Introduction
Approximately 20.6 people per 100,000 people in the
United States are diagnosed with a primary brain tumor each year
(1). GBM constitutes the most
common and aggressive form of malignant glioma, occurring in ~54%
of gliomas (1) or 3.2 per 100,000
US citizens, and carrying a dismal prognosis of a median survival
of around 14 months (2) with
<10% of patients surviving beyond 5 years after diagnosis.
Currently, the standard of care for newly diagnosed GBM patients
consists of maximum surgical resection, followed by radiotherapy
plus concomitant and adjuvant temozolomide. At recurrence, however,
very few therapeutic options exist. Currently, no treatment
regimens have produced considerable therapeutic benefit in
recurrent GBM (3).
Bevacizumab, a monoclonal antibody to VEGF (4) is now a common second-line treatment
option for GBM patients that have failed the standard of care,
particularly due to an apparent progression-free survival benefit
shown in early clinical trials (5–7)
compared with historic controls (2). These early results were based on a
modified Macdonald criteria (8),
which is limited in the evaluation of anti-angiogenic treatments
due to the dramatic effect on vascular permeability resulting in
decreased contrast enhancement (9,10).
Diffusion-sensitive magnetic resonance imaging (MRI) biomarkers
have shown some early promise as predictive tools (11) in bevacizumab therapy at recurrence.
In particular, the functional diffusion map (fDM) technique, which
evaluates voxel-wise changes in the apparent diffusion coefficient
(ADC) over time, has shown utility as an early response biomarker
in bevacizumab therapy in a single institution dataset consisting
of uniform, high-quality diffusion MRI data (11). This technique, however, has not
been evaluated in the context of a large multicenter trial with
mixed quality of diffusion MRI data.
The aim of the present study was to implement
explicit criteria for quality control and evaluate fDM performance
using DWI data collected as part of RTOG-0625, a multicenter,
randomized, phase II trial of bevacizumab with irinotecan or
temozolomide in recurrent GBM.
Materials and methods
The Radiation Therapy Oncology Group (RTOG), in
collaboration with the American College of Radiology Imaging
Network (ACRIN), both funded by the National Cancer Institute,
conducted a prospective, randomized, phase II multi-center trial
comparing bevacizumab with either irinotecan or temozolomide
treatment in recurrent GBM (RTOG 0625/ACRIN 6677; ClinicalTrials.gov #NCT00433381; NCI-2009-00743).
Twenty-four institutions both participated and had diffusion MRI
data available for analysis, each obtaining institutional review
board approval before subject accrual and conducting the trial with
Health Insurance Portability and Accountability Act (HIPAA)
compliance. Informed consent was obtained for all subjects.
Study subjects
A total of 123 patients were enrolled in the current
trial (Table I). All patients had
recurrent histologically proven GBM or gliosarcoma with progression
on MRI within 14 days after registration, ≥42 days after completion
of radiation/temozolomide therapy, ≥28 days after surgical
resection or cytotoxic therapy, as well as imaging or biopsy
confirmation of true progressive disease rather than radiation
necrosis after Gliadel placement or stereotactic radiosurgery.
Detailed inclusion and exclusion criteria are available at
http://www.acrin.org/Portals/0/Protocols/6677/RTOG%20062-ACRIN%206677.pdf
(Section 3.0). Bevacizumab was administered to all patients (10
mg/kg intravenously, days 1 and 15 of a 28-day cycle). In the first
arm, patients received temozolomide (75 mg/m2 per os,
days 1–21 during the first 28-day cycle; 100 mg/m2 for
cycle 2 and beyond in the absence of myelotoxicity). In the second
arm, patients received irinotecan (125 mg/m2
intravenously, days 1 and 15 of a 28-day cycle). Standard of care
MRI occurred at baseline, after every 2 cycles of treatment (every
8 weeks), and after completion or termination of treatment.
Patients demonstrating benefit (stable or responding tumor) were
treated for 12 cycles with optional extension to 24 cycles in the
presence of continued benefit and absence of severe toxicity.
 | Table ISummary of sites and number of
patients enrolled. |
Table I
Summary of sites and number of
patients enrolled.
| Site | No. of patients |
|---|
| 4205 - Barnes Jewish
Hospital | 7 |
| 4212 - Thomas
Jefferson | 2 |
| 4214 - MD
Anderson | 19 |
| 4217 - University of
Iowa | 2 |
| 4219 - Sloan
Kettering | 6 |
| 4220 - University of
Rochester | 1 |
| 4254 - Medical
College of Wisconsin | 3 |
| 4275 - Henry
Ford | 22 |
| 4283 – Akron
General Medical Center | 2 |
| 4372 - St. John’s
Health System | 1 |
| 4399 - St.
Luke’s | 5 |
| 4400 - Tel-Aviv
Medical Center | 13 |
| 4403 - Mt.
