Introduction
Colorectal cancer (CRC), a malignant disease with
high morbidity and mortality, ranks the third most commonly
diagnosed cancer in the world and accounts for 492,000 related
deaths annually (1,2). As a well-studied cancer, CRC is now
recognized as a complex disease resulting from the accumulation of
genetic and epigenetic alterations (3). Treatment strategies for CRC include
surgery, chemotherapy and radiotherapy which have shown great
improvement (4). However, the
prognosis for CRC is unsatisfactory as recurrence and metastasis
frequently occur. Application of chemotherapy to CRC is restricted
due to high incidence of severe side-effects and drug resistance.
Therefore, the research and development of new effective
chemotherapeutic agents for CRC is urgently needed.
β-catenin, the crucial molecule in Wnt/β-catenin
pathway, promotes the transcription of several oncogenic target
genes related to cancer progression (5). β-catenin has been found to drive
cancer development and be active in 80% of CRC (6–9). It
can be induced by phosphorylation and proteasome-mediated
degradation by glycogen synthase kinase 3β (GSK-3β), which acts as
a negative regulator of Wnt/β-catenin signaling pathway and is
implicated in governing cancer cell proliferation and apoptosis
(10,11). Notably, phosphorylation of GSK3β at
Ser9 by phosphorylated AKT (p-AKT) induces GSK3β
inactivation and inhibits its ability to promote the degradation of
β-catenin (12,13). As a result, AKT/GSK-3β/β-catenin
signaling pathway has been indicated as an important therapeutic
target for drug design to inhibit growth and metastasis of cancer
cells.
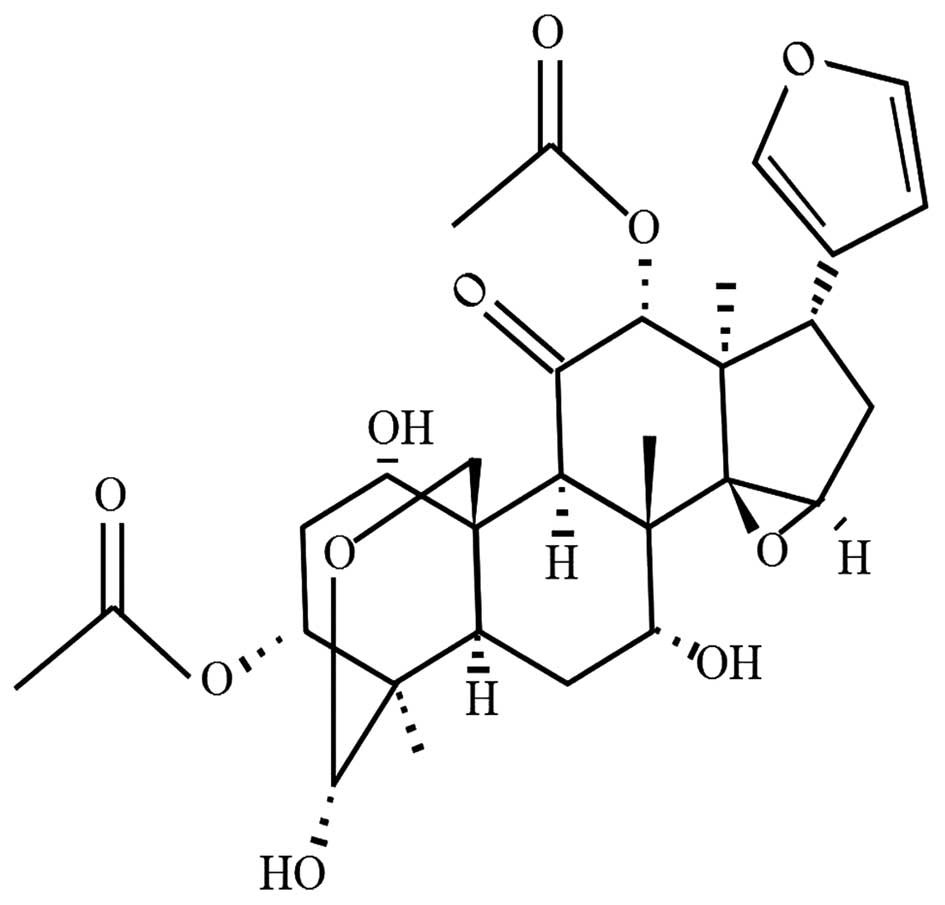
Toosendanin (TSN), a triterpenoid as shown in
Fig. 1, is a colorless and
acicular crystal extracted from the bark or fruits of Melia
toosendan Sieb et Zucc, which mainly grows in China and India
and exhibits analgesic, insecticidal and anti-inflammatory
activities (14). TSN is shown to
possess antitumour effects on various human cancer cells in
vitro, with IC50 values ranging from 5.4 to 900 nM
(15–17). In vivo experiments have
shown that TSN exerts strongly suppressive effects on
hepatocellular carcinoma in BALB/C mice (14). In addition, TSN induces apoptosis
in several types of cancer cells by regulation of the mitochondrial
pathway (14,15). However, sophisticated signaling
pathways involved in TSN regulation of cancer cells including CRC
have not been completely elucidated. Thus, the present study was
performed to investigate the effect of TSN on CRC cells and related
molecular mechanism.
