Introduction
Breast cancer is one of the most common causes of
cancer incidence worldwide and is also the second leading cause of
cancer death among women after lung cancer in the United States
alone (1). Therapeutic failure and
death for breast cancer patients are mainly caused from
invasiveness and metastasis (2,3).
Hypoxia is one of the most common conditions encountered within the
tumor microenvironment that drives cancer development (4,5).
Hypoxia-inducible factor-1 (HIF-1) is a transcription factor that
can be activated by hypoxia. This basic-helix-loop-helix-PAS
heterodimer composed of a HIF-1α subunit and a HIF-1β subunit is
overexpressed in many neoplastic tissues and functions as a key
regulator of the adaptive responses to the hypoxic microenvironment
(4). HIF-1α is stabilized during
periods of hypoxia resulting in its translocation to the nucleus
where HIF-1α heterodimerizes with HIF-1β (6,7), and
then triggers the expression of an array of adaptive genes to
support tumor survival, including carbonic anhydrase IX (CA
IX).
CA IX, with an extracellular active site and an
NH2-terminal proteoglycan-like region (8), is a transmembrane glycoprotein
belonging to a large family of zinc metallic-enzymes that catalyze
the rapid reversible hydration of carbon dioxide (CO2)
to bicarbonate and protons (9–11).
CA IX is observed in very restricted normal tissues (9,12),
but overexpressed in many solid tumors, including breast cancer,
which may be attributed to the hypoxic microenvironments caused by
oxygen deprivation as a result of the uncontrollable proliferation
of cancer cells relative to the inadequate amount of available
oxygen supplied by the blood vessels (13,14).
CA IX has drawn great research concerns because it plays an
important role in the regulation of intracellular pH favoring tumor
cell growth and survival (8,15),
and also contributes to cancer development by increasing cancer
cell motility, invasiveness and metastasis (16,17).
Mechanism involved in the influences of CA IX on such diverse
biological events is very complicated and still remains unclear,
making further mechanism validation necessary (9).
There are two commonly used methods which enable
in vitro cell assays under hypoxia. One is to induce gas at
specific concentration into an air-tight chamber, and the other is
to biochemically make a hypoxic condition within the cell (18). The latter approach relies upon
chemical treatments to induce signaling events associated with
hypoxia. A number of chemicals have been used as chemical inducer
of hypoxia, and cobalt chloride (CoCl2) is most widely
used among them. The role of such inducers is to stabilize the
transcription factor HIF-1α and mimic hypoxia (18,19).
CoCl2 not only acts as iron chelator and replaces iron
by cobalt at the iron-binding center of prolyl hydroxylase to
reduce the hydroxylation activity, but also blocks the von
Hippel-Lindau tumor suppressor protein (pVHL) binding to the PAS
domain of HIF-1α, hence, escaping degradation by a
ubiquitin-proteasomal pathway (20). The mechanism of CoCl2
simulating hypoxia is similar with hypoxic microenvironment in
vivo, because they have identical signal transduction and
transcription regulation (21).
Therefore in this study CoCl2 was used as chemical
inducer of hypoxia.
In this study, we explored the effect of
CoCl2 induced hypoxia on the CA IX protein and mRNA
expression, and the invasive potential in breast cancer cell lines.
Our data indicated that elevated CA IX in hypoxia induced by
CoCl2 promotes breast cancer migration/invasion in
vitro. The expression of E-cadherin, vimentin, matrix
metalloproteinase 2 (MMP2) and MMP9 using western blot analysis
were examined to explore the underlying mechanism. In addition, a
study of 149 clinical cases demonstrated that high level of CA IX
expression is associated with invasive breast cancer progression
and predicts poor patient prognosis.
Materials and methods
Cell culture and hypoxia treatment
Two different breast cancer cell lines, possessing
different invasion abilities, were applied in our experiment: MCF7
cell lines with low invasion abilities and MDA-MB-231 with high
invasion abilities (22,23). The cell lines were obtained from
the cell bank of the Chinese Academy of Sciences and were grown in
DMEM (high glucose) supplemented with 10% fetal bovine serum
(Invitrogen, Carlsbad, CA, USA). Cultured cells were maintained in
a humidified, 5% CO2 atmosphere at 37°C. For hypoxia
experiments, cells were treated with 200 μmol/l CoCl2
(Sigma-Aldrich, St. Louis, MO, USA) for 24, 48 and 72 h, and
maintained in a humidified, 5% CO2 atmosphere at 37°C
(12).
Western blot analysis
Total protein was extracted from cells and fresh
tissues using RIPA lysis buffer. For western blot analyses, equal
amounts of protein from different samples were electrophoresed,
transferred to PVDF membranes (Millipore, Bedford, MA, USA),
blocked in 5% non-fat milk for 2 h, and incubated overnight with
antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), CA IX
(Abcam, Cambridge, UK), HIF-1α (Novus Biologicals, Littleton, CO,
USA), MMP2, MMP9 (Sigma-Aldrich), E-cadherin, vimentin (Cell
Signaling Technology, Beverly, MA, USA), respectively. Then
membranes were incubated for 1 h at room temperature with the
appropriate HRP-conjugated secondary antibodies (ProteinTech Group,
Chicago, IL, USA). Detection by the LAS-3000 Luminescent Imaging
Analyzer (Li-Cor Biosciences, Lincoln, NE, USA) was performed
according to the manufacturer's instructions (Fuji Photo Film Co.,
Ltd., Tokyo, Japan). GAPDH was used as a loading control.
