Introduction
Gambogic acid (GA) is a constructive component of
Garcinia hurburyi, a natural compound derived from brownish
gamboe resin in Southeast Asia countries (1). Gamboge resin is used in conventional
Chinese medicine for the treatment of hemostasis, detoxification
and as a parasiticide (2). GA was
reported to have multiple functions, such as anti-inflammation,
anti-angiogenesis and anti-invasion (3–5). GA
also has potent anticancer activity in numerous types of human
cancers, such as lung cancer, hepatocellular carcinoma, malignant
melanoma, breast carcinoma, and chronic myelogenous leukemia by
targeting NF-κB, thioredoxin reductase, Bcl-2, Akt/mTOR signaling
pathway, and proteasome, respectively (6–10).
Furthermore, it exhibits specific cytotoxic activity on rapidly
dividing cancerous cells with no side effects on normal cells
(11). Despite its anticancer
efficacy, the molecular mechanism of the GA-induced apoptosis in
renal cancer cells is unclear.
TRAIL (tumor necrosis factor (TNF)-related
apoptosis-inducing ligand) belongs to the TNF superfamily, which
can induce apoptosis in a wide variety of tumor cells, but not
normal cells (12). Although TRAIL
has beneficial effects in selectively killing tumor cells, many
cancer cells are resistant to TRAIL (13). The mechanism of TRAIL resistance is
unclear but several studies have reported that TRAIL resistance is
intimately associated with the overexpression of anti-apoptosis
including cellular FADD-like apoptosis regulator (cFLIP),
anti-apoptotic Bcl-2 family proteins (e.g., Bcl-2 and Bcl-xL) and
inhibitor of apoptosis proteins (IAPs) (13,14).
However, a single treatment with TRAIL may not be sufficient for
the treatment of malignant tumor cells. Moreover, TRAIL-resistant
cancer cells can be sensitized by a TRAIL sensitizer, such as
chemotherapeutic drugs and biochemical inhibitors that suppress the
expression of anti-apoptosis-associated proteins, indicating that
combination of drugs rather than just one drug alone appears to be
more effective in cancer therapy. Therefore, the identification of
a novel TRAIL sensitizer is important for effective cancer therapy.
The aim of this study was to examine the anticancer effects of GA,
elucidate the underlying action mechanism of GA, evaluate GA as a
sensitizer of TRAIL, and understand the mechanism of the synergy
between GA and TRAIL against human renal cancer cells. In the
present study, GA was found to induce apoptosis in renal carcinoma
(Caki Cells) through the downregulation of cFLIPL. In
addition, a GA treatment rendered human renal cancer cells more
sensitive to TRAIL.
Materials and methods
Cells and materials
Caki cells were obtained from the American Type
Culture Collection (ATCC, Rockville, MD, USA). Dulbecco's modified
Eagle's medium, containing 10% fetal bovine serum (FBS), 20 mM
HEPES buffer and 100 μg/ml gentamicin was used as the culture
medium in these experiments. PCR primers were purchased from
Bioneer (Daejeon, Korea). The anti-Bcl-2, anti-Mcl-1 and
anti-cIAP-2 were acquired from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). The anti cFLIPL antibody was obtained
from ALEXIS Corp. (San Diego, CA, USA). Anti-PARP, and
anti-caspase-3 antibody were purchased from Cell Signaling
Technology (Beverly, MA, USA). The anti-XIAP antibody was supplied
by R&D systems (Minneapolis, MN, USA). Gambogic acid and the
other chemicals were purchased from Sigma (St. Louis, MO, USA).
Recombinant human TRAIL was obtained from KOMA Biotech (Seoul,
Korea).
Cell count and flow cytometry
analysis
The cell counts were performed using a
hemocytometer. Approximately 0.5×106 Caki cells were
suspended in 100 μl of PBS, and 200 μl of 95% ethanol was added
while vortexing. The cells were incubated at 4°C for 1 h, washed
with PBS, and resuspended in 250 μl of 1.12% sodium citrate buffer
(pH 8.4) together with 12.5 μg of RNase. Incubation was continued
at 37°C for 30 min. The cellular DNA was then stained by applying
250 μl of propidium iodide (50 μg/ml) for 30 min at room
temperature. The stained cells were analyzed by fluorescent
activated cell sorting (FACS) on a BD FACSCanto II flow cytometer
(BD Biosciences, San Jose, CA, USA) to determine the relative DNA
content based on the red fluorescence.
Western blot analysis
The cellular lysates were prepared by suspending
1.2×106 cells in 100 μl of lysis buffer (137 mM NaCl, 15
mM EGTA, 0.1 mM sodium orthovanadate, 15 mM MgCl2, 0.1%
Triton X-100, 25 mM MOPS, 100 μM phenylmethylsulfonyl fluoride, and
20 μM leupeptin, adjusted to pH 7.2). The cells were disrupted by
sonication and extracted at 4°C for 30 min. The proteins were
electro-transferred to Immobilon-P membranes (Millipore Corp.,
Bedford, MA, USA). The detection of specific proteins was carried
out using an ECL Western blotting kit according to the
manufacturer's instructions.
