Gastric cancer refers to a group of heterogeneous
malignant tumors arising in any part of the stomach, with the
potential to spread through the blood/lymph vessels to other
tissues of the body. Gastric carcinoma continues to be one of the
most predominant forms of cancer worldwide (1). Approximately 952,000 new cases of
gastric cancer were reported in the year 2012, accounting for 6.8%
of all cancers, and thus, making it the fifth most common cancer in
the world (2). The incidence rates
of gastric cancer are particularly high in East Asia, South America
and East Europe, whereas lowest incidence rates prevail in North
America, Africa and Eastern Mediterranean region (2). These regional differences reflect
variations in dietary patterns and food habits, as well as the
prevalence of Helicobacter pylori infection (3). Furthermore, gastric cancer accounted
for 723,000 deaths in 2012, making it the third most common cause
of death in both genders (4).
Although the incidence of gastric carcinoma is declining, the
mortality rates are alarming and the need of the hour is to develop
specific and efficacious treatment options in the effort to combat
this dreaded disease.
The stomach is a part of the alimentary tract, which
aids in the digestion of food by secreting hydrochloric acid and
enzymes. Histologically, the stomach contains four distinct layers
with the innermost layer (mucosa) containing the gastric glands
(5). The most common form of
gastric cancer (90–95%) is adenocarcinoma, which arises from the
glandular epithelium of the innermost lining of the stomach
(6). Less common forms of gastric
cancer include lymphoma of the stomach, gastrointestinal stromal
tumor and gastrointestinal carcinoid tumor. Approximately 4% of all
gastric cancer cases are of the lymphoma type arising from immune
cells found in the wall of the stomach (7,8). On
the other hand, gastrointestinal stromal tumor is a rare benign or
malignant tumor arising in the connective tissue of the stomach
(8). Gastrointestinal carcinoid
tumor is a neuroendocrine tumor of hormone-forming cells in the
stomach, accounting for around 3% of all gastric cancers (9).
Under the Lauren classification, gastric carcinoma
can manifest as two main histologic forms, intestinal type or
diffuse type (10–12). The intestinal type of gastric
carcinoma is well differentiated with cells arranged in
glandular/tubular structures, whereas the diffuse type comprises of
scattered poorly cohesive cells with little or no gland formation.
In certain cases, the stomach tumor may exhibit features of both
cancer types (10). The Ming
system of classification of gastric carcinomas is based on the
growth pattern of neoplastic cells, and categorizes tumors into
expanding type or infiltrative type (13,14).
More recently, the WHO system of classification which has also been
adopted, grades adenocarcinomas on the basis of the extent of
metaplastic intestinal tissue. Under this classification system,
five subtypes namely adenocarcinoma (intestinal and diffuse),
papillary, tubular, mucinous and signet-ring cell are identified
(6).
Since gastric cancers grow slowly, the initial
cancer stages are asymptomatic and generally remain undetected
(15). However, the extent of the
tumor as well as outcome of disease is determined by the initial
cancer-causing event. There are numerous risk factors associated
with gastric cancer, ranging from Helicobacter pylori (H.
pylori) infection to age, gender, geographical location and
other lifestyle factors (16).
Infection by H. pylori is the primary risk factor for
gastric cancers, specifically for those arising from the distal
portion of the stomach. H. pylori promotes gastric
inflammation, clinically known as chronic atrophic gastritis, which
subsequently leads to cancerous alterations to the lining of the
stomach (17).
Gastric carcinoma occurs more commonly in older
people, primarily above the age of 60. A combination of aging with
H. pylori infection may have an additive role in the
promotion of gastric carcinoma, and people having been exposed to
H. pylori infection in childhood are at a high risk of
developing adult gastric cancer (18,19).
Concurrently, studies have shown that men are at a higher risk of
developing gastric carcinoma as compared to women; particularly for
H. pylori-induced gastric cancer, due to lifestyle
disparities as well as estrogen-mediated biological differences
(7,20). Geographical features also dictate
incidence rates, with higher number of cases seen in East Asia,
Eastern Europe and parts of South and Central America and lower
rates seen in Northern and Western Africa, South Central Asia, and
North America (21).
Histologically, the diffuse type of gastric adenocarcinoma is
uniformly distributed amongst populations while the intestinal type
of gastric cancer is predominantly found in the high risk
geographical regions (11).
Interestingly, there is also a variation seen in the incidence
rates between different ethnic groups living in the same region
(22).
Furthermore, a low socioeconomic status has been
associated with a high risk of developing gastric cancer (23). Diets including high intake of salt
and preserved or pickled food also increase the chances of a person
developing gastric cancer, and studies have shown that eating fresh
vegetables and fruits may help lower these risk rates (19,24).
Lifestyle factors including smoking has also been associated with
gastric cancers, particularly of those arising in the upper portion
of the stomach (7). An overview of
gastric carcinogenesis is illustrated in Fig. 1.
In addition to the risk factors, studies on the
genomic landscape of gastric adenocarcinoma have revealed that
certain genetic alterations and/or mutations can contribute to the
pathogenesis of gastric cancer (25). Advancement in molecular profiling
technologies have enabled rapid whole genome sequencing, with
better resolution that provides researchers the opportunity to scan
for known genomic mutations, as well as identify novel genomic
aberrations specific to different subsets of gastric carcinoma
patients (26–28).
A high throughput screen has been conducted using
237 gastric adenocarcinoma patient tissues that led to the
identification of 474 ‘hotspot’ mutations in 41 genes (29). Specifically, it was found that 34
(14.4%) of 237 gastric cancer patients harbour mutations in
PlK3CA (5.1%), TP53 (4.6%), APC (2.5%),
STK11 (2.1%), CTNNB1 (1.7%) and CDKN2A (0.8%)
(29). Another study using 15
adenocarcinoma patient tissues along with matched normal tissues
has identified somatic mutations in TP53 (11/15),
PIK3CA (3/15) and ARID1A (3/15) (30). Dulak et al also carried out
genomic profiling analysis of 486 gastrointestinal cancers,
including 296 esophageal and gastric cancers, and identified 64
regions of recurrent amplified/deleted somatic mutations (33). Amplified genes that were identified
included kinases such as ERBB2, FGFR1, FGFR2, EGFR, and
MET, as well as several novel genes not previously known to
be associated with carcinogenesis were recognized. All these
studies provided a foundation for the development of novel
treatment options, specifically targeting genes with ‘hotspot’
mutations in gastric cancer. Since targeted therapy offers several
advantages over the traditionally available treatment alternatives,
many researchers are currently working on translating these genomic
findings to clinical outcomes (Fig.
2).