Quantitative RT-PCR (RT-qPCR)
analysis
TRIzol® reagent (Invitrogen, San Diego,
CA, USA) was used to extract total RNA from cells following the
manufacturer's instructions. RT-PCR was performed using the
one-step qRT-PCR kit (Takara, Otsu, Japan). RT-qPCR was carried out
in 20 ml solution with 1 mg cDNA and 1 mmol/l of each forward and
reverse primer and 2X SYBR-Green mix (Takara). GAPDH was used for
relative quantification. The primers used in the real-time PCR
reactions were designed based on information from the human genomic
database. The following primers were used for the specific
amplification of GAPDH, CA IX, and HIF-1α: GAPDH forward, 5′-CAT
CAA GAA GGT GGT GAA GC-3′ and reverse primer, 5′-GGA AAT TGT GAG
GGA GAT GC-3′; CA IX forward, 5′-TGG AGA ATG TGA GAA GCC A-3′ and
reverse primer, 5′-ATA ATG AGC AGG ACA GGA C-3′; HIF-1α forward,
5′-ATG CTT ACA CAC AGA AAT G-3′ and reverse primer, 5′-ACT GAG GTT
GGT TAC TGT T-3′. A 2−ΔΔCt method was used to evaluate
the relative mRNA expression level for each target gene.
Cell migration and invasion assays
For migration assay, Boyden dual chamber assay was
performed using Transwell chambers with 8 μm pore size membranes
(Corning Costar, Cambridge, MA, USA). A total of 5×104
cells were suspended in serum-free media and added to the upper
chamber, media containing 20% FBS were used as chemoattractant in
the lower chamber. After 48–72 h incubation at 37°C with 5%
CO2, the media and cells remaining in the upper chamber
were removed using a cotton bud. The insert was fixed in methanol
and detected by crystal violet staining. The cells attached to the
lower side of the insert were photographed using an inverted
microscope (Nikon, Tokyo, Japan). The number of migrating cells was
counted in five random fields with 10×20 power and calculating the
mean number of migrating cells. All experiments were performed in
triplicate. The invasive capability of cells was assessed by using
Boyden dual chamber assay as described previously with some
modifications. The Transwell chamber membranes were coated with 40
μl of growth factor-reduced Matrigel (BD Biosciences, San Jose, CA,
USA) for 3 h at 37°C, and the assays were subsequently performed
similarly to those of the cell migration assays.
Patients and tissue samples
All tissues were obtained from Huashan Hospital of
Fudan University (Shanghai, China). The study was approved by the
ethics committee of Huashan Hospital, Fudan University (Shanghai,
China), and all patients provided written informed consent. Eleven
pairs of fresh breast cancer and normal breast tissues were
obtained in 2012, and specimens were instantly acquired in liquid
nitrogen and stored at −80°C until analyzed. Paraffin-embedded
tissue samples from 149 primary breast cancer patients (mean age,
46.36 years; range, 24–84 years) between January 1999 and December
2002 were collected. None of the 149 invasive breast cancer
patients received chemotherapy or radiation therapy prior to
surgery. After successful radical mastectomy, all 149 patients
received chemotherapy of cyclophosphamide, methotrexate and
5-fluorouracil (CMF). All patients were followed after surgery
(until December 31, 2008), and detailed and complete
clinicopathological data were collected on each patient. The
clinical tumor lymph node metastasis (TNM) stage of each cancer was
based on the World Health Organization guidelines (24) and the histological grade was
classified according to Scarff-Bloom-Richardson grading (25). The follow-up time ranged from 2.6
months to 119.6 months, with a median time of 83.1 months. At the
end of the follow-up period, 123 patients were still alive and 26
had died of the disease. The overall survival rates were calculated
from the date of resection to the follow-up deadline or date of
mortality.
Immunohistochemistry
Specimens of tumor tissue were fixed in 10% formalin
and embedded in paraffin wax. Three-micrometer sections were then
cut from the paraffin blocks for immunohistochemical (IHC)
analysis. Slides were dehydrated in xylene and graded alcohols.
Antigen retrieval was performed with 0.01 mol/l citrate buffer at
pH 6.0 at 95°C for 10 min. Then slides were incubated with a
diluted CA IX mouse monoclonal antibody in 1:200 dilution for 12 h
followed by incubations with biotinylated secondary antibody for 1
h, and diaminobenzidine (Dako Corp., Glostrup, Denmark) for 10 min.
Slides were again counterstained with Mayer's hematoxylin. The
saturation and intensity of the immunostained cells were evaluated
over three visual fields, at a power of ×200 under a light
microscope (Carl Zeiss, Oberkochen, Germany). CA IX
immunoreactivity was detected mainly in the cytomembrane. For the
statistical analysis, with reference to a previous study, only
tumors showing a strong membranous staining in ≥10% or more cells
were considered positive for CA IX (26). An immunoglobulin-negative control
was used to rule out non-specific binding. These procedures were
performed by two independent investigators and one pathologist who
were blinded to the model/treatment type for the series of
specimens.
Statistical analysis
Statistics were calculated by SPSS software. All
experiments were repeated at least three times and the results are
presented with mean ± standard errors (SEM). ANOVA and the
Student's t-test were used to determine statistically significant
differences between experimental groups. The Kaplan-Meier method
was used to calculate the overall survival rate, and the prognostic
significance was evaluated by the log-rank test. The correlation of
CA IX immunoreactivity with the patients' clinicopathological
variables was analyzed using Chi-square test and Fisher's exact
test. Differences were considered significant at P<0.05.
