Introduction
Currently, drug repurposing, meaning that is to
discover new applications of existing or abandoned
pharmacotherapies, is an effective approach for developing novel
pharmacotherapies in treating cancer (1). Many existing drugs approved by FDA,
such as metformin (2), aspirin
(3) and disulfiram (4), have demonstrated anticancer effects
in addition to their original uses. Consequently, more approved
drugs need to be found to inhibit the cancer cell growth, leading
to increased choice and enhancement of the effectiveness of
antitumor therapy.
Prostate cancer is one of the most common cancers
worldwide, as well as a frequent cause of cancer-related death
(5). Many patients with prostate
cancer, having a poor prognosis, often developed resistant to the
common therapies, including androgen deprivation treatment and
cytotoxic drugs (6). The
transcription factor STAT3 is constitutively active and has been
associated with prognosis and progression in human prostate cancer
(7). STAT3 is involved in
oncogenesis, cell proliferation, angiogenesis, self-renewal and
drug resistance. Several studies have suggested that inhibition of
STAT3 signaling induces apoptosis, prevents metastasis and
overcomes drug-resistance in prostate cancer (8,9).
Thus, it is indicated that targeting STAT3 activation appears to be
a promising treatment strategy for patients with advanced prostate
cancer.
Pimozide is an FDA-approved compound used to
clinically treat chronic psychosis, Tourette syndrome and resistant
tics (10). Moreover, in previous
studies, pimozide was shown to have anticancer effect on various
carcinomas and leukemia, including melanoma (11), breast cancer (12) and myelogenous leukemia (13,14).
The neuroleptic agents pimozide inhibited cell proliferation and
induced apoptosis in human breast cancer cell line MCF-7 (12,15).
In addition, pimozide suppressed the self-renewal capacity of
chronic myelogenous leukemia cells by inhibiting STAT5 activity
(13). Furthermore, our previous
study showed that pimozide inhibited maintenance and tumorigenicity
of hepatocellular carcinoma stem-like cells through suppressing the
STAT3 activity (16). However,
whether pimozide shows anticancer effect and inhibits the STAT3
activation mechanically in prostate cancer cells have not yet been
fully determined.
The aim of the present study was to investigate the
antitumor effects of the neuroleptic drug pimozide on prostate
cancer cells. The results showed that pimozide inhibited prostate
cancer cell proliferation, colony formation and sphere formation by
inducing G1 phase cell cycle arrest. In addition, pimozide
inhibited cell migration of prostate cancer cells in the Transwell
system. Importantly, pimozide suppressed the basal STAT3 activation
and rescued STAT3 activation induced by IL-6 addition in prostate
cancer cells. Therefore, the antipsychotic agent pimozide may be a
potential treatment strategy for patients with advanced prostate
cancer.
Materials and methods
Cell lines and cell culture
Human prostate cancer cell lines DU145, LNCaP, PC3M,
22RV1 and BHP-1 (The Third Affiliated Hospital of Sun Yat-sen
University, Guangzhou, China) were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) containing 10%
fetal bovine serum (FBS; Sigma, St. Louis, MO, USA). All cancer
cell lines were cultured in incubator with 5% CO2 at
37°C.
Cell proliferation assay using MTT
Cell proliferation was performed by MTT colorimetric
assay. Human prostate cancer cell lines DU145, LNCaP, PC3M, 22RV1
and BHP-1 were seeded into 96-well culture plates with 2,500
cells/well. Subsequently, cells were treated with pimozide at
different concentration for various time intervals (24, 48 and 72
h). Next, each well was added with MTT solution and incubated for 4
h at 37°C. The supernatant fluid was removed, and DMSO solution was
added. Absorbance value was finally measured using the microplate
reader at 490 nm (BioTek Instruments Inc., Winooski, VT, USA).
Cell cycle assay
Cell cycle was determined by propidium iodide (PI;
BD Biosciences Clontech, Palo Alto, CA, USA) staining. Briefly,
equal amounts of cells were seeded in 6-well plates and treated
with pimozide at different concentration for 48 h. The cells were
harvested, washed with phosphate-buffered saline (PBS) containing
0.1% BSA, and then, cold absolute ethanol was added while vortexing
the cells. PI buffer (40 μg/ml, containing 100 μg/ml RNase) was
added, and the cells were analyzed by flow cytometry.