Transfection
For transfection, the cells were plated onto 6-well
plates at a density of 0.5×106 cells/well and grown
overnight. The cells were co-transfected with 2 μg of various
plasmid constructs and 1 μg of pCMV-β-galactosidase plasmid for 5 h
using the Lipofectamine method. After transfection, the cells were
cultured in 10% FBS medium with a vesicle (DMSO) or drug for 24
h.
RNA isolation and reverse
transcriptase-PCR
The expression of cFLIPL mRNA was
determined by RT-PCR. The total cellular RNA was extracted from the
cells using an easyBlue reagent (Life Technologies, Seongnam,
Korea), and the cDNA was prepared using M-MLV reverse transcriptase
(Gibco-BRL, Gaithersburg, MD, USA), according to the manufacturer's
instructions. The cellular RNA sample was reverse-transcribed with
a random primer and then amplified by PCR, the GAPDH primer set was
used as the internal control. The following primers were used to
amplify the cFLIPL and GAPDH. The sequences of the sense
and antisense primer for cFLIPL were 5′-CGG ACT ATA GAG
TGC TGA TGG-3′ and 5′-GAT TAT CAG GCA GAT TCC TAG-3′, respectively.
The sequences of the sense and antisense primer for GAPDH were
5′-AGG TCG GAG TCA ACG GAT TTG-3′ and 5′-GTG ATG GCA TGG ACT GTG
GT-3′, respectively. The PCR products were analyzed by
electrophoresis on a 1.5% agarose gel and detected by UV light.
4′,6′-Diamidino-2-phenylindole (DAPI)
staining for nuclei condensation and fragmentation
The cells were fixed with 1% paraformaldehyde on a
slide glass for 30 min at room temperature. After washing with PBS,
300 nM 4′,6′-diamidino-2-phenylindole (Roche, Mannheim, Germany)
was added to the fixed cells for 5 min, and the cells were examined
by fluorescence microscopy.
Statistical analysis
The data were analyzed by a one-way ANOVA followed
by post-hoc comparisons (Student-Newman-Keuls) using the
Statistical Package for Social Sciences 8.0 (SPSS Inc., Chicago,
IL, USA).
Results
GA induces apoptosis in renal carcinoma
caki cells
To examine the anti-effects of GA in human renal
cancer cells, Caki cells were treated with various concentrations
