Introduction
Pancreatic ductal adenocarcinoma is still an
unresolved therapeutic challenge with nearly similar incidence and
mortality rates. It is the most lethal type of digestive cancer
with an extremely poor prognosis with a 5-year survival rate of
less than 5%. Pancreatic ductal adenocarcinoma represents the
fourth commonest cause of cancer related deaths and its incidence
is rising in most countries (1).
The only potentially curative therapy for pancreatic cancer is
surgical resection. Unfortunately, only 20% of the patients have
resectable cancers at the time of the diagnosis. Even among those
patients who undergo resection, the 5-year survival rate is 10–25%
(2,3). Preclinical and epidemiologic studies
suggest inflammation as a central mediator of the neoplastic
process and a potential driver of pancreatic carcinogenesis
(4,5). Under-pinning this view, activation of
the central signaling module of innate immunity, NF-κB has been
linked to the progression of tumors (6,7); in
this line, tumor immunotherapies could involve strategies that
block activation of innate immune responses. On the other hand,
activation of innate immunity is achieved through stimulation of
pattern recognition receptors (PRRs) (8–10).
Amongst these, Toll-like receptors (TLRs) were the first group to
be identified. TLRs can be activated by a panel of
pathogen-associated molecular patterns (PAMPs) including cell-wall
components like lipopolysaccharide (LPS) as well as by microbial
DNA and RNA (11). Additionally,
damage-associated molecular patterns (DAMPs) which arise from
inflammation and cellular injury and can stimulate TLRs and
subsequently induce TLR signaling (12). Recently, enhanced expression of
TLRs has been described in a variety of different tumors (13). TLRs with their ligands induces
recruitment of the adapter molecule MyD88 (myeloid differentiation
primary response protein 88), leading to activation of the NF-κB
and MAPK-signaling pathways initiating the target products that
prevent cell death by expressing anti-apoptotic proteins such as
Bcl-2 and induce chronic inflammation by producing COX-2
(cyclooxygenase-2) (13,14). COX-2 together with TLR expression
plays a crucial role in transformation of normal cells to cancer
cells and in angiogenesis, reduced apoptosis and immunosuppression
of malignant tumors (15). Our
previous study indicated that endosomally expressed TLR7 and TLR8
are associated with tumor progression in colorectal cancer and
reduced tumor-specific survival amongst patients with high TLR7 and
TLR8 expression in colorectal cancer cells (13). In addition, some research results
suggest that enforcement of innate immunity by targeted TLR
activation has beneficial effects to combat tumor growth, like TLR7
agonist imiquimod, licensed for therapy of basal cell carcinoma.
Other synthetic TLR7 and TLR8 agonists such as resiquimod (R848)
have been developed. R848 is a selective ligand for murine TLR7 and
for TLR7 and TLR8 in humans (16,17).
In the present study, we analyzed the expression of
TLR7, TLR8, NF-κB and COX-2 in pancreatic cancer at different UICC
stages and compared with chronic pancreatitis and healthy controls.
To determine the functional role of TLR7 and TLR8 we generated TLR7
and TLR8 expressing human PANC1 cancer cells and analyzed the
effects of TLR7/8 agonists (R848, resiquimod) in the inflammatory
process on tumor cell proliferation and chemoresistance.
Materials and methods
Patients and human tissue
In a retrospective analysis, 48 out of 112 patients
with a mean age of 69±5.2 years and histologically confirmed
pancreatic cancer of the exocrine pancreas were evaluated in the
present study. We examined only consecutive patients from which
appropriate tumor material for further analysis (tumor border and
tumor center) was available in a period from 06/2003 to 05/2005 in
our Surgical Department approved by the local ethics committee.
Patients were followed up in our Comprehensive Cancer Center
(completeness index 0.96). The classification of pancreatic cancer
was asserted in criterion of the Union Internationale Contre le
Cancer (UICC) for determination of the tumor stage. Cancer
specimens were instantly acquired in liquid nitrogen and stored at
−80°C until analyzed. Tumors were evaluated for localization, tumor
stage, and their differentiation grade in our Institute of
Pathology.
In 69% (n=33/48) of the investigated cases, the
tumor was detected in the head of the pancreas, in the remaining
cases the cancer was diagnosed in the corpus or tail of the
pancreas (19%, n=9/48) or in the head and corpus/tail (2%, n=1/48).
We compared in a subanalysis tumor samples of UICC stage I/II
(n=12) and UICC stage III/IV (n=12) patients with specimens from
individuals operated on histologically confirmed chronic
pancreatitis (n=8) and normal tissue of healthy controls (n=8).
Paraffin sections (5 μm) were stained with haematoxylin and eosin
(H&E) to assess morphology and eosinophilic areas. To determine
eosinophilic areas suspicious for potential viral inclusion bodies
as causative for TLR7 and TLR8 expression within the tumor we
performed additional Phloxine-tartrazine staining.
Animals
Female Balb/c nude mice were purchased from Harlan
Laboratories (Rossdorf, Germany) and maintained under defined
conditions in accordance with institutional guidelines from the
University of Wuerzburg in Germany and the experiments were
performed according to approved experimental protocols. For in
vivo growth studies 2×106 transduced PANC1 cells
(TLR7+ PANC1, n=5; TLR8+ PANC1, n=5; empty
vector PANC1, n=4) were injected subcutaneously into both flanks of
recipient Balb/c nude mice. Mice were sacrificed (day 40) and the
tumor volume was determined (V=π/6 × a × b × c, where a is the
length, b is the width and c is the height).
Immunofluorescence and
immunohistochemistry
The TLR7 antibody was purchased from Imgenex Corp.,
(San Diego, CA, USA), the TLR8 antibody was provided by ProSci Inc.