Diablo | 1 |
| 4404 - JFK | 1 |
| 4405 - LDS | 7 |
| 4406 - Arizona
Oncology Serv @ SJHMC | 1 |
| 4407 - Virginia
Mason Medical Center | 5 |
| 4409 - Carolina’s
Medical Center/Levine Cancer Ctr | 6 |
| 4411 - N. Rockies
Regional Cancer Center | 1 |
| 4413 - Anne Arundel
Medical Center | 3 |
| 4414 - Alta Bates
Comprehensive Cancer Center | 1 |
| 4470 - Yale
University | 1 |
| 4492 - University
of Chicago | 12 |
| 4494 - UCLA | 1 |
| Total | 123 |
Magnetic resonance imaging
Conventional MRI included pre-contrast T1-weighted,
T2-weighted, T2-weighted FLAIR, and diffusion-weighted MRI (DWI).
After intravenous injection of 0.1 mmol/kg of standard
gadolinium-based contrast, an axial 2D spin-echo and 3D volumetric
T1-weighted (T1+C) images were acquired. Patients participating in
the optional advanced component of the trial had dynamic
contrast-enhanced MRI, dynamic susceptibility contrast
perfusion-weighted MRI, and/or MR spectroscopy at baseline, week 2
and after every 2 cycles of treatment.
Diffusion MR acquisition parameters varied widely
across institutions despite specific ACRIN recommendations. Echo
time (TE) varied from 64 to 111.9 ms (~200%), and by as much as 50%
in the same patient during follow-up evaluations. Repetition time
(TR) varied from 6 to 10 sec (~50%), b-values ranged from 0
and 700 to 0 and 1,200 sec/mm2, and in some cases
diffusion tensor imaging (6–12 directions) was also acquired. In
order to ensure relative consistency of ADC calculations across
sites, measures of ADC were obtained from 2 b-values
(typically a single b=0 sec/mm2 image and an
image with higher diffusion weighting, or b=700–1200
sec/mm2. For DTI data, average trace images were used
for this higher diffusion weighted image).
Image registration
All images for each patient were registered to their
own pre-treatment, post-contrast, 3D T1-weighted images with use of
a mutual information algorithm and a 12-degree of freedom
transformation using FSL (FMRIB; http://www.fmrib.ox.ac.uk/fsl/). This was followed by
visual inspection to ensure adequate alignment. All images were
interpolated to the resolution of baseline post-contrast
T1-weighted images using trilinear interpolation. In cases with
significant mass effect, attempts were made to align tumor regions
exclusively. Regions of obvious misregistration (e.g. near
ventricles or edge of the brain) were excluded from final fDM
analysis.
Quantitative quality control evaluation
of diffusion MR data and image registration
Quality control (QC) evaluation was performed on
both the diffusion MR data as well as the alignment between
subsequent scans for use in fDM analysis. DWI at each scan date
were evaluated in terms of the following factors: i) geometric
distortion or artifacts on diffusion MR datasets; ii) ADC values
within normal appearing white matter (NAWM) being within an
acceptable range of ~0.4–1.0 μm2/ms; and iii) ADC values
within cerebrospinal fluid (CSF) being within an acceptable range
of ~2.5–4.0 μm2/ms. A 5-point quantitative scaling
scheme was used for each of these factors as shown in Table II. The final QC score for each
patient was calculated as the minimum QC value from each of the
parameters in Table II.
Additionally, if DWI data were not available for a particular
patient, the QC score was zero.
 | Table IIQuantitative quality control
definitions for diffusion MRI and fDM analysis. |
Table II
Quantitative quality control
definitions for diffusion MRI and fDM analysis.
| Parameter | Score = 1
(Unusable) | Score = 2
(Unusable) | Score = 3
(Usable) | Score = 4
(Good) | Score = 5
(Great) |
|---|
|
Distortion/artifacts | Severe, affecting
tumor | Moderate, affecting
tumor | Moderate, not
affecting tumor | Mild, not affecting
tumor | No distortion or
artifacts |
| ADC values
(NAWM) | Negative
values | Non-physiological
range (0–0.4 μm2/ms) | Lower or higher
than normal, but within physiological range (e.g. 0.4–0.6
μm2/ms; 0.8–1.0 μm2/ms) | | Within normal range
(0.6–0.8 μm2/ms) |
| ADC values
(CSF) | Negative
values | Non-physiological
range (0–1.5 μm2/ms; 4.0+ μm2/ms) | Lower or higher
than normal, but within physiological range | | Within normal range
for CSF |
| Registration of ADC
maps with Baseline ADC maps | Severe
misalignment, tumor not aligned | Moderately
misaligned, tumor not aligned | Moderately
misaligned, but tumor is aligned | Slightly
misaligned, but tumor is largely aligned | Perfectly
aligned |
Region of interest (ROI)
determination
In the present study, we chose to apply fDMs to
regions of contrast-enhancing tumor on pre-treatment, post-contrast
T1-weighted images. This approach has been shown to be the most
predictive in other treatment settings (11,12).
Additionally, this time-point likely contains the largest extent of
contrast enhancing tumor for use in fDM evaluation, since
bevacizumab therapy results in dramatic reduction of the volume of
contrast enhancement in the majority of patients. We used a
semi-automated process of: i) manually defining the relative region
of tumor occurrence; ii) thresholding the post-contrast images
using an empirical threshold combined with a region-growing
algorithm; then iii) manually editing the resulting masks to
exclude any obvious errors. For QC evaluations, a circular ROI
(area, 1.5 cm2 or ~1.4 cm diameter) was placed in the
contra-lateral NAWM and within the contra-lateral, anterior or
posterior lateral ventricles for a measure of normal CSF.