of GA. With increasing GA concentration, the GA-treated Caki cells
progressively showed the typical features of apoptosis, including
cell shrinkage, rounding and detachment of the cell from the plate
(Fig. 1A). Cell death was next
determined by flow cytometry analysis to detect the hypodiploid
cell populations. As shown in Fig.
1B, treatment of Caki cells with GA resulted in a significant
increase in the accumulation of sub-G1 phase cells in a
dose-dependent manner. In addition, the treatment of Caki cells
with GA strongly led to a reduction of the protein levels of 32-kDa
precursor together with the concomitant cleavage of PARP, a
substrate protein of caspases (Fig.
1C).
GA-induced apoptosis is mediated partly
by the AIF translocation
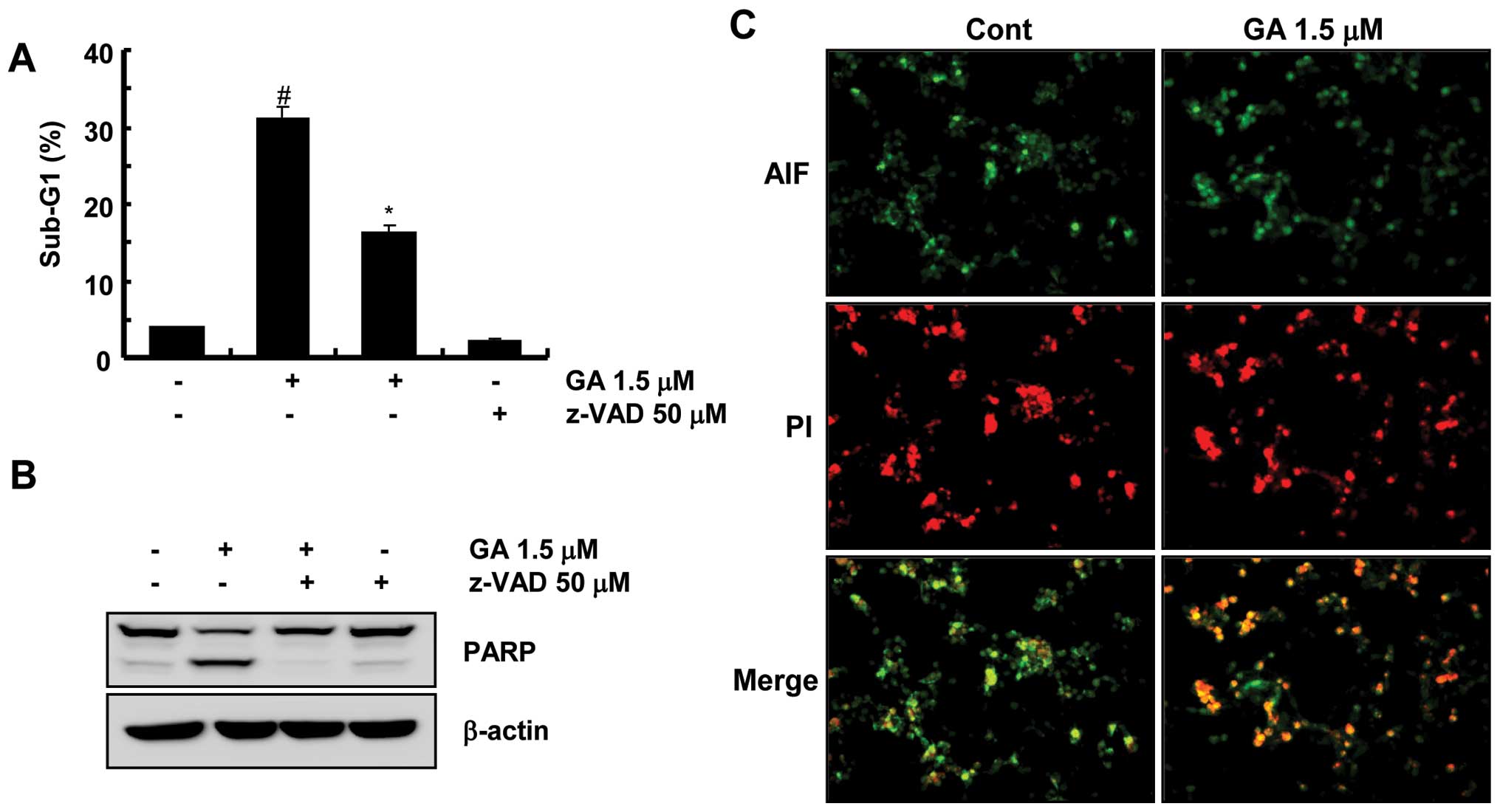
This study next examined whether the activation of
caspase pathway plays a critical role in GA-induced apoptosis. As
shown in Fig. 2A, GA-mediated
apoptosis was prevented partly by a pretreatment with a general and
potent inhibitor of caspases, z-VAD-fmk. In contrast, treatment
with z-VAD-fmk completely prevented these caspase-related events,
such as the cleavage of PARP (Fig.
2B). These results suggest that the GA-induced cell death was
mediated partly by the caspase-dependent pathway and
caspase-independent cell death in the presence of z-VAD-fmk.
Because AIF is involved in the induction of apoptotic cell death
through the caspase-independent pathway, this study examined
whether AIF plays a role in GA-induced apoptotic cell death. The
translocation of AIF was analyzed by the observation of its release
from the mitochondria and translocation to the nucleus by
fluorescence microscopy. As shown in Fig. 2C, fluorescence microscopy showed
that AIF was translocated to the nucleus and caused nuclear
condensation after the treatment with GA. This suggests that
GA-induced apoptotic cell death in Caki cells is mediated by AIF
translocation from the mitochondria into the nucleus via a
caspase-independent pathway.
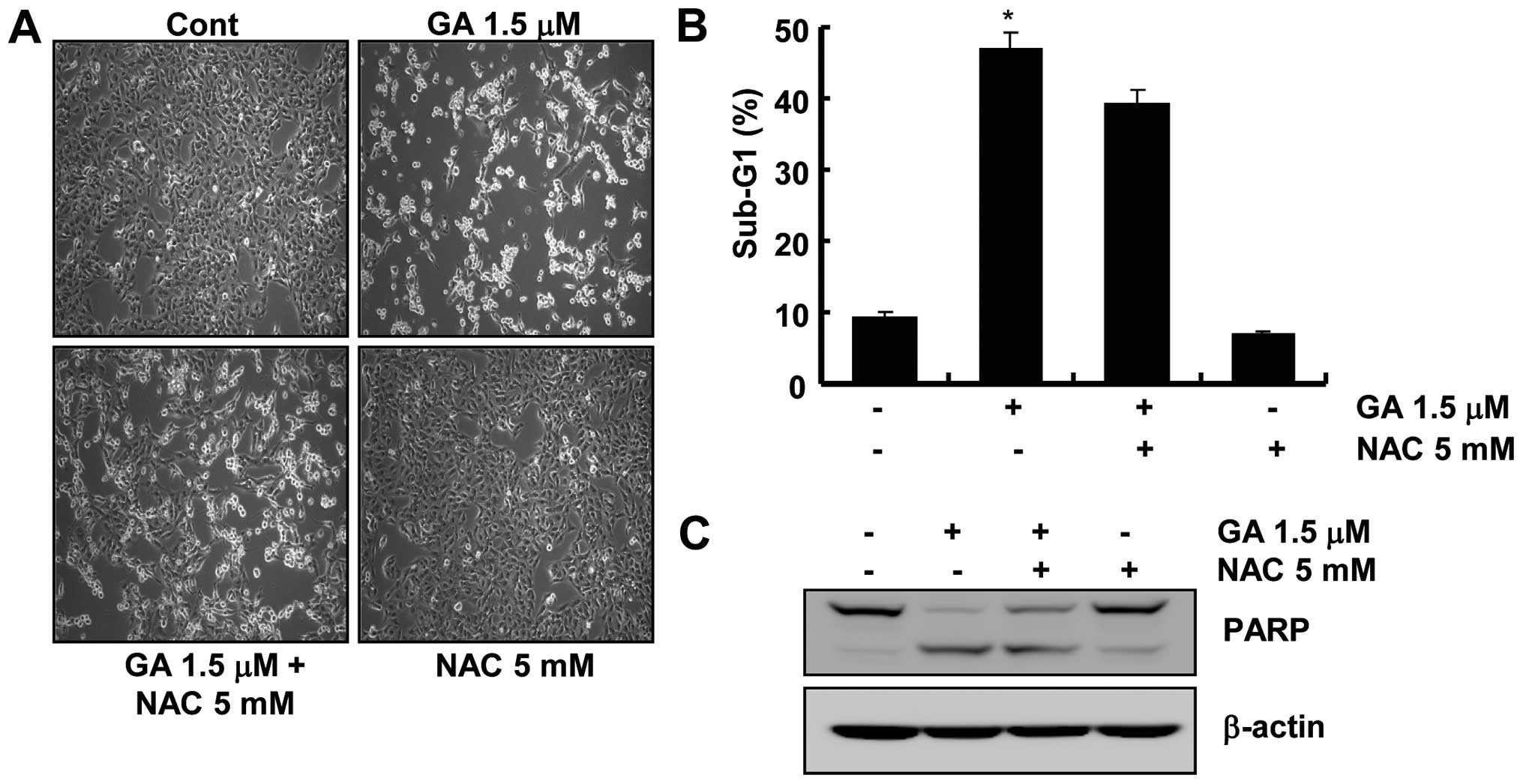
Pretreatment with N-acetylcysteine (NAC)
