Introduction
Rhabdomyosarcoma (RMS) is the most common
soft-tissue sarcoma of adolescence and childhood. RMS accounts for
5% of all malignant tumors in patients under 15 years of age
(1,2) and belongs to the family of so-called
‘small round blue tumor cells’, which often infiltrate bone marrow
(BM) and can resemble hematological blasts. Thus, RMS cells on BM
smears may sometimes be misdiagnosed as acute leukemia cells
(2). There are two major
histological subtypes of this tumor: alveolar rhabdomyosarcoma
(ARMS) and embryonal rhabdomyosarcoma (ERMS). ARMS is associated
with more aggressive behavior and a worse prognosis than ERMS.
At the molecular level, ARMS is characterized by the
t(2;13)(q35;q14) translocation in 70% of cases and the variant
t(1;13)(p36;q14) in a smaller percentage of cases (3). These translocations disrupt the
PAX3 and PAX7 genes on chromosomes 2 and 1,
respectively, and the FOXO1 gene on chromosome 13,
generating PAX3-FOXO1 and PAX7-FOXO1
fusion genes. The resulting fusion proteins, PAX3-FOXO1 and
PAX7-FOXO1, have enhanced transcriptional activity compared with
wild-type PAX3 and PAX7 and are postulated to play a role in cell
survival and dysregulation of the cell cycle in ARMS (1). Recently, we also found that
imprinting of the differentially methylated region (DMR) at the
DLK1-GTL2 locus varies with the histologic subtype:
ERMS tumors have loss of imprinting, whereas ARMS tumors have
erasure of imprinting at this locus (4). This difference provides further
evidence that the cellular origin of these tumors is different.
The erythropoietin receptor (EpoR) is expressed by
cells from the erythroid lineage, although evidence has accumulated
that it is also expressed by several solid tumors (5–13)
including neuroblastoma, Ewing's sarcoma family of tumors,
pediatric brain tumors (medulloblastoma, astrocytoma and
ependymoma), Wilms' tumor, hepatoblastoma, as well as it had been
detected in ERMS but not in ARMS patient cells (14).
Recently our group demonstrated the presence of
functional EpoR in human and murine germline-derived cell lines,
including teratocarcinomas and ovarian cancer cells (15). This observation is intriguing in
the context of the present study, as RMS cells express several
cancer testis antigens (CTAs) (16), which are characteristic of
germline-derived cells. Moreover, 150 years ago, Virchow (17) and Conheim (18) proposed the so-called ‘embryonic
rest hypothesis of cancer development’, in which malignancies may
develop from dormant embryonic or germ cells residing in adult
tissues. Small round blue cell tumors, including RMS, are potential
candidates for such malignancies. Interestingly, a recent study
demonstrated that the PAX7 gene, which plays an important
role in skeletal muscle development, is one of the stem cell
markers in gonads (19). However,
the potential relationship between the germline and target cells
for RMS requires further study.
In the present study, we found expression of EpoR
mRNA in all tested RMS cell lines and patient samples. Importantly,
EpoR was functional in all RMS cell lines tested, responding to
stimulation by erythropoietin (EPO) by an increase in chemotaxis,
adhesion, and phosphorylation of MAPKp42/44 and AKT. Moreover, EPO
stimulates proliferation of RMS cells and may also increase their
resistance to vincristine (VCR). Our results have important
clinical implications for potential EPO therapy in cancer patients
to ameliorate tumor-associated anemia. The presence of functional
EpoR in RMS cells indicates that EPO supplementation may have the
unwanted side effect of facilitating tumor progression in RMS
patients.
Materials and methods
Cell lines
We used several human RMS cell lines (provided by Dr
Peter Houghton, Nationwide Children's Cancer Center, Columbus, OH,
USA), including both fusion-positive (RH28, RH30 and RH41) and
fusion-negative (JR, RD, RH18, RH36 and SMS-CTR) cell lines. All
cell lines used in these studies were authenticated by short tandem
repeat (STR) analysis. STR profiles were compared with those of the
original cell lines, obtained in Dr Peter Houghton's laboratory, or
with published profiles. SMS-CTR and RH36 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS), 100 U/ml penicillin and 10 μg/ml
streptomycin. All other cell lines were maintained in Roswell Park
Memorial Institute (RPMI)-1640 medium, containing 10% FBS, 100 U/ml
penicillin and 10 μg/ml streptomycin. All cells were
cultured in a humidified atmosphere of 5% CO2 at 37°C,
and the medium was changed every 48 h.
Quantitative reverse transcription
(qRT)-PCR to detect EpoR mRNA
Total RNA was isolated from RMS cells with the
RNeasy kit (Qiagen, Inc., Valencia, CA, USA). Human muscle RNA was
obtained from Ambion (Austin, TX, USA). The RNA was
reverse-transcribed with MultiScribe Reverse Transcriptase,
oligo(dT) and a random hexamer primer mix (all from Applied
Biosystems Life Technologies, Foster City, CA, USA). Quantitative
assessment of the mRNA levels of target genes was performed by
qRT-PCR using an ABI Prism 7500 sequence detection system (Applied
Biosystems Life Technologies) with Power SYBR-Green PCR Master mix
reagent and specific primers (hEpoR forward, CCA TGG ACA CTG
TGC CCT G and reverse, CCA TCG GAT AAG CCC CCT T; hEPO
forward, CAC CAC GCC TCA TCT GTG AC and reverse, CAC AGC CCG TCG
TGA TAT TCT). The PCR cycling conditions were as follows: 95°C (15
sec), 40 cycles at 95°C (15 sec) and 60°C (1 min). According to
melting point analysis, only one PCR product was amplified under
these conditions. The relative quantity of a target, normalized to
the endogenous β2-microglobulin gene as control and relative to a
calibrator, is expressed as 2−ΔΔCt (fold difference),
where ΔCt is the threshold cycle, Ct = (Ct of target genes)-(Ct of
the endogenous control gene, β2-microglobulin), and ΔΔCt = (ΔCt for
target gene in test sample)-(ΔCt for target gene in calibrator
sample).
