Introduction
Lung cancer is one of the common cancers in the
world, and also one of the leading causes of cancer-related deaths
worldwide (1–3). Lung cancer metastasizes to the
skeletal system frequently. About 30–40% of lung cancer patients
will develop bone metastasis during the progression of their
disease, which results significant impact on the patients’ quality
of life, morbidity and survival (3–5).
Bone metastasis from lung cancer usually causes osteolytic lesions
characterized by increased osteoclast activity (3,4) and
decreased osteoblast capacity (6–8).
Parathyroid hormone-related protein (PTHrP), produced by lung
cancer cells, will stimulate osteoblasts to express elevated levels
of receptor activator of nuclear factor κB ligand (RANKL) and will
stimulate osteoclastogenesis by binding to the receptor RANK and
activating its downstream signaling pathways in hematopoietic
osteoclast precursors (3,9). Thus, therapy targeting
osteoclast/osteoblast interactions during lung cancer progression
isimportant.
Polycyclic aromatic hydrocarbons (PAHs), are formed
by the incomplete combustion of organic matter. Benzo(a) pyrene
(BaP) is the most commonly measured and studied PAH. They usually
present in the environment at detectable levels in many types of
uncooked food, and cooking process could generate PAHs in food.
Several studies have been conducted to determine the levels of
exposure to PAHs from representative human diet and the proportion
of the overall burden of environmental exposure to PAHs that is
attributable to diet (10–13). Previous studies demonstrated the
association between PAHs and an increased risk of respiratory tract
cancer (14–16). Exposure to BaP enhances the
invasion and metastasis of lung cancer cells and BALB/c 3T3 cells
in vivo and in vitro (17–19).
Moreover, BaP can enhance the expression level of
epithelial-mesenchymal transition-related genes (20) and can promote migration and
invasion of lung cancer cells through upregulating Twist (21).
Tricetin (TCN) (5,7,3′,4′,5′-pentahydroxyflavone), a
flavonoid derivative found in Myrtaceae pollen and
Eucalyptus honey (22–24),
possesses potent anti-inflammatory and anti-cancer activities
(25–27). This study evaluated the effects of
BaP in human non-small cell lung cancer bone metastasis and
investigated the potential role of TCN against the effects from BaP
on human non-small cell lung cancer.
Materials and methods
Chemicals
TCN was obtained from Extrasynthese (Genay, France),
dissolved in dimethyl sulfoxide (DMSO) (Sigma- Aldrich, St. Louis,
MO, USA), and stored at −20°C. Control cultures received the
carrier solvent (0.1% DMSO). All chemicals used were in their
purest form available commercially.
Cell culture and conditioned medium
Human non-small cell lung cancer H460 cells were
obtained from the American Type Culture Collection (HTB-177)
(Manassas, VA, USA) and cultured in RPMI-1640 medium containing 10%
fetal bovine serum (FBS) (both from Gibco-BRL, Gaithersburg, MD,
USA). Human primary osteoblasts were obtained from Lonza
(Walkersville, MD, USA) and cultured in osteoblast medium (OBM)
(Lonza).
To obtain the various conditioned media (CM), H460
cells (2×106/100 mm dish) were treated with various
concentrations of BaP (Sigma-Aldrich) for 6 h. After treatment, the
medium was replaced and the supernatant harvested and filtered
(0.22 mm) after 24 h of incubation.
Measurement of secreted factors
Supernatants from osteoblasts and H460 cells were
collected. Levels of osteoprotegerin (OPG), macrophage
colony-stimulating factor (M-CSF), RANKL and IL-8 were assessed and
quantified using the DuoSet enzyme-linked immunosorbent assay
(ELISA) (R&D Systems, Minneapolis, MN, USA). PTHrP levels were
determined by an ELISA kit (Abnova Corp., Taipei, Taiwan).
Isolation of CD14+ monocytes
and osteoclast differentiation
Monocytes were purified from peripheral blood
mononuclear cells (PBMCs) obtained from healthy donors. Mononuclear
cells were isolated from blood by Ficoll-Hypaque gradient (GE
Healthcare UK, Ltd., Buckinghamshire, UK). CD14+
monocytes were purified using CD14+ monoclonal
antibody-conjugated magnetic beads (MACS MicroBeads; Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany), according to the
manufacturer’s instructions. Osteoclasts were generated by
culturing CD14+ monocytes in medium containing 20%
vehicle control-CM-cultured osteoblasts or BaP-H460-CM-cultured
osteoblasts presented in 100 ng/ml M-CSF and 50 ng/ml RANKL
(R&D Systems) for 14–21 days. The medium was replaced with
fresh medium containing M-CSF and RANKL every 5 days.
Osteoclast formation was measured by quantifying
cells positively stained by TRAP (Sigma-Aldrich). Osteoclasts were
deemed TRAP-positive by light microscopy that revealed staining of
multinuclear (>3 nuclei) cells. The TRAP-positive cells and the
number of nuclei per TRAP-positive cells in each well were counted.