Materials and methods
Chemicals and antibodies
TSN (purity ≥99%) was purchased from Xi'an
Insecticide Biological Projected Co., Ltd. (Xi'an, China) and
dissolved in DMSO; pancreatin and 3-(4,5)-Dimethylthiazol(-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) were from BioSharp (Hefei, China); RPMI-1640 medium
and fetal bovine serum (FBS) were from Life Technologies (Grand
Island, NY, USA); Annexin-V/propidium iodide (PI) apoptosis
detection kit was from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China); TRIzol reagents and Power SYBR-Green PCR Master Mix were
obtained from Invitrogen (Carlsbad, CA, USA); Primescript reverse
transcription reagent kit with gDNA Eraser was from Takara (Dalian,
China); monoclonal mouse β-actin antibody was from Sigma Chemical
Co. (St. Louis, MO, USA); antibodies against Bcl-2, GSK-3β,
pro-caspase-3, pro-PARP, vascular endothelial growth factor-A
(VEGFA) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA);
Antibodies against Bax, AKT, p-AKT Ser473, p-GSK-3β
Ser9, β-catenin were from Cell Signaling Technology
(Beverly, MA, USA).
Cell line and cell culture
Human CRC cell line SW480 was obtained from the Type
Culture Collection, Chinese Academy of Sciences (Shanghai, China),
cultured in RPMI-1640 medium supplemented with 10% heat-inactivated
FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml) at a 37°C
humidified atmosphere containing 5% CO2.
Cell proliferation assay
MTT assay was used to analyze cell proliferation.
Briefly, cells in the logarithmic growth phase were plated in
96-well culture plates at a density of 5×103 cells/well.
After adhering to the plate surface, cells were treated with TSN
(0.5, 0.25, 0.125, 0.063 and 0.031 μM) for 24 and 48 h, followed by
20 μl of MTT (5 mg/ml) incubation for further 4 h. The formazan
crystals formed were dissolved by the addition of DMSO and the
optical densities (ODs) were measured by ELx800 microplate reader
(BioTek Instruments Inc., Winooski, VT, USA) at 490 nm. The cell
growth inhibition rate was calculated using the following formula:
1 - OD (experiment)/OD (control).
Apoptosis assay
Annexin V/PI apoptosis detection kit was used to
assess induction of apoptosis. Briefly, cells (1×106)
treated with or without TSN (0.5 and 0.125 μM) for 48 h, together
with 50 μg/ml 5-fluorouracil (5-FU) for 12 h as a positive control
were harvested and suspended in binding buffer (10 mM Hepes/NaOH,
pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Approximately 5 μl
Annexin V and 5 μl PI were then added to the solution, afterwards
the cells were gently vortexed and incubated for 15 min at room
temperature in the dark for analysis by fluorescent activated cell
sorting (FACS) on a flow cytometer.
Cell cycle distribution analysis
After incubation in serum-free medium for 24 h,
cells were treated with TSN at different concentrations for another
24 h. The cells were then trypsinized, washed with PBS and fixed
with 75% cold ethanol overnight at 4°C. After PBS washing, the
fixed cells were stained with 50 μg/ml PI in the presence of RNase
for 30 min at 4°C in the dark. The stained cells were analyzed by
flow cytometry. The DNA content in cells could be read according to
PI fluorescence.
Hoechst 33342 nuclear staining
The trypsinized cells were plated onto coverslips in
6-well plates at a density of 1.5×105 cells/well. After
incubation for 24 h, the cells were treated with 0.125 or 0.5 μM
TSN for 48 h. The cells were then washed with PBS and incubated
with Hoechst 33342 for 30 min in CO2 incubator.
Following rinsing three times in PBS, the cells were examined for
nuclear changes via fluorescent microscope (normal nuclei was
identified as non-condensed chromatin dispersed over the entire
nucleus and apoptotic nuclei was identified as condensed chromatin,
contiguous with the nuclear membrane and/or fragmented nuclei).
Analysis using confocal laser scanning
microscopy
After incubation on coverslips in 6-well plates at a
density of 1.5×105 cells/well for 24 h, the cells were
treated with 0.5 μM TSN for 48 h. After PBS washing, the cells were
fixed with pre-colling acetone for 15 min, permeabilized by 0.5%
Triton X-100 for 10 min and blocked with PBS containing 5% BSA.
Afterwards, the cells were incubated with primary antibodies
overnight at 4°C. After washing three times with PBS containing 5%
BSA, incubated with secondary antibodies for 1 h and mounted by
mounting liquid containing DAPI, the cells were photographed using
confocal laser scanning microscopy.
Total RNA extraction and real-time PCR
(RT-PCR)
Total RNA was isolated using the TRIzol reagent
following the manufacturer's instructions. Briefly, after treated
with 0.125 and 0.5 μM TSN or 50 μg/ml 5-Fu for 48 h, the cells were
resuspended in 1 ml of TRIzol. The suspension was extracted by 0.2
ml of chloroform, and after centrifugation mixed with 0.5 ml of
isopropyl alcohol, and the resulting pellet was washed with 0.7 ml
of 75% ethanol and finally resuspended in 50 μl RNase-free water.
All total RNA samples were kept at −80°C until use.
Reverse-transcription was carried out using M-MLV and cDNA
amplification was carried out using SYBR-Green Master Mix kit
according to the manufacturer's protocol. Human β-actin expression
was used as internal control. Each sample was tested in triplicate
with the use of the QuantiTect SYBR-Green PCR kit (Qiagen, Hilden,
Germany) for 40 cycles on the ABI 7500 Fast real-time PCR system
(Applied Biosystems, Foster City, CA, USA). The PCR primer
sequences are shown in Table I.