Colony formation assay
Cells with different concentrations of pimozide (7.5
and 15 μM) were plated in 10% FBS medium for 7 days. Cells were
stained with 0.5% crystal violet in 20% ethanol and photographed.
The morphology and the number of colonies were counted under
stereomicroscope.
Sphere formation assay
Sphere formation assay was performed as previously
described (17). To establish
sphere cultures, single cells were cultured in 200 μl of serum-free
DMEM/F12 medium (Gibco) supplemented with 20 ng/ml human
recombinant epidermal growth factor (EGF; PeproTech, Rocky Hill,
NJ, USA), 20 ng/ml human recombinant basic fibroblast growth factor
(bFGF; PeproTech), and B27 (1:50; Gibco). Cells at a density of 500
cells/well were cultured in ultra-low attachment plates. Pimozide
was added to the cells at the beginning. After plating for 7 days,
all spheres in each well were photographed.
Transwell migration assay
Cells (1×105) in serum-free medium were
seed in the upper compartment of a Transwell chamber (Corning
Incorporated, Corning, NY, USA). Pimozide was added at 7.5 and 15
μM. After incubation for 48 h, the migrated cells on the lower
membrane were counted after staining with 0.1% crystal violet.
Results were shown as average from at least three independent
experiments.
Western blot assay
Equal amounts of protein from cells sample harvested
with RIPA lysis buffer were subjected to electrophoresis in
SDS-polyacrylamide gel and transferred to nitrocellulose membrane
(Merck Millipore, Billerica, MA, USA). Blots were detected using
primary antibodies against GAPDH (Ambion, Austin, TX, USA), p21,
Nanog, E-cadherin, N-cadherin, phospho-STAT3(Tyr705) (pY-STAT3),
STAT3 (Cell Signaling Technology, Beverly, MA, USA), cyclin D1,
c-Myc and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Antibody binding was detected with an enhanced chemiluminescence
kit (Sigma).
Statistical analysis
The data were presented as the mean ± SD and
analyzed using the GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Student's t-test was used to compare the
difference between two groups. The level of significance was set at
P<0.05. The statistically significant results are shown as
P<0.05, P<0.01.
Results
The antipsychotic agent pimozide inhibits
cell proliferation of prostate cancer cells in a dose- and
time-dependent manner
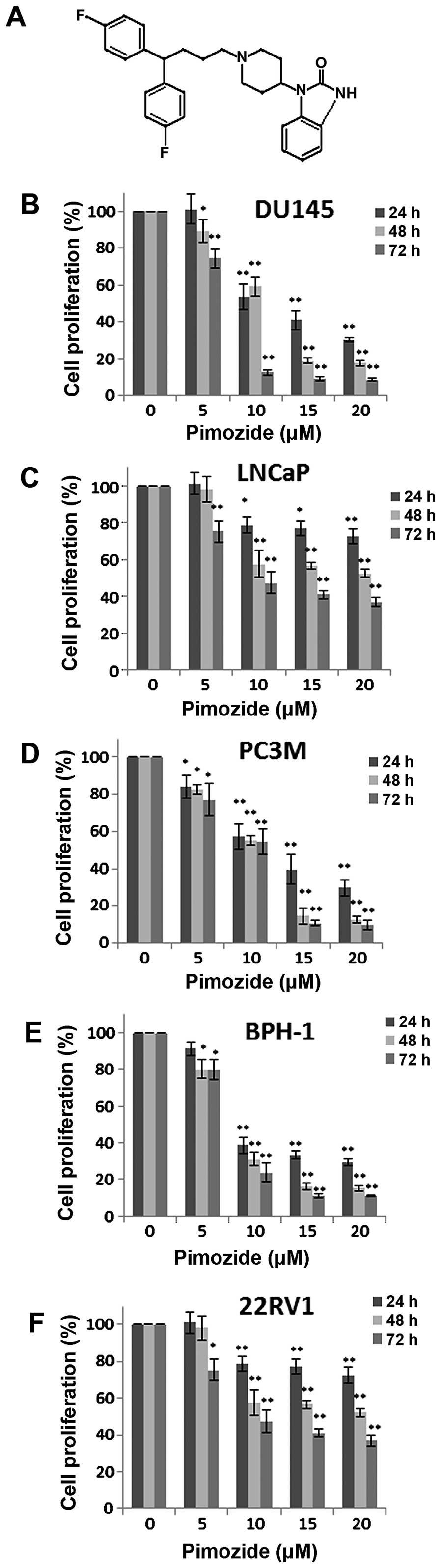
Initially, we examined whether the antipsychotic
agent pimozide (structure shown in Fig. 1A) has anti-proliferative effect in
prostate cancer cells using MTT colorimetric assay. Human prostate
cancer cells DU145, LNCaP, PC3M, 22RV1 and BHP-1 were exposed to a
series of concentrations (0, 5, 10, 15 and 20 μΜ) of pimozide for
24, 48 and 72 h. As shown in Fig.