Conventional RT-PCR
Total RNA from various cells was isolated using the
RNeasy Mini kit, including treatment with DNase I (both from
Qiagen, Inc.). The mRNA (200 ng) was reverse-transcribed with
TaqMan Reverse Transcription Reagents (Applied Biosystems Life
Technologies) according to the manufacturer's instructions. The
resulting cDNA fragments were amplified (1 cycle of 8 min at 95°C,
2 cycles of 2 min at 95°C, 1 min at 60°C, 1 min at 72°C, and
subsequently 35 cycles of 30 sec at 95°C, 1 min at 60°C, 1 min at
72°C, and 1 cycle of 10 min at 72°C) using AmpliTaq Gold with
sequence-specific primers designed using the NCBI/Primer-BLAST
program; one primer was designed to include an exon-intron boundary
(β-actin forward, GGA TGC AGA AGG AGA TCA CTG and reverse,
CGA TCC ACA CGG AGT ACT TG; hEpoR forward, CTC CCT TTG TCT
CCT GCT CG and reverse, TAG GCA GCG AAC ACC AGA AG; hEPO
forward, TCA TCT GTG ACA GCC GAG TC and reverse, GCC ACT GAC GGC
TTT ATC CA; and hCD131 forward, AGG GGC AGA AGA AAC CAT CC
and reverse, GGC CTG TCT GGT TGG AAT GA).
Cell proliferation
Cells were grown in 24-well culture plates at an
initial density of 1.5×104 cells/well. After 24 h, the
medium was changed to new medium supplemented with 10% FBS, and the
cells were cultured in the presence or absence of VCR (1 nM to 1
μM). The complete medium (with 10% FBS) was used as a
control. The cell number was calculated at 24, 48 and 72 h after
the change of medium. At the indicated time points, cells were
harvested from the culture plates by trypsinization.
Chemotaxis assay
Chemotaxis assays were performed in a modified
Boyden's chamber with 8-μm pore polycarbonate membrane
inserts (Costar Transwell; Corning Costar, Lowell, MA, USA) as
previously described (20). In
brief, cells detached with 0.25% trypsin were seeded into the upper
chamber of an insert at a density of 4.5×104 in 100
μl. The lower chamber was filled with pre-warmed culture
medium containing test reagents. Medium supplemented with 0.5% BSA
was used as a negative control. After 24 h, the inserts were
removed from the Transwell supports. The cells that had not
migrated were scraped off with cotton wool from the upper membrane,
and the cells that had transmigrated to the lower side of the
membrane were fixed and stained with HEMA 3 (protocol; Thermo
Fisher Scientific, Pittsburgh, PA, USA) and counted on the lower
side of the membrane using an inverted microscope.
Hypoxia assay
A hypoxic condition was achieved by using a
nitrogen-balanced hypoxia chamber (BioSpherix Ltd., Lacona, NY,
USA) providing a gas mixture containing 5% CO2 and 2%
O2 at 37°C.
Phosphorylation of intracellular pathway
proteins
RMS cell lines were incubated overnight in RPMI-1640
medium containing low levels of bovine serum albumin (BSA, 0.5%) to
render the cells quiescent. After the cells were stimulated with
EPO (0.5 and 20 IU/ml) at 37°C for 5 min, the cells were lysed for
10 min on ice in RIPA lysis buffer containing protease and
phosphatase inhibitors (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). The extracted proteins were separated on a 4–12% SDS-PAGE
gel and transferred to a PVDF membrane. Phosphorylation of the
serine/threonine kinase AKT (phospho-AKT473) and p44/42
mitogen-activated kinase (phospho-p44/42 MAPK) was detected by
phospho-specific p44/42 MAPK mouse and rabbit polyclonal antibodies
(Cell Signaling Technology, Inc., Danvers, MA, USA) with
HRP-conjugated goat anti-mouse and anti-rabbit secondary antibodies
(Santa Cruz Biotechnology, Inc.). Equal loading in the lanes was
evaluated by stripping the blots and reprobing with anti-p42/44
MAPK monoclonal antibody (clone no. 9102) and anti-AKT polyclonal
antibody (both from Cell Signaling Technology, Inc.). The membranes
were developed with an enhanced chemiluminescence (ECL) reagent
(Amersham Life Sciences, Arlington Heights, IL, USA), dried, and
subsequently exposed to film (Hyperfilm; Amersham Life
Sciences).
Adhesion assay to fibronectin
Cells were made quiescent for 3 h with 0.5% BSA in
RPMI-1640 before incubation with EPO (2 and 20 IU/ml).
Subsequently, cell suspensions (2×103/100 μl)
were added directly to 96-well plates covered with fibronectin and
incubated for 5 min at 37°C. The wells were coated with fibronectin
(10 μg/ml) overnight at 4°C and blocked with 0.5% BSA for 1
h before the experiment. Following incubation, the plates were
vigorously washed three times to remove non-adherent cells, and the
adherent cells were counted using an inverted microscope.
Hypoxic assay
To evaluate the effect of hypoxia on expression of
CD131 and endogenous EPO and EpoR in RMS cells, cells were
incubated in hypoxic condition by using a nitrogen-balanced hypoxia
chamber providing a gas mixture containing 5% CO2 and 2%
O2 at 37°C. Cells were treated for 24 h and subsequently
RNA has been extracted for RT-PCR analysis using RNeasy kit
(Qiagen, Inc.).