The bone resorption activity of osteoclasts was assessed by a
48-well plate bone resorption assay (Cosmo Bio Co., Ltd., Tokyo,
Japan), under the same culture conditions as described above.
The Institutional Review Board (IRB) of Kaohsiung
Medical University Chung-Ho Memorial Hospital (Kaohsiung, Taiwan)
approved the study protocol and all of the participants provided
written informed consent in accordance with the Declaration of
Helsinki (IRB nos.: KMUH-IRB-990345, KMUH-IRB-20110377 and
KMUH-IRB-20130054).
Real-time polymerase chain reaction
(RT-qPCR)
The TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) was used for RNA isolation while cDNA was
prepared using an oligo(dT) primer and reverse transcriptase
(Takara Bio, Inc., Shiga, Japan) following standard protocols. The
RT-qPCR was performed using SYBR-Green on the ABI 7500 Real-Time
PCR System (Applied Biosystems, Foster City, CA, USA). Each PCR
reaction mixture contained 200 nM of each primer, 10 μl 2X
SYBR-Green PCR Master Mix (Applied Biosystems), and 5 μl cDNA and
RNase-free water for a total volume of 20 μl. The RT-qPCR was
conducted with a denaturation step at 95°C for 10 min, then for 40
cycles at 95°C for 15 sec, and 60°C for 1 min. All PCRs were
performed in triplicate and normalized to internal control
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The relative
expression level was determined using the 2−ΔΔCT
method.
PTHrP knockdown
H460 cells were transfected with 20 nM non-target or
PTHrP siRNA pool (Dharmacon, Inc., Lafayette, CO, USA) by
DharmaFECT 4 Transfection Reagents, according to the manufacturer’s
instructions. After 24 h transfection, the medium was changed to
whole medium and the cells were treated with BaP. The PTHrP changes
were measured by RT-qPCR.
Statistical analysis
Data are expressed as means ± standard errors.
Statistical comparisons of the results were made using analysis of
variance (ANOVA). Significant differences (p<0.05) between the
means of the test groups were analyzed by Student’s t-test.
Results
BaP induces PTHrP secretion in human
non-small cell lung cancer
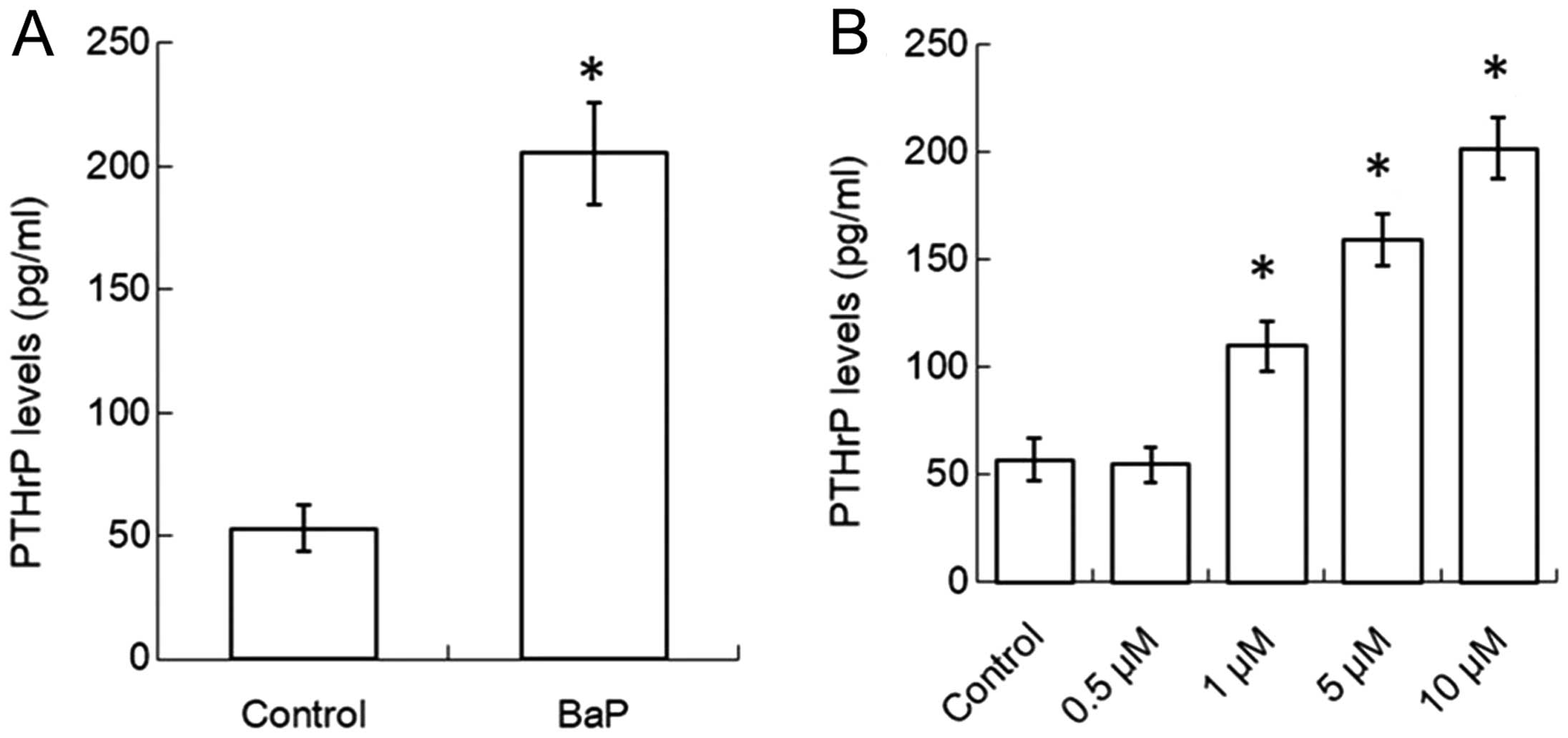
To investigate the effects of BaP on PTHrP secretion
by human non-small cell lung cancer cells, BaP was added to the
culture medium of H460 cells to the concentration of 10 μM for 6 h.