Results
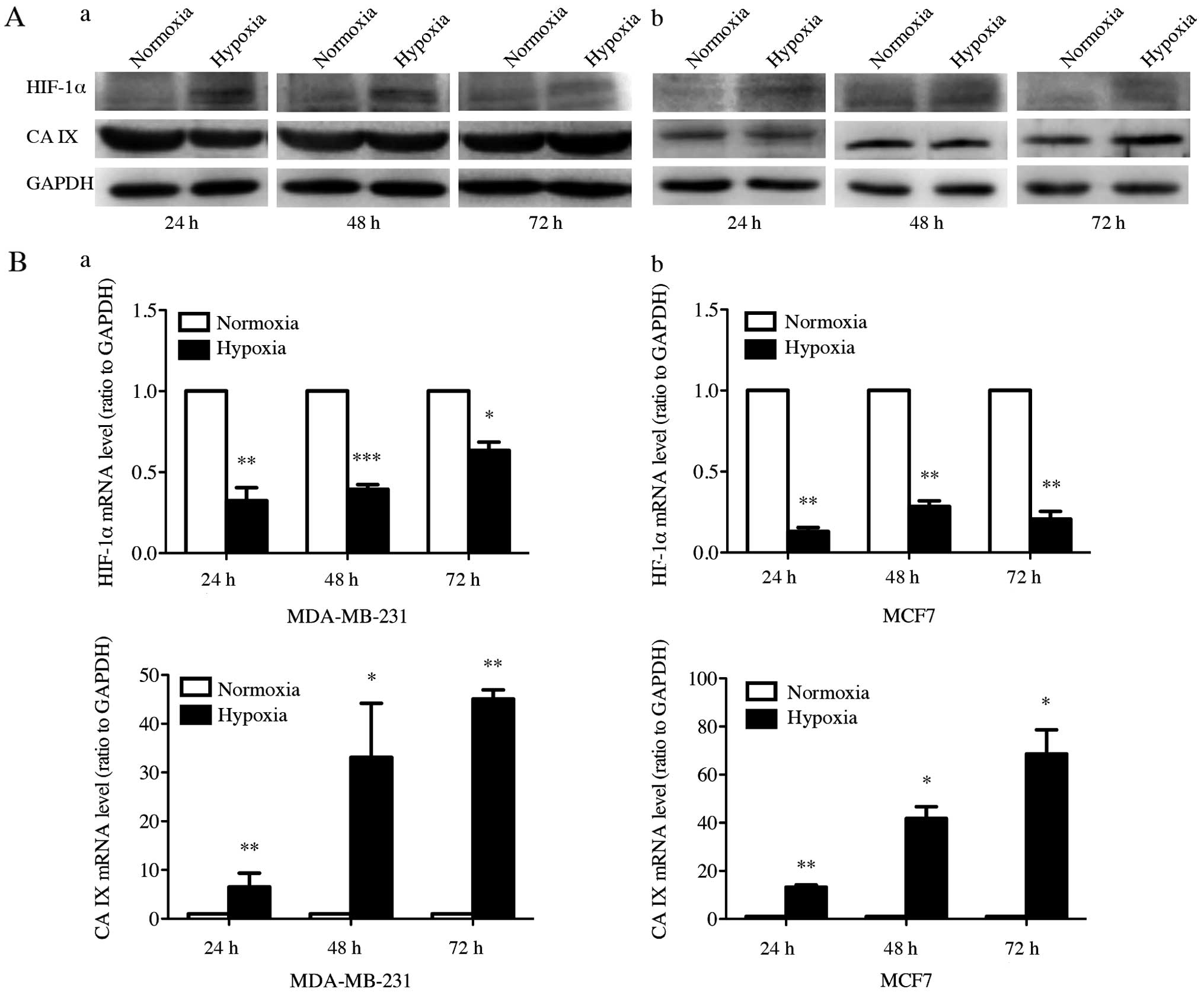
Effect of CoCl2-induced
hypoxia on protein and mRNA expression of HIF-1α and CA IX
Western blot analysis was performed to examine the
expression of HIF-1α and CA IX under normoxia and
CoCl2-induced hypoxia at 24, 48 and 72 h in MDA-MB-231
and MCF7 cell lines (Fig. 1A).
HIF-1α protein expression was induced in both cell lines after
treatment with 200 μmol/l CoCl2 for 24, 48 and 72 h. The
HIF-1α expression level was increased by 156, 110 and 86% in
MDA-MB-231, and 272, 173 and 113% in MCF7, compared to control
cells at 24, 48 and 72 h, respectively. CA IX protein expression
was significantly increased after CoCl2-induced hypoxia
treatment for 72 h, but not for 24 and 48 h. After cultured in
CoCl2 for 72 h, the CA IX expression level in MDA-MB-231
and MCF7 showed an increase of 85 and 36%. Subsequently, we
measured the mRNA expression of HIF-1α and CA IX with RT-qPCR to
further investigate expression of HIF-1α and CA IX at the mRNA
level in CoCl2-induced hypoxia (Fig. 1B). After the hypoxic treatment by
CoCl2 in MDA-MB-231 and MCF7 cells, we detected
remarkable increase of the CA IX mRNA expression level at 24, 48
and 72 h. However, the HIF-1α mRNA expression was reduced by
hypoxic stimulation in both cell lines. These results suggested
that hypoxic stimulation increased both protein and mRNA expression
of CA IX and the protein expression of HIF-1α, whereas HIF-1α mRNA
expression was downregulated by hypoxic stimulation.
Promotion of migration and invasion by
upregulated CA IX under CoCl2-induced hypoxia
In order to verify whether upregulated CA IX under
CoCl2-induced hypoxia modulate the migration and
invasion ability, Transwell assay without and with Matrigel was
performed. Hypoxic MDA-MB-231 and MCF7 cells showed significant
increase in migration and invasion ability compared to the normoxia
groups after CoCl2-induced hypoxia treatment for 72 h
(Fig. 2B), while the changes of
migration and invasion ability for 24 h were quite the opposite
(Fig. 2A).