Functional diffusion map (fDM)
calculation
After proper registration was visually verified,
voxel-wise subtraction was performed between ADC maps acquired
post-treatment and baseline, pre-treatment ADC maps. Individual
voxels were stratified into three categories based on the change in
ADC relative to the baseline ADC map. Red voxels represented areas
where ADC increased beyond a ΔADC threshold of 0.4
μm2/ms, or ADC(+), and blue voxels represented areas
where ADC decreased beyond a ΔADC threshold of 0.4
μm2/ms or ADC(−). These ΔADC thresholds (±0.40
μm2/ms) represent the 95% confidence interval for a
mixture of normal appearing gray and white matter estimated from 69
patients with various tumor grades and follow-up time intervals
ranging from 1 week to 1 year post-baseline (13). The fraction of ADC(+) and ADC(−)
within the pre-treatment, post-contrast T1-weighted images [%ADC(+)
and %ADC(−)] was subsequently used for fDM analysis.
Independent radiological facility
definition of disease progression
All local imaging was retrospectively transmitted to
ACRIN for central review. Two primary readers and one adjudicator,
each with neuroradiology Certificates of Added Qualification and 8,
6 and 3 years of post-fellowship experience, respectively, were
trained via teleconference about 2D measurement techniques. Each
primary reader was assigned 2 similarly trained core laboratory
technologist and conducted independent image assessments. For each
distinct contrast-enhancing target lesion as defined by Macdonald
and RANO criteria (≥1 cm diameter, ≥1 cm from other enhancing
lesions), the largest diameter of contrast enhancement and its
maximum perpendicular diameter in the same plane were measured. 2D
tumor area was computed by summing over all lesions the product of
maximum perpendicular diameters. Each reader determined time of
progression on 2D post-contrast T1-weighted images when there was
>25% increase with respect to nadir in maximal cross-sectional
enhancing areas or the appearance of any new enhancing tumor
(9,14). Similarly, radiologic response was
defined as ≥50% decrease with respect to baseline, confirmed on the
subsequent time-point. Steroid dosage and clinical status were
unavailable to ACRIN readers for the present study. The adjudicator
settled discordant times to progression between primary readers by
selecting the times to progression that were most correct in their
opinion. The final measure of progression-free survival (PFS) for
the present study was defined as the time from the first
post-therapy scan used in fDM analysis until radiographic
progression.
Statistical analysis
A Kruskal-Wallis non-parametric test was used to
compare ADC measurements in normal tissue across sites with 3 or
more patients. Pooled variance two-sample t-tests were used to
compare pre-treatment enhancing tumor volume, %ADC(+), or %ADC(−)
between patients who progressed/expired vs. were progression-free
at 6 months and those who expired at 12 months vs. those who were
alive at 12 months from the first post-treatment MRI. Two-sample
Satterthwaite t-tests were used if group variances were
significantly different. A Cox-regression model was used to
evaluate continuous measures of pre-treatment enhancing volume,
%ADC(+) or %ADC(−) adjusted for age and gender, where the outcome
was either PFS or overall survival (OS). Time-dependent receiver
operating characteristic (ROC) analysis was performed for PFS or OS
to determine the thresholds for %ADC(+) and %ADC(−) that maximized
Youden’s index (sensitivity+specificity-1). The threshold values
were used to divide %ADC(+) or %ADC(−) into two groups. Median PFS
and OS as well as their curves within each group were estimated by
the Kaplan-Meier method. Log-rank tests were conducted to compare
the PFS (or OS) curves between the two groups of %ADC(+) [or
%ADC(−)]. Data were examined separately for all usable DWI cases
(QC ≥3) and cases with high quality DWI data (QC=5) to illustrate
the effects of image quality on fDM analyses. P-values <0.05
were considered significant and P-values <0.1 were considered
trending toward significance. All statistical data analyses were
performed with SAS software, version 9.3 (SAS Institute Inc., Cary,
NC, USA).