does not affect GA-induced apoptosis
Further experiments were conducted to determine if
ROS generation induced by GA is associated directly with the
induction of apoptosis because numerous studies have documented
that GA induces apoptosis through ROS accumulation in human cancer
cells (15,16). On the contrary, as shown in
Fig. 3A and B, pretreatment with
N-acetylcysteine (NAC), a ROS scavenger, did not prevent GA-induced
cell death. In addition, pretreatment with NAC failed to prevent
the cleavage of PARP (Fig. 3C).
These results suggest that ROS generation is not critical for the
induction of apoptosis by GA.
Downregulation of cFLIPL
contributes to GA-induced apoptosis
This study next examined whether the downregulation
of cFLIPL is critical to stimulate GA-induced apoptosis.
The overexpression of cFLIPL in Caki cells attenuated
GA-mediated apoptosis, whereas treatment with GA-induced apoptosis
in Caki/vector cells (Fig. 4C).
This suggests that cFLIPL downregulation also
contributes to GA-induced apoptosis. The cleavage of PARP and
procaspase-3 was also analyzed in both cell lines. As shown in
Fig. 4D, the ectopic expression of
cFLIPL attenuated the cleavage of PARP and procaspase-3
induced by GA treatment in Caki cells. The levels of
cFLIPL and cFLIPs expression were checked after a
treatment with various concentration of GA to determine if GA
specifically downregulates cFLIPL expression without
affecting the levels of cFLIPs. The treatment with GA
decreased the level of cFLIPL expression in Caki cells.
In contrast, no changes in the cFLIPs mRNA and protein levels were
detected in the GA-treated cells (Fig.
4E and F). These results suggest that GA suppressed
cFLIPL expression with little effect on cFLIP expression
in the present system. Interestingly, we found that GA treatment
induced the cleavage of p65 protein, a subunit of nuclear factor-κB
(NF-κB), subsequently reduction of p65 protein level in Caki cells
(Fig. 4G). This result suggested
the possibility that GA-induced cFLIPL downregulation
might be caused by inhibiting NF-κB pathway in our system.