FACS analysis
To evaluate expression of EpoR in fusion positive
and negative RMS cells, the cells were incubated with human EpoR
antibody (APC, clone 38421) for 30 min on ice. After washing, cells
were evaluated using a BD LSR II flow cytometer (BD Biosciences,
San Jose, CA, USA). Representative FACS results are shown. FACS
analysis was also employed in proliferation assay in the presence
of VCR (1 nM-1 μM) or EPO (20 IU/ml) and in apoptosis assay
employing Annexin V-PE.
Apoptosis assay
RMS cells were cultured with or without EPO (20
IU/ml) and with or without VCR (1 μM) in serum-free medium
for 24, 48 and 72 h. Apoptosis was detected by FACS using the
Annexin V-PE Apoptosis Detection kit (BD Pharmingen, San Diego, CA,
USA). Cells were stained for apoptotic cell detection with Annexin
V-PE for 15 min at RT.
Patient samples
Fifty-eight RMS frozen primary tumor specimens (26
PAX3-FOXO1-positive, 7 PAX7-FOXO1-positive, and 25 fusion-negative)
were used for microarray profiling. Total RNA was extracted from
primary tumors using RNA STAT-60 (Tel-Test, Friendswood, TX, USA)
in accordance with manufacturer's instructions. These RNA samples
were analyzed on Affymetrix GeneChip® Human Genome U133
Plus 2.0 Arrays at the University of Pennsylvania Microarray Core
Facility. The expression array data were normalized and log2
transformed with Robus Multiarray Average (RMA) in the Bioconductor
oligo package. The expression level of EPOR was calculated as an
average of three probe sets (probe set ID: 215054_at, 209962_at and
37986_at). Probe set ID 215059_at is the only available probe set
for CD131 on the U133 Plus 2.0 array.
Statistical analysis
The results are presented as mean ± SD. Statistical
analysis of the data was done using Student's t-test for unpaired
samples, with P<0.05 considered significant.
Results
Human RMS cell lines express EpoR
First, we performed RT-PCR studies to evaluate mRNA
expression in three human fusion-positive (RH28, RH30 and RH41) as
well as five human fusion-negative (JR, RD, RH18, RH36 and SMS-CTR)
RMS cell lines. Fig. 1A, shows
that we were able to detect EpoR mRNA by regular RT-PCR in all cell
lines employed in this study, but not in skeletal muscle cells. As
shown in Fig. 1B, all cell lines,
especially RH18 and RH36, expressed high levels of EpoR mRNA as
determined by quantitative RT-PCR. Of note very week signal was
also detected in skeletal muscle cells by employing this highly
sensitive cDNA amplification method. Expression of EpoR was
subsequently confirmed by FACS (Fig.
1C), and RH36 and RH18 highly expressed this receptor at the
protein level. No expression was detected on skeletal muscle cells
(data not shown).
Since it is known that EPO signals via EpoR-EpoR
homodimers in erythroid cells and may signal via EpoR-CD131
heterodimers in non-erythroid cells (21,22),
we evaluated the expression of CD131 in our RMS cell lines by FACS
and RT-PCR, and found that CD131 was not detectably expressed in
these cells (data not shown). However, when we exposed RMS cells to
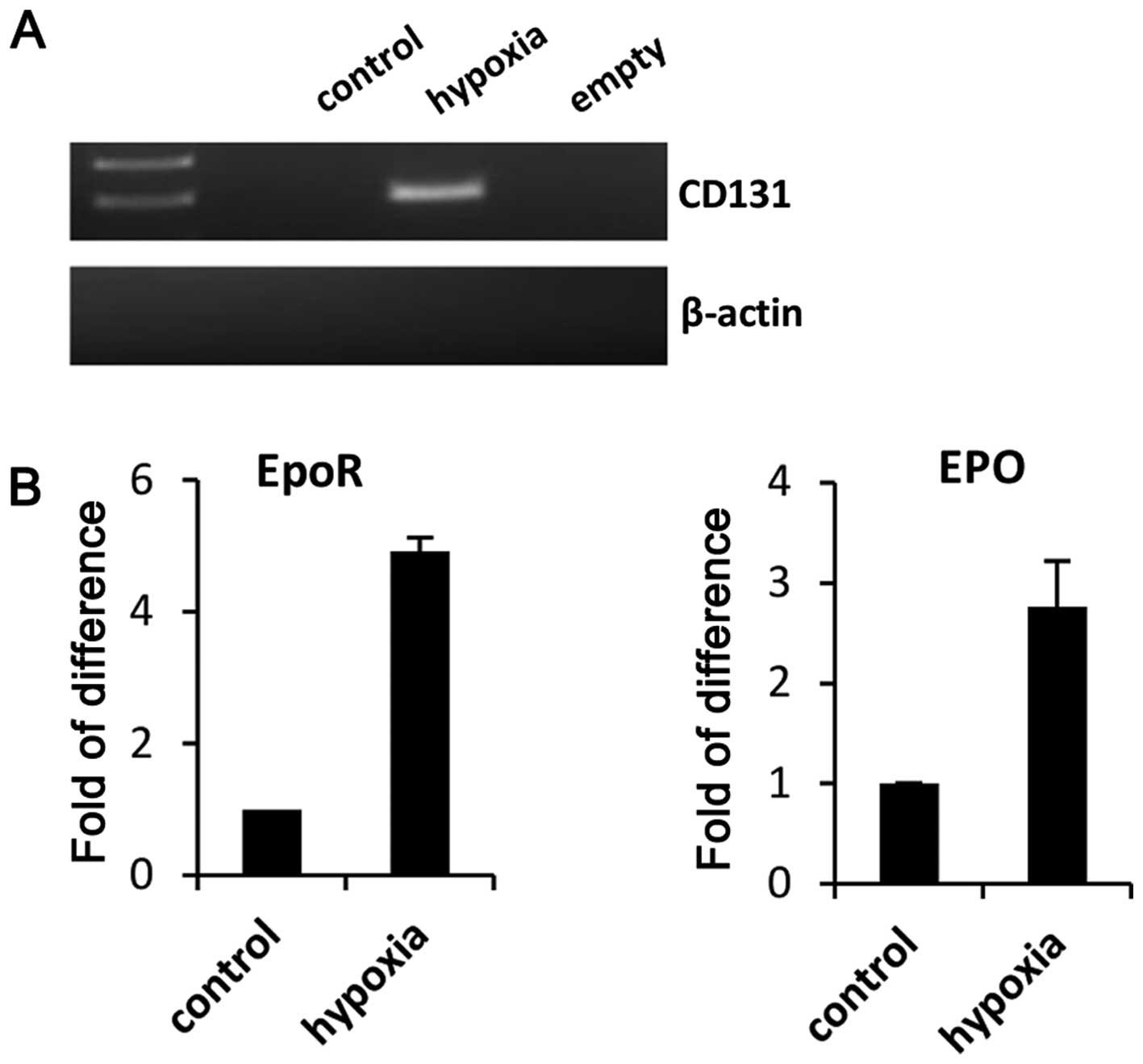
hypoxia, CD133 become detectable (Fig.