Results
Normal tissue ADC and quality control
assessment
The evaluation of pre-treatment ADC measurements
within normal tissues for different sites, MR manufacturers, and
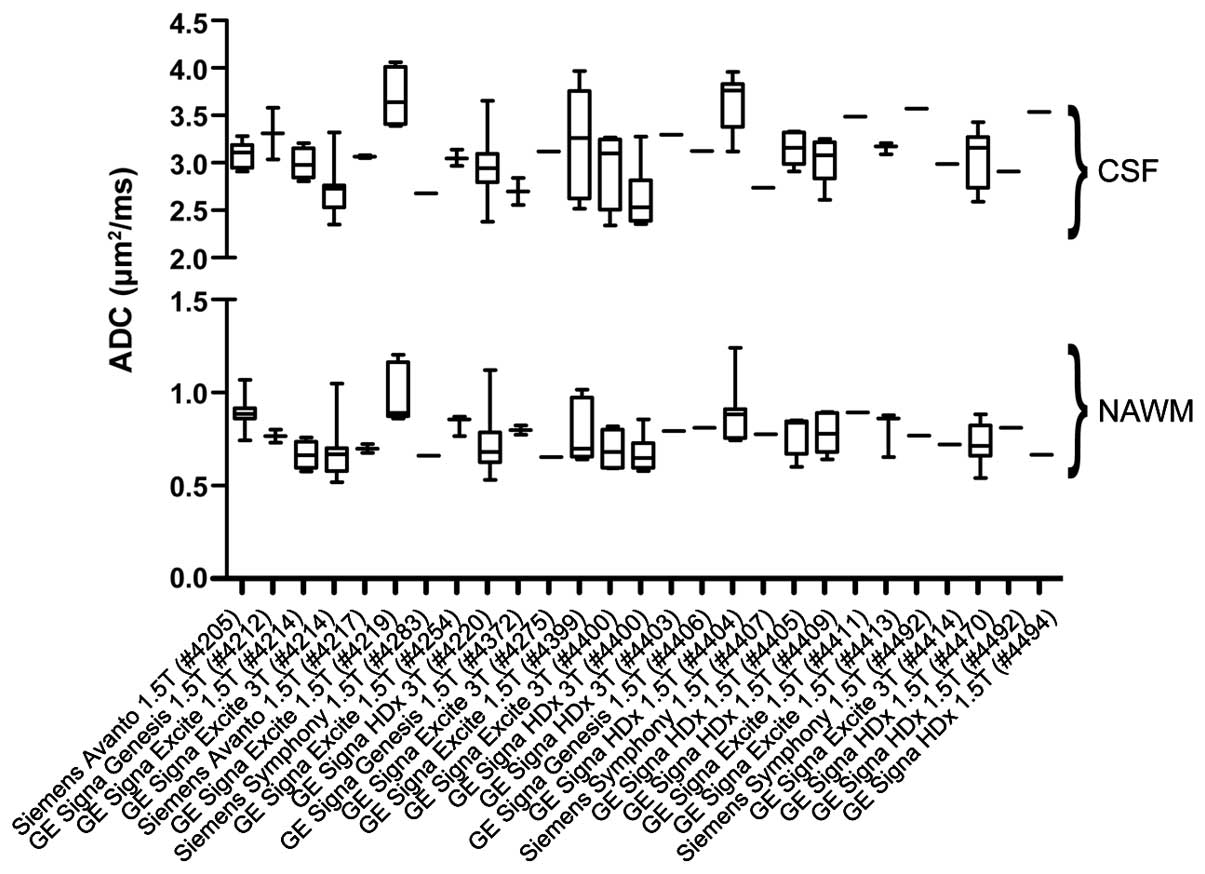
acquisition techniques are shown in Fig. 1. In general, there was a wide
variation in diffusion measurements within the various tissue
types. The average coefficient of variance across all sites was
7.3% for NAWM and 10.5% for CSF. Kruskal-Wallis non-parametric
comparisons of CSF and NAWM in sites with 3 or more patients
suggested ADC varied significantly across sites (NAWM, P<0.001;
CSF, P<0.001). Closer examination suggested that certain sites
had systematically elevated or suppressed estimates of ADC within
normal tissues.
Of the 123 patients with diffusion data available,
84 patients (68%) had adequate image quality (QC score ≥3) and 58
patients (47%) had high quality data (QC score =5). Fig. 2 shows example diffusion MR images
from patients for various QC scores. The average QC score for all
123 patients was 3.37. Of the 84 patients with adequate diffusion
MR information, ACRIN determined 3 cases ineligible for analysis, 3
cases were withdrawn due to no evaluable contrast-enhancing tumor,
2 cases were excluded due to no baseline MR scan after registration
to 6677, and 12 patients progressed prior to the first imaging
time-point, resulting in a total of 64 patients (52%) of total
enrolled patients with evaluable data for fDM analysis (QC score
≥3) and a total of 46 patients (37%) of total enrolled patients
with high quality fDM data (QC score=5).
Study cohort and general fDM
characteristics
Of the 64 patients with diffusion MR data available
for fDM analysis (QC ≥3), 34 patients were male and the mean age
for all patients was 57.3 years old ±11.2 SD. The average
pre-treatment contrast enhancing volume was 18.5±16.9 cc SD,
average %ADC(+) was 17.8±14.4% SD, and average %ADC(−) was
20.6±17.9% SD.
Fig. 3 illustrates
various examples of fDM response to therapy, which in many cases
appeared independent of changes in anatomical images. For example,
the patient in Fig. 3A showed
little change in contrast enhancement after therapy, suggestive of
stable disease or little response to therapy. fDM results in this
patient showed a relatively large proportion of tumor with
decreasing ADC (blue voxels), possibly suggestive of growing tumor
or increasing cell density. Conversely, the patient shown in
Fig. 3B demonstrated a similar
change in anatomical imaging response, but little change on fDMs.
Some patients showed a dramatic decrease in contrast enhancement
following therapy and little change in ADC, such as the patient
shown in Fig. 3C. Other patients
showed a decrease in contrast enhancement that was accompanied by
an increase in ADC (red voxels) similar to the patient shown in
Fig. 3D.