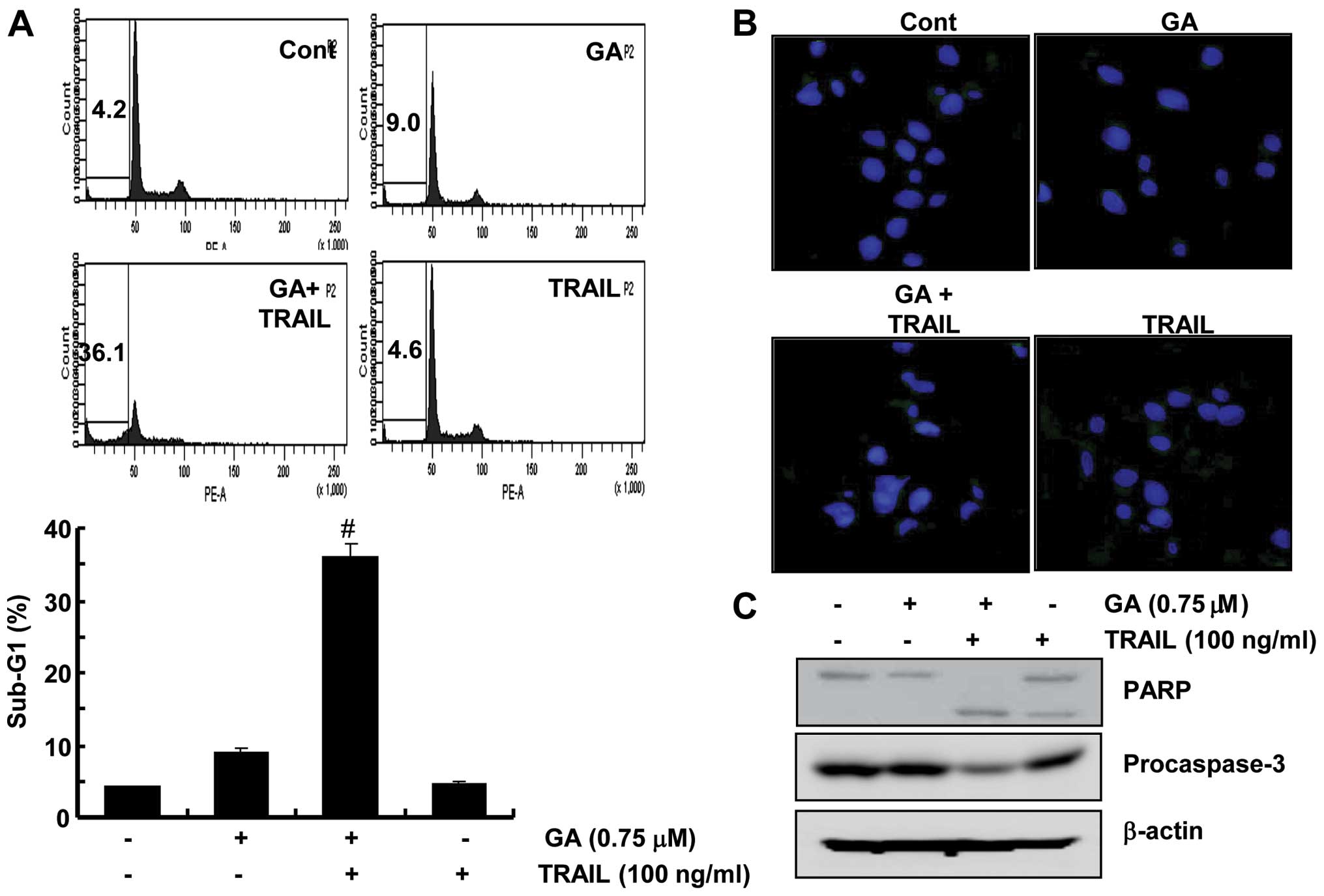
GA sensitizes renal cancer cells for
TRAIL-mediated apoptosis
In an attempt to search for novel strategies to
overcome TRAIL resistance in cancer cells, this study examined the
effects of a combination treatment of GA and TRAIL in Caki cells.
The co-treatment of Caki cells with GA and TRAIL resulted in a
marked increase in the accumulation of sub-G1 phase cells compared
to Caki cells treated with GA or TRAIL alone (Fig. 5A). In addition, a combined
treatment with GA and TRAIL induced chromatin condensation
paralleled by nuclear fragmentation (Fig. 5B). Finally, the combinational
treatment of Caki cells with GA and TRAIL led to a decrease in the
protein levels of procaspase-3 with the concomitant cleavage of
PARP protein (Fig. 5C).
Combination treatment with GA plus
TRAIL-induced apoptosis is mediated via caspase-dependent
pathway
This study next examined whether activation of the
caspase pathway plays a critical role in GA plus TRAIL-induced
apoptosis. As shown in Fig. 5D, GA
plus TRAIL-induced apoptosis was prevented by a pretreatment with
z-VAD-fmk, as determined by FACS analysis. In addition, z-VAD-fmk
prevented these caspase-related events including the cleavage of
procaspase-3 and PARP (data not shown). These results suggest that
a combined treatment of GA and TRAIL-induced apoptosis was mediated
by caspase-dependent apoptosis pathways. Furthermore, NAC partly
prevented GA plus TRAIL-mediated apoptosis in Caki cells (Fig. 5E).
Discussion
In the present study, GA exhibited significant
antitumor activity against human renal cancer cells. GA-induced
apoptosis in human Caki renal cancer cells was mediated by two
different pathways (e.g., caspase-dependent and caspase-independent
pathways). In addition, GA-induced apoptosis was mediated by the
downregulation of cFLIPL expression, and the treatment
of renal cancer cells with GA in combination with TRAIL
synergistically induced apoptosis.
Natural bioactive compounds have attracted
considerable attention as chemotherapeutic agents over the past
year because of their safety and efficacy in overcoming tumor cell
growth by inducing apoptosis by upregulating the pro-apoptotic
proteins or downregulating anti-apoptotic proteins (17–20).
