Introduction
Lung cancer is the most frequent reason for cancer
related death and non-small cell lung cancer (NSCLC) accounts for
~80–85% of all lung cancer cases (1). Although there has been notable
progression in combination chemotherapy and surgical techniques,
the prognosis of NSCLC patients is still unsatisfactory since
5-year survival rate of NSCLC with all stages and histological
types is as low as 11% (2).
Besides the prolonged life time, the survival quality of NSCLC
patients should also be taken care of, while the high toxicity of
anti-cancer drugs adopted in clinical first line to normal tissues
and cells is an impassable barrier for cancer therapy and a heavy
burden for patients to bear. It would be better if there were some
methods that could improve the clinical therapy effects and relieve
the pain of the patients.
Agents derived from various plants with few or no
side effects have been recognized as potential alternative or
auxiliary cure for cancer patients. Flavonoids, one major class of
polyphenols, are well known for their antioxidant activity by
eliminating reactive oxygen species (3) and chelating metal atoms (4,5).
There is also substantial research suggesting that flavonoids have
anticancer effects (6). Phloretin
[3-(4-hydroxyphenyl)-1-(2,4,5-trihydroxyphenyl)] is one of the
major phenolic flavonoid glucosides (structure showed in Fig. 1) found in apples and other plants,
such as Pieris japonica, Hoveniae Lignum and
Loiseleuria procumbens (7,8).
Research has shown that phloretin possesses some pharmacological
activities, such as modulating cytochrome P450 1A1 (9) and inhibiting MRP1-mediated drug
transport (10). Further studies
have revealed that phloretin has other biological benefits
including inhibition of occurrence and progression of cancer
(11).
The main effects of agents from natural products on
cancer cells are correlated with inhibition of cell proliferation
and induction of cell apoptosis. B-cell lymphoma-2 (Bcl-2) protein
family is the best known regulator in modulating cell apoptosis
which could launch an apoptotic signaling cascade (12).
The process of apoptosis is regulated by
pro-apoptotic and anti-apoptotic regulators and their complicated
interactions. Bax and Bcl-2 are the most important pro-apoptotic
regulators which may further activate the PI3K/AKT pathway and
other pathways related to cell survival and death (13). In addition, natural agents may also
affect invasion and metastasis of cancer cells by regulating matrix
metalloproteinases (MMPs). Among which MMP-2 and -9 are of special
concern since it has been confirmed that patients with enhanced
expression of MMP-2 and/or MMP-9 would end up with a poorer
prognosis (14). However, there is
no research on whether phloretin regulates expression of the
apoptotic regulators and MMPs so as to exhibit its anticancer
effects in NSCLC.
Taking all the above into account, we hypothesized
and investigated by a series of biological experiments, in the
present study, that phloretin could induce cell apoptosis in NSCLC
cell lines by regulating apoptotic regulators and downstream
molecules, such as caspase-3 and -9, and inhibit the invasion and
migratory abilities through modulating the expression of MMP-2 and
-9. Importantly, we have confirmed, in the present study, that
phloretin facilitated the anticancer effect of cisplatin on NSCLC
cells by regulating the apoptotic pathway and expression of
MMPs.
Materials and methods
Cell lines and culture
Human lung cancer cell lines, A549, Calu-1, H838 and
H520 (purchased from the American Type Culture Collection), were
maintained in RPMI-1640 or DMEM supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 0.1 mg/ml streptomycin. The cells
were maintained in a humidified incubator at 37°C with 5%
CO2. Cell lines were subcultured by enzymatic digestion
with 0.25% trypsin when cells reached ~70–80% confluency. All cell
culture reagents were purchased from Hyclone Laboratories (Logan,
UT, USA).
Viability assays
Cell viability in A549, Calu-1, H838 and H520 cells
was measured by the mitochondrial conversion of MTT to a colored
product. Briefly, cells were seeded in 96-well plates at a density
of 1×104 cell/well and allowed to attach overnight in a
CO2 incubator. Cells were treated with different dose of
phloretin, cisplatin or combined adoption of the two agents for 24,
48 or 72 h. After that, 10 μl of MTT (5 mg/ml, dissolved in PBS and
filtered through a 0.22-mm membrane) was added into each well and
incubated at 37°C for 4 h before DMSO was added into each well to
dissolve farmazan crystals, and absorbance was determined at 492 nm
on an automated Bio-Rad 550 micro-plate reader (Bio-Rad
Laboratories Ltd., Shanghai, China). The percentage of viable cells
was determined by the formula: ratio = [OD (phloretin / cisplatin /
phloretin + cisplatin) − OD (Blank)] / [OD (Control) − OD (Blank)].
The assay was carried out in 3 independent experiments.