1B–F, pimozide inhibited the proliferation of these five cell
types dose- and time-dependently. The IC50 values at 24,
48 and 72 h were 12.62±2.60, 10.74±1.21 and 6.541±0.85 μΜ for
DU145, 14.10±2.05, 11.90±3.05 and 7.21±0.70 μΜ for LNCaP,
12.07±2.54, 8.90±1.18 and 9.19±1.21 μΜ for PC3M, 10.36±2.60,
7.56±0.90 and 7.78±0.60 μΜ for BPH-1, 38.12±18.68, 18.24±4.80 and
11.12±1.84 μΜ for 22RV1 cells, respectively. The data implied that
the neuroleptic drug pimozide might hold a potential therapeutic
index in treating prostate cancer.
Pimozide induces G0/G1 phase cell cycle
arrest of prostate cancer cells
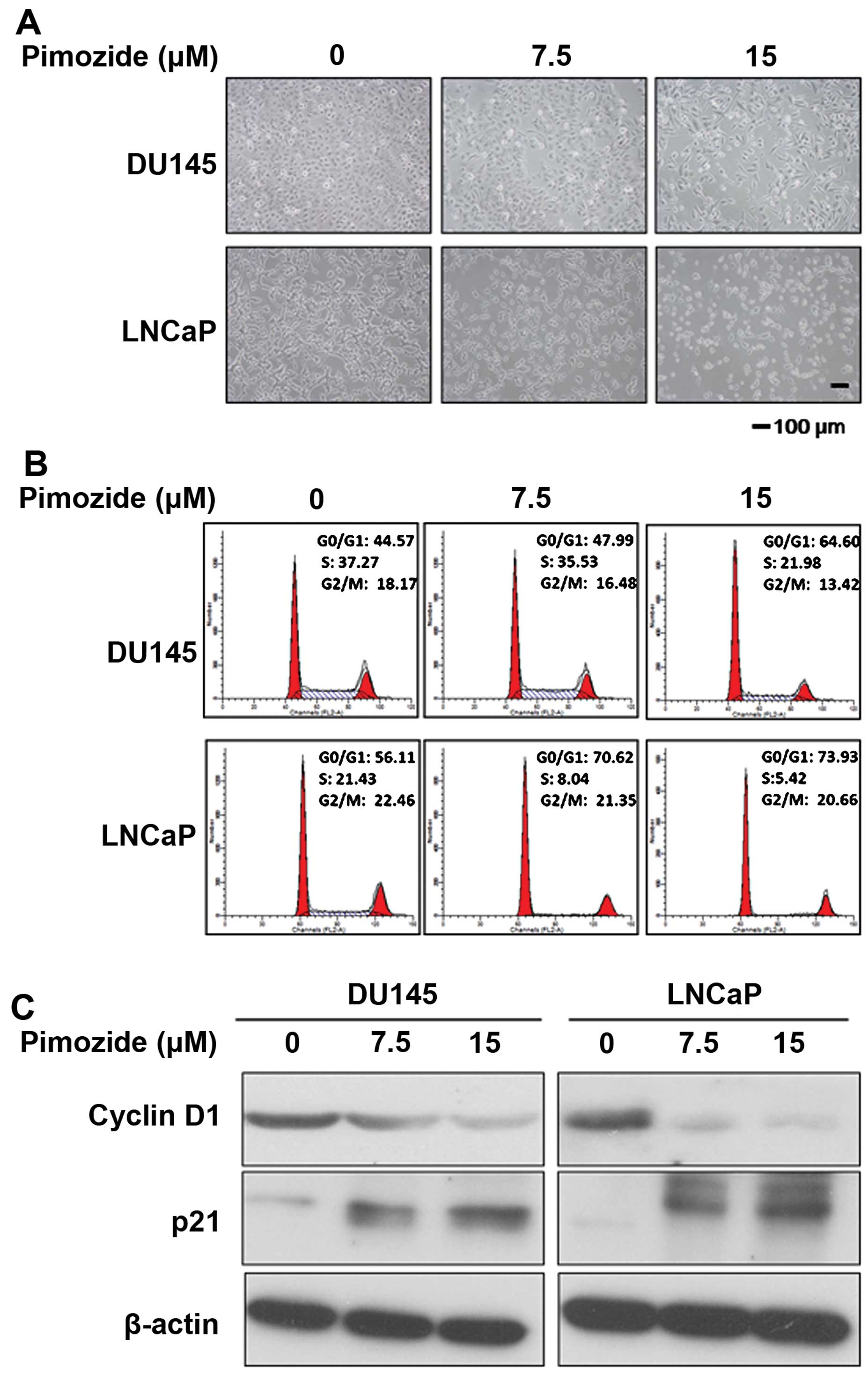
Next, the cellular morphological observation using
light microscopy showed that after pimozide treatment for 24 h the
prostate cancer cells DU145 and LNCaP displayed cytoplasmic
shrinkage and the number of cells was reduced (Fig. 2A). In addition, to determine
whether pimozide could induce cell cycle arrest to inhibit cell
growth, we analyzed the effect of pimozide on the cell cycle
distribution using PI staining. After DU145 and LNCaP cells were
treated with 15 μΜ pimozide for 24 h, the population of cells in
the G0/G1 phase was increased significantly (P<0.01) whereas in
the S phase it was decreased significantly (P<0.01) (Fig. 2B). After treatment with pimozide,
DU145 cells showed a significant increase in the percentage of the
G0/G1 phase cells, from 44.57±2.04 to 64.60±3.07%, while S phase
was reduced from 37.27±1.92 to 21.98±1.38%. Further examination of
relative cell cycle marker showed remarkable increase of p21 levels
and decrease of cyclin D1 level (Fig.
2C), consistent with the G0/G1 arrest phenomenon observed in
the flow cytometric analysis. These results indicated that pimozide
decreased viability of prostate cancer cells in association with
G0/G1 phase cell cycle arrest.
Pimozide inhibits the ability of colony
and sphere forming in prostate cancer cells
Furthermore, we examined whether pimozide inhibited