(Poway, CA, USA). COX-2 antibody was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA) and CD34 antibody from Serotec
(Duesseldorf, Germany). Isotype control antibodies were purchased
by eBioscience (San Diego, CA, USA). Secondary antibodies were
Cy3-conjugated AffiniPure Donkey anti-rabbit IgG (Jackson
ImmunoResearch Laboratories Inc., Suffolk, UK) and Cy5-conjugated
AffiniPure Donkey anti-mouse IgG. The staining was performed on
serial cryostat sections of the snap-frozen specimens of pancreatic
cancers (UICC II and III) with neighbouring normal pancreas (tumor
border) and compared with sections from chronic pancreatitis and
normal pancreas. For nuclear counterstaining slides were treated
with DAPI (4′,6-Diamidino-2-phenylindoledihydrochlorid)
(Sigma-Aldrich, Steinheim, Germany) or haemalaun
(Sigma-Aldrich).
Western blot analysis
Proteins were extracted from tissue samples (250 μg)
using lysis buffer CytoBuster (Merck, Darmstadt, Germany) and
QIAshredder (Qiagen, Hilden, Germany). Normal tissue (protein
lysate) was purchased from BioChain Institute Inc. (Hayward, CA,
USA). Protein samples (50 μg) were resolved by SDS-PAGE and then
transferred to polyvinylidene difluoride (PVDF) membranes
(Invitrogen, Carlsbad, CA, USA). Blots were probed with antibodies
to TLR7 (ProSci), TLR8 (ProSci), β-actin (Santa Cruz Biotechnology)
and COX-2 (Santa Cruz Biotechnology and Novus Biologicals LLC,
Littleton, CO, USA). Anti-mouse IgG and anti-rabbit IgG secondary
antibodies were obtained from Amersham (Braunschweig, Germany) and
anti-goat IgG was purchased from Santa Cruz Biotechnology.
FACS analysis
Cells derived from normal pancreas, chronic
pancreatitis and pancreatic cancer tissues were analyzed on a flow
cytometer (Beckman Coulter, Krefeld, Germany) with a software
package (Coulter, Epics XL-MCL, System II). TLR7 antibody was
purchased from Imgenex, TLR8 was provided by ProSci. CD34-PE
antibody, FITC-conjugated anti-rabbit secondary antibody and
isotype control antibodies were purchased by Beckman Coulter. For
intracellular staining we used IntraPrep kit (Beckman Coulter).
Cell culture
The human pancreatic cancer cell line PANC1 was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) cultured in Dulbecco's modified Eagle's medium
with 10% fetal bovine serum, 1% G418 and 1% penicillin/streptomycin
and incubated in 5% CO2 at 37°C.
In contrast to tumor tissues from patients with
pancreatic cancer or from patients with pancreatitis tumor cell
lines express only very low levels of TLR7 and TLR8. For further
studies it was necessary to overexpress both receptors in those
cells. We chose PANC1, the most common established pancreatic cell
line. The lentiviral transduction of TLR7 and TLR8 PANC1 cells was
performed by Sirion Biotech GmbH (Martinsried, Germany). Cells were
then subjected to antibiotic selection of G418-resistant cells.
Quantitative real-time RT-PCR
Gene expression for TLR7 and TLR8 in pancreatic
cancer was determined using quantitative real-time PCR (RT-qPCR).
Human pancreatic matched cDNA for comparison was purchased from
Pharmingen (Heidelberg, Germany) and used as control. Gene
expression analyzed in pancreatic cancers was compared with normal
tissue of healthy controls (n=8), chronic pancreatitis (n=8). Total
cellular RNA was extracted using RNeasy Mini kit (Qiagen) according
to the manufacturer's instructions. Complementary DNA (cDNA) was
performed using the ImProm-II reverse transcriptase system
(Promega, Mannheim, Germany) and Eppendorf Mastercycler (Eppendorf,
Hamburg, Germany). TLR7 and TLR8 specific primer sets from Qiagen
were used. Housekeeping gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used for relative quantification. PCR
reactions were carried out with a DNA Engine Opticon 2 System (MJ
Research; Biozym, Oldendorf, Germany).
For the experiments performed with the human
pancreatic cancer cell line PANC1 gene quantification was performed
with TaqMan Gene Expression Master Mix (Life Technologies,
Carlsbad, CA, USA) and TaqMan Gene Expression Assays (Life
Technologies) according to the manufacturer's instructions.
Housekeeping genes β-actin, GAPDH, GUSB and HPRT1 were used for
relative quantification. For analysis of PANC1 cells all PCR
reactions were carried out with a Bio-Rad CFX96 Touch Real-Time PCR
detection system.
Reproducibility was confirmed by three independent
PCR runs. The relative quantification value, fold difference, is
expressed as 2−ΔΔCq.
Determination of the median lethal dose
(LD50) for 5-fluorouracil
Empty vector PANC1 cells were cultured at a
concentration of 5×103 cells/well in 96-well plates. The
cells were incubated for 48 h with 5-fluorouracil (5-FU, working
concentration, 10–10,000 μmol/l; Medac, Wedel, Germany). After
medium change and further 24 h at 37°C in 5% CO2
CellTiter 96 AQueous One Solution Cell Proliferation Assay
(Promega) was performed according to the manufacturer's
instructions. The median lethal dose LD50 was defined as
amount of drug resulting in 50% killing within 2 days.
Proliferation and resistance to
chemotherapy assay
To investigate the effect of stimulation with R848
on tumor cell proliferation 2×106 PANC1 cells were
seeded in cell culture flasks, pre-incubated for 24 h following
daily stimulation with 10 μg/ml R848 (InvivoGen, San Diego, CA,
USA) for 3 days. Afterwards cells were detached and seeded 6,000
cells/well in 96-well plates. After additional incubation time of
24 and 72 h cell proliferation assay was performed as described
above.
Then, we analyzed the effect of previous stimulation
with R848 on the chemosensitivity of transduced PANC1 cells. Four
thousand cells/well were seeded in 96-well plates, pre-incubated
for 48 h and then treated with R848 (10 μg/ml). After an additional
incubation of 48 h cells were treated with 500 μmol/l 5-FU and
after another 48-h proliferation assay was performed as described
above.