Molecular profiling has shown that the most
frequently mutated genes in gastric cancer patients are the members
of epidermal growth factor receptor family (ErbB1-4). Of these,
overexpressed ErbB1 has been implicated in 27–64% of gastric cancer
patients (31–33). However, clinical trials where
cetruximab, a monoclonal anti-EGFR antibody, was used along with
capecitabine-cisplatin to treat patients with advanced gastric
cancer have failed to demonstrate a significant improvement
(34). On the other hand, 6–34% of
gastric cancer patients showed increased levels of ErbB2/HER2,
primarily encompassing those belonging to the intestinal type of
tumor (35,36). In the ToGA trial, the inclusion of
trastuzumab (an anti-HER2 monoclonal antibody) to traditional
chemotherapy treatment showed a slight increase in overall survival
(OS) from 11.1 to 13.8 months in patients with HER2 amplified
gastric adenocarcinomas; therefore, providing the potential of
molecular targeted therapy in the treatment of gastric cancer
(37). Overexpression of ErbB3 has
been reported to be associated with tumor progression/invasion and
lower OS rates in gastric cancer patients (38). Analysis of gastric cancer tissues
has shown that significantly higher levels of HER2 and HER3 are
associated with late stage gastric adenocarcinoma (stage III–IV)
compared to stage I–II disease (22–24% vs. 5.8%–7.7%, p<0.05)
(39). Several ongoing clinical
trials are currently evaluating the efficacy of novel EGFR-targeted
treatment options in HER-2 positive/amplified gastric cancers, as
well as on EGFR and HER2 co-expressing tumors (40).
An increased expression of vascular endothelial
growth factor (VEGF) and vascular endothelial growth factor
receptors (VEGFR1-4) is known to promote angiogenesis, and is
therefore associated with more aggressive forms of gastric cancer
(41–43). Despite promising in vitro
results, clinical trials with the VEGF-A monoclonal antibody
bevacizumab failed to show a significant improvement in patient
survival rates when supplemented with first-line chemotherapy
(44,45). On the other hand, the results from
the REGARD clinical trial with ramucirumab, (an anti-VEGFR
monoclonal antibody) showed an increase in OS rates (5.2 versus 3.8
months, p=0.047) in patients with advanced gastric cancer after
first-line chemotherapy (46).
Fibroblast growth factor receptors (FGFR1-4),
another family of receptor tyrosine kinases, have also been
implicated in gastric carcinoma (26). Five to eight percent of gastric
patients show amplified FGFR2 contributing to lymph node
metastasis, and thus, worsening the prognosis (47). Consequently, the clinical studies
of dovitinib targeting this amplified FGFR2 are currently
under phase II clinical trials as a monotherapy in patients with
metastatic or unresectable gastric cancer (48).
The PI3K/Akt/mTOR is a major effector cascade of
receptor tyrosine kinase signaling, and the core components of this
cascade are found to be altered in gastric carcinoma. PIK3CA
is found to be mutated in 5% of gastric cancers in which mutation
of this gene leads to the constitutive activation of the pathway
even in the absence of ligand (29,49,50).
Poor prognosis has also been associated with gastric cancer
patients having amplified PIK3CA in advanced gastric cancer cases
(51). Several clinical trials
targeting molecules involved in this dysregulated signaling pathway
are under investigation. Everolimus, an mTOR inhibitor, has
increased progression-free survival in neuroendocrine tumors and
renal cell carcinomas; however, it failed to show a significant
improvement in survival rates when tested for its efficacy in
previously treated advanced gastric carcinoma patients (52,53).
Phase I trials in gastric cancer patients with altered
PIK3CA or amplified HER2 are being tested with the
isoform specific p110-α inhibitor BYL719 and the heat shock protein
90 (Hsp90) inhibitor AUY922 (NCT01613950) (54). The Akt inhibitor MK2206 is also in
early clinical trials for patients with gastric cancer, as well as
other solid tumors (NCT01260701, NCT00963547) (54).
The treatment of early stage gastric cancer
primarily includes radiation therapy/chemotherapy to reduce tumor
size followed by surgical removal of the tumor mass (55). Advanced forms of gastric carcinoma
are, however, more difficult to target. Several genetic alterations
have been shown to be associated with gastric cancer; and thus,
researchers have been trying to develop therapeutic interventions
targeting these signaling molecules as a novel therapeutic approach
to eradicate these aggressive forms of stomach cancer. Although
targeted therapeutics against the molecules with ‘hotspot’
mutations have provided the potential to treat patients with
gastric cancer, ongoing clinical trials with the drugs have failed
to show a significant improvement in overall survival rates of
patients. Therefore, more effective and promising therapeutics
targeting additional molecules implicated in gastric cancer need to
be designed and developed.
The JAK/STAT (Janus kinase/signal transducer and
activator of transcription) cascade is a principal signal
transduction pathway that is involved in a range of physiological
and cellular processes, such as cellular proliferation, stem cell
self-renewal and immune responses (56–58).
Tightly regulated JAK/STAT signaling is of utmost importance in
maintaining normal cellular homeostasis. Consequently,
dysregulation of this pathway is known to be associated with a
variety of pathological conditions, including immune disorders and
human cancers (59).
The JAK/STAT signaling cascade is highly conserved
across phyla, ranging from slime molds to humans (59). The JAK/STAT cascade was originally
identified in the context of interferon-α (IFNα), IFN-γ and
interleukin-6 (IL-6)-mediated signaling. The binding of these
immune modulators to their receptors triggers the activation and
dimerization of specific receptors, which in turn, causes the
receptor-associated JAKs to come into close proximity, and
subsequently leads to the auto- and/or trans-phosphorylation of the
kinases (60). The cognate
receptor then becomes phosphorylated by the activated JAKs and in
turn, serves as a docking site for the SH2 domain-containing STAT
molecules (5). STATs, which have
been tyrosine-phosphorylated by JAK kinases are released from the
receptor, dimerize and then translocate to the nucleus, where they
act as a transcription factor to modulate the expression of
downstream target genes (56).
This cascade is negatively regulated by three main classes of
molecules; namely protein tyrosine phosphatase (PTP), suppressor of
cytokine signaling (SOCS) and protein inhibitor of activated STAT
(PIAS), at different levels of signaling (62). PTPs dephosphorylate STAT, JAK or
the associated receptors, consequently, inactivating them, whereas
PIAS molecules inhibit the signaling by preventing activated STAT
dimers from binding to their downstream targets or by interfering
with their transactivation capacity (61). SOCS molecules interfere with STAT
recruitment to the receptor, inhibit JAK activation, or promote the
proteasomal degradation of activated JAKs or the associated
receptors. Interestingly, SOCS is transcriptionally regulated by
JAK/STAT signaling, and thus a negative feedback mechanism occurs
(62).