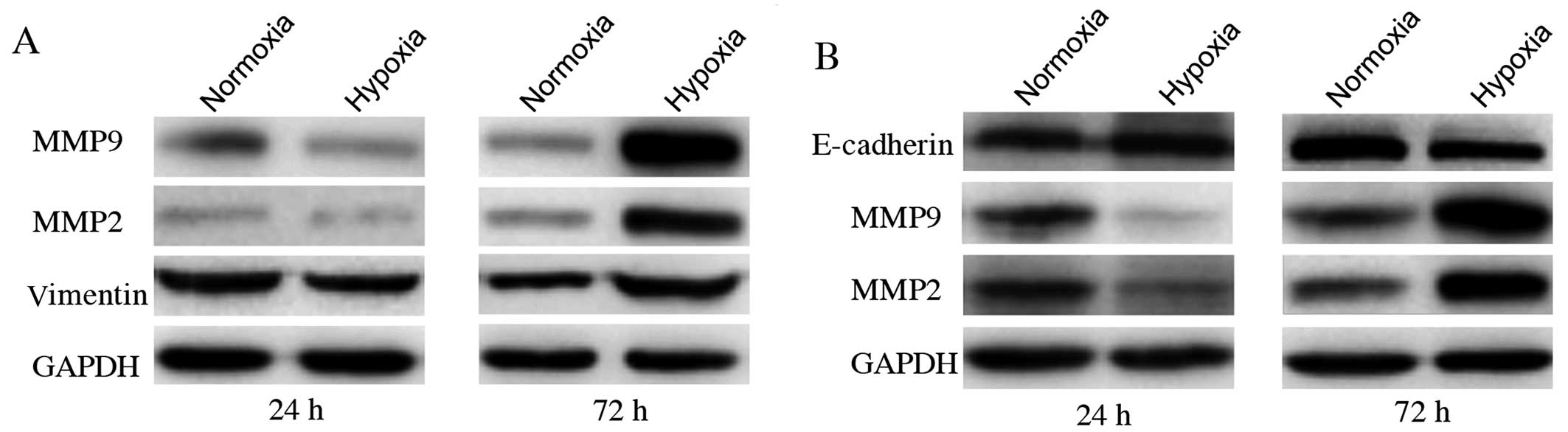
Change of MMP2, MMP9, E-cadherin and
vimentin expression level is accompanied with promoted migration
and invasion
MMP2 and MMP9 proteins have been reported to be
important factors involved in promoting cancer cell migration and
invasion. Cells undergoing epithelial-mesenchymal transition (EMT)
show enhanced invasion. E-cadherin and vimentin proteins are
principal EMT markers. MMP2 and MMP9 levels were significantly
higher in cells exposed to CoCl2 for 72 h than in
control groups (Fig. 3).
E-cadherin was not detected in MDA-MB-231 cells, neither was
vimentin in MCF7. We also observed the upregulation of vimentin in
MDA-MB-231 cells and decreased expression of E-cadherin in MCF7
cells after cultured with 200 μmol/l CoCl2 for 72 h. On
the contrary, cells exposed to CoCl2 for 24 h showed the
opposite results. All these changing trends were in agreement with
those of CA IX protein expression. This correlation suggests that
CA IX upregulation under CoCl2-induced hypoxia may have
a causal role in the promotion of in vitro cell migration
and invasion in both MDA-MB-231 and MCF7 cells.
Expression of CA IX in breast cancer and
normal breast tissues
To investigate the expression pattern of CA IX in
human breast cancer and normal breast tissues, western blot
analysis was used to detect the CA IX expression in 11 pairs of
fresh breast cancer and normal breast tissues (Fig. 4). There was almost no CA IX protein
that can be detected in normal breast tissues. High expression of
CA IX was observed in some breast cancer tissues. Compared to
normal breast tissues, several breast cancer tissues showed
significantly elevated expression levels of CA IX (P<0.001).
Correlation of CA IX expression and poor
prognosis in 149 breast cancer patients
To investigate CA IX expression in human breast
cancer, we performed IHC staining on 149 breast cancer cases. CA IX
immunoreactivity was readily detected in the cytomembrane (Fig. 5A). Only tumors showing a strong
membranous staining in ≥10% or more cells are considered positive
for CA IX. CA IX was positive in 22/139 tumors (15%). Table I shows the patient
clinicopathological characteristics and the correlations of CA IX
expression to clinical factors. We did observe significant
correlations of CA IX with tumor size (P=0.019), lymph node
metastasis (P=0.006), metastatic status (P<0.001), clinical TNM
stage (P=0.003) and ER status (P=0.018). However, no significant
differences were identified between the expression levels of CA IX
and age (P=0.614), menopause (P=0.328), histological grade
(P=0.261), PR status (P=0.131) or HER-2 expression (P=0.557). The
prognostic value of CA IX expression levels was evaluated in 149
breast cancer cases using Kaplan-Meier analysis and the log-rank
test. Among the 149 breast cancer cases, 9/22 (41%) patients with
positive CA IX expression survived, whereas 114/127 (90%) patients
with negative CA IX expression survived. The overall survival rate
was 83%. Among these specimens, a Kaplan-Meier survival analysis of
149 cancer specimens revealed a correlation between higher CA IX
expression levels and shorter survival times (P<0.001) (Fig. 5B). Using univariate survival
analysis, the clinical TNM stage (P=0.001), CA IX expression level
(P=0.002) and distant metastasis (P=0.005) were found to be
significantly associated with prognosis, however, no significant
differences were identified between prognosis and age (P=0.582),
menopause (P=0.839), chemotherapy (P=0.948), tumor size (P=0.215),
lymph node metastasis (P=0.105), histological grade (P=0.667), ER
expression (P=0.331), PR expression (P=0.078) or HER-2 expression
(P=0.068) (Table II).