After washing, cells were cultured with new medium for another 24
h, the conditioned medium of BaP-treated H460 (BaP-H460-CM) was
harvested and PTHrP levels of these CM were assessed. After
exposure to BaP, the production of PTHrP in human non-small cell
lung cancer H460 cells was increased (Fig. 1A). H460 cells were also treated
with different concentrations of BaP, the result revealed that BaP
increased the production of PTHrP in H460 cells in a dose-dependent
manner (Fig. 1B).
Conditioned medium of BaP-treated H460
cells increases M-CSF and RANKL expression, and decreases OPG
expression of osteoblasts
Osteoclastogenesis is regulated by PTHrP by altering
the ratio of osteoclastogenesis activator (M-CSF and
RANKL)/inhibitor (OPG) produced by osteoblasts (28,29).
To determine if BaP influences the interaction between non-small
cell lung cancer cells and the secretion of M-CSF, RANKL, and OPG
by osteoblasts, H460 cells were treated with 0.1% DMSO or 10 μM BaP
for 6 h. Then BaP was removed by washing. H460 cells were cultured
with fresh medium and the culture medium was collected thereafter
(defined as control-CM, H460-CM, and BaP-H460-CM).
Human osteoblasts were cultured with the CM prepared
to assess the effects of BaP on the interaction between non-small
cell lung cancer and osteoblasts (Fig.
2A). H460-CM was found to be able to increase the M-CSF and
RANKL expressions in osteoblasts and such stimulatory effect was
further enhanced when H460 cells were pre-treated with BaP
(Fig. 2B and C). In contrast,
H460-CM decreased the OPG expression in osteoblasts and this
inhibitory effect was strengthened when non-small cell lung cancer
cells were exposed to BaP (Fig.
2D).
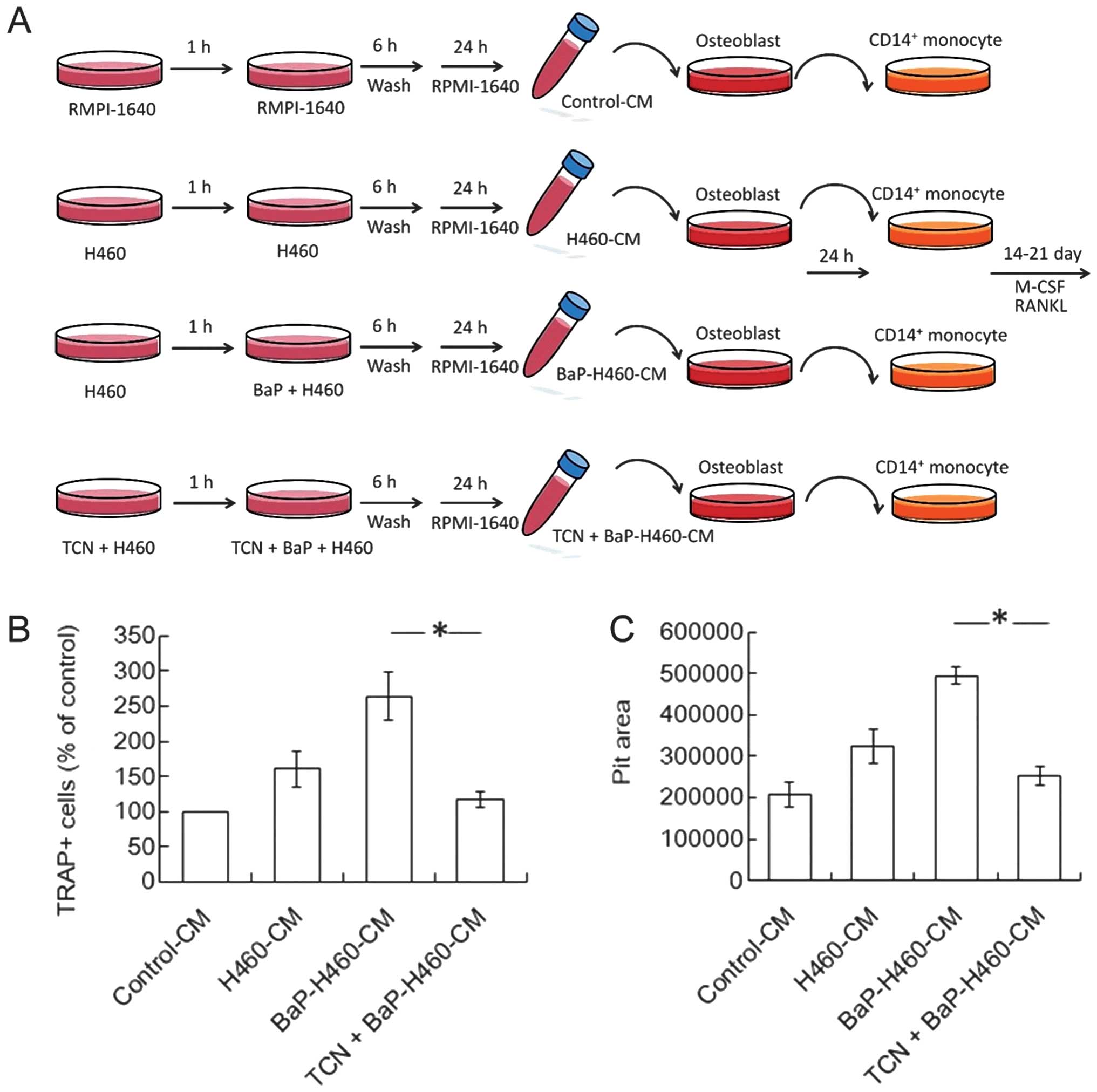
BaP increased human non-small cell lung
cancer H460 cell-mediated osteoclastogenesis and bone
resorption