GA is a natural compound of Garcinia hurburyi, which has
been used for medicinal purpose in Southeast Asia countries since
ancient times (21). As GA has
anticancer effects on cancer cells with very weak activity on the
hematologic system, it has been approved for phase II clinical
trials for solid cancer therapy (10,23).
In addition, caspase activation is required for GA-induced
apoptotic cell death in cancer cells (10,23).
In the present study, GA activated the caspase-dependent apoptotic
pathway in a dose-dependent manner in Caki cells, which was partly
prevented by pretreatment with the pancaspase inhibitor, z-VAD-fmk.
This suggests that GA-induced apoptosis is mediated by
caspase-independent and caspase-dependent apoptotic pathways. To
determine which type of cell death is induced by GA, the
caspase-independent cell death machinery involved in the alternate
pathway activated by GA was first defined. AIF mediates cell death
through a caspase-independent pathway. Mitochondrial AIF
translocates to the nucleus on death stimuli and initiates nuclear
condensation, which leads to large-scale chromatin fragmentation
followed by cell death (24,25).
Fluorescence microscopy revealed the translocation of AIF into the
nucleus of Caki cells, suggesting that the activation of
caspase-independent apoptotic route is mediated by the
translocation of AIF into the nucleus.
Reactive oxygen species (ROS) are used as active
mediators for the regulation of cell death, including
caspase-dependent and caspase-independent pathways and necrosis
(26,27). In addition, exposure of human
bladder cancer cells to GA induces an increase in ROS accumulation,
which causes caspase activation, finally leading to cell death
(28). In the present study, GA
treatment was found to elicit ROS generation (data not shown), but
the inhibition of ROS by NAC did not prevent GA-induced apoptosis
in Caki cells. This suggests that ROS generation is not involved in
GA-mediated cell death in the present system.
GA has a strong cytotoxic effect on a variety of
cancers via repressing the NF-κB pathways, downregulating Bcl-2
expression and inhibiting the proteasome pathways (29–31).
Consistent with previous findings, GA treatment resulted in
downregulation of Bcl-2 proteins in renal cancer cells.
Interestingly, we found that GA downregulated cFLIPL
expression by suppressing transcriptional control as well as that
GA induced cleavage of p65 protein in Caki cells. It is generally
recognized that cFLIPL protein levels can be
transcriptionally regulated through the NF-κB (32,33).
Based on these previous studies and our finding, we postulated the
possibility that GA downregulated cFLIP mRNA expression via
suppressing NF-κB pathway in our system. Furthermore, the ectopic
expression of cFLIPL attenuated GA-induced cell death,
suggesting that cFLIPL downregulation is responsible for
the GA-induced apoptotic cell death in the present system.
The cFLIP gene often generates two splicing
isoforms, cFLIPL and cFLIPs, by alternative splicing in
humans. cFLIPL and cFLIPs play different roles in cell
death, including apoptosis, necroptotic cell death and autophagy
(34–37). Therefore, expression of the cFLIP
isoforms would need to be regulated specifically for cancer
therapy. In the present study, GA specifically downregulated
cFLIPL mRNA expression with no marked change in the
cFLIPs mRNA levels. The FLIPL protein can also block the
recruitment of caspase-8 to DISC, suppressing death
receptor-mediated apoptosis (38).
Previous studies showed that GA could markedly sensitize
chemotherapeutic drug-induced cell death in a range of cancer cells
(6,39–41).
Therefore, based on these studies, this study examined whether
GA-induced cFLIPL downregulation could increase the
sensitivity to the death ligands, including TRAIL, TNF-α and FasL
in human renal cancer cells. This study discovered that GA
increased the sensitivity to TRAIL, suggesting that GA is a
sensitizer of the TRAIL-mediated apoptotic pathways in renal cancer
cells.
In conclusion, we suggest that GA plays an important
role as a proapoptotic agent via the specific downregulation of
cFLIPL expression without affecting the expression of
cFLIPs. In addition, GA treatment rendered human renal cancer cells
more sensitive to TRAIL. These results suggested that a combined
treatment of GA and TRAIL may provide a safe and effective
therapeutic strategy against cancers that are resistant to
conventional treatments. Furthermore, this study provided novel
evidence that the prominent sensitizing effect of GA on
TRAIL-induced apoptosis is due to the downregulation of
cFLIPL.
Acknowledgements
This study was supported by the Yeungnam University
research grants in 2013.
References
|
1
|
Guo QL, You QD, Wu ZQ, Yuan ST and Zhao L:
General gambogic acids inhibited growth of human hepatoma SMMC-7721
cells in vitro and in nude mice. Acta Pharmacol Sin. 25:769–774.
2004.PubMed/NCBI
|
|
2
|
Qin Y, Meng L, Hu C, Duan W, Zuo Z, Lin L,
Zhang X and Ding J: Gambogic acid inhibits the catalytic activity
of human topoisomerase IIalpha by binding to its ATPase domain. Mol
Cancer Ther. 6:2429–2440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geng J, Xiao S, Zheng Z, Song S and Zhang
L: Gambogic acid protects from endotoxin shock by suppressing
pro-inflammatory factors in vivo and in vitro. Inflamm Res.