Progression-free survival (PFS)
Patients with DWI QC ≥3
A total of 60 of 64 patients either progressed or
expired at the time of final evaluation, while 43 of 64 patients
either progressed or expired at 6 months from the first
post-treatment time-point. Patients who were progression-free at 6
months showed no significant differences in pre-treatment volume of
contrast enhancement and fDM characteristics from those who
progressed or expired before 6 months (P>0.05). Continuous
measures of enhancing volume were not significantly correlated with
PFS (Cox regression: age, P=0.153; gender, P=0.214; pre-treatment
enhancing volume, P=0.130); however, stratification of patients by
median pre-treatment volume of contrast enhancement (14.9 cc) did
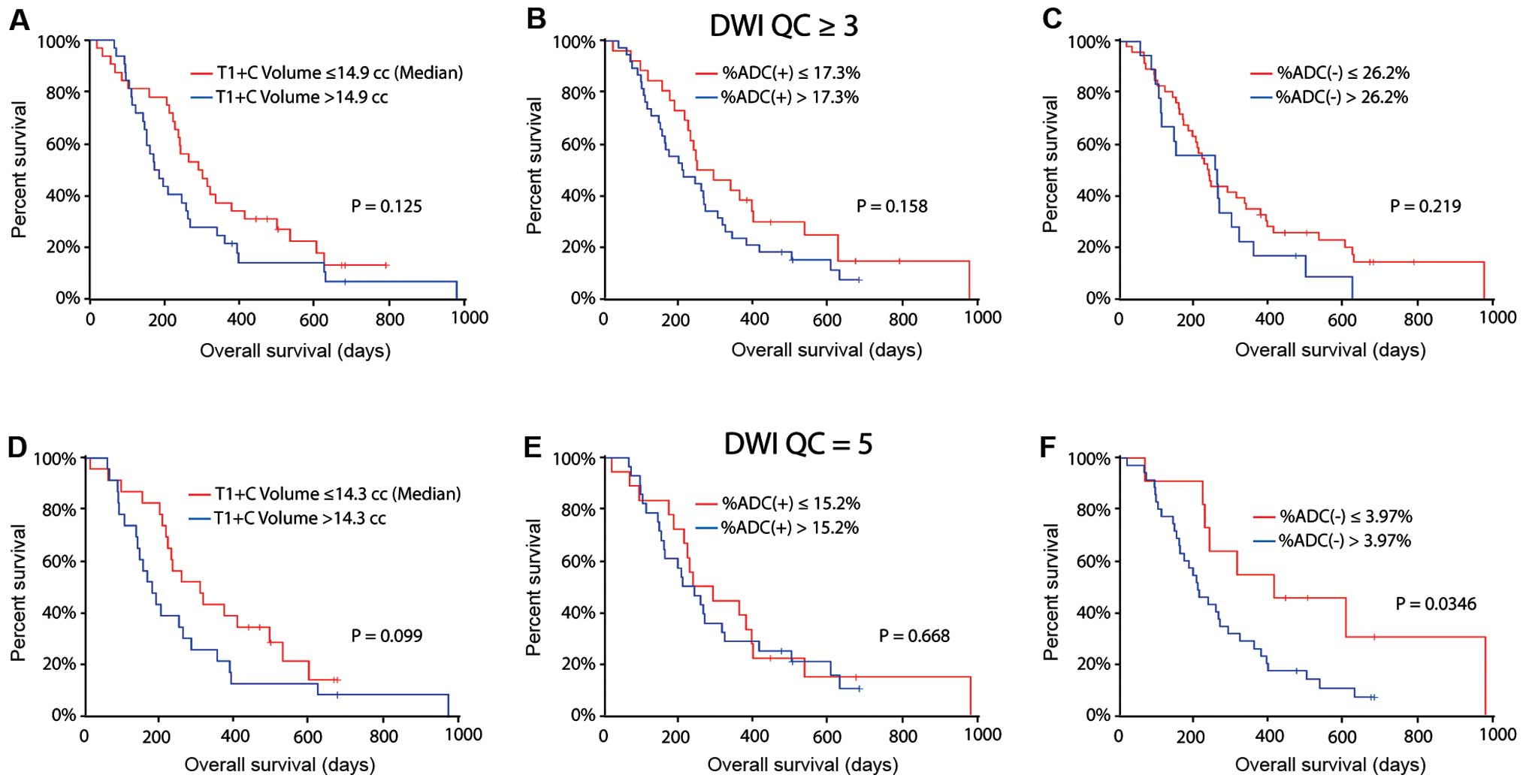
show significant stratification of PFS (Fig. 4A; log-rank, P=0.003). Continuous
measures of %ADC(+) and %ADC(−) from fDM analysis were not
significantly correlated with PFS when adjusted for age and gender
(Cox regression; P>0.05 for both %ADC(+) and %ADC(−)]. Youden’s
index suggested an optimal cutpoint of %ADC(+) of 20.5% and %ADC(−)
of 2.7% for PFS. Using these thresholds, patients with a large
volume fraction of pre-treatment enhancing tumor with increasing
ADC, or %ADC(+) >20.5 cc, had slightly worse PFS (median PFS =
167 vs. 98 days); however, this was not statistically significant
(Fig. 4B; log-rank, P=0.103).