Analysis of apoptosis by flow
cytometry
A549 and Calu-1 cells stimulated by phloretin,
cisplatin or combination of the two agents were analyzed by Annexin
V-FITC/PI staining (Keygen Biotech, Jiangsu, China) in order to
confirm their apoptotic rate. Briefly, 2×105 cells in
the exponential growth phase were seeded in 6-well plates. Cells
were treated with or without specified drugs for 24 h at 37°C.
Then, both adherent and floating cells were collected and analyzed
by flow cytometry according to the manufacturer's instructions.
Pelleted cells were gently washed with PBS 3 times and resuspended
in Annexin binding buffer. Then, cells were incubated with Annexin
V-FITC and PI for 15 min at room temperature. The stained cells
were analyzed by using flow cytometry (Becton-Dickinson, San Jose,
CA, USA). Cells with no drug treatment were taken as controls.
Wound healing assays
H838 and H520 cells were seeded into a 6-well plate
and cultured to a confluent monolayer. A sterile 200-μl pipette tip
was used to scratch the monolayer to form wounds with equal width
in each well. H838 and 520 cells were then incubated in
serum-containing medium (2% serum) with phloretin (40 and 50 μg/ml)
for 24 h. Images of wound areas were taken at 0 and 24 h at ×100
magnification.
Invasion assay
The in vitro invasive ability of H838 and
H520 cells was tested by the 6.5-mm Transwell with an 8.0-μm pore
polycarbonate membrane insert (Corning Co., USA). The Matrigel
(Becton-Dickinson and Co., USA) was incubated in the upper chamber
of the Transwell system and allowed to form a gel at 37°C. Cells
(3×105) suspended in 300 μl of serum-free medium were
seeded into the upper compartments of each chamber in the presence
of phloretin, while the lower compartments of each chamber were
filled with 500 μl medium with 10% FBS. The non-invasive cells were
gently removed away from the upper surface of the membrane after 24
h of incubation at 37°C. Cells on the reverse side were stained
with 0.1% crystal violet and counted in five randomly selected
fields under a microscope at ×400 magnification.
Real-time polymerase chain reaction (PCR)
analysis
Total mRNA was isolated from H838 and H520 cells in
the exponential growth phase with TRIzol reagent (Invitrogen, USA)
according to the manufacturer's instructions. RNAs (1 μg per
reaction) were reverse-transcribed to yield first-strand cDNA using
transcriptase (Takara Biotechnology Co., Dalian, China), diluted
cDNA was then mixed with pairs of specific primers (sequences
showed in Table I) and SYBR Green
PCR Master Mix (Takara) in a 20-μl total volume. The PCR cycling
conditions were as follows: 5 min at 95°C for 1 cycle, 30 sec at
95°C, and 1 min at 60°C for 45 cycles. Every cDNA sample was run
three times independently and the primers of β-actin mRNA, and the
expression of the gene of interest was determined by the
2−ΔΔCT method.
 | Table IThe sequence of primers used in the
present research. |
Table I
The sequence of primers used in the
present research.
| Sequence | Primers |
|---|
| β-actin-F |
5′-CCTGTACGCCAACACAGTGC-3′ |
| β-actin-R |
5′-ATACTCCTGCTTGCTGATCC-3′ |
| Bcl-2-F |
5′-CCAGGCCGGCGACGACTTCTC-3′ |
| Bcl-2-R |
5′-ATCTCCCGGTTGACGCTCTCCACA-3′ |
| MMP-2-F |
5′-CACGCTGGGCCCTGTCACTCCT-3′ |
| MMP-2-R |
5′-TGGGGCCTCGTATACCGCATCAAT-3′ |
| MMP-9-F |
5′-TGCCCGGACCAAGGATACAGTTT-3′ |
| MMP-9-R |
5′-AGGCCGTGGCTCAGGTTCAGG-3′ |
Western blot analysis
Cytosolic and nuclear proteins from H838 and H520
cells were extracted according to instructions of protein
extraction kit (Beyotime Institute of Biotechnology, Jiangsu,
China), and concentration of these proteins was determined by BCA
method (Beyotime). Equal amount of protein from each group was
separated on 8% SDS-polyacrylamide gel electrophoresis, and
transferred to PVDF membrane (Millipore, MA, USA). The membranes
were soaked in blocking buffer [5% skimmed milk melted in TBS-T [25
mM Tris (pH 7.6), 138 mM NaCl and 0.05% Tween-20) for 2 h and then
probed with Bax, cleaved caspase-3, cleaved caspase-9 and β-actin
(1:1,000–1:5,000) (Santa Cruz, CA, USA) overnight at 4°C. The
membranes were further incubated with anti-rabbit IgG peroxidase
conjugated secondary antibody (1:5,000) conjugated with HRP, then
the immune-reactive signals were detected by ECL detection system
(Amersham Pharmacia Biotech).
Statistical analysis
Numeric variables are expressed as means ± SD.
Statistical differences among experimental groups were performed by
one-way analysis of variance (ANOVA) followed by Dunnett's test.