Each sample was tested in triplicate. Cycle threshold (Ct) values
were obtained graphically for the target genes and β-actin. ΔCt =
Ct (target genes) − Ct (endogenous reference gene). ΔΔCt = ΔCt
(treated samples) − ΔCt (control samples). The relative fold change
in gene expression were calculated using the 2−ΔΔCt
method.
 | Table ISequences of the primers used in the
real-time PCR amplifications. |
Table I
Sequences of the primers used in the
real-time PCR amplifications.
| Gene primer | Sequence
(5′-3′) | Length of PCR
product (bp) |
|---|
| Bax | Forward:
TTTGCTTCAGGGTTTCATCC
Reverse: GCCACTCGGAAAAAGACCTC | 213 |
| Bak | Forward:
ACGTGTCAGAAGCCTCCAAG
Reverse: TGAGAGCCTTCACCTGTAGTG | 110 |
| Bcl-2 | Forward:
TCGCCCTGTGGATGACTGAG
Reverse: CAGAGTCTTCAGAGACAGCCAGGA | 143 |
| Bcl-xL | Forward:
ATGAACTCTTCCGGGATGG
Reverse: TGGATCCAAGGCTCTAGGTG | 166 |
| Survivin | Forward:
TTCTCAAGGACCACCGCATC
Reverse: GCCAAGTCTGGCTCGTTCTC | 127 |
| ACTB | Forward:
GGCCAACCGCGAGAAGAT
Reverse: CGTCACCGGAGTCCATCA | 134 |
| β-catenin | Forward:
GGCCATATCCACCAGAGTGAA
Reverse: GCCAATGGCTTGGAATGAGA | 119 |
| GSK-3β | Forward:
GACTAAGGTCTTCCGACCCC
Reverse: AAGAGTGCAGGTGTGTCTCG | 177 |
| c-myc | Forward:
CACCAGCAGCGACTCTGA
Reverse: GATCCAGACTCTGACCTTTTGC | 250 |
| VEGFA | Forward:
CCCTGATGAGATCGAGTACATCTT
Reverse: CTTGTCTTGCTCTATCTTTCTTTGGTCT | 224 |
| Cyclin D1 | Forward:
GAAGTTGCAAAGTCCTGGAGC
Reverse: ATGGTTTCCACTTCGCAGCA | 221 |
| Cyclin D2 | Forward:
CCGCAGTGCTCCTACTTCAA
Reverse: GCCAAGAAACGGTCCAGGTA | 152 |
| Cyclin D3 | Forward:
TGCACATGATTTCCTGGCCT
Reverse: CTGTAGCACAGAGGGCCAAA | 107 |
| COX-2 | Forward:
ATACGACTTGCAGTGAGCGT
Reverse: GGGTGGGAACAGCAAGGATT | 200 |
Western blot analysis
Cells treated with TSN or 50 μg/ml 5-Fu for 48 h
were harvested with a cell scraper. Proteins were extracted with
RIPA buffer containing protease inhibitor cocktail and protein
concentrations were determined using Bradford assay. Equal amounts
of protein (20 μg) from each sample was separated by 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis and transferred
onto polyvinylidene fluoride (PVDF) membranes (Millipore,
Billerica, MA, USA), which were then blocked with 5% skim milk for
at least 30 min. The primary antibodies were diluted according to
the manufacturer's instructions. Afterwards, the membranes were
incubated with appropriate primary antibodies overnight at 4°C,
washed three times with PBST and incubated with horseradish
peroxidase-linked secondary antibodies at a dilution ratio of
1:1,000 for 1 h at room temperature. Then the immunoreactive bands
were detected using an ECL detection kit (Millipore). β-actin was
used as a loading control. Three separate experiments were
performed for each sample.
Animals and in vivo tumor xenograft
studies
Male BALB/c/nu/nu nude mice weighing 18–22 g were
obtained from Shanghai Experimental Animal Center of the Chinese
Academy of Sciences. All animal experiments were approved by the
Animal Ethics and Research Committee of Shanghai Jiaotong
University. SW480 cells (5×107) were injected
subcutaneously into the right flank of mice (n=30). When the tumor
diameter reached ~5 mm, mice were randomly divided into 3 groups:
control (mice receiving PBS; n=10), low-dose TSN (0.15 mg/kg;
n=10), and high-dose TSN (0.30 mg/kg; n=10). The PBS and TSN were
intraperitoneally given once daily for 14 days. When the treatment
began, the mean tumor volumes were calculated every 3 days with a
caliper, using the formula volume = (length × width2)/2.
The mice were sacrificed 24 h after the final dose and tumors were
resected aseptically for weight and volume calculation.
Statistical analysis
The data are expressed as the mean ± standard
deviation (SD). The statistical differences of experimental data
between the groups were determined by SPSS 17.0 software using
one-way ANOVA test. Significance was defined as P<0.05 or
P<0.01.