To assess the effect of BaP on non-small cell lung
cancer-mediated osteoclastogenesis, TRAP and bone resorption assays
were tested. H460-CM increased osteoclastogenesis induced by H460
cells, and such effect was strengthened when H460 cells were
pre-treated with BaP (Fig. 3A).
Similarly, H460-CMs enhanced bone resorption activity and such
enhancement further intensified once H460 cells were pre-treated
with BaP (Fig. 3B).
PTHrP/IL-8 autocrine loop is involved in
the stimulation of BaP on non-small cell lung cancer-mediated
osteoclastogenesis
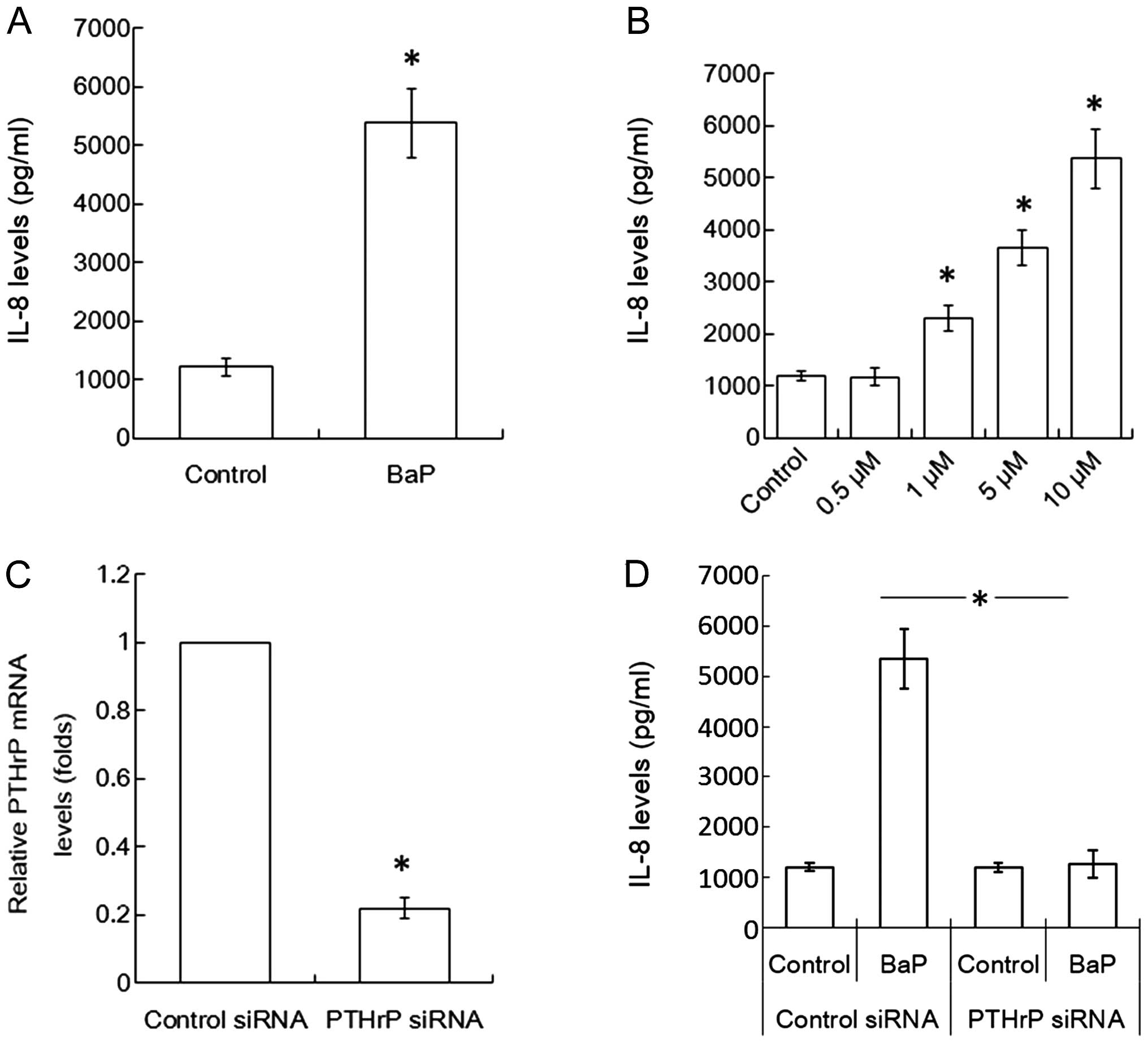
Since PTHrP was reported to be able to increase IL-8
expression of cancer cells (30),
this study assessed whether BaP increased the effect of H460 cells
on osteoclastogenesis is through the PTHrP/IL-8 loop or not. The
level of IL-8 was higher in BaP-treated H460 cells than in the
control group (Fig. 4A). Also, the
effect of BaP to increase IL-8 expression from H460 cells was
demonstrated to be in a dose-dependent (Fig. 4B).
To confirm the role of PTHrP in the upregulation of
IL-8 induced by BaP, H460 cells were transfected with PTHrP siRNA.
The expression of PTHrP mRNA in H460 cells was decreased ~78% when
the cells were transfected with PTHrP siRNA (Fig. 4C). PTHrP silencing prevented the
upregulation effect from BaP on the expression of IL-8 in H460
cells (Fig. 4D).
TCN suppresses BaP-mediated bone
resorption
To access the effects of TCN on BaP-mediated
non-small cell lung cancer bone metastasis, BaP-induced PTHrP and
IL-8 secretion by non-small cell lung cancer H460 cells were tested
again, both PTHrP and IL-8 secretion were decreased by 1 μM TCN
treatment (Fig. 5A and B).
Similarly, the CM of BaP-treated H460 cells enhanced RANKL
upregulation in osteoblasts, and 1 μM TCN treatment blocked such
upregulation (Fig. 5C). The
activity of TCN on human non-small cell lung cancer-mediated