All statistical analysis was carried out with SPSS 17.0. A value of
P<0.05 was considered to be statistically significant.
Results
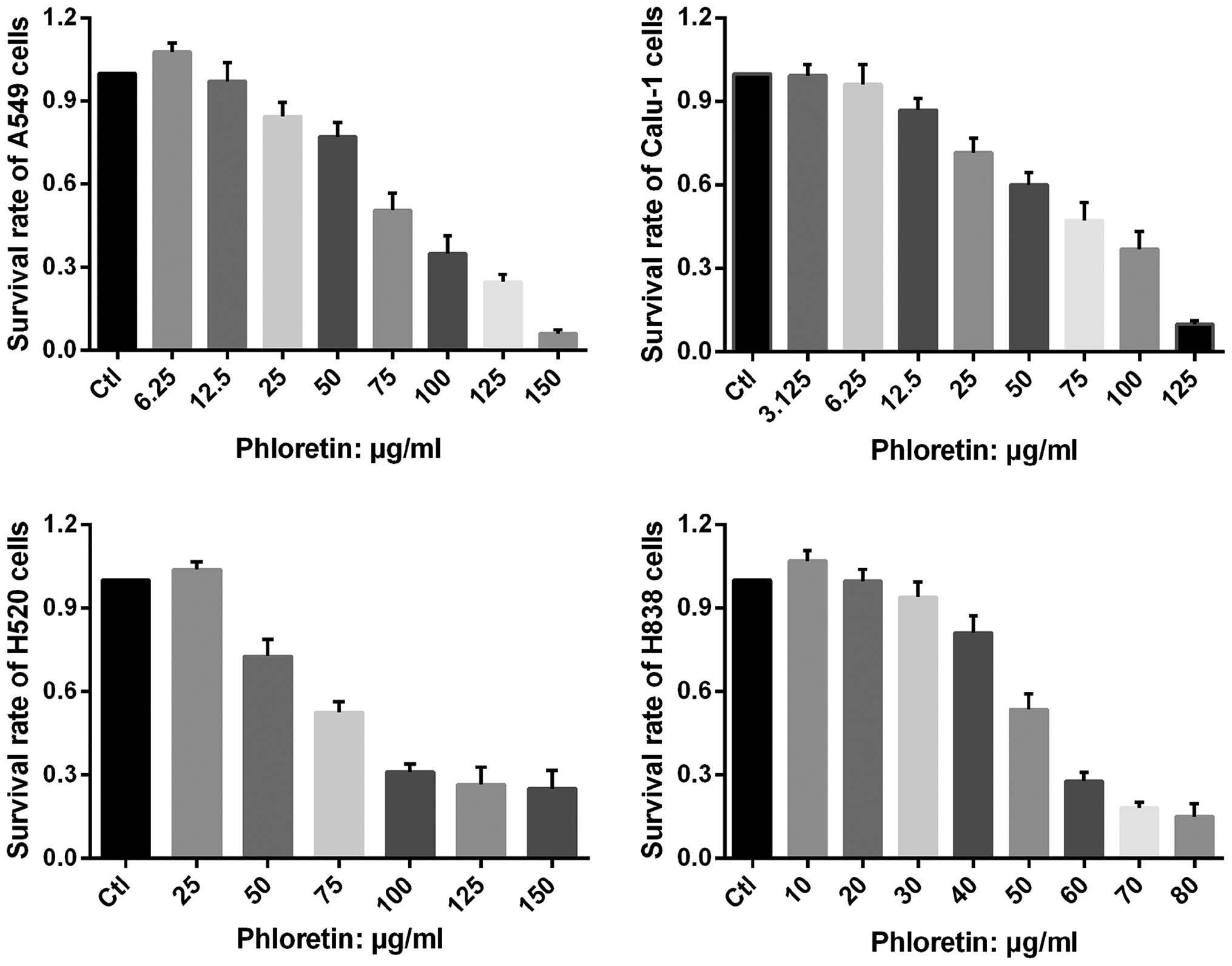
Phloretin inhibits proliferation of NSCLC
cells
The vitality of A549, Calu-1, H838 and H520 cells
treated with different dose of phloretin was determined by MTT
assay. As shown in Fig. 2,
extremely low dose of phloretin enhanced proliferation of the
cells, while it could also inhibit proliferation of the cells in a
dose-dependent manner with varied minimum effective
concentration.
Phloretin induces cell apoptosis in A549
and Calu-1 cells
In order to determine whether the cytotoxicity of
phloretin was associated with cell apoptosis, we measured the
percentage of Annexin V-positive and PI-positive cells in each
group. As shown in Fig. 3,
phloretin treatment resulted in an increased number of early-stage
apoptotic H838 and H520 cells compared with that of control. The
number of Annexin V and PI positive cells in experimental groups
increased in a dose-dependent manner.