In mammals, the JAK family includes four members,
namely JAK1, JAK2, JAK3 and TYK2. JAK1, JAK2 and TYK2 are known to
be ubiquitously expressed, whereas JAK3 expression is restricted to
the hematopoietic cells, suggesting its essential role in
hematopoietic development (63).
On the other hand, the STAT protein family has 7 key players namely
STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6, each of which
is known to regulate diverse cellular processes (56). STAT proteins act as important
transcription factors that regulate the transcription of many key
molecules involved in cell differentiation, proliferation,
inflammation and apoptosis. Recently, an indirect transcriptional
role of STAT has been reported, and this non-canonical JAK/STAT
signaling has been shown to be associated with hetero-chromatin
stability and the epigenetic regulation of global transcriptional
state, particularly by DNA methylation and chromatin remodelling
(64).
Constitutive activation of JAK/STAT signaling is
well-established in cancers. It may occur as a result of an
increased cytokine/cytokine receptor production or a decreased
expression of the negative regulators of the pathway (65). Activating somatic mutations in
JAK2 or MPL (encoding thrombopoietin receptor) have
been implicated in certain cases of myeloproliferative disorders,
resulting in the persistent activation of STAT3/5 (66,67).
In-frame deletions in gp130 were shown to lead to the
constitutive activation of STAT3, even in the absence of a ligand
during the pathogenesis of hepatocellular carcinomas (68). Furthermore, sphingosine-1-phosphate
receptor-1 (S1PR1) was reported to upregulate JAK2/STAT3 signaling
in different epithelial cancers via increasing STAT3 signaling,
which in turn transcriptionally regulates itself, S1PR1 and
IL-6 gene in a positive feed forward loop, contributing to
the process of tumorigenesis in these cancers (69). Novel somatic mutations in
LNK, encoding a negative regulator of the JAK/STAT pathway,
was also found to be a driving force in a subset of
myeloproliferative neoplasms by activating STAT signaling (70). Additionally, the tumor suppressor
PTP delta, which is known to dephosphorylate STAT3, was found to be
frequently mutated in human glioblastoma, head and neck cancers and
lung cancers, thus, reinforcing the causative role of dysregulated
STAT signaling in a wide variety of cancers (71).
The first report describing STAT3 as an oncogene was
published more than a decade ago. Constitutively-active STAT3
produced by substituting two cysteine residues for alanine and
asparagine respectively, in the C-terminal loop of the SH2 domain
was demonstrated to have the ability to transform immortalized
fibroblasts and induce tumors in nude mice (72). This study provided the foundation
for exploring the role of STAT3 as an oncogene in various human
cancers. Subsequent studies have linked dysregulated JAK/STAT
signaling to the tumor initiation and progression of a variety of
solid cancers and hematopoietic malignancies (66–69).
STAT3 has been shown to prevent apoptosis by increasing the
expression levels of the anti-apoptotic proteins of Bcl-2 family
proteins (73). In particular,
STAT3 mediates its pro-survival functions via Survivin, which not
only prevents apoptosis but also promotes the mitogenic activity of
cells (73,74). Studies have also shown that STAT3
controls the expression of the master EMT
(epithelial-to-mesenchymal) transcriptional regulators, thus,
contributing to the process of metastasis in cancers (75). An aberrant expression of STAT3 was
also shown to contribute to the tumor progression via facilitating
cell motility and invasion (76).
Moreover, STAT3 is also known to promote the formation of new blood
vessels by increasing the levels of VEGF and hypoxia-inducible
factor (HIF)-1α (77–79). Several immunomodulatory molecules
are also regulated by the JAK/STAT cascade. Specifically, reduced
Th1-dominated antitumor response on aberrant STAT3 activation
contributes to cancer cell survival and proliferation, suggesting
the role of STAT3 in the maintenance of the tumor microenvironment
by contributing to the process of inflammation and angiogenesis
(77,80–82).
One hundred gastric adenocarcinoma patient tissues
after gastrectomy were also analysed to examine the expression of
STAT3 by immunohistochemical staining (88). It was found that STAT3 expression
was highly associated with TNM staging and survival, thus,
suggesting that it functions as a biomarker predicting poor
prognosis of gastric cancer. Similarly, Deng et al evaluated
the association of STAT3, phospho-STAT3, SOCS1 as well as other
clinicopathological factors with overall survival rates in 53
gastric cancer patient tissue samples. Univariate and multivariate
analysis revealed that phospho-STAT3 and lymph node metastasis are
independent predictors of OS in gastric cancer, and STAT3
expression correlates with lymph node metastasis status in these
patients (89). Deng et al
also analysed 107 gastric cancer patient tissue samples by
immunohistochemistry to elucidate the role of SOCS3 expression in
gastric cancer, and found that SOCS3 was the best indicator for
lymph node metastasis; and thus, further studies are warranted to
explore its role as a predictor of lymph node metastasis in gastric
cancer (90).
The role of STAT3 in inflammation-mediated gastric
tumorigenesis has also been extensively explored. A study using
loss- and gain-of-function of STAT3 mice in a colitis-associated
cancer (CAC) model showed that gp130/STAT3 signaling cascade
provides a link between inflammation and gastrointestinal cancers.
STAT3 was found to function in tumor progression by promoting
intestinal epithelial cell (IEC) survival and proliferation through
G1 and G2/M cell-cycle progression (91). Furthermore, it was shown that
low-grade intraepithelial lesions in Stat3-deficient mice progress
to advanced tubular tumors, thus, affirming the critical role of
STAT3 in IEC proliferation and survival in CAC carcinogenesis
(92). Additionally, more
aggressive tumors have been observed in relation to STAT3
activation, either by epithelial-specific SOCS3 ablation or
introduction of SOCS3 binding-deficient gp130Y757F
mutation (93,94). These reports have provided valuable
insights into STAT3-driven inflammation and gastric cancer
(94).
Constitutive activation of JAK/STAT signaling is a
common occurrence in human cancers. Tumors may arise either due to
an increased autocrine/paracrine cytokine signaling via the
associated receptors or due to an increased transcription of
downstream STAT-dependent target genes, such as pro-survival Bcl-2
family members, angiogenic factors (HIF-1α), inflammation promoting
genes (IL-10, TGF-β;, COX-2) or metastasis regulators (MMP1/3/9,
ICAM-1) (77,95–97).