Multivariate analyses were then used to determine whether CA IX
expression levels were an independent prognostic predictor of
clinical outcomes. The results revealed that CA IX expression
(HR=5.758; 95% CI, 2.286–14.502; P<0.001) and clinical TNM stage
(HR=7.256; 95% CI, 3.293–15.987; P<0.001) (Table II) showed significant prognostic
effects on overall survival. These results suggested that CA IX is
an independent prognostic factor for breast cancer.
 | Table ICorrelations of CA IX and major
clinicopathological factors (n=149). |
Table I
Correlations of CA IX and major
clinicopathological factors (n=149).
| | CA IX
expression | |
|---|
| |
| |
|---|
| Factors | Total | Positive | Negative | P-valuea |
|---|
| Age (year) |
| Mean (SD) | 55.34 (11.318) | 22 | 127 | 0.614 |
| Menopause |
| No | 91 | 16 | 75 | 0.328 |
| Yes | 58 | 6 | 52 | |
| Tumor size |
| ≤3 | 124 | 14 | 110 | 0.019 |
| >3 | 25 | 8 | 17 | |
| Lymph node
metastasis |
| 0–3 | 131 | 15 | 116 | 0.006 |
| >3 | 18 | 7 | 11 | |
| Metastatic
status |
| No | 138 | 15 | 123 | <0.001 |
| Yes | 11 | 7 | 4 | |
| Histological
grade |
| I–II | 126 | 17 | 109 | 0.480 |
| III | 23 | 5 | 18 | |
| Clinical TNM
stage |
| I–II | 115 | 11 | 104 | 0.003 |
| III–IV | 34 | 11 | 23 | |
| ER |
| Negative | 64 | 15 | 49 | 0.018 |
| Positive | 85 | 7 | 78 | |
| PR |
| Negative | 83 | 16 | 67 | 0.131 |
| Positive | 66 | 6 | 60 | |
| HER2 |
| Negative | 56 | 10 | 46 | 0.557 |
| Positive | 93 | 12 | 81 | |
| TNBC |
| No | 126 | 13 | 113 | 0.001 |
| Yes | 23 | 9 | 14 | |
 | Table IIUnivariable and multivariate survival
analysis of the influencing factors (n=149). |
Table II
Univariable and multivariate survival
analysis of the influencing factors (n=149).
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variable | P-valuea | P-valuea | HR | 95% CI |
|---|
| Age (years) | 0.582 | | | |
| Menopause | 0.839 | | | |
| Chemotherapy | 0.948 | | | |
| T | 0.215 | | | |
| N | 0.105 | | | |
| Histological
grading | 0.667 | | | |
| Clinical TNM
stage | 0.001 | <0.001 | 7.256 | 3.293–15.987 |
| CA IX | 0.002 | <0.001 | 5.758 | 2.286–14.502 |
| ER | 0.331 | | | |
| PR | 0.078 | | | |
| HER2 | 0.068 | | | |
| Distant
metastasis | 0.005 | 0.279 | 1.806 | 0.619–5.267 |
Discussion
Hypoxia is a common consequence of solid tumor
growth in breast cancer and other cancers, which serves to
propagate a cascade of molecular pathways known as hypoxic
response, including angiogenesis, glycolysis, and alterations in
microenvironmental pH (27).
Recent experimental and clinical studies demonstrated that
intra-tumor hypoxia may be a key factor in tumor microenvironment
promoting invasive growth and metastasis (21).
The present study utilized CoCl2 to mimic
hypoxia, which provides an easily performed platform to investigate
the hypoxic response. Hypoxia-inducible factors provide critical
regulation of this response (27).
HIF-1 is a transcription factor found in mammalian cells cultured
under reduced oxygen tension, and plays an essential role in
cellular and systemic homeostatic responses to hypoxic
microenvironments. HIF-1α is rapidly degraded by the proteasome
under normal conditions but is stabilized by hypoxic conditions
(28). In the present study,
CoCl2-induced hypoxia caused increases in expressions of
HIF-1α protein, CA IX mRNA, and CA IX protein in both breast cancer
cell lines. However, in contrast to the elevation of HIF-1α protein
expression after cultured in CoCl2 for 24, 48 and 72 h,
downregulations of HIF-1α mRNA were observed for the same periods.
This phenomenon may be due to the tight regulation at the
post-transcriptional level of HIF-1α by a mechanism that ensures
the capacity for brisk induction and decay of this key central
regulator of the hypoxia response (29). Chen et al (12) have reported that HIF-1α expression
is responsive to proliferation-induced hypoxia in the initial stage
and then modulates tumor progression in later stages. According to
the western blot analysis at different incubation times with
CoCl2, the elevation of CA IX tends to lag behind HIF-1α
elevation, which is more obvious in earlier hypoxic period. We
speculate that the initial downregulated CA IX expression may be
attributed to the toxicity of CoCl2. Cobalt itself is
cytotoxic, and CoCl2 affects cell division and
morphology, while in some cases, inducing mitochondrial DNA damage
and apoptosis (18,21,30).
The asynchronous changes between HIF-1α and CA IX expression in our
study are probably because of their different half-lives (31). Previous findings have found that
HIF-1α was undetectable within minutes after re-oxygenation,
whereas CA IX protein was relatively stable and persisted longer
than HIF-1α (12,29,32).
CA IX is a target gene of HIF-1α (33), and transcription of the CA IX gene
is tightly regulated by a hypoxic-responsive element (HRE) in the
5′ promoter region (4,34), which is the binding site of HIF-1.
Although previous studies showed that the expression of CA IX could
also be regulated by mechanisms other than HIF-1α, for instance,
the AP-1 transcription factor (29), the phosphatidylinositol-3-kinase
(PI3K) pathway, and the mitogen-activated protein kinase (MAPK)
pathway (13), an accumulation of
evidence revealed that HIF-1α is the exclusive regulator of CA IX
activity, others are likely secondary to the modulation of HIF-1α.