Results also suggest patients with a large volume fraction of
pre-treatment enhancing tumor with decreasing ADC at follow-up, or
%ADC(−) >2.7, had a slightly shorter PFS (median PFS = 107 vs.
240 days), but this was also not statistically significant
(Fig. 4C; log-rank, P=0.116).
Patients with DWI QC=5
For patients with high quality DWI data, a
significant difference in pre-treatment contrast enhancing volume
was observed between patients who were progression-free at 6 months
and those who expired or progressed before 6 months (11.6 vs. 19.9
cc, P=0.027), but no significant differences were found in fDM
characteristics between these patients (P>0.05). Continuous
measures of pre-treatment contrast-enhancing tumor volume were
significantly correlated with PFS (Cox regression: age, P=0.196;
gender, P=0.810; pre-treatment enhancing volume, P=0.012).
Consistent with these trends, stratification of patients by median
pre-treatment volume of contrast enhancement (14.3 cc) demonstrated
significant stratification of PFS (Fig. 4D; log-rank, P=0.011). Continuous
measures of %ADC(+) and %ADC(−) from fDM analysis were not
significant predictors for PFS when accounting for age and gender
[Cox regression: P>0.05 for both %ADC(+) and %ADC(−)]. Youden’s
index suggested a threshold of %ADC(+) of 27.4% and %ADC(−) of 2.7%
for PFS in patients with high quality DWI. Results suggest patients
with a large volume fraction of pre-treatment enhancing tumor with
increasing ADC or %ADC(+) >27.4%, had significantly shorter PFS
(Fig. 4E; median PFS =77 vs. 120
days; log-rank, P=0.042). Results also suggest patients with a
large volume fraction of pre-treatment enhancing tumor with
decreasing ADC at follow-up or %ADC(−) >2.7%, had a slightly
shorter PFS (median PFS = 107 vs. 240 days), but this was not
statistically significant (Fig.
4F; log-rank, P=0.121).
Overall survival (OS)
A total of 56 of 64 patients with evaluable DWI
expired by the end of the study, while 45 of 64 patients expired by
12 months from the first post-treatment time-point. No difference
in mean pre-treatment contrast enhancing volume or fDM
characteristics were observed between patients alive at 12 months
compared with those who expired at 12 months (P>0.05 for all
metrics).
Patients with DWI QC ≥3
Neither continuous measures of enhancing volume or
fDM characteristics were significant predictors for OS [Cox,
P>0.05 for volume, %ADC(+) and %ADC(−)]. When patients were
stratified by median pre-treatment contrast-enhancing tumor volume
(14.9 cc), no significant difference in OS was observed (Fig. 5A; log-rank, P=0.125). Th optimal
cutpoints for %ADC(+) and %ADC(−) were 17.3 and 26.2% when the
outcome was OS; however, neither %ADC(+) (Fig. 5B; log-rank, P=0.158) nor %ADC(−)
(Fig. 5C; log-rank, P=0.219)
significantly separated these groups in terms of OS.
Patients with DWI QC=5
For patients with high quality DWI data available,
continuous measures of pre-treatment contrast-enhancing tumor was
significantly correlated with OS (Cox, P=0.006 for volume, P=0.080
for age and 0.575 for gender). When patients were stratified by
median pre-treatment enhancing volume (14.3 cc), a trend toward a
difference in OS was observed (Fig.
5D; log-rank, P=0.099). Continuous measures of %ADC(+) and
%ADC(−) were not significantly associated with OS (Cox, P>0.05
for fDM metrics). The optimal cutpoints for %ADC(+) and %ADC(−) in
patients with high quality DWI data were 15.2 and 3.97%,
respectively. The Kaplan-Meier curves between the two groups of
%ADC(+) were not significantly different (Fig. 5E; log-rank, P=0.668). On the other
hand, patients with a large volume fraction of pre-treatment
enhancing tumor with decreasing ADC at follow-up, or %ADC(−)
>3.97%, had a significantly shorter OS (Fig. 5F; median OS = 210 vs. 413 days;
log-rank, P=0.035).
Discussion
To the best of our knowledge, this is one of the
first studies to define and implement specific diffusion MRI
quality control criteria in the setting of a multicenter clinical
trial in brain cancer. Results from the present study showed
~7.3–10.5% coefficient of variance in measurement of ADC across
various sites. These results appear consistent with the
measurements obtained by Chenevert et al (15), who estimated the variability of ADC
in an ideal setting of an ice water phantom at ~5% when evaluated
across vendors and platforms. It is important to note, however,
that measures of ADC within a water phantom is monoexponential,
thus, measurements of ADC may be quite resilient to the number of
b-values and maximum b-value chosen, which may not be
the case with normal neural tissues. More importantly, only 84 of
the original 123 (68%) patients had usable DWI data free of
distortion around the areas of tumor and only 58 of the original
123 (47%) patients had high quality DWI data with no distortions or
ADC abnormalities. [In the end, only 64 patients (52%) had usable
DWI data and 46 patients (37%) had high quality DWI data after
patients were excluded based on other factors]. This degree of
unusable data is particularly discouraging if diffusion MRI is to
be considered a secondary response biomarker or a potential imaging
endpoint in future prospective multicenter clinical trials.