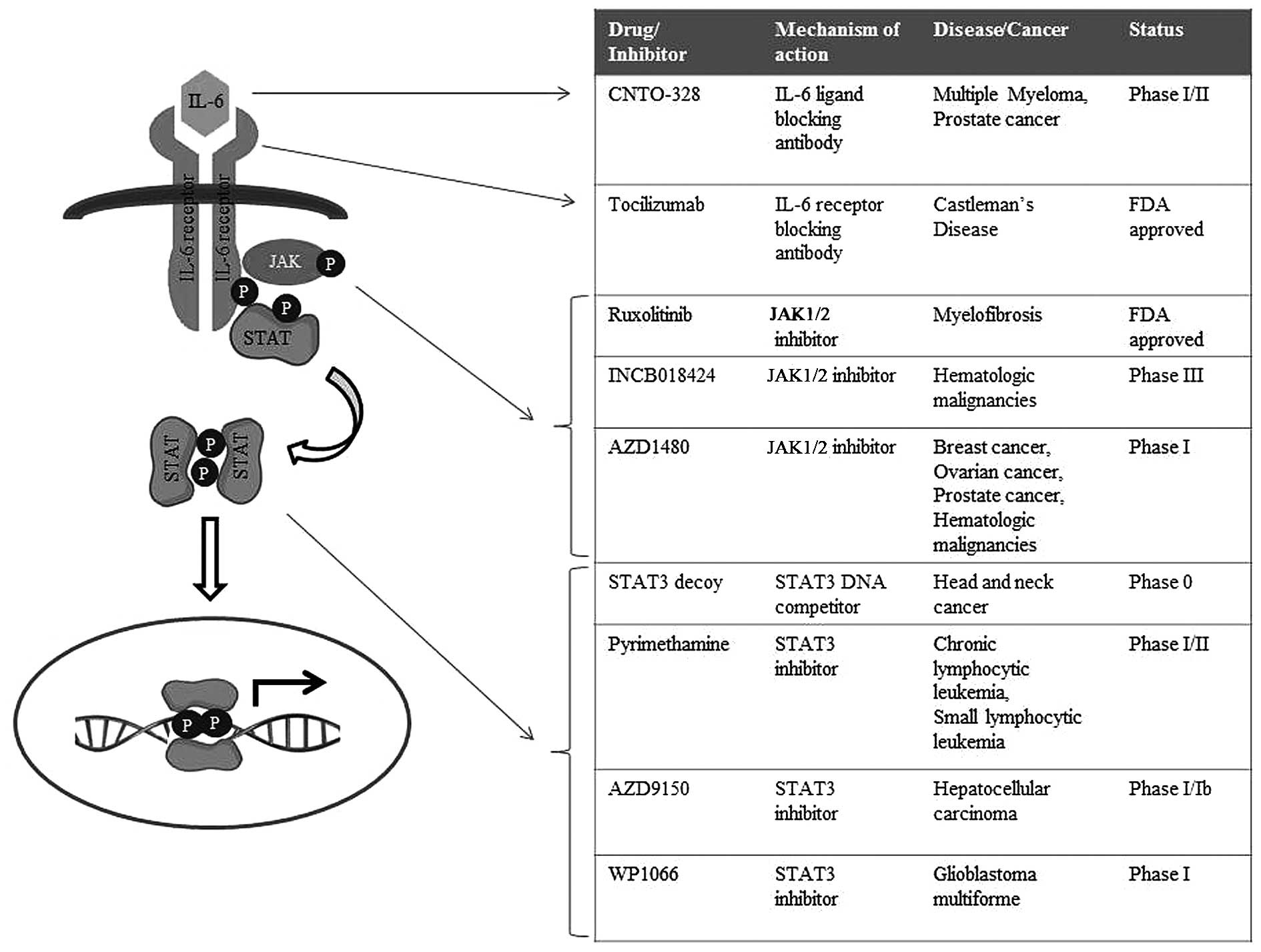
Tocilizumab, a monoclonal antibody against IL-6
receptors, has proven to be effective in inflammatory conditions,
including Rheumatoid arthritis as well as Castleman's disease, with
recent clinical trials testing the efficacy of this antibody in the
context of cancers (98–100). Tocilizumab has been tested for
its efficacy in various IL-6-driven cancer models for large-cell
carcinoma of the lung, ovarian cancer as well as pancreatic ductal
adenocarcinoma (101–103). In vitro and in vivo
studies have shown that treatment of these tumors with this IL-6
receptor antibody significantly decreases the size of the tumor
mass as well as invasion/metastasis, and ongoing clinical trials
are testing its efficacy in patients with recurrent ovarian cancer
and chronic lymphocytic leukemia (104,105). In addition, the IL-6 ligand
antibody CNTO-328 is currently under phase I/II clinical trials for
myeloma and prostate cancer (106–108).
Due to their multifaceted roles as transcription
factors, STAT family proteins are vital mediators of tumorigenesis,
in the context of both solid tumors as well as hematopoietic
malignancies. Hence, inhibitors abrogating STAT signaling are also
extensively being studied (114–116). A STAT3 decoy, a double-stranded
DNA containing STAT3-binding site, has been shown to successfully
act in sequestering dimeric STAT3 away from its endogenous targets
(117). Preliminary results with
the STAT3 decoy in head and neck cancer showed that it successfully
results in the apoptosis of cancer cells and a reduction in tumor
growth; and thus, this decoy is being tested clinically in patients
with head and neck squamous carcinoma (118,119). Peptidomemetics and designed small
molecules specifically targeting STAT3 have also shown promising
outcomes in preclinical acute cancer models for human breast
cancer, pancreatic cancer, prostate cancer and non-small cell lung
cancer, as well as in hematopoietic disorders such as acute myeloid
leukemia (120–122). Nifuroxazide, which was initially
identified as a treatment drug for diarrhea, was shown to
effectively inhibit the survival of multiple myeloma cells by
suppressing JAK2 and TYK2 directly (123). Similarly, the malarial drug
pyrimethamine was found to inhibit STAT3, and is currently under
investigation as a treatment option for chronic lymphocytic
leukemia and small lymphocytic leukemia (124).
As compared to other cancer types, a large
proportion of gastric carcinoma patients even after curative
surgery suffer from relapse and secondary diseases. The use of
histopathological features such as depth of primary tumor and lymph
node metastasis status have improved prognosis in these cancers.
However, the need for better and new molecular markers still
exists. Ongoing studies on important signaling molecules, including
ErbB, VEGFR and PI3K/mTOR/Akt, as potential prognostic markers have
not yet led to translation into clinical practices hitherto.