interaction of osteoblasts and osteoclasts was further investigated
(Fig. 6A). Osteoclastogenesis and
bone resorption were both significantly decreased by TCN treatment
(Fig. 6B and C).
 | Figure 5Tricetin (TCN) decreases the effects
of benzo(a)pyrene (BaP) on parathyroid hormone-related protein
(PTHrP), IL-8, and receptor activator of nuclear factor κB ligand
(RANKL) expression. TCN (1 μM) decreased the upregulation of BaP on
(A) PTHrP and (B) IL-8 in H460 cells. (C) TCN decreased the effects
of BaP on RANKL expression of osteoblasts. For (A) and (B), H460
cells were pre-treated with TCN for 1 h, then incubated with BaP
(10 μM) for another 6 h for PTHrP, or 24 h for IL-8 analysis. After
washing and a 24-h culture, the culture media were collected and
PTHrP levels were assessed by enzyme-linked immunosorbent assay
(ELISA). (C), H460 cells were pre-treated with TCN for 1 h, then
incubated with BaP (10 μM) for another 6 h. After washing and a
24-h culture, the culture media were collected, then added to
osteoblasts for another 24 h. The levels of RANKL in the
supernatants of osteoblasts were assessed by ELISA. Each value is
the mean ± SD of three independent experiments.
*P<0.05, or significant difference between the
control and test groups. |
Discussion
Bone metastasis is a devastating event for lung
cancer patients because once it occurs, the morbidity and mortality
will increase (31,32). Sone and Yano have demonstrated that
several compounds, including bisphosphonates and reveromycin A,
able to suppress the activity of osteoclast, are beneficial for the
treatments of lung cancer patients with bone metastasis (33). However, 30–50% of lung cancer
patients still developed new bone metastasis or skeletal
complications while they are receiving such therapy, which
emphasizes the necessity for new therapies (34,35).