Results
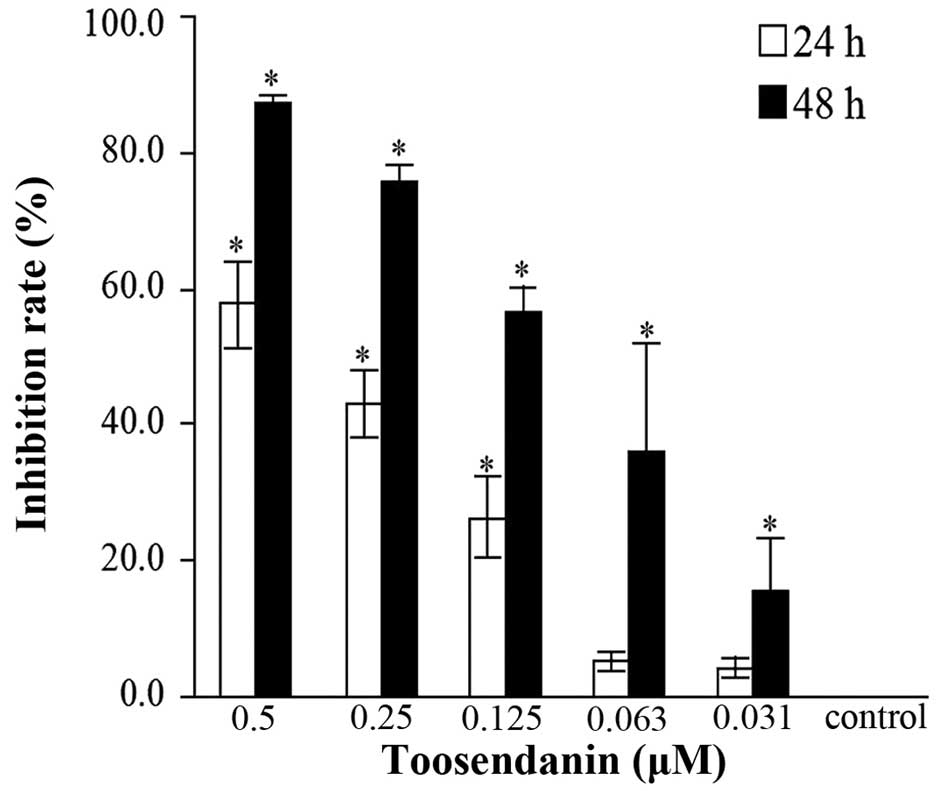
Effects of TSN on cell proliferation
In order to examine the effects of TSN on CRC SW480
cell proliferation in vitro, MTT assay was used. As a
result, it was found that cell proliferation of SW480 cells was
significantly suppressed when treated by TSN and the inhibition
rates were both dose- and time-dependent (Fig. 2). The IC50 value was
0.3493 μM for 24 h and 0.1059 μM for 48 h.
TSN induces cell apoptosis
Analysis of Annexin V/PI double staining cells by
flow cytometry showed apoptosis of SW480 cells by TSN. After
treatment for 48 h, the early apoptosis (lower right quadrant)
rates were 21.80 and 35.67% for TSN of 0.125 and 0.5 μM, but 6.21
and 26.80% for negative control and positive control (5-Fu),
respectively (Fig. 3A). Hoechst
33342 nuclear staining was used to explore the morphological
alterations of cells after TSN (0.125 and 0.5 μM) treatment for 48
h. As indicated in Fig. 3B, the
features of apoptosis including nuclear shrinkage, chromatin
condensation and nucleolus fragmentation in TSN treated cells were
investigated as compared with the control. Taken together, the
above results demonstrated that TSN was able to effectively induce
apoptosis of SW480 cell in dose-dependent manner.
 | Figure 3(A) Apoptosis and (B) apoptotic
morphological changes in SW480 cells after TSN treatment. a,
control; b, 0.125 μM TSN; c, 0.5 μM TSN; d, 50 μg/ml 5-Fu. Lower
left (LL) quadrants, living cells (AV negative/PI negative); lower
right (LR) quadrants, early apoptotic cells (AV positive/PI
negative); upper right (UR) quadrants, late apoptotic cells (AV
positive/PI positive); upper left (UL) quadrants, necrotic cells
(AV negative/PI positive). The numbers represent the percentage of
the cells in the sum of LR quadrants. For apoptotic morphological
changes, apoptotic cells were detected by Hoechst 33342 staining
and measured by fluorescence microscopy (magnification, ×200). |
TSN induced cycle arrest
The cell cycle of SW480 cells treated with TSN was
analyzed by flow cytometric analysis. The proportion of cells in
the S phase increased while that in the G0/G1 phase decreased in a
dose-dependent manner resulting from treatment with TSN (0.125 and
0.5 μM) (Fig. 4). The results
indicated that inhibitory effect on SW480 cell proliferation by TSN
is possibly mediated by blocking cellular progress through the S
phase.
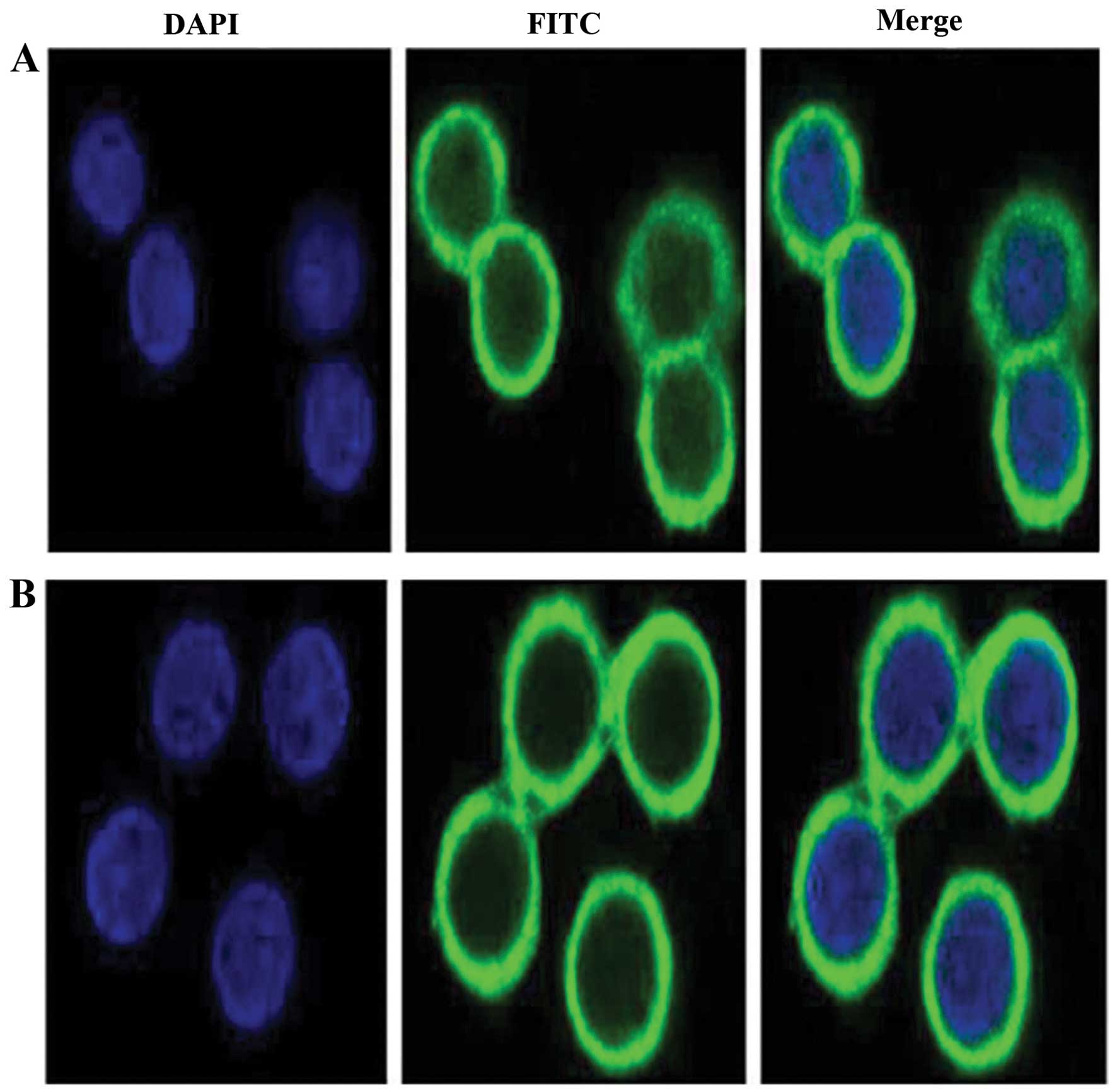
Effects of TSN on the nucleus
translocation of β-catenin in SW480 cell
Confocal laser scanning microscope was used to
observe the nucleus translocation of β-catenin after TSN treatment