Studies have demonstrated dysregulated JAK/STAT
signaling in patient cohorts with gastric carcinoma. In particular,
aberrant STAT3 expression has been implicated in gastric
adenocarcinoma patients, making STAT3 a promising candidate as a
prognostic marker in gastric cancers (79,85,125,126). In gastric cancer, the abnormal
STAT3 expression not only contributes to cancer cell proliferation
and survival, but also functions in promoting inflammation, EMT
transition and metastasis (94,97,127,128). Various in vitro and in
vivo studies have confirmed the role of STAT3 in the
precancerous pathology of the stomach, indicating that STAT3 may
serve as a useful prognostic/diagnostic biomarker for the early
detection of gastric cancer and that limiting STAT3 activity could
help prevent malignancy (125).
Altogether, these findings suggest that targeting the aberrant
JAK/STAT signaling in gastric carcinoma may hold great potential as
a novel therapeutic intervention for the treatment of patients with
gastric cancer; and that the drugs/inhibitors of JAK/STAT cascade
currently in clinical trials for solid tumors and hematopoietic
malignancies (Fig. 3) should also
be tested for their efficacy as therapeutics in gastric
carcinoma.
The authors would like to thank Ms. Song Lin Bay
from the Department of Anatomy, National University of Singapore
for technical assistance. This study was supported by the NUS
start-up grant R-181-000-142-133.
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owen DA: Normal histology of the stomach.
Am J Surg Pathol. 10:48–61. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dicken BJ, Bigam DL, Cass C, Mackey JR,
Joy AA and Hamilton SM: Gastric adenocarcinoma: Review and
considerations for future directions. Ann Surg. 241:27–39.
2005.
|
|
7
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allum WH: Tumours of the stomach. Surgery.
29:575–580. 2011.
|
|
9
|
Gilligan CJ, Lawton GP, Tang LH, West AB
and Modlin IM: Gastric carcinoid tumors: The biology and therapy of
an enigmatic and controversial lesion. Am J Gastroenterol.
90:338–352. 1995.PubMed/NCBI
|
|
10
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
11
|
Munoz N, Correa P, Cuello C and Duque E:
Histologic types of gastric carcinoma in high- and low-risk areas.
Int J Cancer. 3:809–818. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006.PubMed/NCBI
|
|
13
|
Davessar K, Pezzullo JC, Kessimian N, Hale
JH and Jauregui HO: Gastric adenocarcinoma: Prognostic significance
of several pathologic parameters and histologic classifications.
Hum Pathol. 21:325–332. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ming SC: Gastric carcinoma. A
pathobiological classification. Cancer. 39:2475–2485. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rugge M, Capelle LG, Cappellesso R, Nitti
D and Kuipers EJ: Precancerous lesions in the stomach: From biology
to clinical patient management. Best Pract Res Clin Gastroenterol.
27:205–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forman D and Burley VJ: Gastric cancer:
Global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pizzi M, Saraggi D, Fassan M, Megraud F,
Di Mario F and Rugge M: Secondary prevention of epidemic gastric
cancer in the model of Helicobacter pylori-associated gastritis.
Dig Dis. 32:265–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levi E, Sochacki P, Khoury N, Patel BB and
Majumdar AP: Cancer stem cells in Helicobacter pylori infection and
aging: Implications for gastric carcinogenesis. World J
Gastrointest Pathophysiol. 5:366–372. 2014.PubMed/NCBI
|
|
19
|
Compare D, Rocco A and Nardone G: Risk
factors in gastric cancer. Eur Rev Med Pharmacol Sci. 14:302–308.
2010.PubMed/NCBI
|
|
20
|
Sheh A, Ge Z, Parry NM, Muthupalani S,
Rager JE, Raczynski AR, Mobley MW, McCabe AF, Fry RC, Wang TC, et
al: 17β-estradiol and tamoxifen prevent gastric cancer by
modulating leukocyte recruitment and oncogenic pathways in
Helicobacter pylori-infected INS-GAS male mice. Cancer Prev Res
(Phila). 4:1426–1435. 2011. View Article : Google Scholar
|
|
21
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: a global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Curado M-P, Edwards B, Shin HR, et al:
Cancer incidence in five continents. IX. IARC Press, International
Agency for Research on Cancer; Lyon: 2007
|
|
23
|
Howson CP, Hiyama T and Wynder EL: The
decline in gastric cancer: Epidemiology of an unplanned triumph.
Epidemiol Rev. 8:1–27. 1986.PubMed/NCBI
|
|
24
|
De Stefani E, Correa P, Boffetta P,
Deneo-Pellegrini H, Ronco AL and Mendilaharsu M: Dietary patterns
and risk of gastric cancer: a case-control study in Uruguay.
Gastric cancer. 7:211–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng N, Goh LK, Wang H, Das K, Tao J, Tan
IB, Zhang S, Lee M, Wu J, Lim KH, et al: A comprehensive survey of
genomic alterations in gastric cancer reveals systematic patterns
of molecular exclusivity and co-occurrence among distinct
therapeutic targets. Gut. 61:673–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Chiodini R, Badr A and Zhang G:
The impact of next-generation sequencing on genomics. J Genet
Genomics. 38:95–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grada A and Weinbrecht K: Next-generation
sequencing: Methodology and application. J Invest Dermatol.
133:e112013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee J, van Hummelen P, Go C, Palescandolo
E, Jang J, Park HY, Kang SY, Park JO, Kang WK, MacConaill L, et al:
High-throughput mutation profiling identifies frequent somatic
mutations in advanced gastric adenocarcinoma. PLoS One.
7:e388922012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zang ZJ, Cutcutache I, Poon SL, Zhang SL,
McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A, et al:
Exome sequencing of gastric adenocarcinoma identifies recurrent
somatic mutations in cell adhesion and chromatin remodeling genes.
Nat Genet. 44:570–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK
and Kim WH: EGFR in gastric carcinomas: Prognostic significance of
protein overexpression and high gene copy number. Histopathology.
52:738–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langer R, Von Rahden BH, Nahrig J, Von
Weyhern C, Reiter R, Feith M, Stein HJ, Siewert JR, Höfler H and
Sarbia M: Prognostic significance of expression patterns of
c-erbB-2, p53, p16INK4A, p27KIP1, cyclin D1
and epidermal growth factor receptor in oesophageal adenocarcinoma:
A tissue microarray study. J Clin Pathol. 59:631–634. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dulak AM, Schumacher SE, van Lieshout J,
Imamura Y, Fox C, Shim B, Ramos AH, Saksena G, Baca SC, Baselga J,
et al: Gastrointestinal adenocarcinomas of the esophagus, stomach,
and colon exhibit distinct patterns of genome instability and
oncogenesis. Cancer Res. 72:4383–4393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin, and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: a new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yano T, Doi T, Ohtsu A, Boku N, Hashizume
K, Nakanishi M and Ochiai A: Comparison of HER2 gene amplification
assessed by fluorescence in situ hybridization and HER2 protein
expression assessed by immunohistochemistry in gastric cancer.