the ability of colony and sphere forming in prostate cancer cells.
Colony and sphere formation assay showed that pimozide inhibited
the self-renewal capacity of prostate cancer cell lines DU145 and
LNCaP in a dose-dependent manner (Figs. 3 and 4). After treatment with 7.5 μΜ pimozide
for a week, DU145 cells had a decrease of 77.90±1.11% of the
colonies (Fig. 3A and B). Sphere
formation assay showed that the inhibition rate was 94.48±0.28% and
99.39±0.03% in DU145 cells treated with pimozide at the
concentration of 7.5 and 15 μM, respectively (Fig. 4). Similar results, evaluated by
colony and sphere formation assay, were shown in LNCaP cells with
pimozide. In addition, western blot assay showed that prostate
cancer cells DU145 and LNCaP treated with pimozide for 48 h
significantly downregulated the expression level of the stemness
genes Nanog and c-Myc (Fig. 3C).
These results indicated pimozide inhibited the ability of colony
and sphere forming in prostate cancer cells.
Pimozide suppresses cell migration in
prostate cancer cells
As demonstrated in Fig.
5A and B, both DU145 and LNCaP cells suppressed the capacity of
cell migration after treatment with 7.5 and 15 μΜ pimozide compare
to control without treatment (P<0.01, respectively). Using
Transwell migration assay, the ability to migrate assessed in
chambers without a matrix was also significantly reduced by
49.72±2.49% and 15.15±7.58% in the case of 7.5 and 15 μΜ pimozide
treated DU145 cells, respectively. In addition, LNCaP cells treated
with 15 μΜ pimozide had a decrease of 96.19±1.90% of migrated cells
compare to control (P<0.01). We assessed whether pimozide
inhibited cell migration through manipulating relative marker of
epithelial-mesenchymal transition (EMT). Western blot assay showed
that pimozide downregulated N-cadherin expression and upregulated
E-cadherin expression in prostate cancer cells DU145 and LNCaP
(Fig. 5C), suggesting that
pimozide inhibited prostate cell migration through suppression of
the EMT marker.