in SW480 cells. In the negative control, β-catenin was accumulated
in the nucleus, whereas β-catenin transferred to the outside of the
nucleus when SW480 cells were treated by TSN of 0.5 μM for 48 h
(Fig. 5).
Effects of TSN on the mRNA expression of
genes related to apoptosis and AKT/GSK-3β/β-catenin pathway
RT-PCR was used to investigate the effects of TSN on
the mRNA changes of molecules related to apoptosis and
AKT/GSK-3β/β-catenin pathway. TSN could significantly increase the
mRNA levels of Bax, Bak and GSK-3β while decrease those of Bcl-2,
Bcl-xL, Survivin, cyclin Dl, cyclin D2, cyclin D3, β-catenin,
VEGFA, c-myc and COX-2 in a dose-dependent manner (Fig. 6A). The results demonstrated that
the apoptosis-induction effects on SW480 cells by TSN might be
associated with regulation of mRNA expressions of genes in Bcl-2
family and AKT/GSK-3β/β-catenin pathway.
 | Figure 6Effects of TSN on the mRNA and
protein expression of genes related top apoptosis and
AKT/GSK-3β/β-catenin pathway. (A) RT-PCR analysis of genes related
to apoptosis and AKT/GSK-3β/β-catenin signaling. After SW480 cells
were treated for 48 h with or without TSN (0.125 and 0.5 μM), the
mRNA levels of genes (Bcl-2, Bcl-xL, Bax, Bak, β-catenin, GSK-3β,
c-myc, VEGFA, cyclin Dl, cyclin D2, cyclin D3, COX-2 and survivin)
were determined by RT-PCR. β-actin was taken as an internal control
and 5-Fu (50 μg/ml) as a positive control. *P<0.05
compared with control. The data are representative of three
independent experiments. (B) Western blot analysis of genes related
to apoptosis and AKT/GSK-3β/β-catenin signaling. After SW480 cells
were treated for 48 h with or without TSN (0.125 and 0.5 μM), the
protein levels of genes (Bax, Bcl-2, Pro-caspase-3, Pro-PARP, AKT,
P-AKT Ser473, β-catenin, GSK-3β, P-GSK-3β
Ser9 and VEGFA) were determined by western blot
analysis. β-actin was taken as an internal control and 5-Fu (50
μg/ml) as a positive control. |
Effects of TSN on the protein expression
related to apoptosis and AKT/GSK-3β/β-catenin pathway
Western blot analysis was performed to analyze the
changes of proteins related to apoptosis and AKT/GSK-3β/β-catenin
pathway. After treatment with TSN for 48 h, protein levels of
Bcl-2, pro-caspase-3 and Pro-PARP decreased while that of Bax
increased in a dose-dependent manner. Besides, a significant
decrease of AKT, P-AKT Ser473, P-GSK-3β Ser9,
β-catenin and VEGFA and increase of GSK-3β was observed after TSN
treatment (Fig. 6B). These results
indicated that TSN induced the apoptosis of SW480 cells through
regulating Bcl-2 family and AKT/GSK-3β/β-catenin pathway as well as
activating the caspase-cascade response.