Oncol Rep. 15:65–71. 2006.
|
|
37
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al; ToGA Trial Investigators. Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayashi M, Inokuchi M, Takagi Y, Yamada H,
Kojima K, Kumagai J, Kawano T and Sugihara K: High expression of
HER3 is associated with a decreased survival in gastric cancer.
Clin Cancer Res. 14:7843–7849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang XL, Yang YS, Xu DP, Qu JH, Guo MZ,
Gong Y and Huang J: Comparative study on overexpression of HER2/neu
and HER3 in gastric cancer. World J Surg. 33:2112–2118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang W, Raufi A and Klempner SJ: Targeted
therapy for gastric cancer: Molecular pathways and ongoing
investigations. Biochim Biophys Acta. 1846:232–237. 2014.PubMed/NCBI
|
|
41
|
Kim SE, Shim KN, Jung SA, Yoo K and Lee
JH: The clinicopathological significance of tissue levels of
hypoxia-inducible factor-1alpha and vascular endothelial growth
factor in gastric cancer. Gut Liver. 3:88–94. 2009. View Article : Google Scholar
|
|
42
|
Cabuk D, Basaran G, Celikel C, Dane F,
Yumuk PF, Iyikesici MS, Ekenel M and Turhal NS: Vascular
endothelial growth factor, hypoxia-inducible factor 1 alpha and
CD34 expressions in early-stage gastric tumors: Relationship with
pathological factors and prognostic impact on survival. Oncology.
72:111–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jüttner S, Wissmann C, Jöns T, Vieth M,
Hertel J, Gretschel S, Schlag PM, Kemmner W and Höcker M: Vascular
endothelial growth factor-D and its receptor VEGFR-3: Two novel
independent prognostic markers in gastric adenocarcinoma. J Clin
Oncol. 24:228–240. 2006. View Article : Google Scholar
|
|
44
|
Shah MA, Ramanathan RK, Ilson DH, Levnor
A, D'Adamo D, O'Reilly E, Tse A, Trocola R, Schwartz L, Capanu M,
et al: Multicenter phase II study of irinotecan, cisplatin, and
bevacizumab in patients with metastatic gastric or gastroesophageal
junction adenocarcinoma. J Clin Oncol. 24:5201–5206. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry
DR, et al; REGARD Trial Investigators. Ramucirumab monotherapy for
previously treated advanced gastric or gastro-oesophageal junction
adenocarcinoma (REGARD): An international, randomised, multicentre,
placebo-controlled, phase 3 trial. Lancet. 383:31–39. 2014.
View Article : Google Scholar
|
|
47
|
Su X, Zhan P, Gavine PR, Morgan S, Womack
C, Ni X, Shen D, Bang YJ, Im SA, Ho Kim W, et al: FGFR2
amplification has prognostic significance in gastric cancer:
Results from a large international multicentre study. Br J Cancer.
110:967–975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie L, Su X, Zhang L, Yin X, Tang L, Zhang
X, Xu Y, Gao Z, Liu K, Zhou M, et al: FGFR2 gene amplification in
gastric cancer predicts sensitivity to the selective FGFR inhibitor
AZD4547. Clin Cancer Res. 19:2572–2583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu JF, Zhou XK, Chen JH, Yi G, Chen HG,
Ba MC, Lin SQ and Qi YC: Up-regulation of PIK3CA promotes
metastasis in gastric carcinoma. World J Gastroenterol.
16:4986–4991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shi J, Yao D, Liu W, Wang N, Lv H, Zhang
G, Ji M, Xu L, He N, Shi B, et al: Highly frequent PIK3CA
amplification is associated with poor prognosis in gastric cancer.
BMC Cancer. 12:502012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dong M, Phan AT and Yao JC: New strategies
for advanced neuroendocrine tumors in the era of targeted therapy.
Clin Cancer Res. 18:1830–1836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung
HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al: Everolimus
for previously treated advanced gastric cancer: Results of the
randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol.
31:3935–3943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang W, Raufi A and Klempner SJ: Targeted
therapy for gastric cancer: Molecular pathways and ongoing
investigations. Biochim Biophys Acta. 1846:232–237. 2014.PubMed/NCBI
|
|
55
|
Proserpio I, Rausei S, Barzaghi S,
Frattini F, Galli F, Iovino D, Rovera F, Boni L, Dionigi G and
Pinotti G: Multimodal treatment of gastric cancer. World J
Gastrointest Surg. 6:55–58. 2014.PubMed/NCBI
|
|
56
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Aaronson DS and Horvath CM: A road map for
those who don't know JAK-STAT. Science. 296:1653–1655. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rawlings JS, Rosler KM and Harrison DA:
The JAK/STAT signaling pathway. J Cell Sci. 117:1281–1283. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Harrison DA: The Jak/STAT pathway. Cold
Spring Harb Perspect Biol. 4:42012. View Article : Google Scholar
|
|
60
|
Kiu H and Nicholson SE: Biology and
significance of the JAK/ STAT signalling pathways. Growth Factors.
30:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Espert L, Dusanter-Fourt I and Chelbi-Alix
MK: Negative regulation of the JAK/STAT: Pathway implication in
tumorigenesis. Bull Cancer. 92:845–857. 2005.(In French).
PubMed/NCBI
|
|
62
|
Valentino L and Pierre J: JAK/STAT signal
transduction: Regulators and implication in hematological
malignancies. Biochem Pharmacol. 71:713–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kisseleva T, Bhattacharya S, Braunstein J
and Schindler CW: Signaling through the JAK/STAT pathway, recent
advances and future challenges. Gene. 285:1–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li WX: Canonical and non-canonical
JAK-STAT signaling. Trends Cell Biol. 18:545–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Scott LM: The JAK2 exon 12 mutations: A
comprehensive review. Am J Hematol. 86:668–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kralovics R, Passamonti F, Buser AS, Teo
SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M and Skoda RC: A
gain-of-function mutation of JAK2 in myeloproliferative disorders.
N Engl J Med. 352:1779–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rebouissou S, Amessou M, Couchy G, Poussin
K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P and
Zucman-Rossi J: Frequent in-frame somatic deletions activate gp130
in inflammatory hepatocellular tumours. Nature. 457:200–204. 2009.