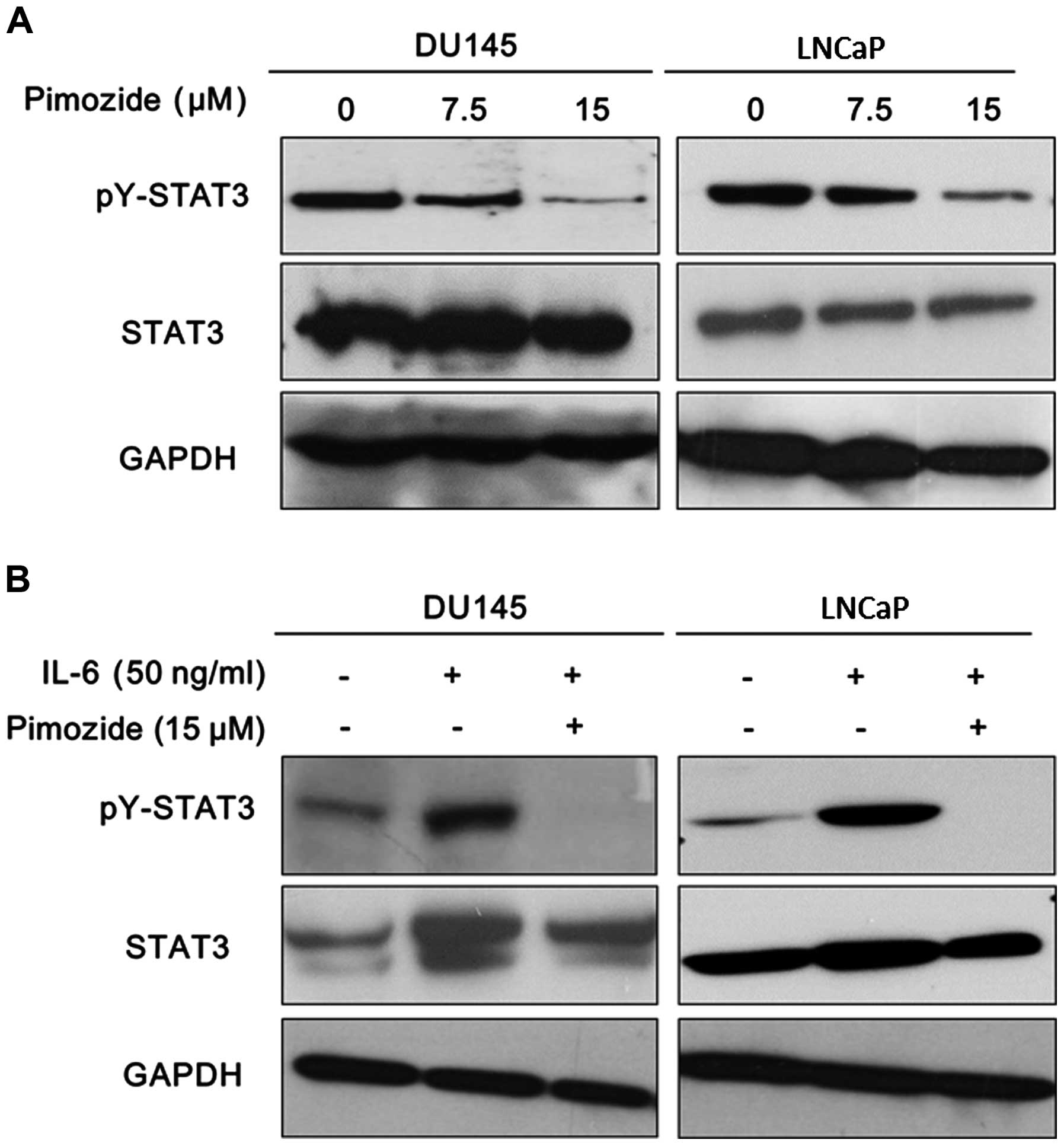
Pimozide suppresses the basal STAT3
activation and reverses the STAT3 activation induced by IL-6 in
prostate cancer cells
It is clear that STAT3 signaling is of prime
importance for promoting tumor progression and drug resistance as
well as its potential as a therapeutic target in prostate cancer
cells (18,19). The activation of STAT3 signaling
was reported with high expression of STAT3 phosphorylation at
tyrosine 705 (pY-STAT3). Western blot analysis was performed to
validate the expression of pY-STAT3. The results showed that
pimozide reduced the basal expression of pY-STAT3 in both DU145 and
LNCaP cells (Fig. 6A). Moreover,
it is well known that IL-6 can activate STAT3 signaling to exert
function in prostate cancer cells presenting high expression of
pY-STAT3 (Fig. 6B) (20,21).