Statistical analysis
Results were expressed as mean ± SEM in groups of
patients with normal pancreatic tissue, chronic pancreatitis and
pancreatic cancer. Comparisons were performed by ANOVA or paired
and unpaired t-test when appropriate. Bonferroni's correction for
multiple comparisons was used to determine the level of
significance; P<0.05 was considered significant.
Results
TLR7 and TLR8 are expressed in pancreatic
cancer
TLR7 and TLR8 expression in pancreatic cancer,
chronic pancreatitis, and normal pancreatic tissue was analyzed by
immunohistochemistry in pancreatic tissue from patients with
pancreatic cancer (UICC II and UICC III, n=48), chronic
pancreatitis (n=8) and in normal pancreas (n=8). In general, TLR7
expression of pancreatic cells in all analyzed subjects with
pancreatic cancer and with chronic pancreatitis was more intense
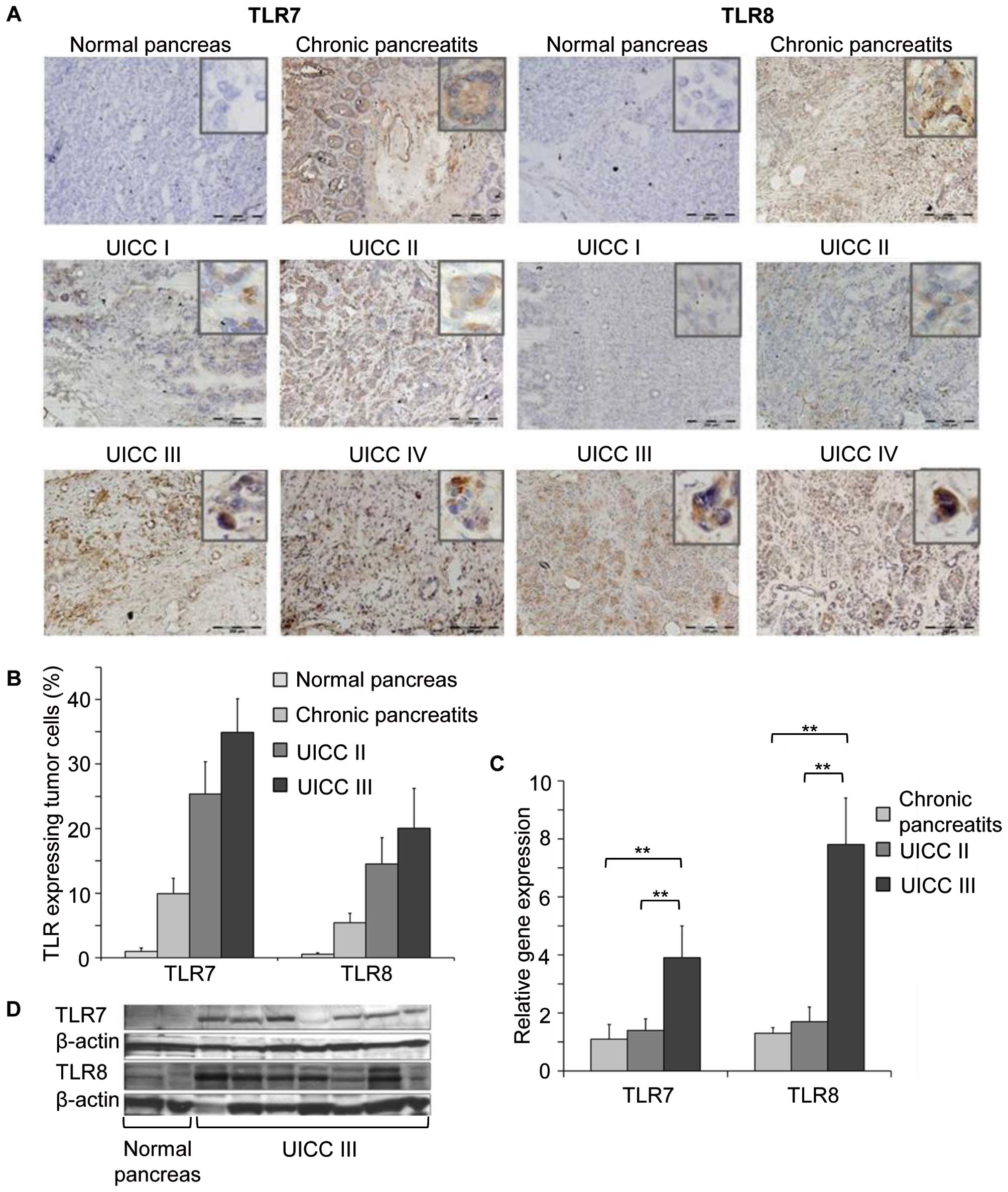
than TLR8. Fig. 1A shows examples
of positive TLR7 and TLR8 tumor cell expression in pancreatic
cancer of different stages and chronic pancreatitis. In contrast,
no or only occasionally low TLR7 or TLR8 expression was detected in
normal pancreatic cells (Fig. 1A),
an observation that we believe to be novel. Quantification of TLR
expressing cells also demonstrated strong expression of TLR7 or
TLR8 in pancreatic cells from patients with chronic pancreatitis
and pancreatic cancer, compared to no, or occasionally low
expression in normal pancreatic tissue (Fig. 1B). Notably, similar results were
also observed by western blot analysis and gene analysis of the
tumor tissues. Significant TLR7 and TLR8 protein and gene
expression was observed in tissues from patients with pancreatic
cancer (UICC III) compared with normal pancreatic tissue (Fig. 1C and D, respectively). These
observations indicated inflammation within the tumor, which could
be mediated not only through infiltrating inflammatory cells but
also through TLR7 and TLR8 expression of pancreatic cancer
cells.