The present study clearly demonstrates the
importance of performing semi-quantitative QC in the context of
advanced imaging in multicenter clinical trials. Functional
diffusion mapping using high quality diffusion MRI acquired before
and after administration of bevacizumab is a valuable imaging
biomarker for predicting survival in recurrent glioblastoma
patients treated with bevacizumab. Almost all fDM metrics showed
improved stratification of short- and long-term PFS and OS when
examining the highest quality DWI data (QC=5) compared with usable
DWI data (QC ≥3). In particular, examination of high quality DWI
data showed significant stratification of short- and long-term PFS
when examining the volume fraction of pre-treatment enhancing tumor
with increasing ADC [%ADC(+)], while the volume fraction of
enhancing tumor with decreasing ADC [%ADC(−)] showed significant
stratification of short- and long-term OS. When examining only the
usable DWI data (QC ≥3), these trends were not statistically
significant.
Although only a subset of data was evaluable in the
present multicenter study, fDM results appeared to show some trends
that were consistent and other trends that were inconsistent with
previous studies. For example, previous fDM studies involving
radiochemotherapy (12,16,17)
in newly diagnosed malignant gliomas and bevacizumab (11) in recurrent GBM showed that patients
with a low volume fraction of tumor with decreasing ADC [%ADC(−)]
were more likely to have a longer PFS and OS. In the present study,
we observed the same trend, however, results only showed
statistical significance when examining %ADC(−) in terms of OS the
subset of patients with high quality DWI data. Contrary to previous
fDM reports, patients exhibiting a large volume fraction of
enhancing tumor demonstrating an increase in ADC at first follow-up
[%ADC(+)] appeared more likely to progress earlier than patients
with a small volume fraction. Since all these patients were treated
with bevacizumab, which tends to rapidly reduce the amount of
vasogenic edema, it is conceivable that tumors demonstrating an
increase in ADC following bevacizumab may represent those tumors to
which vascular permeability has increased, indicating ineffective
anti-angiogenic therapy.
It is important to point out that pre-treatment
contrast enhancing tumor volume was one of the strongest correlates
of survival in recurrent GBM patients treated with bevacizumab and
chemotherapy. Results from the present study suggest that
continuous measures of pre-treatment enhancing tumor were
significantly correlated with PFS and OS when accounting for
clinical covariates, particularly when examining patients with the
highest quality MR data. This observation is consistent with a
recent study (18) examining
contrast enhancing tumor before and after bevacizumab treatment in
a similarly structured phase II multicenter study in recurrent GBM
patients treated with bevacizumab monotherapy or bevcizumab and
irinotecan. As measures of contrast enhancing tumor remain the gold
standard for response assessment and estimating tumor burden in
malignant gliomas, it is important to compare emerging imaging
biomarkers with this standard to determine if they truly add
clinical benefit.
A number of limitations and possible explanations
for the relatively poor fDM performance should be addressed. First,
the present study involved calculation of ADC given only 2
b-values, while the National Cancer Institute recommends
that at least 3 b-values be acquired (0, >100 and >500
sec/mm2) for estimation of perfusion-insensitive ADC
(19). Additionally, many sites
did not comply with the recommended diffusion MRI protocols, nor
was there a mechanism in place for real-time feedback of image
quality as diffusion MRI was considered a secondary measurement to
standard anatomic imaging techniques. Another potential limitation
was the potential influence of geometric distortions on ADC
measurements. Woodworth et al (20) recently showed that post hoc
non-linear distortion correction of diffusion MR images to
high-resolution T2-weighted images can improve diffusion
measurements in brain tumors, demonstrating that subtle distortions
can cause significant differences in ADC measurements. A similar
approach could have been used in the present study to improve ADC
measurements, even in patients with usable data (QC ≥3). Similarly,
the use of a rigid-body image registration algorithm to align
serial ADC maps to baseline ADC maps poses another potential
limitation. Significant changes in mass effect from tumor growth or
shrinkage, or intracranial pressure changes induced by changes in
the extent of vasogenic edema may cause inaccuracies in the
alignment between the diffusion MR datasets. A recent study by
Ellingson et al (21)
showed improved fDM performance in the context of bevacizumab
therapy by using non-linear registration of ADC maps over time. It
is conceivable that a similar approach may also have improved fDM
performance in the context of the current study, which also
involved similar therapies and registration challenges.
In conclusion, the present study suggests diffusion
MRI data collected as part of a multicenter trial for brain tumors
may be of limited value, due particularly to the wide variety in
image quality across sites, vendors and acquisition protocols. In
data deemed usable, fDM results showed similar trends but lower
correlations compared with previous single-institution trials
involving relatively high-quality diffusion data with homogeneous
acquisition protocols. Stratification of survival using fDM metrics
were substantially improved by examining a subset of patients with
high quality DWI data, suggesting image quality may have a
significant impact on fDM performance. Future studies aimed at
improving the consistency of image quality in multicenter trials
are necessary for further advancement of diffusion MR
biomarkers.