Furthermore, pimozide treatment reversed the expression level of
phosphorylation STAT3 at tyrosine 705 induced by IL-6 addition in
DU145 and LNCaP cells (Fig. 6B).
These data further suggested that pimozide may inhibit STAT3
signaling activity to suppress cell growth in prostate cancer
cells.
Discussion
Currently, serious efforts are made in drug
discovery and development for clinical treatment of prostate cancer
(22). In our previous study, it
is reported that the neuroleptic drug pimozide had antitumor
activity against hepatocellular carcinoma cells or stem-like cells
through suppressing the STAT3 activity and may be a novel candidate
drug for treating advanced hepatocellular carcinoma (16). Since STAT3 signaling is important
to cell proliferation, angiogenesis and drug resistance in prostate
cancer, we concluded that pimozide also inhibited cellular
proliferation of prostate cancer cells. In the present study, our
results showed that pimozide inhibited cell proliferation of
prostate cancer cells with G0/G1 phase cell cycle arrest, evaluated
by MTT, colony and sphere formation assay. Additionally, pimozide
suppressed cell migration in prostate cancer cells. The mechanism
of action of pimozide was considered to be due to inhibition of
STAT3 activation. It indicated that the antipsychotic agent
pimozide may be a potential and novel therapeutic for patients with
advanced prostate cancer.
Pimozide is a clinical drug approved by FDA to treat
neuroleptic disorders. Since pimozide had relatively low
side-effect and a broad spectrum of molecular targets, pimozide has
been used to treat other diseases during the past 20 years,
including monosymptomatic hypochondriacal psychoses, body
dysmorphic disorder, metastatic melanoma, trichotillomania, and
trigeminal and postherpetic neuralgia (23). Previous studies show that pimozide
is a well-known antagonist of serotonin 5HT7 receptors
(Ki=0.5 nM) for treating anti-depressant effect and of
dopamine receptor D2 (D2R) (Ki=0.33 nM) for treating
schizophrenia (24–26). However, in the present study, it is
reported that pimozide shows potential for use as a new anticancer
agent for treating prostate cancer.
Numerous studies have demonstrated constitutive
activation of STAT3 in a wide variety of human malignancies,
including head and neck, breast, lung, gastric, hepatocellular,
colorectal and prostate cancers (27–29).
Aberrantly STAT3 activation contributes to oncogenesis by
preventing apoptosis, inducing cell proliferation, angiogenesis,
invasion, and metastasis as well as suppressing antitumor immune
responses (30,31). Phosphorylated STAT3 dimerizes and
translocates into the nucleus to bind to specific DNA response
elements to induce the transcription of downstream genes, such as
BCL-xL, MCL1 and c-Myc (32). Targeting STAT3 signaling results in
suppression of the proliferation of various cancer cells in
vitro and tumorigenicity in vivo (33,34).
Therefore, the identification and development of novel drugs
targeting deregulated STAT3 activation effectively remains an
important scientific and clinical challenge (35,36).
As yet, no direct STAT3 inhibitor has been approved for clinical
use (18,36). A previous study showed that
pimozide suppressed the self-renewal capacity of chronic
myelogenous leukemia cells by inhibiting the cellular transcription
factor STAT5 activity (15).
Surprisingly, our data showed that pimozide can inhibit STAT3
activation in prostate cancer, presenting that pimozide reduced the
basal expression of pY-STAT3 in both DU145 and LNCaP cells and
reversed the expression level of phosphorylation STAT3 at tyrosine
705 induced by IL-6 addition. Similar results, reported by our
previous work, were shown in hepatocellular carcinoma cells
(16). Furthermore, another study
showed that pimozide reduced STAT3 tyrosine phosphorylation in
multiple myeloma cells (37).
These further suggest that pimozide may be a novel and potential
STAT3 inhibitor for anticancer treatment.