Furthermore, we analyzed TLR7 and TLR8 in
dissociated cells derived from the same patient tissues together
with CD34, a marker for endothelial cells and known to be expressed
by cancer cells with neoangiogenetic potential, by FACS and
immunohistochemical analysis (cytospins). Indeed TLR7, TLR8 and
CD34 were positively expressed in pancreatic cancer and pancreatic
cells from chronic pancreatitis cells (Figs. 2B and C and 3A), but not or at very low levels in
normal pancreatic cells (Figs. 2A
and 3A). Comparison of the
cellular co-localization of TLR7 or TLR8 with CD34 analyzed by
immunofluorescence double staining revealed increased coexpression
of TLR7 or TLR8 with CD34 in tumor cells (Fig. 3B), indicating that those cells were
indeed cancer cells expressing the angiogenic surface molecule.
COX-2 is expressed in pancreatic cancer
cells
To analyze whether inflammation in pancreatic cancer
was associated with TLR7 and TLR8 expressing cancer cells, we
dissected the expression of COX-2 in the pancreatic tumor cells by
immunohistochemical staining and western blot analysis. Increased
COX-2 expression together with TLR7 and TLR8 positivity in
pancreatic cancer cells was detected (Fig. 4A, top and below right, and B,
respectively). No positivity was observed in normal pancreatic
cells (Fig. 4A, top and below
left, and B, respectively). These data demonstrate inflammation in
pancreatic cancer in association with TLR7 and TLR8 expressing
cancer cells.
TLR7 and TLR8 are expressed by human
pancreatic cancer cell lines
We characterized the expression of TLR7 and TLR8 in
several purchased human pancreatic cancer cell lines. In contrast
to tumor cells derived from our patients with pancreatic cancer,
acquired tumor cell lines expressed only very low levels of TLR7
and TLR8. This may be due to artificial, non-inflammatory culture
conditions of the cell lines. Therefore, for further in
vitro studies both receptors were successfully transduced in
the most common pancreatic cell line, PANC1, using a
Lentivirus-mediated stable gene expression as described in
Materials and methods. As controls, PANC1 cells transduced with
empty vector construct as well as peripheral blood mono-nuclear
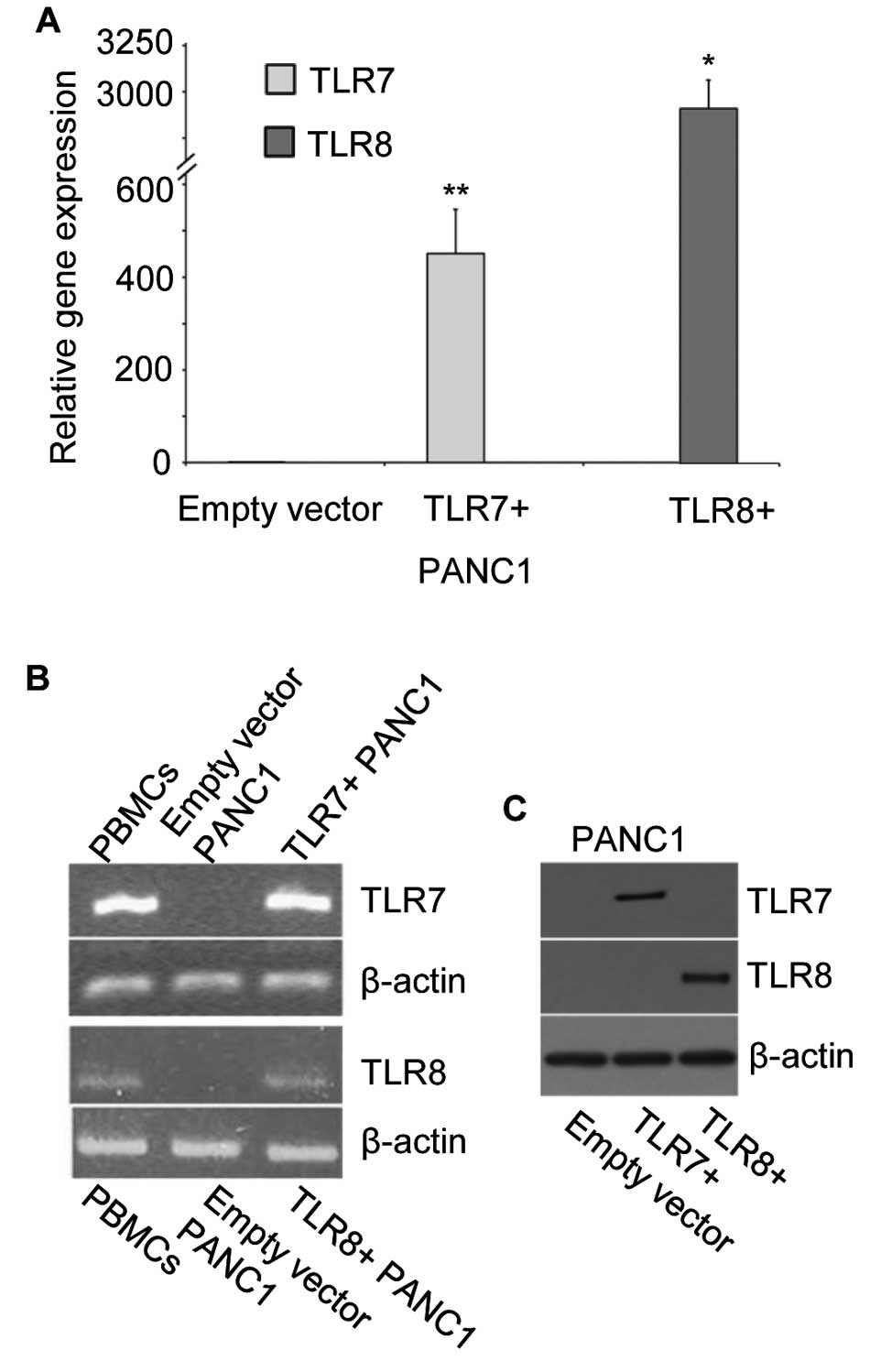
cells (PBMCs), were used. Indeed, increased gene expression of TLR7
and TLR8 was observed in the transduced PANC1 cells
(TLR7+ and TLR8+ PANC1 cells) by qRT-PCR and
following agarose gel electrophoresis (Fig. 5A and B). In Fig. 5C successful protein expression of
TLR7 or TLR8 by transduced PANC1 cells was demonstrated by western
blot analysis.