Phloretin inhibited migratory ability of
H838 and H520 cells
Wound healing assay was carried out to determine the
effects of phloretin on migration of lung cancer cells. As shown in
Fig. 4, H520 cells migrated and
covered the wound area after 24 h of incubation, while phloretin
treatment inhibited the migration of H520 cells from each edge of
the wound leaving an uncovered area in the image (Fig. 4A and B). Moreover, similar results
were obtained from H838 cells and quantitative data indicating that
phloretin inhibited the migration in H838 cells (Fig. 4C and D).
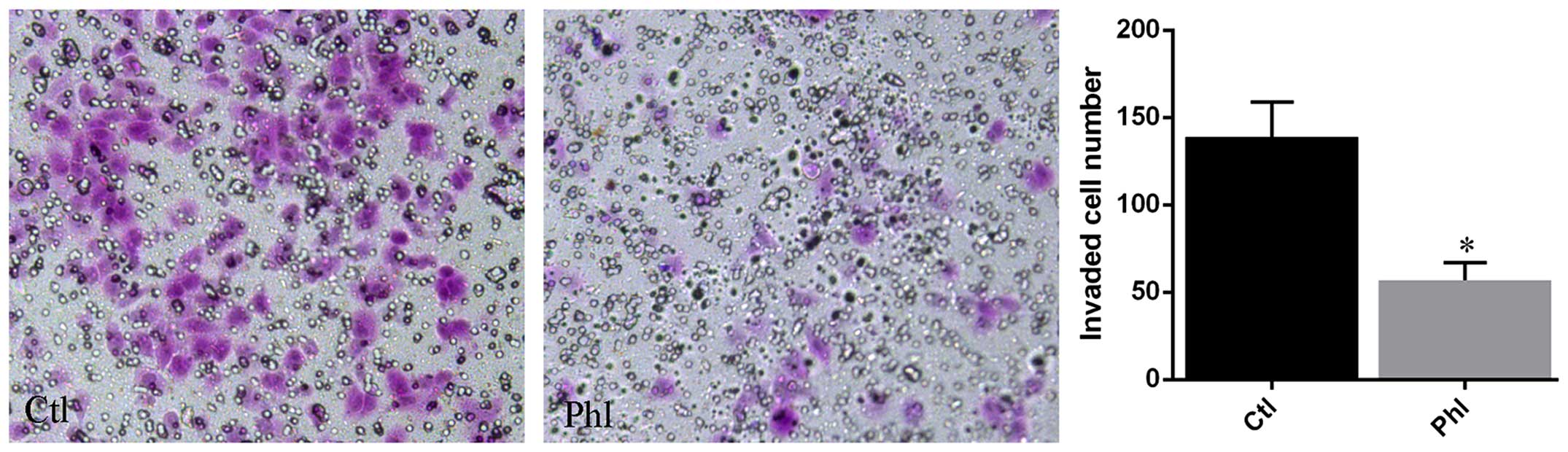
Phloretin inhibits invasion of lung
cancer cells
Transwell membrane coated with Matrigel was used to
determine the invasion of lung cancer cells treated with or without
phloretin. The results (Fig. 5)
showed that large amount of H520 cells from control group invaded
into the lower chamber of the Transwell system, while much fewer
cells treated with phloretin invaded into the lower chamber
compared with that of control.
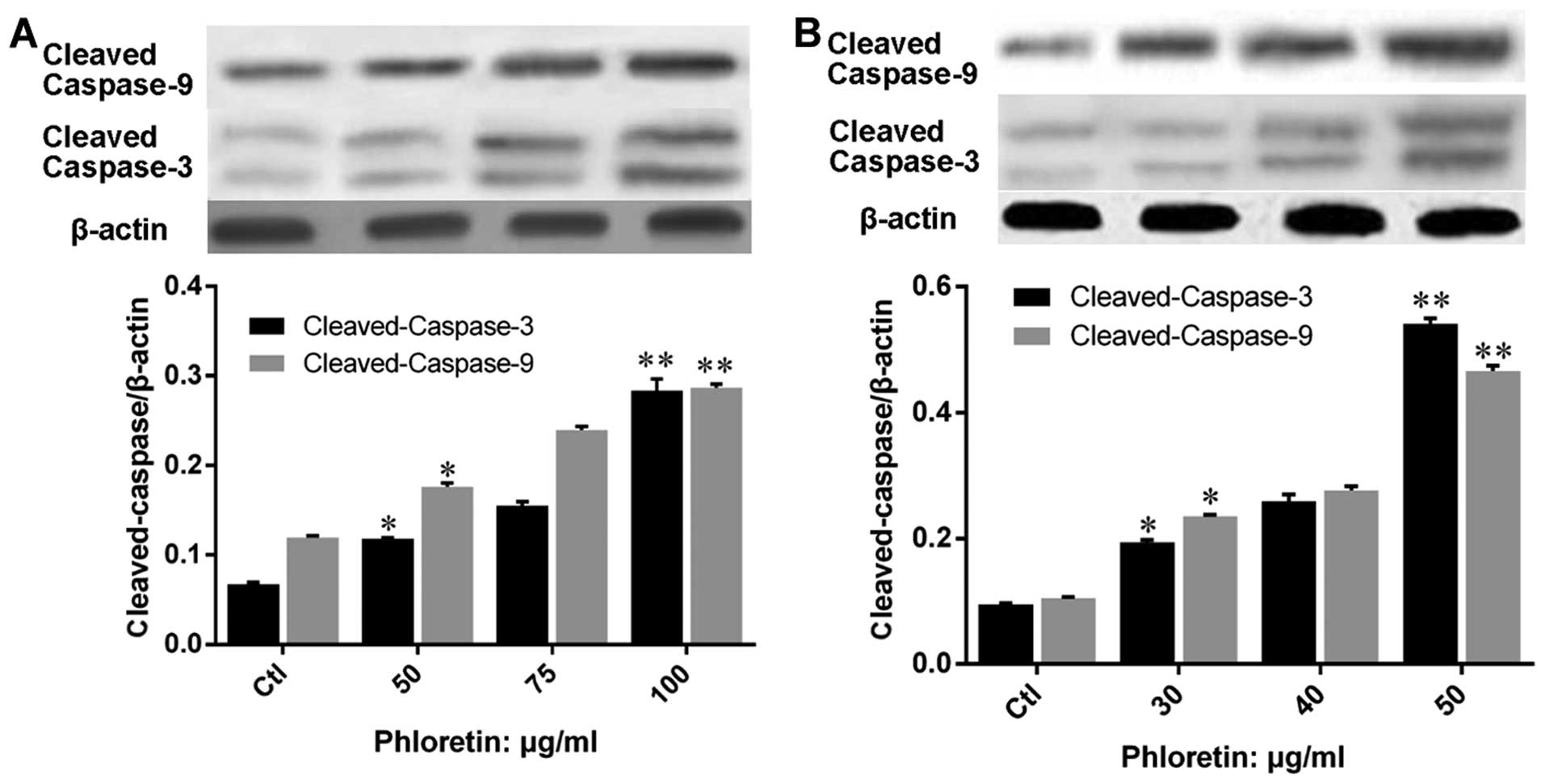
Phloretin modulated expression of
apoptotic regulators and executors in lung cancer cells
In order to understand the mechanism of how
phloretin induced cell apoptosis in lung cancer cells, we examined
the expression of apoptotic regulators on gene and protein levels.
We investigated the expression of Bcl-2 (Fig. 6). The results showed that phloretin
significantly decreased the expression of Bcl-2 at both gene and
protein levels in H520 (Fig. 6A and
B) and H838 (Fig. 6C and D)
cells. Importantly, the changes of Bcl-2 gene and protein
expression was obviously dose-dependent in the two cell lines
treated with phloretin. Further, we evaluated the expression of
apoptosis executors, caspase-3 and -9, and results showed that