Although pimozide can induce cardiac toxicity, it
has still not been reported to have adverse effects on other normal
functional cells, such as hepatic or haematopoietic cells. A
previous study showed that pimozide has almost no effect on
haematopoietic progenitors derived from healthy donors (14). In addition, pimozide treatment was
well tolerated with no significant effects on body weight in
vivo (14). The lethal dose of
pimozide is unknown for human. The LD50 is 228 mg/kg in mice and
5120 mg/kg in rats. In our previous study, we adopted 25 mg/kg dose
pimozide for in vivo treatment of hepatocellular carcinoma
cells by oral gavage (PO), showing no significant effects on body
weight (16). The dose of pimozide
in the present study is relatively lower compared to the commonly
used dose for treating clinical disease. Therefore, pimozide
possibly is a safe drug for treating cancer. Besides in clinical
application for many years, pimozide has the significance of a
translational medicine in the clinical treatment of cancer.
In conclusion, the present study is to demonstrate
whether the neuroleptic drug pimozide has antitumour activity
against prostate cancer cells. Here, our results showed that
pimozide inhibited cell proliferation of prostate cancer cells with
G0/G1 phase cell cycle arrest, as well as suppressed cells
migration. Moreover, pimozide can inhibit STAT3 activation in
prostate cancer. Thus, an antipsychotic agent pimozide may be a
potential and novel therapeutic for patients with advanced prostate
cancer.
Acknowledgements
We thank members of Professor Qi Zhang laboratory,
the Third Affiliated Hospital of Sun Yat-sen University for their
critical comments. We also thank Vaccine Research Institute of Sun
Yat-sen University for technical and laboratory apparatus support.
The present study was supported by the China Postdoctoral Science
Foundation funded project (2014M562239 to J.-J.C.) and the PhD
Start-up Fund of National Natural Science Foundation of Guangdong
Province of China (to J.-J.C.).
References
|
1
|
Boguski MS, Mandl KD and Sukhatme VP: Drug
discovery. Repurposing with a difference. Science. 324:1394–1395.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Del Barco S, Vazquez-Martin A, Cufí S,
Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B
and Menendez JA: Metformin: Multi-faceted protection against
cancer. Oncotarget. 2:896–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon
JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, et al: Aspirin
induces apoptosis in vitro and inhibits tumor growth of human
hepatocellular carcinoma cells in a nude mouse xenograft model. Int
J Oncol. 40:1298–1304. 2012.
|
|
4
|
Triscott J, Lee C, Hu K, Fotovati A, Berns
R, Pambid M, Luk M, Kast RE, Kong E, Toyota E, et al: Disulfiram, a
drug widely used to control alcoholism, suppresses the self-renewal
of glioblastoma and over-rides resistance to temozolomide.
Oncotarget. 3:1112–1123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bashir MN: Epidemiology of prostate
cancer. Asian Pac J Cancer Prev. 16:5137–5141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karantanos T, Corn PG and Thompson TC:
Prostate cancer progression after androgen deprivation therapy:
Mechanisms of castrate resistance and novel therapeutic approaches.
Oncogene. 32:5501–5511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barton BE, Karras JG, Murphy TF, Barton A
and Huang HF: Signal transducer and activator of transcription 3
(STAT3) activation in prostate cancer: Direct STAT3 inhibition
induces apoptosis in prostate cancer lines. Mol Cancer Ther.
3:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni Z, Lou W, Leman ES and Gao AC:
Inhibition of constitutively activated Stat3 signaling pathway
suppresses growth of prostate cancer cells. Cancer Res.
60:1225–1228. 2000.PubMed/NCBI
|
|
9
|
Bishop JL, Thaper D and Zoubeidi A: The
multifaceted roles of STAT3 signaling in the progression of
prostate cancer. Cancers (Basel). 6:829–859. 2014. View Article : Google Scholar
|
|
10
|
Egolf A and Coffey BJ: Current
pharmacotherapeutic approaches for the treatment of Tourette
syndrome. Drugs Today (Barc). 50:159–179. 2014. View Article : Google Scholar
|
|
11
|
Taub RN and Baker MA: Treatment of
metastatic malignant melanoma with pimozide. Lancet. 1:6051979.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strobl JS, Kirkwood KL, Lantz TK, Lewine
MA, Peterson VA and Worley JF III: Inhibition of human breast
cancer cell proliferation in tissue culture by the neuroleptic
agents pimozide and thioridazine. Cancer Res. 50:5399–5405.