View Article : Google Scholar :
|
|
69
|
Lee H, Deng J, Kujawski M, Yang C, Liu Y,
Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, et al:
STAT3-induced S1PR1 expression is crucial for persistent STAT3
activation in tumors. Nat Med. 16:1421–1428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Oh ST, Simonds EF, Jones C, Hale MB,
Goltsev Y, Gibbs KD Jr, Merker JD, Zehnder JL, Nolan GP and Gotlib
J: Novel mutations in the inhibitory adaptor protein LNK drive
JAK-STAT signaling in patients with myeloproliferative neoplasms.
Blood. 116:988–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Veeriah S, Brennan C, Meng S, Singh B,
Fagin JA, Solit DB, Paty PB, Rohle D, Vivanco I, Chmielecki J, et
al: The tyrosine phosphatase PTPRD is a tumor suppressor that is
frequently inactivated and mutated in glioblastoma and other human
cancers. Proc Natl Acad Sci USA. 106:9435–9440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Stephanou A, Brar BK, Knight RA and
Latchman DS: Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and
Bcl-x promoters. Cell Death Differ. 7:329–330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
O'Connor DS, Grossman D, Plescia J, Li F,
Zhang H, Villa A, Tognin S, Marchisio PC and Altieri DC: Regulation
of apoptosis at cell division by p34cdc2 phosphorylation of
survivin. Proc Natl Acad Sci USA. 97:13103–13107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wendt MK, Balanis N, Carlin CR and
Schiemann WP: STAT3 and epithelial-mesenchymal transitions in
carcinomas. JAK-STAT. 3:e289752014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Teng Y, Ross JL and Cowell JK: The
involvement of JAK-STAT3 in cell motility, invasion, and
metastasis. JAK-STAT. 3:e280862014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, et al: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kujawski M, Kortylewski M, Lee H, Herrmann
A, Kay H and Yu H: Stat3 mediates myeloid cell-dependent tumor
angiogenesis in mice. J Clin Invest. 118:3367–3377. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Gong W, Wang L, Yao JC, Ajani JA, Wei D,
Aldape KD, Xie K, Sawaya R and Huang S: Expression of activated
signal transducer and activator of transcription 3 predicts
expression of vascular endothelial growth factor in and angiogenic
phenotype of human gastric cancer. Clin Cancer Res. 11:1386–1393.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang T, Niu G, Kortylewski M, Burdelya L,
Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola
D, et al: Regulation of the innate and adaptive immune responses by
Stat-3 signaling in tumor cells. Nat Med. 10:48–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang F, Arun P, Friedman J, Chen Z and Van
Waes C: Current and potential inflammation targeted therapies in
head and neck cancer. Curr Opin Pharmacol. 9:389–395. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang Z, Si X, Xu A, Meng X, Gao S, Qi Y,
Zhu L, Li T, Li W and Dong L: Activation of STAT3 in human gastric
cancer cells via interleukin (IL)-6-type cytokine signaling
correlates with clinical implications. PLoS One. 8:e757882013.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Giraud AS, Menheniott TR and Judd LM:
Targeting STAT3 in gastric cancer. Expert Opin Ther Targets.
16:889–901. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sekikawa A, Fukui H, Fujii S, Ichikawa K,
Tomita S, Imura J, Chiba T and Fujimori T: REG Ialpha protein
mediates an anti-apoptotic effect of STAT3 signaling in gastric
cancer cells. Carcinogenesis. 29:76–83. 2008. View Article : Google Scholar
|
|
87
|
Jackson CB, Judd LM, Menheniott TR,
Kronborg I, Dow C, Yeomans ND, Boussioutas A, Robb L and Giraud AS:
Augmented gp130-mediated cytokine signalling accompanies human
gastric cancer progression. J Pathol. 213:140–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim DY, Cha ST, Ahn DH, Kang HY, Kwon CI,
Ko KH, Hwang SG, Park PW, Rim KS and Hong SP: STAT3 expression in
gastric cancer indicates a poor prognosis. J Gastroenterol Hepatol.
24:646–651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Deng JY, Sun D, Liu XY, Pan Y and Liang H:
STAT-3 correlates with lymph node metastasis and cell survival in
gastric cancer. World J Gastroenterol. 16:5380–5387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Deng J, Jiao X, Liu H, Wu L, Zhang R, Wang
B, Pan Y, Hao X and Liang H: Lymph node metastasis is mediated by
suppressor of cytokine signaling-3 in gastric cancer. Tumour Biol.
34:3627–3636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bollrath J, Phesse TJ, von Burstin VA,
Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T,
Canli O, Schwitalla S, et al: gp130-mediated Stat3 activation in
enterocytes regulates cell survival and cell-cycle progression
during colitis-associated tumorigenesis. Cancer Cell. 15:91–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L, et al: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rigby RJ, Simmons JG, Greenhalgh CJ,
Alexander WS and Lund PK: Suppressor of cytokine signaling 3
(SOCS3) limits damage-induced crypt hyper-proliferation and
inflammation-associated tumorigenesis in the colon. Oncogene.
26:4833–4841. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ernst M and Putoczki TL: Stat3: Linking
inflammation to (gastrointestinal) tumourigenesis. Clin Exp
Pharmacol Physiol. 39:711–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Leonard WJ: Role of Jak kinases and STATs
in cytokine signal transduction. Int J Hematol. 73:271–277. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ihle JN: The Stat family in cytokine
signaling. Curr Opin Cell Biol. 13:211–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers (Basel). 6:897–925. 2014.
View Article : Google Scholar
|
|
98
|
Nishimoto N and Kishimoto T: Interleukin
6: From bench to bedside. Nat Clin Pract Rheumatol. 2:619–626.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nakashima Y, Kondo M, Harada H, Horiuchi
T, Ishinishi T, Jojima H, Kuroda K, Miyahara H, Nagamine R,
Nakashima H, et al: Clinical evaluation of tocilizumab for patients
with active rheumatoid arthritis refractory to anti-TNF biologics:
tocilizumab in combination with methotrexate. Mod Rheumatol.
20:343–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Garnero P, Thompson E, Woodworth T and
Smolen JS: Rapid and sustained improvement in bone and cartilage
turnover markers with the anti-interleukin-6 receptor inhibitor
tocilizumab plus methotrexate in rheumatoid arthritis patients with
an inadequate response to methotrexate: Results from a substudy of
the multi-center double-blind, placebo-controlled trial of
tocilizumab in inadequate responders to methotrexate alone.