Evaluation of the differentiation of osteoclast is important in
bone metastasis of lung cancer. Multimodality therapy is necessary
to improve the efficacy of therapy against lung cancer bone
metastasis (31–33). This is the first study to
demonstrate that BaP increases the stimulatory effect of non-small
cell lung cancer on osteoclastogenesis and osteoclastic bone
resorption activity directly via PTHrP/IL-8 and by interfering in
the osteoblast-osteoclast interaction.
PTHrP, a potent activator of osteoclastic bone
resorption, is an important pathologic factor for hypercalcemia
among cancer patients (36–38).
PTHrP stimulates osteoclastogenesis by increasing RANKL expression
and by reducing OPG expression in osteoblasts. However, it does not
act directly on the precursors of the osteoclasts (39,40).
RANKL binds with the RANK receptor of the precursors of osteoclasts
and induces the formation of mature osteoclasts in the presence of
M-CSF (41,42).
OPG is a soluble decoy receptor for RANKL, with the
ability to decrease osteoclastogenesis (43). Increase of RANKL/OPG ratio by
cancer-derived PTHrP results in osteoclastic bone resorption
(3,44). This study shows that BaP
upregulates the secretion of PTHrP in non-small cell lung cancer
cells. Besides upregulation of PTHrP, BaP can reinforce the effects
of non-small cell lung cancer on osteoblasts, including increased
M-CSF and RANKL, and decreased OPG. These results suggest that BaP
may worsen bone metastasis in non-small cell lung cancer.
Many studies have reported that non-small cell lung
cancer expresses high levels of IL-8, which enhances both
osteoclastogenesis and bone resorption (45,46).
PTHrP has been reported to enhance osteoclastogenesis by increasing
the expression of osteoclast stimulatory factors such as IL-8
(3,44). The present study demonstrates that
BaP increases IL-8 expression in non-small cell lung cancer cells.
Inhibition of PTHrP by siRNA transfection prevents the upregulation
effects from BaP on IL-8 secretion. These results suggest that
PTHrP is a major mediator involved in the stimulatory effect of BaP
on IL-8 production. Furthermore, BaP enhances the stimulatory
effect of non-small cell lung cancer on osteoclastogenesis and
their bone resorption activity. Thus, the regulation of IL-8
expression by BaP through PTHrP is a key point in BaP-induced
osteoclastogenesis and bone resorption.
Current therapy for bone metastases have limited
efficacy and are only palliative. The side-effects of these
treatments, renal toxicity and osteonecrosis of the jaw potentially
will decrease the quality of life of these lung cancer patients
(47,48). In addition, eliminating all BaP
exposure may be impossible because BaP is widely present in modern
life. It is therefore important that strategies be developed for
preventing bone metastasis in non-small cell lung cancer.
Our data show that TCN, a flavonoid derivative found
in Myrtaceae pollen and Eucalyptus honey, exhibits effects
to decrease PTHrP expression in non-small cell lung cancer H460
cells. Simultaneously, TCN also decreases IL-8 expression,
resulting in the inhibition of H460-mediated osteoclastogenesis and
bone resorption. Moreover, TCN also decreases the stimulatory
effect from non-small cell lung cancer on RANKL espression of
osteoblasts, suggesting that TCN may be a potential agent to
prevent the aggravating effect from BaP on non-small cell lung
cancer bone metastasis.
In conclusion, there are two novel findings in this
study: it is the first study to demonstrate that BaP increases the
stimulatory effect of human non-small cell lung cancer on
osteoclastogenesis and their activity of osteoclast bone resorption
directly by PTHrP/IL-8 and by interfering in the
osteoblast-osteoclast interaction, and it is also the first to
reveal that TCN, a flavonoid derivative found in Myrtaceae pollen
and Eucalyptus honey, reverses BaP-mediated bone resorption
(Fig. 7).
Acknowledgements
This study was supported by grants from the National
Science Council (NSC 101-2628-B-037-001-MY3; NSC
101-2320-B-037-043-MY3), the Ministry of Science and Technology
(MOST 103-2320-B-037-006-MY3; MOST 103- 2314-B-037-052; MOST
103-2320-B-037-032), the Kaohsiung Medical University ‘Aim for the
Top 500 Universities Grant, Grant no. KMU-DT103008’, the Kaohsiung
Medical University ‘Aim for the Top Universities Grant, Grant nos.
KMU-TP103A19 and KMU-TP103A20’, and the Kaohsiung Medical
University Hospital Research Foundation (KMUH101-1M08). The authors
thank the Center for Research Resources and Development of
Kaohsiung Medical University for its support with the
instrumentation.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zükin M: Epidermal growth factor receptor
inhibitors in non-small cell lung cancer: Current status and future
perspectives. Rev Assoc Med Bras. 58:263–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuo PL, Liao SH, Hung JY, Huang MS and Hsu
YL: Micro-RNA-33a functions as a bone metastasis suppressor in lung
cancer by targeting parathyroid hormone related protein. Biochim
Biophys Acta. 1830:3756–3766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al Husaini H, Wheatley-Price P, Clemons M
and Shepherd FA: Prevention and management of bone metastases in
lung cancer: A review. J Thorac Oncol. 4:251–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirsh V, Major PP, Lipton A, Cook RJ,
Langer CJ, Smith MR, Brown JE and Coleman RE: Zoledronic acid and
survival in patients with metastatic bone disease from lung cancer
and elevated markers of osteoclast activity. J Thorac Oncol.