TLR7 and TLR8 expression increases tumor
growth in Balb/c nude mice
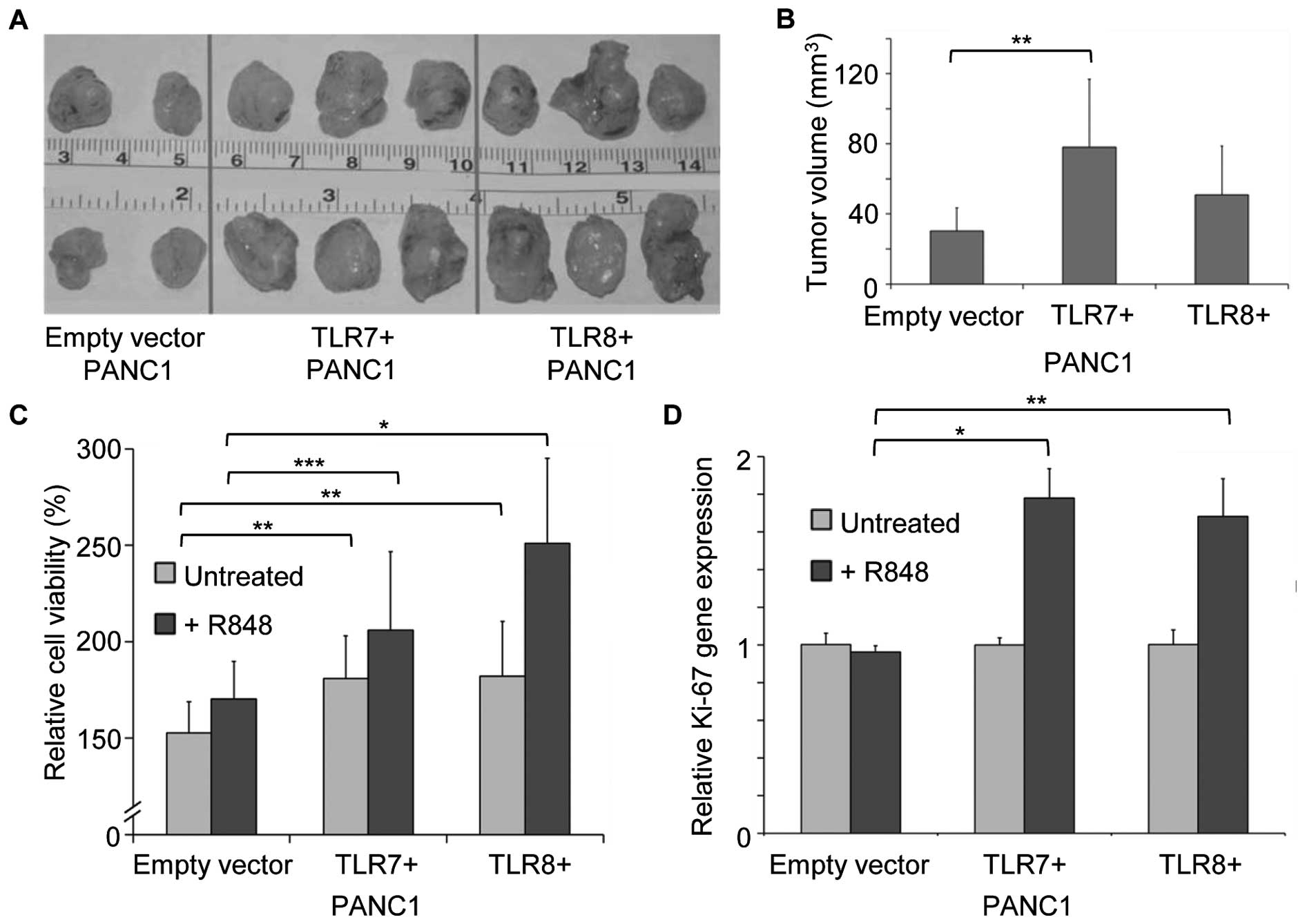
Tumor xenograft growth of TLR7 and TLR8 transduced
human PANC1 cancer cells in Balb/c nude mice was examined. Tumor
growth in vivo was found to be enhanced when compared to
controls with empty vector PANC1 cells (Fig. 6A; TLR7+ and
TLR8+, each n=5 vs. empty vector, n=4). Determination of
the tumor growth showed a significant increase in tumor volume of
TLR7+ PANC1 pancreatic tumors in contrast to empty
vector PANC1 tumors (Fig. 6B;
P<0.005).
TLR7 and TLR8 expression and stimulation
induces proliferation of PANC1 cells
The promoting effect of TLR7 and TLR8 expression on
PANC1 cancer cell proliferation was analyzed using MTS
proliferation assays. Untreated TLR7+ and
TLR8+ PANC1 cells showed significantly increased tumor
cell proliferation when compared to controls at 72 h after seeding
(Fig. 6C; TLR7, 181% and TLR8,
182% vs. empty vector, 153%; P<0.002 and P<0.005).
We examined whether TLR7 and TLR8 stimulation with
the agonist R848 further increases proliferation of
TLR7+ and TLR8+ PANC1 cancer cells.
Stimulation with the TLR7/TLR8 ligand R848 induced a relative
increase in proliferation in TLR7+ and TLR8+
in PANC1 cancer cells compared to empty vector treated PANC1 cells
(Fig. 6C; TLR7+, 206%
and TLR8, 251% vs. empty vector, 170%; P<0.02 and P<0.0001).
Gene expression of the proliferation marker Ki-67 in R848 treated
TLR7+ and TLR8+ PANC1 cancer cells confirmed
these proliferative effects. (Fig.
6D; P<0.0001 and P<0.0005).