1990.PubMed/NCBI
|
|
13
|
Nelson EA, Walker SR, Weisberg E,
Bar-Natan M, Barrett R, Gashin LB, Terrell S, Klitgaard JL, Santo
L, Addorio MR, et al: The STAT5 inhibitor pimozide decreases
survival of chronic myelogenous leukemia cells resistant to kinase
inhibitors. Blood. 117:3421–3429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nelson EA, Walker SR, Xiang M, Weisberg E,
Bar-Natan M, Barrett R, Liu S, Kharbanda S, Christie AL, Nicolais
M, et al: The STAT5 inhibitor pimozide displays efficacy in models
of acute myelogenous leukemia driven by FLT3 mutations. Genes
Cancer. 3:503–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strobl JS, Melkoumian Z, Peterson VA and
Hylton H: The cell death response to gamma-radiation in MCF-7 cells
is enhanced by a neuroleptic drug, pimozide. Breast Cancer Res
Treat. 51:83–95. 1998. View Article : Google Scholar
|
|
16
|
Chen JJ, Cai N, Chen GZ, Jia CC, Qiu DB,
Du C, Liu W, Yang Y, Long ZJ and Zhang Q: The neuroleptic drug
pimozide inhibits stem-like cell maintenance and tumorigenicity in
hepatocellular carcinoma. Oncotarget. May 27–2015.Epub ahead of
print.
|
|
17
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: Cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
19
|
Shodeinde AL and Barton BE: Potential use
of STAT3 inhibitors in targeted prostate cancer therapy: Future
prospects. Onco Targets Ther. 5:119–125. 2012.PubMed/NCBI
|
|
20
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin D, Wyatt AW, Xue H, Wang Y, Dong X,
Haegert A, Wu R, Brahmbhatt S, Mo F, Jong L, et al: High fidelity
patient-derived xenografts for accelerating prostate cancer
discovery and drug development. Cancer Res. 74:1272–1283. 2014.
View Article : Google Scholar
|
|
23
|
Lorenzo CR and Koo J: Pimozide in
dermatologic practice: A comprehensive review. Am J Clin Dermatol.
5:339–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seeman P: Atypical antipsychotics:
Mechanism of action. Can J Psychiatry. 47:27–38. 2002.PubMed/NCBI
|
|
25
|
Muscat R, Sampson D and Willner P:
Dopaminergic mechanism of imipramine action in an animal model of
depression. Biol Psychiatry. 28:223–230. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Ji ZL and Chen YZ: TTD:
Therapeutic target database. Nucleic Acids Res. 30:412–415. 2002.
View Article : Google Scholar :
|
|
27
|
Jing N and Tweardy DJ: Targeting Stat3 in
cancer therapy. Anticancer Drugs. 16:601–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wendt MK, Balanis N, Carlin CR and
Schiemann WP: STAT3 and epithelial-mesenchymal transitions in
carcinomas. JAKSTAT. 3:e289752014.PubMed/NCBI
|
|
29
|
Teng Y, Ross JL and Cowell JK: The
involvement of JAK-STAT3 in cell motility, invasion, and
metastasis. JAKSTAT. 3:e280862014.PubMed/NCBI
|
|
30
|
Al Zaid Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Crowe PJ, Goldstein D and Yang JL:
STAT3 inhibition, a novel approach to enhancing targeted therapy in
human cancers (Review). Int J Oncol. 41:1181–1191. 2012.PubMed/NCBI
|
|
32
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: Role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar
|
|
33
|
Gurbuz V, Konac E, Varol N, Yilmaz A,
Gurocak S, Menevse S and Sozen S: Effects of AG490 and S3I-201 on
regulation of the JAK/STAT3 signaling pathway in relation to
angiogenesis in TRAIL-resistant prostate cancer cells in vitro.
Oncol Lett. 7:755–763. 2014.PubMed/NCBI
|
|
34
|
Han Z, Wang X, Ma L, Chen L, Xiao M, Huang
L, Cao Y, Bai J, Ma D, Zhou J, et al: Inhibition of STAT3 signaling
targets both tumor-initiating and differentiated cell populations
in prostate cancer. Oncotarget. 5:8416–8428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao M, Jiang B and Gao FH: Small molecule
inhibitors of STAT3 for cancer therapy. Curr Med Chem.
18:4012–4018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mankan AK and Greten FR: Inhibiting signal
transducer and activator of transcription 3: Rationality and
rationale design of inhibitors. Expert Opin Investig Drugs.
20:1263–1275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nelson EA, Sharma SV, Settleman J and
Frank DA: A chemical biology approach to developing STAT
inhibitors: Molecular strategies for accelerating clinical
translation. Oncotarget. 2:518–524. 2011. View Article : Google Scholar : PubMed/NCBI
|