2A). In parallel we observed upregulation of EpoR and EpO
(Fig. 2B). Furthermore, since our
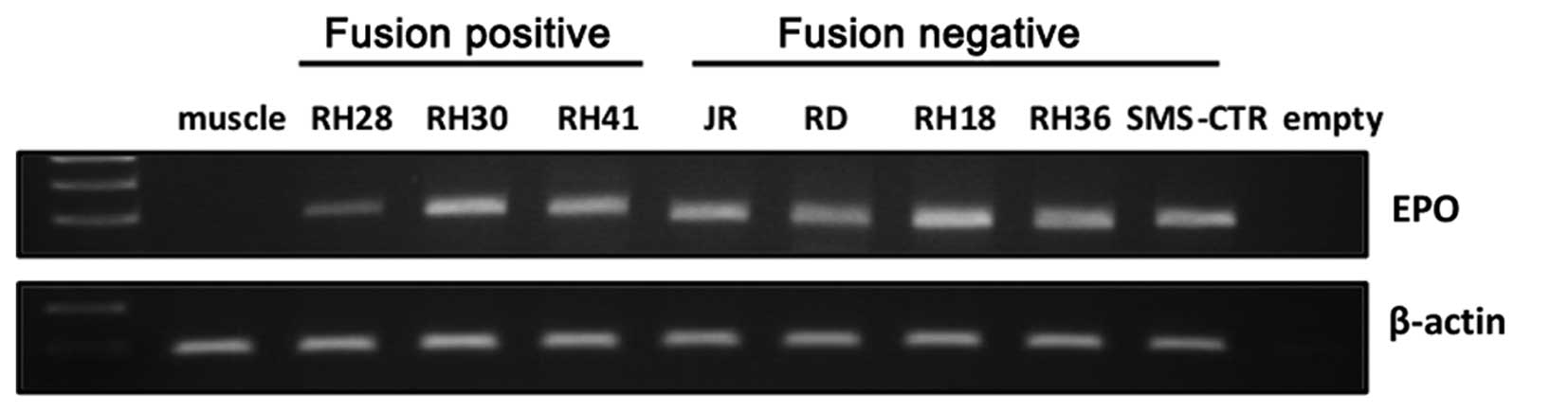
RT-PCR analysis revealed expression of endogenous EpO in studied
RMS cell lines (Fig. 3), we cannot
exclude contribution of autocrine EpO-EpoR interactions, and this
phenomenon is currently investigated in our laboratory.