expression of cleaved-caspase-3 and -9 in H520 (Fig. 7A) and H838 (Fig. 7B) cells were upregulated by
phloretin dose-dependently.
Phloretin decreases the expression of
MMP-2 and -9
In order to illustrate the mechanisms underlying the
suppression of migration and invasion by phloretin in H838 and H520
cells, we evaluated the expression of MMP-2 and -9 on both gene and
protein levels in cells treated with different doses of phloretin.
The results showed that phloretin inhibited the gene expression of
MMP-2 and -9 in H520 (Fig. 8A and
B) and H838 (Fig. 8C and D)
cells, and the results revealed a clear dose-dependent manner in
the changes of MMP-2 and -9 gene expression. Moreover, results of
MMP-2 and -9 protein expressions in phloretin treated H520 and H838
cells revealed a similar trend to that of gene expression.
Phloretin enhances the effects of
cisplatin on cell proliferation and apoptosis
After confirming that phloretin could inhibit
proliferation of cancer cells in a dose-dependent manner, we
investigated whether phloretin could facilitate the anticancer
effects of cisplatin. MTT test was carried out in cells treated
with phloretin, cisplatin and combined adoption of the two agents.
The results showed that cisplatin inhibited the proliferation of
A549, Calu-1, H838 and H520 cells in a dose-dependent manner
(Fig. 9A–D), more importantly,
combined adoption of cisplatin and phloretin exhibited a much
better effects than that of individual usage of the two agents
(Fig. 9E and F).
Furthermore, the results from flow cytometry showed
that combined adoption of the two agents led to more apoptosis in
A549 and Calu-1 cells than individual usage of phloretin and
cisplatin (Fig. 10), which was in
consistent with results from MTT assay.