Moreover, additional transcription factor binding sites present in
the CA IX promoter appear to coordinate with the HRE to promote or
amplify the HIF-1 response (15).
However, this point of view still remains to be elucidated in
future.
We observed that the migration and invasion of
MDA-MB-231 and MCF7 cells was inhibited after treatment with
CoCl2 for 24 h, but when the incubation time increased
to 72 h, cell migration and invasion are significantly enhanced
compared to control cells. The activities of migration and invasion
was coinciding with the expression level of CA IX. It's well known
that hypoxic state inhibits tumor cells from gaining energy by
mitochondrial respiratory chain. In order to adapt to the hypoxic
microenvironment and satisfy the demand for energy, tumor cells
employ the mechanism that consumes the least oxygen, aerobic
glycolysis (13), leading to
increased glucose consumption, and excessive lactic acid production
and secretion by highly upregulated lactate and ATP-driven proton
pumps (35). This metabolic
alteration results in the intracellular acidosis, and the
expression of CA IX increases to prevent intracellular acidosis by
catalyzing the reversible hydration of CO2 to
bicarbonate and protons with the extracellular active domain. Then
the bicarbonate is shuttled into the cytoplasm to buffer
intracellular pH, while the proton remains in the extracellular
space, thus generating an increasingly acidic extracellular space
(15). In addition, CO2
production by CA IX also causes acidosis in the tumor environment,
which may be a significant source of acidity in tumors (4,36).
Moellering et al have hypothesized that microenvironmental
acidosis leads to more aggressive invasive behavior during
carcinogenesis (37). Estrella
et al have reported that the acidity generated by the tumor
microenvironment drives local invasion (38). The extracellular acidification by
CA IX was suggested to be related to the induced cell growth
factor, tumorigenic transformation, extracellular matrix breakdown,
and protease activation in hypoxia (33), hence, facilitating tumor
invasion/metastasis. In fact, the action of CA IX in hypoxic tumors
extends further beyond the control of intra-tumor pH. There is
evidence that CA IX also contributes to cell processes such as cell
adhesion, which is vital for metastatic progression in human cancer
(9,15). It was reported that by interaction
with β-catenin, CA IX could reduce E-cadherin-mediated cell
adhesion in MDCK cells, which is associated with tumorigenesis and
invasion (11).
One of our important observations was that the
hypoxia induced by CoCl2 led to a significant reduction
in the expression of the epithelial marker (E-cadherin) and an
increase in the expression of the mesenchymal marker (vimentin),
suggesting that cells with elevation of CA IX expression under
hypoxia showed overt EMT, leading to the formation of more
aggressive and metastatic tumors. EMT is a critical step in cancer
cell migration and invasion (3).
During the process, cell adhesions were weakened, cell motility was
augmented, and then cell metastatic potential was enhanced. It is
reasonable to assume that one mechanism by which upregulated CA IX
in hypoxic microenvironment contributes to cancer progression may
be through induction of EMT. In human cervical carcinoma cell line
C33A, Shin et al showed that CA IX overexpression strongly
activated the EMT process through inhibition of Rho-GTPase activity
(10). They further reported CA
IX-DKK1 interaction and transcriptional regulation of the β-catenin
signaling pathway may be involved in the effect of CA IX
overexpression on the enhanced metastatic potential (39).
The MMP family (e.g. MMP2 and MMP9) could degrade
most of the components of the extracellular matrix (3). Several studies have described the
presence and role of MMPs in many types of cancers including breast
cancer and MMPs have been found to be involved in breast cancer
progression and metastasis (40).
Mehner et al showed that MMP9 is specifically needed for
breast cancer invasion and pulmonary metastasis, while MMP2
knockdown can reduce cellular invasion (41). We found that in both MDA-MB-231 and
MCF7 cells, CoCl2 treatment for 72 h induced a
significant increase in the expression of MMP2 and MMP9.
Additionally, the study showed that the changing trend of MMP2/MMP9
was correlated with CA IX expression, indicating the participation
of MMP2/MMP9 in the enhancement of cell migration and invasion
associated with upregulated CA IX expression in hypoxia.
Here it was verified using western blot analysis
that CA IX is not present in normal breast tissues, but is
upregulated in invasive tumors. There was almost no CA IX protein
that can be detected in normal breast tissues, while the incidence
and level of CA IX in some breast cancer tissues were considerably
higher (42,43). Additionally, we studied the
expression of CA IX in 149 clinical primary breast cancer cases
using IHC staining. In agreement with previous reports that
overexpression of CA IX is an important predictor of breast cancer
(31,44–46),
our IHC examination of 149 breast cancer patients showed that the
increase in CA IX expression is correlated with tumor size, lymph
node metastasis, metastatic status and clinical TNM stage, which
are known indicators of a relatively poor prognosis in breast
cancer patients. Following the analysis of CA IX expression in 149
invasive breast cancers, the correlation between CA IX and patient
prognosis was determined. The results showed that CA IX could
potentially be used as a factor in predicting poor outcomes for
invasive breast cancer patients. This result was confirmed using
univariate and multivariate survival analyses, whereby high CA IX
expression was found to be an independent prognostic predictor of
poor survival. The data from the clinical cases were consistent
with the results from the cell lines in vitro.
In conclusion, this study suggested that
CoCl2-induced hypoxia can effectively upregulate CA IX
expression. We showed that the elevated CA IX expression is closely
related to in vitro cell migration and invasion, and the
underlying mechanism of this process may be associated with EMT.