TLR7 or TLR8 stimulation of human PANC1
cells induces gene expression of NF-κB and COX-2
To determine whether TLR7 and TLR8 stimulation
activates intracellular signaling pathways and the synthesis of
proinflammatory cytokines, we analyzed gene expression levels of
NF-κB and COX-2 in response to stimulation of TLR7+ and
TLR8+ PANC1 cells with R848. Stimulation with R848 for 6
h induced an ~4-fold increase in gene expression levels of NF-κB in
TLR7+ and TLR8+ PANC1 cancer cells compared
with untreated cells (Fig. 7A and
B; P<0.0001). Seventy-two hours after stimulation with R848
NF-κB expression returned to background levels in both
TLR7+ and TLR8+ PANC1 cancer cells.
Additionally, stimulation with R848 induced an ~60-fold increased
gene expression of COX-2 in TLR7+ PANC1 cancer cells (12
h after stimulation, Fig. 7C) and
an ~34-fold increased level in TLR8+ PANC1 cells (24 h
after stimulation, Fig. 7D)
compared with untreated cells (Fig. 7C
and D; P<0.005 and 0.0001). Even 72 h post-stimulation COX-2
expression levels remained significantly elevated in stimulated
TLR7+ and TLR8+ cancer cells in comparison to
untreated cancer cells.
TLR7 or TLR8 stimulation induces
chemoresistance in PANC1 cells
To analyze the influence on chemoresistance in R848
stimulated and non-stimulated TLR7+ and TLR8+
PANC1 cancer cells 5-fluorouracil was used. 5-FU is amongst other
chemotherapeutics used as treatment for pancreatic cancer (18) and thus herein used as
representative chemotherapeutic agent. We first determined the
LD50 concentration for 5-FU (500 μmol/l) using
non-stimulated empty vector PANC1 cells in MTS assays (Fig. 8A).
To investigate the effects of induced TLR7 and TLR8
expression in PANC1 cancer cells on chemosensitivity transduced
tumor cells were treated with two different concentrations of 5-FU
(100 and 1000 μmol/l) as approximated concentrations for
LD50. For both concentrations increased cell viability
of TLR7+ and TLR8+ PANC1 cancer cells was
demonstrated when compared to empty vector PANC1 cells, pointing to
an increased chemoresistance in the cells. At a concentration of
100 μmol/l of 5-FU relative cell viability of TLR7+ and
TLR8+ PANC1 tumor cells was less reduced when compared
with empty vector PANC1 tumor cells (Fig. 8B; 62 and 73% vs. 58% for empty
vector cells; P<0.05 and P<0.0001). This effect was confirmed
at a concentration of 1000 μmol/l of 5-FU (TLR7+ and
TLR8+ cells, 49 and 56% vs. 46% in empty vector cells
(Fig. 8B; P<0.05 and
P<0.0001).
Stimulation of TLR7+ and TLR8+
PANC1 cancer cells for 48 h with the agonist R848 prior to
treatment with 500 μmol/l of 5-FU (LD50 for empty vector
PANC1 cells) increased cell viability of TLR7+ and
TLR8+ cells in contrast to empty vector PANC1 cells
(Fig. 8C; TLR7+, 75%
and TLR8+, 81% vs. empty vector PANC1 cells, 52%; both
P<0.0001).
Discussion
We previously reported that TLR7 and TLR8 expression
is upregulated in tumor cells of patients with colorectal cancer.
Interestingly, this expression was related to cancer cells but
rarely detected in stromal-tumor-infiltrating leukocytes. Moreover,
our results indicated that both TLR7 and TLR8 expression is
associated with tumor progression in patients with colorectal
cancer and reduced tumor-specific survival among patients with high
TLR7 and TLR8 expression in their cancer cells (13).
In the present study, we demonstrated that TLR7 and
TLR8 expression are highly expressed by primary human ductal
pancreatic cancer. We showed that stimulation of both receptors
TLR7 and TLR8 in pancreatic cancer cells results in increased tumor
cell proliferation and reduced chemosensitivity.
To analyze the impact of the intracellular TLR7 and
TLR8 expression in mediating inflammation in pancreatic cancer
cells we first examined in the present study their expression in
human tissues from primary pancreatic cancers. We observed that
tumor cells in pancreatic cancer strongly expressed stage-dependent
TLR7 and TLR8. This was intensified when compared to pancreatic
cells in chronic pancreatitis. Whether intracellular TLR7 and TLR8
expression, known to be associated with single stranded RNA (virus)
infection in this context may be associated with recognition of
pathogenic viruses in the investigated human pancreatic cancers
remains speculative. In our investigated human pancreatic cancers
we did not find any evidence from medical records or from virus
genome analysis. These data suggest that inflammation within the
tumor tissues could be mediated through TLR7 and TLR8 expressing
pancreatic cancer cells. Thus, intracellular TLR7 and TLR8
signaling pathways in TLR7+ and TLR8+
expressing pancreatic cancer cells may have the potential to
sustain cancer progression. CD34 is a known marker for endothelial
cells and is expressed by cancer cells with neoangiogenetic
potential. Cell morphology of cancer cells and positive staining
for CD34 indicated that cells expressing TLR7 and TLR8 were indeed
cancer cells.
Invasion and angiogenesis of gastric cancer cells
was described to be mediated by cyclooxygenase-2 (COX-2) after TLR2
and TLR9 activation, leading to inflammation and cancer progression
(19). Moreover, increased COX-2
expression in human pancreatic carcinomas supports the suggestion
that these tumors share common features of chronic inflammatory
processes in parallel to all essential features of carcinogenesis
(mutagenesis, mitogenesis, angiogenesis, reduced apoptosis,
metastasis and immunosuppression). All these events are linked to
COX-2-driven prostaglandin (PGE-2) biosynthesis (20–22).
TLR8 signaling was recently described to strongly promote
inflammatory lipid mediator biosynthesis PGE2 and thromboxane A2
(TXA2) through the COX-2 pathway. These data provide novel insights
into the innate immune response to viral infections and raise the
possibility that the immune response to single-stranded RNA viruses
via the TLR8 pathway may implicate the lipid mediators of
inflammation (23).