EpoR induces motility and adhesion of
cells in human RMS cell lines
Next, to evaluate whether EpoR is functional in
human RMS cell lines, we performed chemotaxis and cell adhesion
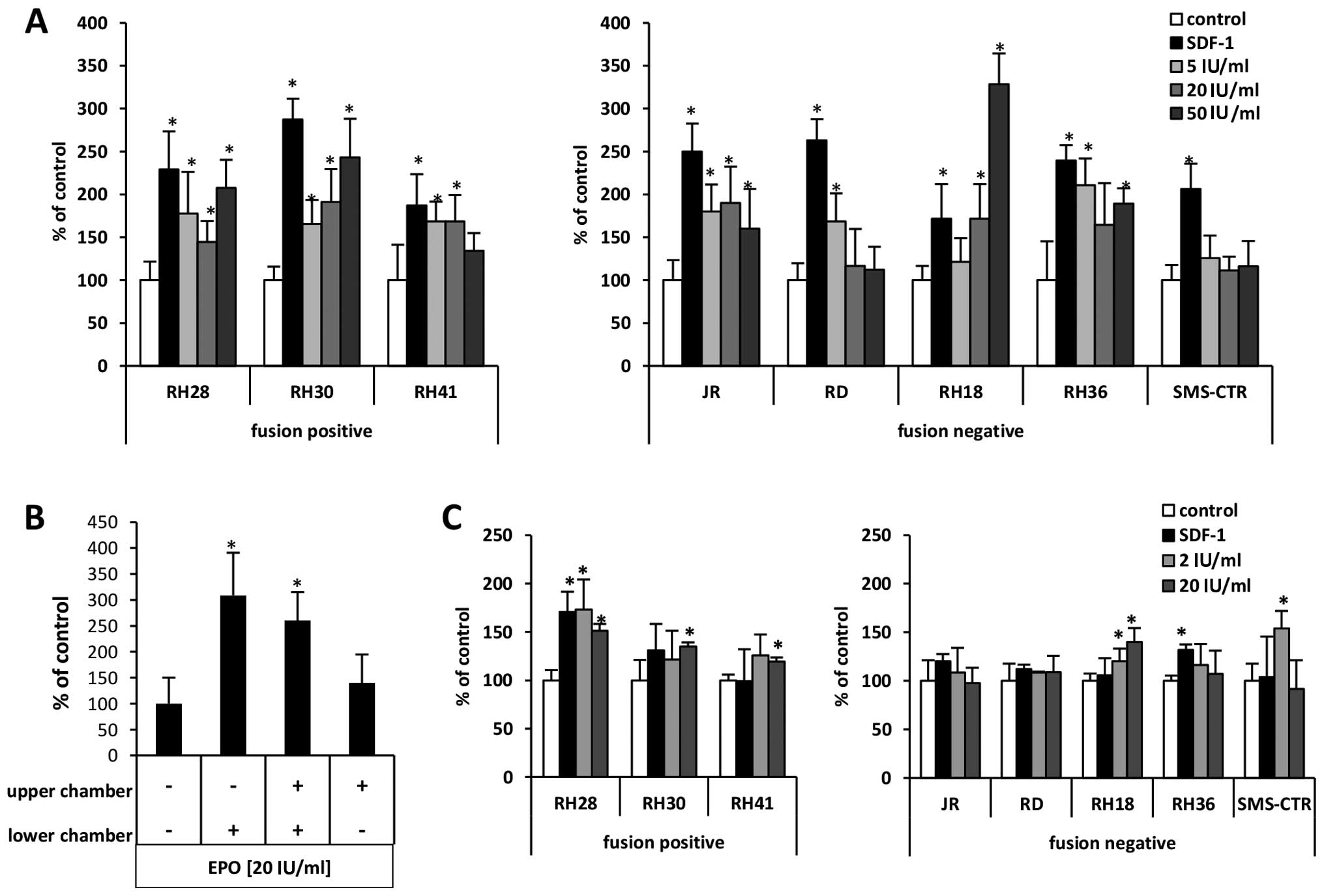
studies. In Transwell chemotaxis assays (Fig. 4A) we employed EPO at doses of 5, 20
and 50 U/ml as chemoattractant and compared the responsiveness of
RMS cells to 300 ng/ml of the α-chemokine stromal-derived factor 1
(SDF-1), which, as we previously described (23,24),
is a known chemoattractant for these cells. We observed that EPO
induced motility of all RMS cell lines evaluated in our studies.
The highest responsiveness was observed for RH30 (fusion-positive)
cell lines and for RH18, RH30, RH36 and RH28 cell lines. The
specificity of EpO-EpoR interaction has been confirmed by employing
EpoR blocking antibodies (data not shown).
The motility of cancer cells plays a crucial role in
the process of tumor metastasis, and to address whether the
observed increase in motility of RMS cells in response to EPO is
the result of a chemotactic or a chemokinetic response, we
performed a checkerboard assay in which EPO was added at the same
time into the upper and the lower Transwell chambers so that there
was no EPO gradient between the chambers. Fig. 4B, demonstrates that the migration
of RH30 cells in response to EPO in the upper and lower chambers
was not significantly changed when it was in both chambers compared
to only one, the lower chamber. This finding indicates that EPO is
mainly a chemokinetic rather than a chemotactic factor for RMS
cells.
Another important feature of metastasizing cancer
cells is their adhesive properties at the site of metastasis.
Therefore, we next studied the effect of EPO on adhesion of RMS
cell lines to fibronectin. Depending on the dose employed, we found
that EPO may induce adhesion of RMS cells to fibronectin (Fig. 4C), and this effect was particularly
visible for RH28 and SMS-CTR cells. Again, the specificity of
EpO-EpoR interaction was confirmed by employing EpoR blocking
antibodies (data not shown).
EPO may induce proliferation of RMS cells
and increase their resistance to VCR
EPO is a known growth factor for erythroid cells
(22) as well as for some tumor
cell lines (5–14). Therefore, based on receptor
expression studies (Fig. 1), we
exposed RH18 cell line and the RH36 cell line to 20 IU/ml EPO and
evaluated its effect on proliferation of these cells. We found that
EPO stimulated proliferation of both RH18 (Fig. 5A) and RH36 cells (Fig. 5B). Western blot analysis confirmed
that both cell lines responded to EPO stimulation by
phosphorylation of MAPKp44/42 and to a lesser degree
also by phosphorylation of Aktser473.
Since EPO has pro-proliferative effects on RMS
cells, to assess its effect on the potential resistance of RMS
cells to chemotherapy, we performed proliferation assays of these
cells in medium supplemented with EPO and different doses of VCR.
As demonstrated in Fig. 6, we
observed an increase in survival of RH18 (Fig. 6A) and RH36 cells (Fig. 6B) in the presence of VCR (25). While a significant beneficial
effect was observed for RH18 cells after and 72 h of treatment,
some beneficial effect for RH36 cells was observed after only 24 h
of treatment. The cell proliferation data are supported by the
effect of EpO on inhibiting apoptosis in VCR treated cells (data
not shown).