62:165–172. 2013. View Article : Google Scholar
|
|
4
|
Lu N, Hui H, Yang H, Zhao K, Chen Y, You
QD and Guo QL: Gambogic acid inhibits angiogenesis through
inhibiting PHD2-VHL-HIF-1α pathway. Eur J Pharm Sci. 49:220–226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xin ZF, Shen CC, Tao LJ, Yan SG and Wu HB:
Gambogic acid inhibits invasion of osteosarcoma via upregulation of
TIMP-1. Int J Mol Med. 31:105–112. 2013.
|
|
6
|
Wang LH, Yang JY, Yang SN, Li Y, Ping GF,
Hou Y, Cui W, Wang ZZ, Xiao W and Wu CF: Suppression of NF-κB
signaling and P-glycoprotein function by gambogic acid
synergistically potentiates adriamycin-induced apoptosis in lung
cancer. Curr Cancer Drug Targets. 14:91–103. 2014. View Article : Google Scholar
|
|
7
|
Duan D, Zhang B, Yao J, Liu Y, Sun J, Ge
C, Peng S, Fang J, Xiao W and Wu CF: Gambogic acid induces
apoptosis in hepatocellular carcinoma SMMC-7721 cells by targeting
cytosolic thioredoxin reductase. Free Radic Biol Med. 69:15–25.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu X, Liu Y, Wang L, He J, Zhang H, Chen
X, Li Y, Yang J and Tao J: Gambogic acid induces apoptosis by
regulating the expression of Bax and Bcl-2 and enhancing caspase-3
activity in human malignant melanoma A375 cells. Int J Dermatol.
48:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Qi Q, Lu N, Dai Q, Li F, Wang X, You
Q and Guo Q: Gambogic acid promotes apoptosis and resistance to
metastatic potential in MDA-MB-231 human breast carcinoma cells.
Biochem Cell Biol. 90:718–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi X, Chen X, Li X, Lan X, Zhao C, Liu S,
Huang H, Liu N, Liao S, Song W, et al: Gambogic acid induces
apoptosis in imatinib-resistant chronic myeloid leukemia cells via
inducing proteasome inhibition and caspase-dependent Bcr-Abl
downregulation. Clin Cancer Res. 20:151–163. 2014. View Article : Google Scholar :
|
|
11
|
Guo Q, Qi Q, You Q, Gu H, Zhao L and Wu Z:
Toxicological studies of gambogic acid and its potential targets in
experimental animals. Basic Clin Pharmacol Toxicol. 99:178–184.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan ML, Ooi JP, Ismail N, Moad AI and
Muhammad TS: Programmed cell death pathways and current antitumor
targets. Pharm Res. 26:1547–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu GS: TRAIL as a target in anti-cancer
therapy. Cancer Lett. 285:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Refaat A, Abd-Rabou A and Reda A: TRAIL
combinations: The new ‘trail’ for cancer therapy (Review). Oncol
Lett. 7:1327–1332. 2014.PubMed/NCBI
|
|
15
|
Yang LJ and Chen Y: New targets for the
antitumor activity of gambogic acid in hematologic malignancies.
Acta Pharmacol Sin. 34:191–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nie F, Zhang X, Qi Q, Yang L, Yang Y, Liu
W, Lu N, Wu Z, You Q and Guo Q: Reactive oxygen species
accumulation contributes to gambogic acid-induced apoptosis in
human hepatoma SMMC-7721 cells. Toxicology. 260:60–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Gao Y, Zhang L, Zeng J, He D and Sun
Y: Silibinin inhibits cell growth and induces apoptosis by caspase
activation, downregulating survivin and blocking EGFR-ERK
activation in renal cell carcinoma. Cancer Lett. 272:61–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spagnuolo C, Cerella C, Russo M,
Chateauvieux S, Diederich M and Russo GL: Quercetin downregulates
Mcl-1 by acting on mRNA stability and protein degradation. Br J
Cancer. 105:221–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu YL, Wan Y, Jin XJ, OuYang BQ, Bai T,
Zhao YQ and Nan JX: 25-OCH3-PPD induces the apoptosis of activated
t-HSC/Cl-6 cells via c-FLIP-mediated NF-κB activation. Chem Biol
Interact. 194:106–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu HY, Jin CY, Kim KS, Lee YC, Park SH,
Kim GY, Kim WJ, Moon HI, Choi YH and Lee JH: Oleifolioside A
mediates caspase-independent human cervical carcinoma HeLa cell
apoptosis involving nuclear relocation of mitochondrial apoptogenic
factors AIF and EndoG. J Agric Food Chem. 60:5400–5406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He D, Xu Q, Yan M, Zhang P, Zhou X, Zhang
Z, Duan W, Zhong L, Ye D and Chen W: The NF-kappa B inhibitor,
celastrol, could enhance the anti-cancer effect of gambogic acid on
oral squamous cell carcinoma. BMC Cancer. 9:3432009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Márquez F, Babio N, Bulló M and
Salas-Salvadó J: Evaluation of the safety and efficacy of
hydroxycitric acid or Garcinia cambogia extracts in humans. Crit
Rev Food Sci Nutr. 52:585–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahman MA, Kim NH and Huh SO: Cytotoxic
effect of gambogic acid on SH-SY5Y neuroblastoma cells is mediated
by intrinsic caspase-dependent signaling pathway. Mol Cell Biochem.