Phloretin facilitats the modulation of
cisplatin on apoptotic regulators and executors
Based on the knowledge that phloretin would enhance
the effects of cisplatin on cell proliferation and apoptosis, we
further investigated the expression of Bcl-2 in cells treated with
cisplatin, phloretin and combined usage of these agents. The
results (Fig. 11) showed that
both cisplatin and phloretin decreased expression of Bcl-2, while
combined adoption of the two agents decreased Bcl-2 much more
strongly in H520 (Fig. 11A) and
H838 (Fig. 11B) cells. Further,
we evaluated the effects of phloretin and cisplatin on the
expression cleaved-caspase-3 and -9. The results showed that
combined adoption of the two agents also exhibited a better effect
than individual usage of cisplatin and phloretin in H520 (Fig. 12A) and H838 (Fig. 12B) cells.
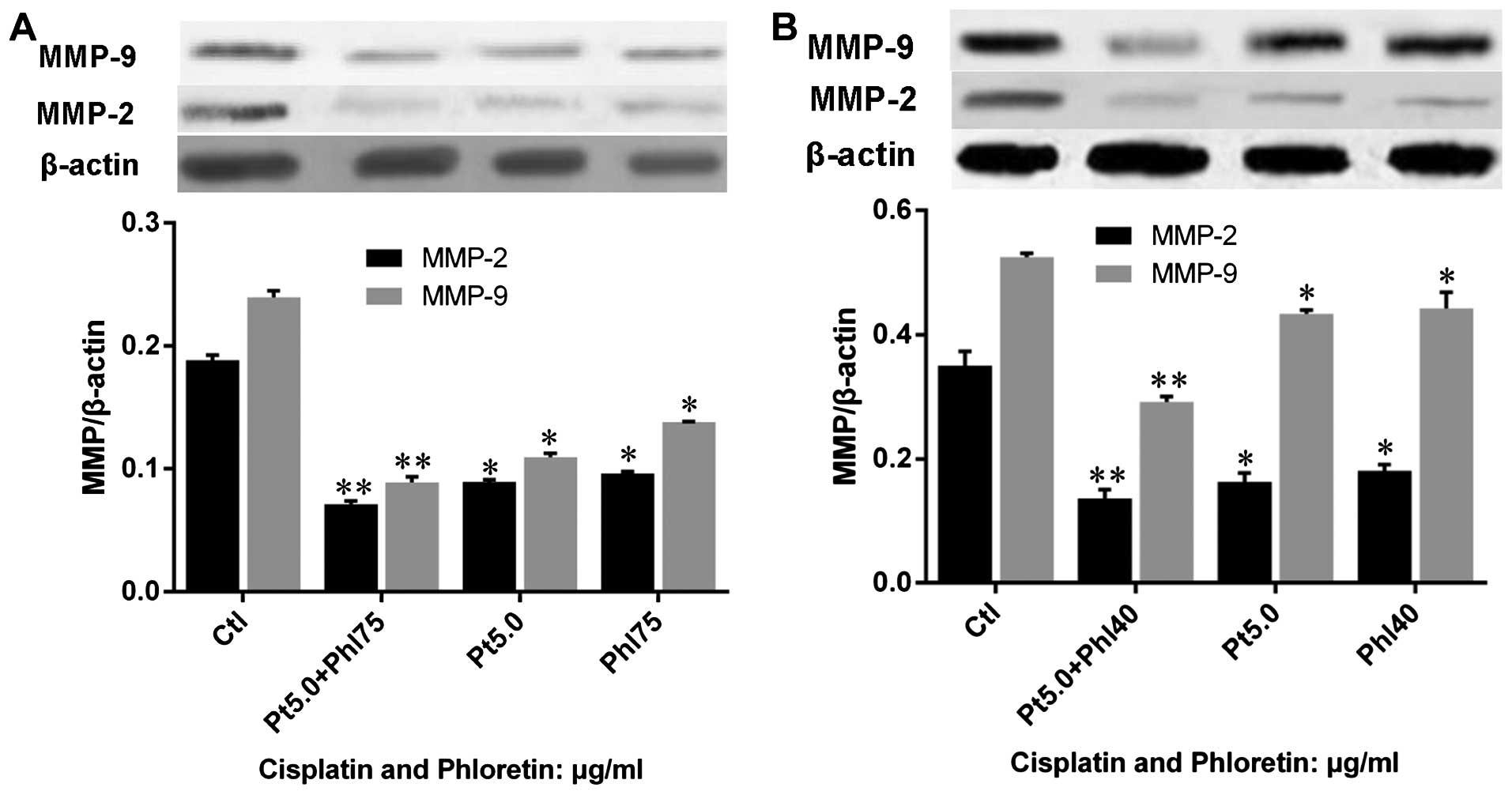
Phloretin enhances modulation of
cisplatin on MMP-2 and -9
We also investigated the effects of phloretin on the
regulation of cisplatin on MMP-2 and -9 by western blot analysis.