Notably, COX-2 expression was indeed upregulated in
our investigated patient tumors and was associated with TLR7 and
TLR8 positivity in specimens of pancreatic cancer and after
stimulation of human PANC1 cancer cells. These data clearly
indicate that inflammation in pancreatic cancer is associated
stage-dependently with upregulated TLR7 and TLR8 expression in the
cancer cells. Moreover, TLR7 and TLR8 stimulation in human PANC1
cancer cells led to the release of inflammatory mediators, mainly
through the activation of the NF-κB pathway. It is known, that
pancreatic carcinogenesis is attributed to the deregulated
expression of many signaling elements, such as NF-κB. This
signaling pathway leads to activation of mitotic and survival
pathways (Bcl-2, bcl-XL), as it was described for EGF-EGFR
signaling (24). In our so far
unpublished preliminary data studying inflammatory cells and tumor
cells within the tumor microenvironment in pancreatic cancer
resulted from SABiosciences RT2 pathway array analysis, we observed
strong regulation of several genes. This includes Bcl-2 in PANC1
pancreatic cancer cells stimulated with an agonist for TLR7 and
TLR8. We also found in response to TLR7 and TLR8 stimulation an
upregulation of several genes involved in angiogenesis as well as
proinflammatory cytokines such as IL-8 and IL-12. Further studies
are needed to confirm these first data.
Chemotherapy is a conventional regimen for
unresectable cases of pancreatic cancer. However, treatment with
chemotherapy drugs, like 5-FU or gemcitabine, merely results in a
median survival of 5.65 months and 1-year survival rate of 18%
(25). The main reason for
chemotherapy failure lies in the intrinsic and acquired
chemoresistance of pancreatic cancer cells (26). Recent data pointed to the role of
the Notch-2 receptor in the increasing of chemoresistance in the
pancreatic cancer (27). TLR7 and
TLR8 seems to stimulate the expression of Notch-2 receptor
(28). It seems that there is a
link between TLR7 and TLR8 expression and the activation of Notch.
Notably, stimulation of TLR7 and TLR8 in the present study also
resulted in a more robust chemoresistance in PANC1 cancer cells
against 5-fluorouracil. Further studies must be performed to
confirm our hypothesis.
To date, several agonists have been characterized as
TLR7 and/or TLR8 ligands. Resiquimod (R848) exerts its
immunostimulatory activities via activation of mouse TLR7 and human
TLR7 and TLR8 (29,30). The agonist R848 has now also been
tested as an immune response modifier in preclinical models and in
clinical trials (31,32). It has been shown that TLR agonists
can promote cancer cell survival and migration and tumor
progression. For example, TLR agonists have been shown to increase
tumor viability and metastasis of human lung cancer cells (10), proliferation in human myeloma cells
(TLR3) (33), adhesion and
metastasis in human colorectal cancer cells (TLR4) (34), and migration in human gliobastoma
(TLR4) or human breast cancer cells (TLR2) (35). We hypothesized that these
contradictory results are due to the complex nature of the tumor
microenvironment. Interestingly, in the present study in pancreatic
cancer we observed that TLR7 or TLR8 stimulation increased tumor
cell survival and resistance to the chemotherapeutic substance
5-fluorouracil. Further studies are necessary to dissect which
cells and pathways are involved in these effects.
We conclude that inflammation-mediated progression,
tumor survival, metastatic potential and mediation of
chemoresistance are closely associated with TLR7 and TLR8
expressing pancreatic cancer cells. Therefore, targeting of TLR
signaling might be a potential mechanism to reduce chemoresistance,
tumor surveillance and COX-2 induced carcinogenesis. However, the
direct effects of immune response modifiers on tumor cells include
induction of apoptosis and sensitization to killing mediated by
chemotherapeutic agents. On the other hand, TLR activation can be
advantageous for the proliferation, invasiveness, and/or survival
of tumor cells. These effects of TLR7 and TLR8 agonists on tumor
cells depend on the tumor cell type, and need to be carefully taken
into account in preclinical studies.
Acknowledgements
We thank Mrs. Sabine Mueller-Morath, Mrs. Nadine
Gutermuth and Mrs. Mariola Dragan for their excellent technical
assistance as well as Mrs. Ingrid Strauss and Mrs. Dipl.-Ueb.
Ulrike Faber. The present study was support by the Deutsche
Bundesstiftung Umwelt grant (no. DBU 16011) for Scientific
Research, Germany.
Abbreviations:
|
DAMPs
|
damage-associated molecular
patterns
|
|
COX-2
|
cyclooxygenase-2
|
|
NF-κB
|
nuclear factor
kappa-light-chain-enhancer of activated B cells
|
|
PAMPs
|
pathogen-associated molecular
patterns
|
|
PRR
|
pattern recognition receptor
|
|
UICC
|
Union International Contre le
Cancer
|
|
TLR
|
Toll-like receptor
|
|
5-FU
|
5-fluorouracil
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saif MW: Controversies in the adjuvant
treatment of pancreatic adenocarcinoma. JOP. 8:545–552.
2007.PubMed/NCBI
|
|
3
|
Saif MW: Pancreatic neoplasm in 2011: An
update. JOP. 12:316–321. 2011.PubMed/NCBI
|
|
4
|
Balkwill F and Coussens LM: Cancer: An
inflammatory link. Nature. 431:405–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greten FR and Karin M: The IKK/NF-kappaB
activation pathway-a target for prevention and treatment of cancer.