377:187–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joza N, Susin SA, Daugas E, Stanford WL,
Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, et al:
Essential role of the mitochondrial apoptosis-inducing factor in
programmed cell death. Nature. 410:549–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EA, Jang JH, Lee YH, Sung EG, Song IH,
Kim JY, Kim S, Sohn HY and Lee TJ: Dioscin induces
caspase-independent apoptosis through activation of
apoptosis-inducing factor in breast cancer cells. Apoptosis.
19:1165–1175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jacobson MD: Reactive oxygen species and
programmed cell death. Trends Biochem Sci. 21:83–86. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang YH, Yi MJ, Kim MJ, Park MT, Bae S,
Kang CM, Cho CK, Park IC, Park MJ, Rhee CH, et al:
Caspase-independent cell death by arsenic trioxide in human
cervical cancer cells: Reactive oxygen species-mediated
poly(ADP-ribose) polymerase-1 activation signals apoptosis-inducing
factor release from mitochondria. Cancer Res. 64:8960–8967. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishaq M, Khan MA, Sharma K, Sharma G,
Dutta RK and Majumdar S: Gambogic acid induced oxidative stress
dependent caspase activation regulates both apoptosis and autophagy
by targeting various key molecules (NF-κB, Beclin-1, p62 and NBR1)
in human bladder cancer cells. Biochim Biophys Acta.
1840:3374–3384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palempalli UD, Gandhi U, Kalantari P,
Vunta H, Arner RJ, Narayan V, Ravindran A and Prabhu KS: Gambogic
acid covalently modifies IkappaB kinase-beta subunit to mediate
suppression of lipopolysaccharide-induced activation of NF-kappaB
in macrophages. Biochem J. 419:401–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Guo QL, You QD, Wu ZQ and Gu HY:
Gambogic acid induces apoptosis and regulates expressions of Bax
and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol
Pharm Bull. 27:998–1003. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Liu S, Huang H, Liu N, Zhao C, Liao
S, Yang C, Liu Y, Zhao C, Li S, et al: Gambogic acid is a
tissue-specific proteasome inhibitor in vitro and in vivo. Cell
Rep. 3:211–222. 2013. View Article : Google Scholar
|
|
32
|
Kreuz S, Siegmund D, Scheurich P and
Wajant H: NF-kappaB inducers upregulate cFLIP, a
cycloheximide-sensitive inhibitor of death receptor signaling. Mol
Cell Biol. 21:3964–3973. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Micheau O, Lens S, Gaide O, Alevizopoulos
K and Tschopp J: NF-kappaB signals induce the expression of c-FLIP.
Mol Cell Biol. 21:5299–5305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tenev T, Bianchi K, Darding M, Broemer M,
Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K,
et al: The Ripoptosome, a signaling platform that assembles in
response to genotoxic stress and loss of IAPs. Mol Cell.
43:432–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feoktistova M, Geserick P, Kellert B,
Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Häcker G
and Leverkus M: cIAPs block Ripoptosome formation, a RIP1/caspase-8
containing intracellular cell death complex differentially
regulated by cFLIP isoforms. Mol Cell. 43:449–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JS, Li Q, Lee JY, Lee SH, Jeong JH,
Lee HR, Chang H, Zhou FC, Gao SJ, Liang C, et al: FLIP-mediated
autophagy regulation in cell death control. Nat Cell Biol.
11:1355–1362. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
He MX and He YW: A role for c-FLIP(L) in
the regulation of apoptosis, autophagy, and necroptosis in T
lymphocytes. Cell Death Differ. 20:188–197. 2013. View Article : Google Scholar :
|
|
38
|
Safa AR, Day TW and Wu CH: Cellular
FLICE-like inhibitory protein (C-FLIP): A novel target for cancer
therapy. Curr Cancer Drug Targets. 8:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su J, Cheng H, Zhang D, Wang M, Xie C, Hu
Y, Chang HC and Li Q: Synergistic effects of 5-fluorouracil and
gambogenic acid on A549 cells: Activation of cell death caused by
apoptotic and necroptotic mechanisms via the ROS-mitochondria
pathway. Biol Pharm Bull. 37:1259–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S, Wang L, Chen M and Wang Y:
Gambogic acid sensitizes resistant breast cancer cells to
doxorubicin through inhibiting P-glycoprotein and suppressing
survivin expression. Chem Biol Interact. 235:76–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao W, You CC, Zhuang JP, Zu JN, Chi ZY,
Xu GP and Yan JL: Viability inhibition effect of gambogic acid
combined with cisplatin on osteosarcoma cells via
mitochondria-independent apoptotic pathway. Mol Cell Biochem.
382:243–252. 2013. View Article : Google Scholar : PubMed/NCBI
|