Arthritis Rheum. 62:33–43. 2010. View Article : Google Scholar
|
|
101
|
Ando K, Takahashi F, Motojima S, Nakashima
K, Kaneko N, Hoshi K and Takahashi K: Possible role for
tocilizumab, an anti-interleukin-6 receptor antibody, in treating
cancer cachexia. J Clin Oncol. 31:e69–e72. 2013. View Article : Google Scholar
|
|
102
|
Isobe A, Sawada K, Kinose Y, Ohyagi-Hara
C, Nakatsuka E, Makino H, Ogura T, Mizuno T, Suzuki N, Morii E, et
al: Interleukin 6 receptor is an independent prognostic factor and
a potential therapeutic target of ovarian cancer. PLoS One.
10:e01180802015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Goumas FA, Holmer R, Egberts JH, et al:
Inhibition of IL-6 signaling significantly reduces primary tumor
growth and recurrencies in orthotopic xenograft models of
pancreatic cancer. Int J Cancer. Jan 21–2015.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Dijkgraaf EM, Welters MJ, Nortier JW, van
der Burg SH and Kroep JR: Interleukin-6/interleukin-6 receptor
pathway as a new therapy target in epithelial ovarian cancer. Curr
Pharm Des. 18:3816–3827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yao X, Huang J, Zhong H, Shen N, Faggioni
R, Fung M and Yao Y: Targeting interleukin-6 in inflammatory
autoimmune diseases and cancers. Pharmacol Ther. 141:125–139. 2014.
View Article : Google Scholar
|
|
106
|
Wallner L, Dai J, Escara-Wilke J, Zhang J,
Yao Z, Lu Y, Trikha M, Nemeth JA, Zaki MH and Keller ET: Inhibition
of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal
antibody, inhibits conversion of androgen-dependent prostate cancer
to an androgen-independent phenotype in orchiectomized mice. Cancer
Res. 66:3087–3095. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Puchalski T, Prabhakar U, Jiao Q, Berns B
and Davis HM: Pharmacokinetic and pharmacodynamic modeling of an
anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in
patients with metastatic renal cell carcinoma. Clin Cancer Res.
16:1652–1661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Dorff TB, Goldman B, Pinski JK, Mack PC,
Lara PN Jr, Van Veldhuizen PJ Jr, Quinn DI, Vogelzang NJ, Thompson
IM Jr and Hussain MH: Clinical and correlative results of SWOG
S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal
antibody against interleukin-6, in chemotherapy-pretreated patients
with castration-resistant prostate cancer. Clin Cancer Res.
16:3028–3034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mascarenhas J and Hoffman R: Ruxolitinib:
the first FDA approved therapy for the treatment of myelofibrosis.
Clin Cancer Res. 18:3008–3014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ganetsky A: Ruxolitinib: A new treatment
option for myelofibrosis. Pharmacotherapy. 33:84–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Meydan N, Grunberger T, Dadi H, Shahar M,
Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, et
al: Inhibition of acute lymphoblastic leukaemia by a Jak-2
inhibitor. Nature. 379:645–648. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Quintás-Cardama A, Vaddi K, Liu P,
Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, et
al: Preclinical characterization of the selective JAK1/2 inhibitor
INCB018424: Therapeutic implications for the treatment of
myeloproliferative neoplasms. Blood. 115:3109–3117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Hedvat M, Huszar D, Herrmann A, Gozgit JM,
Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et
al: The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and
oncogenesis in solid tumors. Cancer Cell. 16:487–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Munoz J, Dhillon N, Janku F, Watowich SS
and Hong DS: STAT3 inhibitors: Finding a home in lymphoma and
leukemia. Oncologist. 19:536–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bar-Natan M, Nelson EA, Xiang M and Frank
DA: STAT signaling in the pathogenesis and treatment of myeloid
malignancies. JAK-STAT. 1:55–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Frank DA: STAT signaling in the
pathogenesis and treatment of cancer. Mol Med. 5:432–456.
1999.PubMed/NCBI
|
|
117
|
Sen M, Tosca PJ, Zwayer C, Ryan MJ,
Johnson JD, Knostman KA, Giclas PC, Peggins JO, Tomaszewski JE,
McMurray TP, et al: Lack of toxicity of a STAT3 decoy
oligonucleotide. Cancer Chemother Pharmacol. 63:983–995. 2009.
View Article : Google Scholar
|
|
118
|
Leong PL, Andrews GA, Johnson DE, Dyer KF,
Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF, et
al: Targeted inhibition of Stat3 with a decoy oligonucleotide
abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA.
100:4138–4143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Xi S, Gooding WE and Grandis JR: In vivo
antitumor efficacy of STAT3 blockade using a transcription factor
decoy approach: Implications for cancer therapy. Oncogene.
24:970–979. 2005. View Article : Google Scholar
|
|
120
|
Zhao W, Jaganathan S and Turkson J: A
cell-permeable Stat3 SH2 domain mimetic inhibits Stat3 activation
and induces antitumor cell effects in vitro. J Biol Chem.
285:35855–35865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB
and Tweardy DJ: Stat3 signaling in acute myeloid leukemia:
Ligand-dependent and -independent activation and induction of
apoptosis by a novel small-molecule Stat3 inhibitor. Blood.
117:5701–5709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang X, Yue P, Fletcher S, Zhao W,
Gunning PT and Turkson J: A novel small-molecule disrupts Stat3 SH2
domain-phosphotyrosine interactions and Stat3-dependent tumor
processes. Biochem Pharmacol. 79:1398–1409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Nelson EA, Walker SR, Kepich A, Gashin LB,
Hideshima T, Ikeda H, Chauhan D, Anderson KC and Frank DA:
Nifuroxazide inhibits survival of multiple myeloma cells by
directly inhibiting STAT3. Blood. 112:5095–5102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Nelson EA, Sharma SV, Settleman J and
Frank DA: A chemical biology approach to developing STAT
inhibitors: Molecular strategies for accelerating clinical
translation. Oncotarget. 2:518–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Jackson CB and Giraud AS: STAT3 as a
prognostic marker in human gastric cancer. J Gastroenterol Hepatol.
24:505–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai
AH, Lo AW, Chu SH, Tong JH, Lo KW, et al: Constitutional activation
of IL-6-mediated JAK/STAT pathway through hypermethylation of
SOCS-1 in human gastric cancer cell line. Br J Cancer.
91:1335–1341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tye H, Kennedy CL, Najdovska M, McLeod L,
McCormack W, Hughes N, Dev A, Sievert W, Ooi CH, Ishikawa TO, et
al: STAT3-driven upregulation of TLR2 promotes gastric
tumorigenesis independent of tumor inflammation. Cancer Cell.
22:466–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Deng J, Liang H, Zhang R, Sun D, Pan Y,
Liu Y, Zhang L and Hao X: STAT3 is associated with lymph node
metastasis in gastric cancer. Tumour Biol. 34:2791–2800. 2013.
View Article : Google Scholar : PubMed/NCBI
|