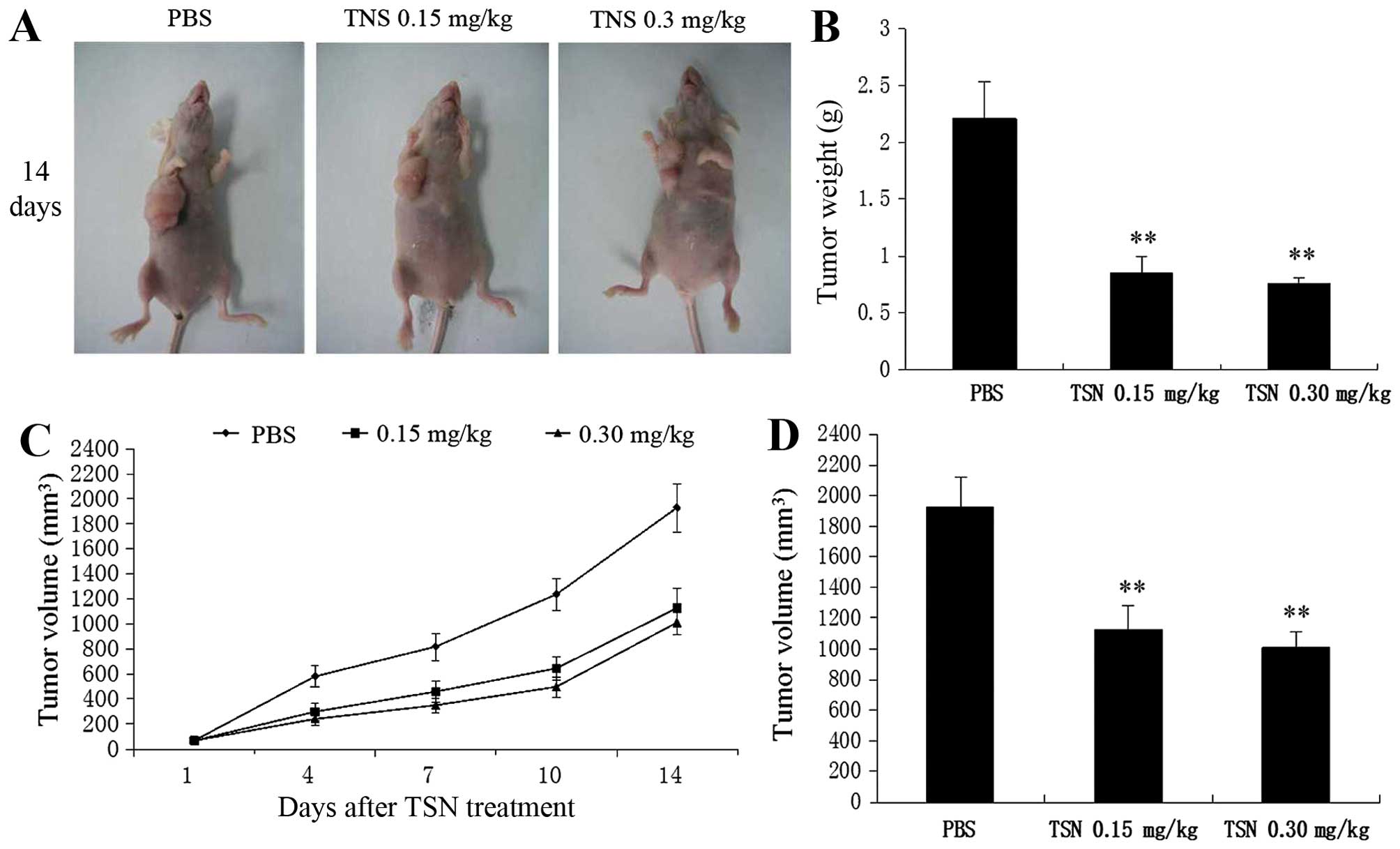
Effects of TSN administration on
xenograft tumor growth
The effects of TSN on xenograft tumor growth were
investigated in vivo. During the whole tumor growth period,
the tumor volume was measured. Tumors of mice in low or high-dose
TSN group grew more slowly compared with those in control group
(Fig. 7A and C). The average
weight and volume of the finally resected tumors in the TSN-treated
groups were significantly lower than those of the control group
(Fig. 7B and D). Nevertheless, a
significant difference in tumor weight and volume was not found
between the low- and high-dose TSN groups. This result strongly
suggested that TSN administration inhibited CRC growth in
vivo.
Discussion
Although chemotherapy is an important therapeutic
strategy for CRC, the prognosis is still poor due to low response
rates to most chemotherapeutic agents and severe toxicities.
Natural plants and their effective components have been studied by
pharmacologists and chemists for their diversity of chemical
structure and promising therapeutic applications to cancer
(18,19). Toosendanin is such a plant
component which has significant inhibitory effects on various
cancers including pheochromocytoma (15), hepatoma (14), leukemia (20), glioblastoma, neuroblastoma,
prostate adenocarcinoma and lymphoma (21). Herein, we reported that TSN
exhibited suppressive activity of the viability of CRC SW480 cells
in a time- and dose-dependent manner with IC50 values of
0.349 μM for 24 h and 0.1059 μM for 48 h, respectively. The
abnormal regulation of cell cycle is suggested to be the driving
factor of tumor formation and regulators in cell cycle are often
considered to be potential therapeutic targets (22). Cyclin D1, a recognized oncogene,
can affect G1/S phase control point in the cell cycle. The
overexpression of cyclin D1 results in disorder regulation of cell
cycle and abnormal cell proliferation (23). Cyclin D2 and cyclin D3 act in
dysregulating normal cell cycle and promoting the proliferation of
cancer cells (24). Our results
indicated that TSN suppressed proliferation of SW480 cell possibly
by blocking the cell cycle through S phase. RT-PCR assay further
showed that the molecular mechanism might be associated with
downregulating the mRNA expressions of cyclin Dl, cyclin D2 and
cyclin D3.
