JB, Dyer MJ
and Cohen GM: Conformational change and mitochondrial translocation
of Bax accompany proteasome inhibitor-induced apoptosis of chronic
lymphocytic leukemic cells. Oncogene. 22:2643–2654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pajic A, Spitkovsky D, Christoph B,
Kempkes B, Schuhmacher M, Staege MS, Brielmeier M, Ellwart J,
Kohlhuber F, Bornkamm GW, Polack A and Eick D: Cell cycle
activation by c-myc in a Burkitt lymphoma model cell line. Int J
Cancer. 87:787–793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mori N: Cell signaling modifiers for
molecular targeted therapy in ATLL. Front Biosci (Landmark Ed).
14:1479–1489. 2009. View
Article : Google Scholar
|
|
27
|
Smolewski P and Robak T: Inhibitors of
apoptosis proteins (IAPs) as potential molecular targets for
therapy of hematological malignancies. Curr Mol Med. 11:633–649.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawakami H, Tomita M, Matsuda T, Ohta T,
Tanaka Y, Fujii M, Hatano M, Tokuhisa T and Mori N: Transcriptional
activation of survivin through the NF-κB pathway by human T-cell
leukemia virus type I tax. Int J Cancer. 115:967–974. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawakami A, Nakashima T, Sakai H, Urayama
S, Yamasaki S, Hida A, Tsuboi M, Nakamura H, Ida H, Migita K,
Kawabe Y and Eguchi K: Inhibition of caspase cascade by HTLV-I tax
through induction of NF-κB nuclear translocation. Blood.
94:3847–3854. 1999.PubMed/NCBI
|
|
30
|
Nicot C, Mahieux R, Takemoto S and
Franchini G: Bcl-xL is up-regulated by HTLV-I and
HTLV-II in vitro and in ex vivo ATLL samples. Blood. 96:275–281.
2000.PubMed/NCBI
|
|
31
|
Krueger A, Fas SC, Giaisi M, Bleumink M,
Merling A, Stumpf C, Baumann S, Holtkotte D, Bosch V, Krammer PH
and Li-Weber M: HTLV-1 Tax protects against CD95-mediated apoptosis
by induction of the cellular FLICE-inhibitory protein (c-FLIP).
Blood. 107:3933–3939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwanaga R, Ozono E, Fujisawa J, Ikeda MA,
Okamura N, Huang Y and Ohtani K: Activation of the cyclin D2 and
cdk6 genes through NF-κB is critical for cell-cycle progression
induced by HTLV-I Tax. Oncogene. 27:5635–5642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwanaga R, Ohtani K, Hayashi T and
Nakamura M: Molecular mechanism of cell cycle progression induced
by the oncogene product Tax of human T-cell leukemia virus type I.
Oncogene. 20:2055–2067. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duyao MP, Kessler DJ, Spicer DB,
Bartholomew C, Cleveland JL, Siekevitz M and Sonenshein GE:
Transactivation of the c-myc promoter by human T cell leukemia
virus type 1 tax is mediated by NF κB. J Biol Chem.
267:16288–16291. 1992.PubMed/NCBI
|
|
35
|
Maioli E, Greci L, Soucek K, Hyzdalova M,
Pecorelli A, Fortino V and Valacchi G: Rottlerin inhibits ROS
formation and prevents NFκB activation in MCF-7 and HT-29 cells. J
Biomed Biotechnol. 2009:7429362009. View Article : Google Scholar
|
|
36
|
Lu ZG, Liu H, Yamaguchi T, Miki Y and
Yoshida K: Protein kinase Cδ activates RelA/p65 and nuclear
factor-κB signaling in response to tumor necrosis factor-α. Cancer
Res. 69:5927–5935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Wang X and Evers BM: Induction of
cIAP-2 in human colon cancer cells through PKCδ/NF-κB. J Biol Chem.
278:51091–51099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Page K, Li J, Zhou L, Iasvovskaia S,
Corbit KC, Soh JW, Weinstein IB, Brasier AR, Lin A and Hershenson
MB: Regulation of airway epithelial cell NF-κB-dependent gene
expression by protein kinase Cδ. J Immunol. 170:5681–5689. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minami T, Abid MR, Zhang J, King G, Kodama
T and Aird WC: Thrombin stimulation of vascular adhesion molecule-1
in endothelial cells is mediated by protein kinase C (PKC)-δ-NF-κB
and PKC-ζ-GATA signaling pathways. J Biol Chem. 278:6976–6984.
2003. View Article : Google Scholar
|
|
40
|
Torricelli C, Fortino V, Capurro E,
Valacchi G, Pacini A, Muscettola M, Soucek K and Maioli E:
Rottlerin inhibits the nuclear factor κB/cyclin-D1 cascade in MCF-7
breast cancer cells. Life Sci. 82:638–643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ogasa M, Miyazaki Y, Hiraoka S, Kitamura
S, Nagasawa Y, Kishida O, Miyazaki T, Kiyohara T, Shinomura Y and
Matsuzawa Y: Gastrin activates nuclear factor κB (NFκB) through a
protein kinase C dependent pathway involving NFκB inducing kinase,
inhibitor κB (IκB) kinase, and tumour necrosis factor receptor
associated factor 6 (TRAF6) in MKN-28 cells transfected with
gastrin receptor. Gut. 52:813–819. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lindholm PF, Tamami M, Makowski J and
Brady JN: Human T-cell lymphotropic virus type 1 Tax1
activation of NF-κB: involvement of the protein kinase C pathway. J
Virol. 70:2525–2532. 1996.PubMed/NCBI
|
|
43
|
Mori N, Fujii M, Ikeda S, Yamada Y,
Tomonaga M, Ballard DW and Yamamoto N: Constitutive activation of
NF-κB in primary adult T-cell leukemia cells. Blood. 93:2360–2368.
1999.PubMed/NCBI
|
|
44
|
Mori N, Yamada Y, Ikeda S, Yamasaki Y,
Tsukasaki K, Tanaka Y, Tomonaga M, Yamamoto N and Fujii M: Bay
11-7082 inhibits transcription factor NF-κB and induces apoptosis
of HTLV-I-infected T-cell lines and primary adult T-cell leukemia
cells. Blood. 100:1828–1834. 2002. View Article : Google Scholar : PubMed/NCBI
|