Acknowledgements
The present study was funded by NIH/NCI U01 CA079778
(ACRIN); NIH/NCI U01 CA080098 (ACRIN); the ACRIN Young Investigator
Initiative Grant (BME); the National Brain Tumor Society Research
Grant (BME); NIH/NCI 1 R21 CA167354-01 (BME); the UCLA Institute
for Molecular Medicine Seed Grant (BME); the UCLA Radiology
Exploratory Research Grant (BME); the University of California
Cancer Research Coordinating Committee Grant (BME); and the Siemens
Technical Research Grant (BME).
References
|
1
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14(Suppl 5): v1–v49. 2012. View Article : Google Scholar :
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. New Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weller M, Cloughesy T, Perry JR and Wick
W: Standards of care for treatment of recurrent glioblastoma - are
we there yet? Neuro Oncol. 15:4–27. 2013. View Article : Google Scholar :
|
|
4
|
Duda DG, Batchelor TT, Willett CG and Jain
RK: VEGF-targeted cancer therapy strategies: current progress
hurdles and future prospects. Trends Mol Med. 13:223–230. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nghiemphu PL, Liu W, Lee Y, et al:
Bevacizumab and chemotherapy for recurrent glioblastoma: a
single-institution experience. Neurology. 72:1217–1222. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vredenburgh JJ, Desjardins A, Herndon JE,
et al: Phase II trial of bevacizumab and irinotecan in recurrent
malignant glioma. Clin Cancer Res. 13:1253–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman AH, Prados MD, Wen PY, et al:
Bevacizumab alone and in combination with irinotecan in recurrent
glioblastoma. J Clin Oncol. 27:4733–4740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Macdonald DR, Cascino TL, Schold SC Jr and
Cairncross JG: Response criteria for phase II studies of
supratentorial malignant glioma. J Clin Oncol. 8:1277–1280.
1990.PubMed/NCBI
|
|
9
|
Iwamoto FM, Abrey LE, Beal K, et al:
Patterns of relapse and prognosis after bevacizumab failure in
recurrent glioblastoma. Neurology. 73:1200–1206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Norden AD, Young GS, Setayesh K, et al:
Bevacizumab for recurrent malignant gliomas: efficacy, toxicity,
and patterns of recurrence. Neurology. 70:779–787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellingson BM, Cloughesy TF, Lai A, et al:
Graded functional diffusion map-defined characteristics of apparent
diffusion coefficients predict overall survival in recurrent
glioblastoma treated with bevacizumab. Neuro Oncol. 13:1151–1161.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ellingson BM, Cloughesy TF, Zaw T, et al:
Functional diffusion maps (fDMs) evaluated before and after
radiochemotherapy predict progression-free and overall survival in
newly diagnosed glioblastoma. Neuro Oncol. 14:333–343. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellingson BM, Malkin MG, Rand SD, et al:
Validation of functional diffusion maps (fDMs) as a biomarker for
human glioma cellularity. J Magn Reson Imaging. 31:538–548. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen PY, Macdonald DR, Reardon DA, et al:
Updated response assessment criteria for high-grade gliomas:
response assessment in neuro-oncology working group. J Clin Oncol.
28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chenevert TL, Galban CJ, Ivancevic MK, et
al: Diffusion coefficient measurement using a
temperature-controlled fluid for quality control in multicenter
studies. J Magn Reson Imaging. 34:983–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamstra DA, Chenevert TL, Moffat BA, et
al: Evaluation of the functional diffusion map as an early
biomarker of time-to-progression and overall survival in high-grade
glioma. Proc Natl Acad Sci USA. 102:16759–16764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moffat BA, Chenevert TL, Lawrence TS, et
al: Functional diffusion map: a noninvasive MRI biomarker for early
stratification of clinical brain tumor response. Proc Natl Acad Sci
USA. 102:5524–5529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellingson BM, Kim HJ, Woodworth DC, et al:
Recurrent glioblastoma treated with bevacizumab: contrast-enhanced
T1-weighted subtraction maps improve tumor delineation and aid
prediction of survival in a multicenter clinical trial. Radiology.
271:200–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Padhani AR, Liu G, Koh DM, et al:
Diffusion-weighted magnetic resonance imaging as a cancer
biomarker: consensus and recommendations. Neoplasia. 11:102–125.
2009.PubMed/NCBI
|
|
20
|
Woodworth DC, Pope WB, Liau LM, et al:
Nonlinear distortion correction of diffusion MR images improves
quantitative DTI measurements in glioblastoma. J Neurooncol.
116:551–558. 2014. View Article : Google Scholar :
|
|
21
|
Ellingson BM, Cloughesy TF, Lai A,
Nghiemphu PL and Pope WB: Nonlinear registration of
diffusion-weighted images improves clinical sensitivity of
functional diffusion maps in recurrent glioblastoma treated with
bevacizumab. Magn Reson Med. 67:237–245. 2012. View Article : Google Scholar
|