EpoR is expressed in patient RMS
samples
Finally, we assessed EpoR expression from expression
microarray analyses of 26 PAX3-FOXO1-positive, 7
PAX7-FOXO1-positive, and 25 fusion-negative patient samples. In all
these tumor samples, we were able to detect EpoR expression;
however, expression of EpoR was higher in PAX3-FOXO1-positive and
in fusion-negative samples than in PAX7-FOXO1-positive samples
(Fig. 7A). Interestingly, in
contrast to established RMS cell lines (data not shown), CD131
expression was detected in all human RMS samples (Fig. 7B), and similar to our EpoR
findings, expression of CD133 was higher in PAX3-FOXO1-positive as
compared to PAX7-FOXO1-positive tumor samples.
Discussion
It is well-known that systemic anemia is an
independent prognostic factor for poor survival in cancer patients
(26). Therefore, it has been
proposed that recombinant human EPO be used in clinical settings to
treat this complication. Unfortunately, such treatment has often
resulted in rapid tumor progression and reduced survival (26). EpoR has been reported to be
expressed by several solid tumors (5–13)
including several pediatric malignancies such as neuroblastoma,
Ewing's sarcoma, Wilms' tumor, hepatoblastoma and what is relevant
to this study was also detected in ERMS patient tumor samples
(14). The salient observation of
our work is that not only human ERMS cells (14), but all types of RMS express EpoR
and that this receptor may regulate pro-metastatic behavior of RMS
cells. Therefore EPO is a previously unrecognized pro-metastatic
and growth-promoting factor for this tumor, which indicates a
potential risk in treating RMS patient anemia with human
recombinant EPO.
EpoR is expressed in cell lines derived from several
malignancies; however, to our surprise, no studies on its
expression and the role of the EPO-EpoR axis have been performed in
RMS cell lines and patient samples. By contrast, it has been shown
that EpoR is a negative prognostic factor in gastric, renal,
breast, and head and neck cancer and in melanoma (8–11,26).
For example, in breast cancer cells EPO stimulates proliferation by
involving Egr1 and Fos transcription factors (7), activates the survival of stem-like
breast cancer cells to protect them from chemotherapy (6), and antagonizes trastuzumab treatment
of breast cancer cells via Jak-2-mediated activation of Src and
PTEN inactivation (5). In
agreement with these observations, EPO in our hands enhanced the
resistance of human RMS cells to VCR treatment.
In some malignancies it has been shown that EPO can
be upregulated in a hypoxia-dependent manner and influence tumor
cell growth by involving an EPO-EpoR autocrine loop. In support of
this mechanism, autocrine-paracrine EPO-EpoR signaling has been
described in cervical cancer cells (12). In these tumor cells, EPO, in
cooperation with the stem cell factor (SCF), induces migration of
malignant cells (13). An
autocrine EPO-EpoR axis has also been reported to be involved in
the progression of neuroblastoma (27–29).
In contrast to our data with RMS cells, EPO did not directly affect
cell proliferation in this pediatric cancer (29), but was involved in increasing
vascularity of the growing tumor and thus contributed indirectly to
its progression (27,28).
Thus, accumulating evidence indicates that EpoR is
functional in cells in several human malignancies. It has also been
reported that, in contrast to erythroid cells in which EPO signals
via EpoR-EpoR homodimers, in non-erythroid cells EPO may signal by
employing EpoR-CD131 heterodimers (22). CD131 is known as a the common β
chain of the high affinity receptor for interleukin-3 (IL-3),
interleukin-5 (IL-5) and granulocyte-macrophage colony stimulating
factor (GM-CSF) (22). Based on
these findings, we evaluated the expression of CD131 in established
human RMS cell lines by FACS, but the expression of CD131 was not
detected. However, when RMS cells were cultured in hypoxic
conditions CD133 as well as EpoR became upregulated, as expected.
In contrast CD131 was expressed in RMS patient samples isolated
from hypoxic tumor tissue. The role of CD133 that is a common
receptor subunit to several receptors for hematopoietic cytokines
(GM-CSF, IL-3 and IL-5) in signaling within RMS cells requires
further studies.
The recurrence of tumor growth after successful
initial treatment and the fatal tendency of cancerous cells to
spread and metastasize to different vital organs are major problems
affecting the survival of cancer patients. The ability to
metastasize is one characteristic of highly malignant and primitive
tumors, including RMS. The tropism of cancer cells to metastasize
to selected organs requires the involvement of organ-specific
factors that direct metastasis. These factors may promote the
formation of a pre-metastatic niche that provides metastasizing
tumor cells with a favorable growth and survival environment.
Here, for the first time, we identified EPO as a
potential pro-metastatic factor for RMS cells. The metastasis of
RMS cells, however, is directed by several other factors, including
growth factors (e.g., hepatocyte growth factor, insulin-like growth
factors 1 and 2) (24), chemokines
(stromal-derived factor 1 α and macrophage inhibitory factor)
(30,31), cytokines (leukemia inhibitory
factor) (32), as well as several
bioactive lipids, such as sphingosine-1-phosphate,
ceramide-1-phosphate-1 (33),
lysophosphatidylcholine and lysophosphatidic acid (34). In our studies we have shown for the
first time that EPO increases the chemokinesis of RMS cells, and
thus it may promote their egress from the primary tumor and direct
their spread to distant locations.
It is known that the EpoR is expressed by cells from
the erythroid lineage and is expressed at very early stages of
embryogenesis. Interestingly, we recently demonstrated the presence
of functional EpoR in human and murine germ-line-derived cell
lines, including teratocarcinomas and ovarian cancer cells
(15). RMS cells also express
several CTAs, which are characteristic of germline-derived cells.