The results showed that both cisplatin and phloretin decreased
MMP-2 and -9 protein expression, and combined adoption of cisplatin
and phloretin would lead to a much sharper decrease of MMP-2 and -9
in H520 (Fig. 13A) and H838
(Fig. 13B) cells than that of
individual treatment with cisplatin, or phloretin.
Discussion
Chemotherapy is one of the most important options
for NSCLC and this therapeutic process is also a heavy burden for
patients. Agents derived from various plants have been considered
as potential alternative or auxiliary cure for cancer patients
because research has shown that these therapeutic agents had no
side effects, or at least the side effects were much more moderate
compared with that of clinical first line chemotherapeutics. The
anticancer effects of phloretin have been confirmed in various
cancers (15–17), while its anti-cancer effects and
underlying mechanisms on NSCLC is still uncertain. The aim of the
present study was to evaluate the anticancer effects of phloretin
on NSCLC cell lines and the results revealed that phloretin could
exert anticancer effects on NSCLC cell lines and it also enhanced
the anticancer effects of cisplatin.
In order to elucidate the effects of phloretin on
cell vitality, MTT assay and flow cytometry were, respectively,
carried out. MTT results showed that phloretin inhibited
proliferation of A549, Calu-1, H838 and H520 cells in a
dose-dependent manner while flow cytometry also indicated its
apoptosis-induction ability in H838 and H520 cells, these results
were consistent with previous research reporting the anticancer
effects of phloretin on colon cancer cells (18). As we know, the imbalance between
proliferation and apoptosis leads to limitless cell proliferation
which is the hallmark of cancers. Induction of apoptosis in cancer
cells has been one of the main targets for cancer therapy (19). Bcl-2 family is a pivotal regulator
of cell survival and apoptosis, Bcl-2 was the first discovered
death regulator which could enhance cell survival and proliferation
and inhibited expression of Bcl-2 indicating restricted cell
proliferation and accelerated cell apoptosis (20). Our results have shown that
phloretin induced apoptosis in NSCLC H838 and H520 cells
accompanied with dose-dependently decreased Bcl-2 expression.
Decreased expression of Bacl-2 would lead to upregulation of
proapoptotic molecules, such as Bax, and release of cytochrome
c from mitochondria into cytosol followed by activation of
caspase, especially caspase-3 and -9 (21). The results from the present study
showed that phloretin treatment enhanced the expression of
caspase-3 and -9 followed by upgraded cell apoptosis, which was
consistent with previous research indicating that caspases played a
pivotal part in final common pathway of cell apoptosis (22). These results indicated that
phloretin triggered cell apoptosis in NSCLC cells by inhibiting the
expression of Bcl-2 and enhancing the activity of caspase-3 and
-9.
Metastasis is a remarkable characteristic of
malignant tumors and also the primary cause of cancer-related death
in most cancer patients. Previous studies have indicated that
complex mechanisms were involved in the metastasis of cancers
(23), among which the changed
expression of MMPs, especially MMP-2 and -9, were leading causes
(24,25). Enhanced expression and upgraded
activity of MMP-2 and -9 resulted in degradation of extracellular
matrix and destruction of basilar membrane, which facilitated the
migration of cancer cells (26,27).
On the contrary, restricted expression of MMP-2 and -9 in cancer
cells leads to a better prognosis (28–30).
The results from the present study showed that exposure of NSCLC
H520 cells to phloretin led to decreased invasion and migratory
ability. In order to illustrated the underlying mechanism of this
phenomenon, we detected the expression of MMP-2 and -9 on both gene
and protein levels. The results showed that phloretin treatment
decreased the expression of MMP-2 and -9 in H838 and H520 cell
lines, which illustrated the primary role of MMP-2 and -9 in the
inhibition effects of phloretin on lung cancer metastasis.
Based on the findings that phloretin exhibited its
anticancer effects by regulating apoptotic regulators and MMPs, we
were interested whether this natural product could enhance the
therapeutic effects of first line drugs. We then evaluated the
vitality of NSCLC A549, Calu-1, H838 and H520 cells treated by
cisplatin combined with or without phloretin. The MTT results
showed that cisplatin treatment suppressed proliferation of the
cells in a dose-dependent manner, and combined adoption of
cisplatin and phloretin exhibited a much greater inhibition effect
on proliferation of H838 and H520 cells than that of single
adoption of the two agents. Furthermore, combined usage of
cisplatin and phloretin also led to more apoptosis in H838 and H520
cells compared with that of cells treated with cisplatin or
phloretin alone. Further examinations on expressions of apoptotic
regulators showed that combined usage of cisplatin and phloretin
led to sharper decrease in Bcl-2 expression compared with that of
cells treated with cisplatin or phloretin. Besides, combined
adoption of cisplatin and phloretin showed stronger effects on
deregulating MMP-2 and -9 expression compared with the results from
cells treated with cisplatin alone.