Apoptosis, which differs from necrosis, is a pattern
of programmed death. Lack of apoptosis is closely related to
tumorigenesis and death of cancer cells occurs chiefly via
apoptosis rather than necrosis (25). Apoptosis occurs in the phase in
which cellular progress is blocked. The present study using flow
cytometry and Hoechst 33342 nuclear staining showed that TSN
treatment of 0.125 and 0.5 μM for 48 h increased apoptosis rates of
SW480 cells and revealed the typical apoptotic morphological
alterations. All the above results indicated that induction of cell
apoptosis and cell cycle arrest might contribute to the growth
inhibition effects on CRC cells by TSN.
Caspases are a family of cysteine proteases which
play a central part in the initiation and execution of apoptosis
(14). Caspase-3, activated only
by upstream initiator caspases, is an executioner caspase and
induces apoptosis (26). PARP is
one of the substrates of caspase-3 and plays an role in repairing
DNA damage induced by anticancer agents or radiation. During
apoptosis, caspase-3 restrains the activity of PARP by cleaving it
into two fragments, p89 and p24 (27). Caspase-cascade is regulated by
various factors, such as Bcl-2 family proteins involved in
promoting (Bcl-2, Bcl-xL) or inhibiting (Bax and Bak) apoptosis
(28). RT-PCR and western blot
analysis in the present study revealed that the expressions of Bax
and Bak increased while those of Pro-caspase-3, Pro-PARP, Bcl-2 and
Bcl-xL decreased in TSN-treated CRC cells. Therefore, we
hypothesized that TSN induced SW480 cell apoptosis through
activating the caspase-cascade and modulating Bcl-2 family
molecules. Nevertheless, further research is needed to identify
whether apoptosis-induction in SW480 cell by TSN depends on the
extrinsic (death receptor), or the intrinsic (mitochondrial)
pathway, or both.
A large body of evidence exists indicating that
dysregulation of PI3K/AKT and Wnt signaling pathways plays
important roles in the progression of CRC (29). The interplay between the two
pathways through AKT-GSK-3β-β-catenin axis is found to participate
in vitality of cancer stem cells (30). In the axis, β-catenin enters the
nucleus and binds with T cell factor/lymphoid enhancer
factor(TCF/LEF)-1 proteins, followed by the transactivation of
downstream target oncogenes such as cyclin D, surviving, VEGF,
c-Myc and COX-2 (31–33). As a result, β-catenin serves as a
powerful transcription factor that promotes cell proliferation in
this way (34). Moreover, GSK-3β
leads to phosphorylation and then proteasome-mediated degradation
of β-catenin and activity of GSK-3β can be inhibited resulting from
phosphorylation in serine-9 of GSK-3β by p-AKT (10,13).
Thus, AKT phosphorylation of GSK-3β results in β-catenin
stabilization and translocation to the nucleus. In the present
study, we provide evidence that TSN treatment translocated
β-catenin outside the nucleus and attenuated levels of AKT, p-AKT
Ser473, p-GSK-3β Ser9, β-catenin as well as
survivin, cyclin D, VEGFA, c-Myc and COX-2 which are
β-catenin-activated genes. The expression of GSK-3β was upregulated
by TSN. All the findings suggested that TSN inhibited the activity
of β-catenin through suppressing p-AKT, activating GSK-3β and then
inducing degradation of β-catenin. Importantly, mounting research
has confirmed that downstream genes of β-catenin such as survivin,
cyclin D, VEGFA, c-Myc and COX-2 are involved in promoting tumor
growth and antiapoptosis (35,36).
Bcl-2 activity can be regulated by β-catenin pathway in CRC
progression (37). Therefore, the
results in the present study suggested that the molecular mechanism
of inhibiting proliferation and inducing apoptosis by TSN may lie
on suppression of the AKT/GSK-3β/β-catenin pathway. In vivo
study demonstrated that TSN administration significantly inhibited
CRC growth in the mouse tumor xenograft model. In summary, our
research might provide an experimental basis for TSN as a new
chemotherapy drug against CRC.
In conclusion, our findings indicate that TSN
inhibits growth and induces apoptosis in CRC cells through
suppression of the AKT/GSK-3β/β-catenin pathway, suggesting that
TSN may possess potential for use in CRC treatment.
Acknowledgements
The present study is supported by the National
Nature Science Foundation of China (nos. 81302093 and
81272752).
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grady WM and Carethers JM: Genomic and
epigenetic instability in colorectal cancer pathogenesis.
Gastroenterology. 135:1079–1099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KY, Cha IH, Ahn JB, Kim NK, Rha SY,
Chung HC, Roh JK and Shin SJ: Estimating the adjuvant chemotherapy
effect in elderly stage II and III colon cancer patients in an
observational study. J Surg Oncol. 107:613–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kolligs FT, Bommer G and Göke B:
Wnt/beta-catenin/tcf signaling: A critical pathway in
gastrointestinal tumorigenesis. Digestion. 66:131–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta N, Schmitt F, Grebhardt S and Mayer
D: Beta-catenin is a positive regulator of estrogen receptor-alpha
function in breast cancer cells. Cancers (Basel). 3:2990–3001.
2011. View Article : Google Scholar
|
|
7
|
Yuan G, Wang C, Ma C, Chen N, Tian Q,
Zhang T and Fu W: Oncogenic function of DACT1 in colon cancer
through the regulation of β-catenin. PLoS One. 7:e340042012.