Interestingly, 150 years ago, Virchow (17) and Conheim (18) proposed the so-called ‘embryonic
rest hypothesis of cancer development’, in which malignancies may
develop from dormant embryonic or germ cells residing in adult
tissues. Small round blue cell tumors, including RMS, are
candidates for such malignancies. In support of this possibility,
the PAX7 gene, which plays an important role in skeletal
muscle development and is involved in one of the characteristic
gene fusions in RMS, has recently been established as a novel
marker for gonadal stem cells in testes (19).
In conclusion, since recombinant human EPO is
frequently employed to ameliorate chemotherapy-related anemia in
cancer patients, our in vitro data have important clinical
implications. The presence of functional EpoR in RMS cells
indicates that EPO supplementation may have, in the same way as in
other malignancies (27), the
unwanted side effect of tumor progression. Finally, the presence of
functional EpoR in RMS cells and germline-derived tumors as well as
expression of CTA antigens by these cells is consistent with the
hypothesis that some pediatric sarcomas may develop in adult
tissues from embryonic remnants that are endowed with germline
characteristics.
Acknowledgements
The present study was supported by NIH grants (nos.
2R01 DK074720 and R01HL112788), the Stella and Henry Endowment, and
the Maestro grant 2011/02/A/NZ4/00035 awarded to M.-Z.R. Work by
F.-G.B. and W.S. was supported by the Intramural Research Program
of the National Cancer Institute.
References
|
1
|
Collins MH, Zhao H, Womer RB and Barr FG:
Proliferative and apoptotic differences between alveolar
rhabdomyosarcoma subtypes: A comparative study of tumors containing
PAX3-FKHR or PAX7-FKHR gene fusions. Med Pediatr Oncol. 37:83–89.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandberg AA, Stone JF, Czarnecki L and
Cohen JD: Hematologic masquerade of rhabdomyosarcoma. Am J Hematol.
68:51–57. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis RJ, D'Cruz CM, Lovell MA, Biegel JA
and Barr FG: Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14)
translocation in alveolar rhabdomyosarcoma. Cancer Res.
54:2869–2872. 1994.PubMed/NCBI
|
|
4
|
Schneider G, Bowser MJ, Shin DM, Barr FG
and Ratajczak MZ: The paternally imprinted DLK1-GTL2 locus is
differentially methylated in embryonal and alveolar
rhabdomyosarcomas. Int J Oncol. 44:295–300. 2014.
|
|
5
|
Liang K, Esteva FJ, Albarracin C,
Stemke-Hale K, Lu Y, Bianchini G, Yang CY, Li Y, Li X, Chen CT, et
al: Recombinant human erythropoietin antagonizes trastuzumab
treatment of breast cancer cells via Jak2-mediated Src activation
and PTEN inactivation. Cancer Cell. 18:423–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Todaro M, Turdo A, Bartucci M, Iovino F,
Dattilo R, Biffoni M, Stassi G, Federici G, De Maria R and Zeuner
A: Erythropoietin activates cell survival pathways in breast cancer
stem-like cells to protect them from chemotherapy. Cancer Res.
73:6393–6400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trost N, Stepisnik T, Berne S, Pucer A,
Petan T, Komel R and Debeljak N: Recombinant human erythropoietin
alters gene expression and stimulates proliferation of MCF-7 breast
cancer cells. Radiol Oncol. 47:382–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morais C, Johnson DW, Vesey DA and Gobe
GC: Functional significance of erythropoietin in renal cell
carcinoma. BMC Cancer. 13:142013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seibold ND, Schild SE, Gebhard MP, Noack
F, Schröder U and Rades D: Prognosis of patients with locally
advanced squamous cell carcinoma of the head and neck. Impact of
tumor cell expression of EPO and EPO-R. Strahlenther Onkol.
189:559–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Li HG, Xia ZS, Wen JM and Lv J:
Prognostic significance of erythropoietin and erythropoietin
receptor in gastric adenocarcinoma. World J Gastroenterol.
17:3933–3940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar SM, Zhang G, Bastian BC, Arcasoy MO,
Karande P, Pushparajan A, Acs G and Xu X: Erythropoietin receptor
contributes to melanoma cell survival in vivo. Oncogene.
31:1649–1660. 2012. View Article : Google Scholar :
|
|
12
|
Lopez TV, Lappin TR, Maxwell P, Shi Z,
Lopez-Marure R, Aguilar C and Rocha-Zavaleta L: Autocrine/paracrine
erythropoietin signalling promotes JAK/STAT-dependent proliferation
of human cervical cancer cells. Int J Cancer. 129:2566–2576. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aguilar C, Aguilar C, Lopez-Marure R,
Jiménez-Sánchez A and Rocha-Zavaleta L: Co-stimulation with stem
cell factor and erythropoietin enhances migration of c-Kit
expressing cervical cancer cells through the sustained activation
of ERK1/2. Mol Med Rep. 9:1895–1902. 2014.PubMed/NCBI
|
|
14
|
Batra S, Perelman N, Luck LR, Shimada H
and Malik P: Pediatric tumor cells express erythropoietin and a
functional erythropoietin receptor that promotes angiogenesis and
tumor cell survival. Lab Invest. 83:1477–1487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suszynska M, Poniewierska-Baran A, Gunjal
P, Ratajczak J, Marycz K, Kakar SS, Kucia M and Ratajczak MZ:
Expression of the erythropoietin receptor by germline-derived cells
- further support for a potential developmental link between the
germline and hematopoiesis. J Ovarian Res. 7:662014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacobs JF, Brasseur F, Hulsbergen-van de
Kaa CA, van de Rakt MW, Figdor CG, Adema GJ, Hoogerbrugge PM,
Coulie PG and de Vries IJ: Cancer-germline gene expression in
pediatric solid tumors using quantitative real-time PCR. Int J
Cancer. 120:67–74. 2007. View Article : Google Scholar
|
|
17
|
Virchow R: Archiv fuer pathologische
anatomie und physiologie und fuer klinische. Medizin. 8:23–54.