The study of clinical tissue samples also demonstrated that CA IX
is an independent prognostic marker that may serve as a useful
clinical biomarker for predicting tumor progression and the
invasion/metastasis of breast cancer. These results provide further
insight into the role of CA IX in tumor development and put forward
further strong evidence and aspects for CA IX target therapy.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (nos. 81272387, 81470857 and
81502272), and the Shanghai Natural Science Foundation (no.
134119b1100). We thank members of our laboratory for helpful
discussions.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
2
|
Fu OY, Hou MF, Yang SF, Huang SC and Lee
WY: Cobalt chloride-induced hypoxia modulates the invasive
potential and matrix metalloproteinases of primary and metastatic
breast cancer cells. Anticancer Res. 29:3131–3138. 2009.PubMed/NCBI
|
|
3
|
Geng J, Fan J, Ouyang Q, Zhang X, Zhang X,
Yu J, Xu Z, Li Q, Yao X, Liu X, et al: Loss of PPM1A expression
enhances invasion and the epithelial-to-mesenchymal transition in
bladder cancer by activating the TGF-β/Smad signaling pathway.
Oncotarget. 5:5700–5711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. Mar 17–2015.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu H, Luo SF, Wang J, Li X, Wang H, Pu
WY, Zhang H and Zhuang ZX: Effect of environmental factors on
chemoresistance of HepG2 cells by regulating hypoxia-inducible
factor-1α. Chin Med J (Engl). 125:1095–1103. 2012.
|
|
6
|
Semenza GL: Oxygen sensing,
hypoxia-inducible factors, and disease pathophysiology. Annu Rev
Pathol. 9:47–71. 2014. View Article : Google Scholar
|
|
7
|
Wenger RH, Stiehl DP and Camenisch G:
Integration of oxygen signaling at the consensus HRE. Sci STKE.
2005:re122005.PubMed/NCBI
|
|
8
|
Swietach P, Hulikova A, Vaughan-Jones RD
and Harris AL: New insights into the physiological role of carbonic
anhydrase IX in tumour pH regulation. Oncogene. 29:6509–6521. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ilardi G, Zambrano N, Merolla F, Siano M,
Varricchio S, Vecchione M, De Rosa G, Mascolo M and Staibano S:
Histopathological determinants of tumor resistance: A special look
to the immunohistochemical expression of carbonic anhydrase IX in
human cancers. Curr Med Chem. 21:1569–1582. 2014. View Article : Google Scholar :
|
|
10
|
Shin HJ, Rho SB, Jung DC, Han IO, Oh ES
and Kim JY: Carbonic anhydrase IX (CA9) modulates tumor-associated
cell migration and invasion. J Cell Sci. 124:1077–1087. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Svastová E, Žilka N, Zat'ovicová M,
Gibadulinová A, Čiampor F, Pastorek J and Pastoreková S: Carbonic
anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via
interaction with beta-catenin. Exp Cell Res. 290:332–345. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CL, Chu JS, Su WC, Huang SC and Lee
WY: Hypoxia and metabolic phenotypes during breast carcinogenesis:
Expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch.
457:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi J, Jung WH and Koo JS:
Metabolism-related proteins are differentially expressed according
to the molecular subtype of invasive breast cancer defined by
surrogate immunohistochemistry. Pathobiology. 80:41–52. 2013.
View Article : Google Scholar
|
|
14
|
Kwon JE, Jung WH and Koo JS: The
expression of metabolismrelated proteins in phyllodes tumors.
Tumour Biol. 34:115–124. 2013. View Article : Google Scholar
|
|
15
|
McDonald PC, Winum JY, Supuran CT and
Dedhar S: Recent developments in targeting carbonic anhydrase IX
for cancer therapeutics. Oncotarget. 3:84–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swietach P, Patiar S, Supuran CT, Harris
AL and Vaughan-Jones RD: The role of carbonic anhydrase 9 in
regulating extracellular and intracellular ph in three-dimensional
tumor cell growths. J Biol Chem. 284:20299–20310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parks SK, Chiche J and Pouysségur J: pH
control mechanisms of tumor survival and growth. J Cell Physiol.
226:299–308. 2011. View Article : Google Scholar
|
|
18
|
Byrne MB, Leslie MT, Gaskins HR and Kenis
PJA: Methods to study the tumor microenvironment under controlled
oxygen conditions. Trends Biotechnol. 32:556–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu D and Yotnda P: Induction and testing
of hypoxia in cell culture. J Vis Exp. 54:e28992011.
|
|
20
|
Shweta, Mishra KP, Chanda S, Singh SB and
Ganju L: A comparative immunological analysis of CoCl2
treated cells with in vitro hypoxic exposure. Biometals.
28:175–185. 2015. View Article : Google Scholar
|
|
21
|
Dai ZJ, Gao J, Ma XB, Yan K, Liu XX, Kang
HF, Ji ZZ, Guan HT and Wang XJ: Up-regulation of hypoxia inducible
factor-1α by cobalt chloride correlates with proliferation and
apoptosis in PC-2 cells. J Exp Clin Cancer Res. 31:282012.
View Article : Google Scholar
|
|
22
|
Hunakova L, Sedlakova O, Cholujova D,
Gronesova P, Duraj J and Sedlak J: Modulation of markers associated
with aggressive phenotype in MDA-MB-231 breast carcinoma cells by
sulforaphane. Neoplasma. 56:548–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartusik D, Tomanek B, Lattová E,
Perreault H and Fallone G: Combined treatment of human MCF-7 breast
carcinoma with antibody, cationic lipid and hyaluronic acid using
ex vivo assays. J Pharm Biomed Anal. 51:192–201. 2010. View Article : Google Scholar
|
|
24
|
Böcker W: WHO classification of breast
tumors and tumors of the female genital organs: Pathology and
genetics. Verh Dtsch Ges Pathol. 86:116–119. 2002.In German.
|
|
25
|
Allred DC, Wu Y, Mao S, Nagtegaal ID, Lee
S, Perou CM, Mohsin SK, O'Connell P, Tsimelzon A and Medina D:
Ductal carcinoma in situ and the emergence of diversity during
breast cancer evolution. Clin Cancer Res. 14:370–378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan EY, Campo L, Han C, Turley H, Pezzella
F, Gatter KC, Harris AL and Fox SB: Cytoplasmic location of
factor-inhibiting hypoxia-inducible factor is associated with an
enhanced hypoxic response and a shorter survival in invasive breast
cancer. Breast Cancer Res. 9:R892007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goonewardene TI, Sowter HM and Harris AL:
Hypoxia-induced pathways in breast cancer. Microsc Res Tech.