3:228–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sterling JA, Edwards JR, Martin TJ and
Mundy GR: Advances in the biology of bone metastasis: How the
skeleton affects tumor behavior. Bone. 48:6–15. 2011. View Article : Google Scholar
|
|
7
|
Sims NA and Gooi JH: Bone remodeling:
Multiple cellular interactions required for coupling of bone
formation and resorption. Semin Cell Dev Biol. 19:444–451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller RE, Jones JC, Tometsko M, Blake ML
and Dougall WC: RANKL inhibition blocks osteolytic lesions and
reduces skeletal tumor burden in models of non-small-cell lung
cancer bone metastases. J Thorac Oncol. 9:345–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esposito M and Kang Y: Targeting
tumor-stromal interactions in bone metastasis. Pharmacol Ther.
141:222–233. 2014. View Article : Google Scholar :
|
|
10
|
Phillips DH: Polycyclic aromatic
hydrocarbons in the diet. Mutat Res. 443:139–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anastasio A, Mercogliano R, Vollano L,
Pepe T and Cortesi ML: Levels of benzo[a]pyrene (BaP) in
‘mozzarella di bufala campana’ cheese smoked according to different
procedures. J Agric Food Chem. 52:4452–4455. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domingo JL: Influence of cooking processes
on the concentrations of toxic metals and various organic
environmental pollutants in food: A review of the published
literature. Crit Rev Food Sci Nutr. 51:29–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guillén MD, Sopelana P and Partearroyo MA:
Food as a source of polycyclic aromatic carcinogens. Rev Environ
Health. 12:133–146. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rota M, Bosetti C, Boccia S, Boffetta P
and La Vecchia C: Occupational exposures to polycyclic aromatic
hydrocarbons and respiratory and urinary tract cancers: An updated
systematic review and a meta-analysis to 2014. Arch Toxicol.
88:1479–1490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burstyn I, Boffetta P, Heederik D, et al:
Mortality from obstructive lung diseases and exposure to polycyclic
aromatic hydrocarbons among asphalt workers. Am J Epidemiol.
158:468–478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boffetta P, Burstyn I, Partanen T, et al:
Cancer mortality among European asphalt workers: An international
epidemiological study. I. Results of the analysis based on job
titles. Am J Ind Med. 43:18–27. 2003. View Article : Google Scholar
|
|
17
|
Iizasa T, Momiki S, Bauer B, Caamano J,
Metcalf R, Lechner J, Harris CC and Klein-Szanto AJ: Invasive
tumors derived from xenotransplanted, immortalized human cells
after in vivo exposure to chemical carcinogens. Carcinogenesis.
14:1789–1794. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Melchiori A, Colacci A, Lollini PL, De
Giovanni C, Carlone S, Grilli S, Parodi S and Albini A: Induction
of invasive and experimental metastasis potential in BALB/c 3T3
cells by benzo(a)pyrene transformation. Invasion Metastasis.
12:1–11. 1992.PubMed/NCBI
|
|
19
|
Ueng TH, Chang YL, Tsai YY, Su JL, Chan
PK, Shih JY, Lee YC, Ma YC and Kuo ML: Potential roles of
fibroblast growth factor-9 in the benzo(a)pyrene-induced invasion
in vitro and the metastasis of human lung adenocarcinoma. Arch
Toxicol. 84:651–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshino I, Kometani T, Shoji F, Osoegawa
A, Ohba T, Kouso H, Takenaka T, Yohena T and Maehara Y: Induction
of epithelial-mesenchymal transition-related genes by
benzo[a]-pyrene in lung cancer cells. Cancer. 110:369–374. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Zhai W, Wang H, Xia X and Zhang C:
Benzo(a)pyrene promotes A549 cell migration and invasion through
up-regulating Twist. Arch Toxicol. May 22–2014.(Epub ahead of
print).
|
|
22
|
Martos I, Ferreres F and Tomás-Barberán
FA: Identification of flavonoid markers for the botanical origin of
Eucalyptus honey. J Agric Food Chem. 48:1498–1502. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martos I, Ferreres F, Yao L, D’Arcy B,
Caffin N and Tomás-Barberán FA: Flavonoids in monospecific
eucalyptus honeys from Australia. J Agric Food Chem. 48:4744–4748.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao L, Jiang Y, D’Arcy B, Singanusong R,
Datta N, Caffin N and Raymont K: Quantitative high-performance
liquid chromatography analyses of flavonoids in Australian
Eucalyptus honeys. J Agric Food Chem. 52:210–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geraets L, Moonen HJ, Brauers K, Wouters
EF, Bast A and Hageman GJ: Dietary flavones and flavonoles are
inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial
cells. J Nutr. 137:2190–2195. 2007.PubMed/NCBI
|
|
26
|
Hsu YL, Uen YH, Chen Y, Liang HL and Kuo
PL: Tricetin, a dietary flavonoid, inhibits proliferation of human
breast adenocarcinoma mcf-7 cells by blocking cell cycle
progression and inducing apoptosis. J Agric Food Chem.