1855.(In German).
|
|
18
|
Conheim J: Congenitales, quergestreiftes
Muskelsarkon der Nireren. Virchows Arch. 65:64–69. 1875.(In
German). View Article : Google Scholar
|
|
19
|
Aloisio GM, Nakada Y, Saatcioglu HD, Peña
CG, Baker MD, Tarnawa ED, Mukherjee J, Manjunath H, Bugde A,
Sengupta AL, et al: PAX7 expression defines germline stem cells in
the adult testis. J Clin Invest. 124:3929–3944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grymula K, Tarnowski M, Wysoczynski M,
Drukala J, Barr FG, Ratajczak J, Kucia M and Ratajczak MZ:
Overlapping and distinct role of CXCR7-SDF-1/ITAC and CXCR4-SDF-1
axes in regulating metastatic behavior of human rhabdomyosarcomas.
Int J Cancer. 127:2554–2568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rölfing JH, Baatrup A, Stiehler M, Jensen
J, Lysdahl H and Bünger C: The osteogenic effect of erythropoietin
on human mesenchymal stromal cells is dose-dependent and involves
non-hematopoietic receptors and multiple intracellular signaling
pathways. Stem Cell Rev. 10:69–78. 2014. View Article : Google Scholar
|
|
22
|
Broxmeyer HE: Erythropoietin: Multiple
targets, actions, and modifying influences for biological and
clinical consideration. J Exp Med. 210:205–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Libura J, Drukala J, Majka M, Tomescu O,
Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG,
Janowska-Wieczorek A, et al: CXCR4-SDF-1 signaling is active in
rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and
adhesion. Blood. 100:2597–2606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jankowski K, Kucia M, Wysoczynski M, Reca
R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J, et
al: Both hepatocyte growth factor (HGF) and stromal-derived
factor-1 regulate the metastatic behavior of human rhabdomyosarcoma
cells, but only HGF enhances their resistance to radiochemotherapy.
Cancer Res. 63:7926–7935. 2003.PubMed/NCBI
|
|
25
|
Kang MH, Smith MA, Morton CL, Keshelava N,
Houghton PJ and Reynolds CP: National Cancer Institute pediatric
preclinical testing program: Model description for in vitro
cytotoxicity testing. Pediatr Blood Cancer. 56:239–249. 2011.
View Article : Google Scholar
|
|
26
|
Maxwell P, Melendez-Rodríguez F, Matchett
KB, Aragones J, Ben-Califa N, Jaekel H, Hengst L, Lindner H,
Bernardini A, Brockmeier U, et al: Novel antibodies directed
against the human erythropoietin receptor: Creating a basis for
clinical implementation. Br J Haematol. 168:429–442. 2015.
View Article : Google Scholar
|
|
27
|
Stolze I, Berchner-Pfannschmidt U, Freitag
P, Wotzlaw C, Rössler J, Frede S, Acker H and Fandrey J:
Hypoxia-inducible erythropoietin gene expression in human
neuroblastoma cells. Blood. 100:2623–2628. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ribatti D, Poliani PL, Longo V, Mangieri
D, Nico B and Vacca A: Erythropoietin/erythropoietin receptor
system is involved in angiogenesis in human neuroblastoma.
Histopathology. 50:636–641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sartelet H, Fabre M, Castaing M, Bosq J,
Racu I, Lagonotte E, Scott V, Lecluse Y, Barette S, Michiels S, et
al: Expression of erythropoietin and its receptor in
neuroblastomas. Cancer. 110:1096–1106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tarnowski M, Grymula K, Reca R, Jankowski
K, Maksym R, Tarnowska J, Przybylski G, Barr FG, Kucia M and
Ratajczak MZ: Regulation of expression of stromal-derived factor-1
receptors: CXCR4 and CXCR7 in human rhabdomyosarcomas. Mol Cancer
Res. 8:1–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tarnowski M, Grymula K, Liu R, Tarnowska
J, Drukala J, Ratajczak J, Mitchell RA, Ratajczak MZ and Kucia M:
Macrophage migration inhibitory factor is secreted by
rhabdomyosarcoma cells, modulates tumor metastasis by binding to
CXCR4 and CXCR7 receptors and inhibits recruitment of
cancer-associated fibroblasts. Mol Cancer Res. 8:1328–1343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wysoczynski M, Miekus K, Jankowski K,
Wanzeck J, Bertolone S, Janowska-Wieczorek A, Ratajczak J and
Ratajczak MZ: Leukemia inhibitory factor: A newly identified
metastatic factor in rhabdomyosarcomas. Cancer Res. 67:2131–2140.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schneider G, Bryndza E, Abdel-Latif A,
Ratajczak J, Maj M, Tarnowski M, Klyachkin YM, Houghton P, Morris
AJ, Vater A, et al: Bioactive lipids S1P and C1P are prometastatic
factors in human rhabdomyosarcoma, and their tissue levels increase
in response to radio/chemotherapy. Mol Cancer Res. 11:793–807.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schneider G, Sellers ZP, Abdel-Latif A,
Morris AJ and Ratajczak MZ: Bioactive lipids, LPC and LPA, are
novel prometastatic factors and their tissue levels increase in
response to radio/chemotherapy. Mol Cancer Res. 12:1560–1573. 2014.
View Article : Google Scholar : PubMed/NCBI
|