Cancer Lett. 206:193–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ketloy C, Engering A, Srichairatanakul U,
Limsalakpetch A, Yongvanitchit K, Pichyangkul S and Ruxrungtham K:
Expression and function of Toll-like receptors on dendritic cells
and other antigen presenting cells from non-human primates. Vet
Immunol Immunopathol. 125:18–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ochi A, Graffeo CS, Zambirinis CP, Rehman
A, Hackman M, Fallon N, Barilla RM, Henning JR, Jamal M, Rao R, et
al: Toll-like receptor 7 regulates pancreatic carcinogenesis in
mice and humans. J Clin Invest. 122:4118–4129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cherfils-Vicini J, Platonova S, Gillard M,
Laurans L, Validire P, Caliandro R, Magdeleinat P, Mami-Chouaib F,
Dieu-Nosjean MC, Fridman WH, et al: Triggering of TLR7 and TLR8
expressed by human lung cancer cells induces cell survival and
chemoresistance. J Clin Invest. 120:1285–1297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janeway CA Jr: Approaching the asymptote?
Evolution and revolution in immunology. Cold Spring Harb Symp Quant
Biol. 54:1–13. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rubartelli A and Lotze MT: Inside,
outside, upside down: Damage-associated molecular-pattern molecules
(DAMPs) and redox. Trends Immunol. 28:429–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grimm M, Kim M, Rosenwald A, Heemann U,
Germer CT, Waaga-Gasser AM and Gasser M: Toll-like receptor (TLR) 7
and TLR8 expression on CD133+ cells in colorectal cancer
points to a specific role for inflammation-induced TLRs in
tumourigenesis and tumour progression. Eur J Cancer. 46:2849–2857.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bowie A and O'Neill LA: The interleukin-1
receptor/Toll-like receptor superfamily: Signal generators for
pro-inflammatory interleukins and microbial products. J Leukoc
Biol. 67:508–514. 2000.PubMed/NCBI
|
|
15
|
Harris RE: Cyclooxygenase-2 (cox-2) and
the inflammogenesis of cancer. Subcell Biochem. 42:93–126. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bong AB, Bonnekoh B, Franke I, Schön M,
Ulrich J and Gollnick H: Imiquimod, a topical immune response
modifier, in the treatment of cutaneous metastases of malignant
melanoma. Dermatology. 205:135–138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunne A, Marshall NA and Mills KH: TLR
based therapeutics. Curr Opin Pharmacol. 11:404–411. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al; Groupe Tumeurs Digestives of Unicancer;
PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang YJ, Wu MS, Lin JT and Chen CC:
Helicobacter pylori-induced invasion and angiogenesis of gastric
cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9
and promoter regulation. J Immunol. 175:8242–8252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Moraes E, Dar NA, de Moura Gallo CV and
Hainaut P: Crosstalks between cyclooxygenase-2 and tumor suppressor
protein p53: Balancing life and death during inflammatory stress
and carcinogenesis. Int J Cancer. 121:929–937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dvorak HF: Angiogenesis: Update 2005. J
Thromb Haemost. 3:1835–1842. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yip-Schneider MT, Barnard DS, Billings SD,
Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS
and Sweeney CJ: Cyclooxygenase-2 expression in human pancreatic
adenocarcinomas. Carcinogenesis. 21:139–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hattermann K, Picard S, Borgeat M, Leclerc
P, Pouliot M and Borgeat P: The Toll-like receptor 7/8-ligand
resiquimod (R-848) primes human neutrophils for leukotriene B4,
prostaglandin E2 and platelet-activating factor biosynthesis. FASEB
J. 21:1575–1585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meteoglu I, Erdogdu IH, Meydan N, Erkus M
and Barutca S: NF-KappaB expression correlates with apoptosis and
angiogenesis in clear cell renal cell carcinoma tissues. J Exp Clin
Cancer Res. 27:532008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997.PubMed/NCBI
|
|
26
|
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi
AS, Kong D and Sarkar FH: Pancreatic cancer: Understanding and
overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 8:27–33.
2011. View Article : Google Scholar
|
|
27
|
Güngör C, Zander H, Effenberger KE,
Vashist YK, Kalinina T, Izbicki JR, Yekebas E and Bockhorn M: Notch
signaling activated by replication stress-induced expression of
midkine drives epithelial-mesenchymal transition and
chemoresistance in pancreatic cancer. Cancer Res. 71:5009–5019.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji
JD, Tateya T, Kang YJ, Han J, Gessler M, et al: Integrated
regulation of Toll-like receptor responses by Notch and
interferon-gamma pathways. Immunity. 29:691–703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hemmi H, Noike M, Nakayama T and Nishino
T: Change of product specificity of hexaprenyl diphosphate synthase
from Sulfolobus solfataricus by introducing mimetic mutations.
Biochem Biophys Res Commun. 297:1096–1101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jurk M, Heil F, Vollmer J, Schetter C,
Krieg AM, Wagner H, Lipford G and Bauer S: Human TLR7 or TLR8
independently confer responsiveness to the antiviral compound
R-848. Nat Immunol. 3:4992002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scheel B, Aulwurm S, Probst J, Stitz L,
Hoerr I, Rammensee HG, Weller M and Pascolo S: Therapeutic
anti-tumor immunity triggered by injections of immunostimulating
single-stranded RNA. Eur J Immunol. 36:2807–2816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sauder DN, Smith MH, Senta-McMillian T,
Soria I and Meng TC: Randomized, single-blind, placebo-controlled
study of topical application of the immune response modulator
resiquimod in healthy adults. Antimicrob Agents Chemother.
47:3846–3852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiron D, Pellat-Deceunynck C, Amiot M,
Bataille R and Jego G: TLR3 ligand induces NF-{kappa}B activation
and various fates of multiple myeloma cells depending on
IFN-{alpha} production. J Immunol. 182:4471–4478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsu RY, Chan CH, Spicer JD, Rousseau MC,
Giannias B, Rousseau S and Ferri LE: LPS-induced TLR4 signaling in
human colorectal cancer cells increases beta1 integrin-mediated
cell adhesion and liver metastasis. Cancer Res. 71:1989–1998. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thuringer D, Hammann A, Benikhlef N,
Fourmaux E, Bouchot A, Wettstein G, Solary E and Garrido C:
Transactivation of the epidermal growth factor receptor by heat
shock protein 90 via Toll-like receptor 4 contributes to the
migration of glioblastoma cells. J Biol Chem. 286:3418–3428. 2011.
View Article : Google Scholar :
|