59:41–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Q, Xu Z, Mao S, Chen W, Zeng R, Zhou S
and Liu J: Effect of hypoxia on hypoxia inducible factor-1α,
insulin-like growth factor I and vascular endothelial growth factor
expression in hepatocellular carcinoma HepG2 cells. Oncol Lett.
9:1142–1148. 2015.PubMed/NCBI
|
|
29
|
Tomes L, Emberley E, Niu Y, Troup S,
Pastorek J, Strange K, Harris A and Watson PH: Necrosis and hypoxia
in invasive breast carcinoma. Breast Cancer Res Treat. 81:61–69.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simonsen LO, Harbak H and Bennekou P:
Cobalt metabolism and toxicology - a brief update. Sci Total
Environ. 432:210–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan EY, Yan M, Campo L, Han C, Takano E,
Turley H, Candiloro I, Pezzella F, Gatter KC, Millar EK, et al: The
key hypoxia regulated gene CAIX is upregulated in basal-like breast
tumours and is associated with resistance to chemotherapy. Br J
Cancer. 100:405–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bruckner BA, Ammini CV, Otal MP, Raizada
MK and Stacpoole PW: Regulation of brain glucose transporters by
glucose and oxygen deprivation. Metabolism. 48:422–431. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thiry A, Dogné JM, Masereel B and Supuran
CT: Targeting tumor-associated carbonic anhydrase IX in cancer
therapy. Trends Pharmacol Sci. 27:566–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colpaert CG, Vermeulen PB, Fox SB, Harris
AL, Dirix LY and Van Marck EA: The presence of a fibrotic focus in
invasive breast carcinoma correlates with the expression of
carbonic anhydrase IX and is a marker of hypoxia and poor
prognosis. Breast Cancer Res Treat. 81:137–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mitra S, Stemke-Hale K, Mills GB and
Claerhout S: Interactions between tumor cells and microenvironment
in breast cancer: A new opportunity for targeted therapy. Cancer
Sci. 103:400–407. 2012. View Article : Google Scholar
|
|
36
|
Li Y, Wang H, Oosterwijk E, Tu C,
Shiverick KT, Silverman DN and Frost SC: Expression and activity of
carbonic anhydrase IX is associated with metabolic dysfunction in
MDA-MB-231 breast cancer cells. Cancer Invest. 27:613–623. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moellering RE, Black KC, Krishnamurty C,
Baggett BK, Stafford P, Rain M, Gatenby RA and Gillies RJ: Acid
treatment of melanoma cells selects for invasive phenotypes. Clin
Exp Metastasis. 25:411–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Estrella V, Chen T, Lloyd M, Wojtkowiak J,
Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg
JM, Sloane BF, et al: Acidity generated by the tumor
microenvironment drives local invasion. Cancer Res. 73:1524–1535.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim BR, Shin HJ, Kim JY, Byun HJ, Lee JH,
Sung YK and Rho SB: Dickkopf-1 (DKK-1) interrupts FAK/PI3K/mTOR
pathway by interaction of carbonic anhydrase IX (CA9) in
tumorigenesis. Cell Signal. 24:1406–1413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Merdad A, Karim S, Schulten HJ, Dallol A,
Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM and
Al-Qahtani MH: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer: MMP-9 as a potential biomarker for
cancer invasion and metastasis. Anticancer Res. 34:1355–1366.
2014.PubMed/NCBI
|
|
41
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DC and Radisky ES: Tumor cell-produced matrix
metalloproteinase 9 (MMP-9) drives malignant progression and
metastasis of basallike triple negative breast cancer. Oncotarget.
5:2736–2749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartosová M, Parkkila S, Pohlodek K,
Karttunen TJ, Galbavý S, Mucha V, Harris AL, Pastorek J and
Pastoreková S: Expression of carbonic anhydrase IX in breast is
associated with malignant tissues and is related to overexpression
of c-erbB2. J Pathol. 197:314–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Span PN, Bussink J, Manders P, Beex LV and
Sweep CG: Carbonic anhydrase-9 expression levels and prognosis in
human breast cancer: Association with treatment outcome. Br J
Cancer. 89:271–276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Eom KY, Jang MH, Park SY, Kang EY, Kim SW,
Kim JH, Kim JS and Kim IA: The expression of carbonic anhydrase
(CA) IX/XII and lymph node metastasis in early breast cancer.
Cancer Res Treat. Mar 3–2015.Epub ahead of print. View Article : Google Scholar
|
|
45
|
Trastour C, Benizri E, Ettore F, Ramaioli
A, Chamorey E, Pouysségur J and Berra E: HIF-1α and CA IX staining
in invasive breast carcinomas: Prognosis and treatment outcome. Int
J Cancer. 120:1451–1458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chia SK, Wykoff CC, Watson PH, Han C, Leek
RD, Pastorek J, Gatter KC, Ratcliffe P and Harris AL: Prognostic
significance of a novel hypoxia-regulated marker, carbonic
anhydrase IX, in invasive breast carcinoma. J Clin Oncol.
19:3660–3668. 2001.PubMed/NCBI
|