57:8688–8695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsu YL, Hou MF, Tsai EM and Kuo PL:
Tricetin, a dietary flavonoid, induces apoptosis through the
reactive oxygen species/c-Jun NH2-terminal kinase pathway in human
liver cancer cells. J Agric Food Chem. 58:12547–12556. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Casimiro S, Mohammad KS, Pires R, et al:
RANKL/RANK/MMP-1 molecular triad contributes to the metastatic
phenotype of breast and prostate cancer cells in vitro. PLoS One.
8:e631532013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krishnan V, Vogler EA, Sosnoski DM and
Mastro AM: In vitro mimics of bone remodeling and the vicious cycle
of cancer in bone. J Cell Physiol. 229:453–462. 2014. View Article : Google Scholar
|
|
30
|
Manenti G, Peissel B, Gariboldi M, et al:
A cancer modifier role for parathyroid hormone-related protein.
Oncogene. 19:5324–5328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu X, Liu T, Fang O, Leach LJ, Hu X and
Luo Z: miR-194 suppresses metastasis of non-small cell lung cancer
through regulating expression of BMP1 and p27(kip1). Oncogene.
33:1506–1514. 2014. View Article : Google Scholar
|
|
32
|
Hernández I, Moreno JL, Zandueta C,
Montuenga L and Lecanda F: Novel alternatively spliced ADAM8
isoforms contribute to the aggressive bone metastatic phenotype of
lung cancer. Oncogene. 29:3758–3769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sone S and Yano S: Molecular pathogenesis
and its therapeutic modalities of lung cancer metastasis to bone.
Cancer Metastasis Rev. 26:685–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coleman RE and McCloskey EV:
Bisphosphonates in oncology. Bone. 49:71–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hiraki A, Ueoka H, Bessho A, Segawa Y,
Takigawa N, Kiura K, Eguchi K, Yoneda T, Tanimoto M and Harada M:
Parathyroid hormone-related protein measured at the time of first
visit is an indicator of bone metastases and survival in lung
carcinoma patients with hypercalcemia. Cancer. 95:1706–1713. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu YL, Tsai EM, Hou MF, Wang TN, Hung JY
and Kuo PL: Obtusifolin suppresses phthalate esters-induced breast
cancer bone metastasis by targeting parathyroid hormone-related
protein. J Agric Food Chem. 62:11933–11940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen X and Falzon M: PTH-related protein
upregulates integrin alpha6beta4 expression and activates Akt in
breast cancer cells. Exp Cell Res. 312:3822–3834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kitazawa S and Kitazawa R: RANK ligand is
a prerequisite for cancer-associated osteolytic lesions. J Pathol.
198:228–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thomas RJ, Guise TA, Yin JJ, Elliott J,
Horwood NJ, Martin TJ and Gillespie MT: Breast cancer cells
interact with osteoblasts to support osteoclast formation.
Endocrinology. 140:4451–4458. 1999.PubMed/NCBI
|
|
41
|
Yasuda H, Shima N, Nakagawa N, et al: A
novel molecular mechanism modulating osteoclast differentiation and
function. Bone. 25:109–113. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jansen ID, Vermeer JA, Bloemen V, Stap J
and Everts V: Osteoclast fusion and fission. Calcif Tissue Int.
90:515–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lacey DL, Timms E, Tan HL, et al:
Osteoprotegerin ligand is a cytokine that regulates osteoclast
differentiation and activation. Cell. 93:165–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jun AY, Kim HJ, Park KK, Son KH, Lee DH,
Woo MH and Chung WY: Tetrahydrofurofuran-type lignans inhibit
breast cancer-mediated bone destruction by blocking the vicious
cycle between cancer cells, osteoblasts and osteoclasts. Invest New
Drugs. 32:1–13. 2014. View Article : Google Scholar
|
|
45
|
Bendre MS, Margulies AG, Walser B, et al:
Tumor-derived interleukin-8 stimulates osteolysis independent of
the receptor activator of nuclear factor-kappaB ligand pathway.
Cancer Res. 65:11001–11009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hsu YL, Hung JY, Ko YC, Hung CH, Huang MS
and Kuo PL: Phospholipase D signaling pathway is involved in lung
cancer-derived IL-8 increased osteoclastogenesis. Carcinogenesis.
31:587–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lewiecki EM: Safety of long-term
bisphosphonate therapy for the management of osteoporosis. Drugs.
71:791–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Papapetrou PD: Bisphosphonate-associated
adverse events. Hormones (Athens). 8:96–110. 2009. View Article : Google Scholar
|