View Article : Google Scholar
|
|
8
|
Satow R, Shitashige M, Jigami T, Fukami K,
Honda K, Kitabayashi I and Yamada T: β-catenin inhibits
promyelocytic leukemia protein tumor suppressor function in
colorectal cancer cells. Gastroenterology. 142:572–581. 2012.
View Article : Google Scholar
|
|
9
|
Rowan AJ, Lamlum H, Ilyas M, Wheeler J,
Straub J, Papadopoulou A, Bicknell D, Bodmer WF and Tomlinson IP:
APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’
and interdependence of the ‘two hits’. Proc Natl Acad Sci USA.
97:3352–3357. 2000. View Article : Google Scholar
|
|
10
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477.
2009.PubMed/NCBI
|
|
11
|
Mills CN, Nowsheen S, Bonner JA and Yang
ES: Emerging roles of glycogen synthase kinase 3 in the treatment
of brain tumors. Front Mol Neurosci. 4:472011. View Article : Google Scholar
|
|
12
|
Cohen P and Goedert M: GSK3 inhibitors:
Development and therapeutic potential. Nat Rev Drug Discov.
3:479–487. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He Y, Wang J, Liu X, Zhang L, Yi G, Li C,
He X, Wang P and Jiang H: Toosendanin inhibits hepatocellular
carcinoma cells by inducing mitochondria-dependent apoptosis.
Planta Med. 76:1447–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang MZ, Wang ZF and Shi YL: Involvement
of cytochrome c release and caspase activation in
toosendanin-induced PC12 cell apoptosis. Toxicology. 201:31–38.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu JC, Min ZD and Ip NY: Melia toosendan
regulates PC12 Cell differentiation via the activation of protein
kinase A and extracellular signal-regulated kinases. Neurosignals.
13:248–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B, Wang ZF, Tang MZ and Shi YL:
Growth inhibition and apoptosis-induced effect on human cancer
cells of toosendanin, a triterpenoid derivative from chinese
traditional medicine. Invest New Drugs. 23:547–553. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clardy J and Walsh C: Lessons from natural
molecules. Nature. 432:829–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mishra BB and Tiwari VK: Natural products:
An evolving role in future drug discovery. Eur J Med Chem.
46:4769–4807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ju J, Qi Z, Cai X, Cao P, Huang Y, Wang S,
Liu N and Chen Y: The apoptotic effects of toosendanin are
partially mediated by activation of deoxycytidine kinase in HL-60
cells. PLoS One. 7:e525362012. View Article : Google Scholar
|
|
21
|
Shi YL and Li MF: Biological effects of
toosendanin, a triterpenoid extracted from Chinese traditional
medicine. Prog Neurobiol. 82:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamgue O, Chai CS, Hao L, Zambe JC, Huang
WW, Zhang B, Lei M and Wei YM: Triptolide inhibits histone
methyltransferase EZH2 and modulates the expression of its target
genes in prostate cancer cells. Asian Pac J Cancer Prev.
14:5663–5669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji AJ, Liu SL, Ju WZ and Huang XE:
Anti-proliferation effects and molecular mechanisms of action of
tetramethypyrazine on human SGC-7901 gastric carcinoma cells. Asian
Pac J Cancer Prev. 15:3581–3586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song YA, Park YL, Kim KY, Myung E, Chung
CY, Cho SB, Lee WS, Jung YD, Kweon SS and Joo YE: RON is associated
with tumor progression via the inhibition of apoptosis and cell
cycle arrest in human gastric cancer. Pathol Int. 62:127–136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arends MJ, Morris RG and Wyllie AH:
Apoptosis. The role of the endonuclease. Am J Pathol. 136:593–608.
1990.PubMed/NCBI
|
|
26
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gambi N, Tramontano F and Quesada P:
Poly(ADPR)polymerase inhibition and apoptosis induction in
cDDP-treated human carcinoma cell lines. Biochem Pharmacol.
75:2356–2363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu W, Jia G, Meng X, Zhao C, Zhang L, Ren
Y, Pan H and Ni Y: Beta-catenin mediates the apoptosis induction
effect of celastrol in HT29 cells. Life Sci. 91:279–283. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar
|
|
31
|
Cai C and Zhu X: The Wnt/β-catenin pathway
regulates self-renewal of cancer stem-like cells in human gastric
cancer. Mol Med Rep. 5:1191–1196. 2012.PubMed/NCBI
|
|
32
|
Nuñez F, Bravo S, Cruzat F, Montecino M
and De Ferrari GV: Wnt/β-catenin signaling enhances
cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer
cells. PLoS One. 6:e185622011. View Article : Google Scholar
|
|
33
|
Park EJ, Chung HJ, Park HJ, Kim GD, Ahn YH
and Lee SK: Suppression of Src/ERK and GSK-3/β-catenin signaling by
pinosylvin inhibits the growth of human colorectal cancer cells.
Food Chem Toxicol. 55:424–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kharbanda S, Pandey P, Schofield L,
Israels S, Roncinske R, Yoshida K, Bharti A, Yuan ZM, Saxena S,
Weichselbaum R, et al: Role for Bcl-xL as an inhibitor of cytosolic
cytochrome C accumulation in DNA damage-induced apoptosis. Proc
Natl Acad Sci USA. 94:6939–6942. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Q, Dashwood WM, Zhong X, Nakagama H and
Dashwood RH: Bcl-2 overexpression in PhIP-induced colon tumors:
Cloning of the rat Bcl-2 promoter and characterization of a pathway
involving beta-catenin, c-Myc and E2F1. Oncogene. 26:6194–6202.
2007. View Article : Google Scholar : PubMed/NCBI
|