This study laid emphasis on evaluating the
anticancer effects of phloretin on NSCLC cell lines and elucidated
the possible mechanisms in H838 and H520 cell lines. Importantly,
we proved the enhancement of phloretin on the anticancer effects of
cisplatin. However, this study has several limitations. First, this
study only assessed the anticancer effects of phloretin in
vitro, it would be better if there were results from in
vivo experiments. Second, it has been confirmed, in the present
research, that phloretin exhibited its inhibition on invasion,
however, there was no visual results indicating phloretin
facilitated the inhibition effects of cisplatin on invasion and
migration since combined adoption of cisplatin and phloretin would
result in too much cell death.
In conclusion, our results confirmed for the first
time that phloretin treatment could suppress cell proliferation,
induce apoptosis and inhibit the invasive and migratory ability of
NSCLC cells probably through regulating expression of apoptotic
regulators and downstream molecules, such as caspase-3 and -9, and
modulating the activities of MMPs, especially MMP-2 and -9. Above
all, results from this study indicated that phloretin enhanced the
anticancer ability of cisplatin at least partly through this
pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81071933).
References
|
1
|
Subramaniam S, Thakur RK, Yadav VK, Nanda
R, Chowdhury S and Agrawal A: Lung cancer biomarkers: State of the
art. J Carcinog. 12:32013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I; EUROCARE-4 Working
Group. Recent cancer survival in Europe: A 2000–02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Boer A, Vos E and Bast A:
Implementation of the nutrition and health claim regulation - the
case of antioxidants. Regul Toxicol Pharmacol. 68:475–487. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zicker SC, Wedekind KJ and Jewell DE:
Antioxidants in veterinary nutrition. Vet Clin North Am Small Anim
Pract. 36:1183–1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mandel S, Amit T, Reznichenko L, Weinreb O
and Youdim MB: Green tea catechins as brain-permeable, natural iron
chelators-antioxidants for the treatment of neurodegenerative
disorders. Mol Nutr Food Res. 50:229–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang DS, Li ZL, Peng WB, Yang YP, Wang X,
Liu KC, Li XL and Xiao WL: Three new prenylated flavonoids from
Macaranga denticulata and their anticancer effects. Fitoterapia.
103:165–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rezk BM, Haenen GR, van der Vijgh WJ and
Bast A: The anti-oxidant activity of phloretin: The disclosure of a
new antioxidant pharmacophore in flavonoids. Biochem Biophys Res
Commun. 295:9–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S and Morris ME: Effects of the
flavonoids biochanin A, morin, phloretin, and silymarin on
P-glycoprotein-mediated transport. J Pharmacol Exp Ther.
304:1258–1267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pohl C, Will F, Dietrich H and Schrenk D:
Cytochrome P450 1A1 expression and activity in Caco-2 cells:
Modulation by apple juice extract and certain apple polyphenols. J
Agric Food Chem. 54:10262–10268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nguyen H, Zhang S and Morris ME: Effect of
flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharm Sci.
92:250–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boyer J and Liu RH: Apple phytochemicals
and their health benefits. Nutr J. 3:52004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xin M and Deng X: Nicotine inactivation of
the proapoptotic function of Bax through phosphorylation. J Biol
Chem. 280:10781–10789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bayramoglu A, Gunes HV, Metintas M,
Değirmenci I, Mutlu F and Alataş F: The association of MMP-9 enzyme
activity, MMP-9 C1562T polymorphism, and MMP-2 and -9 and TIMP-1,
-2, -3, and -4 gene expression in lung cancer. Genet Test Mol
Biomarkers. 13:671–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH,
Chen JH, Wu CH and Ho YS: Apple polyphenol phloretin potentiates
the anticancer actions of paclitaxel through induction of apoptosis
in human hep G2 cells. Mol Carcinog. 48:420–431. 2009. View Article : Google Scholar
|
|
16
|
Nelson JA and Falk RE: Phloridzin and
phloretin inhibition of 2-deoxy-D-glucose uptake by tumor cells in
vitro and in vivo. Anticancer Res. 13A:2293–2299. 1993.
|
|
17
|
Nelson JA and Falk RE: The efficacy of
phloridzin and phloretin on tumor cell growth. Anticancer Res.
13A:2287–2292. 1993.
|
|
18
|
Zhu SP, Liu G, Wu XT, Chen FX, Liu JQ,
Zhou ZH, Zhang JF and Fei SJ: The effect of phloretin on human γδ T
cells killing colon cancer SW-1116 cells. Int Immunopharmacol.
15:6–14. 2013. View Article : Google Scholar
|
|
19
|
Karunagaran D, Joseph J and Kumar TR: Cell
growth regulation. Adv Exp Med Biol. 595:245–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rong Y and Distelhorst CW: Bcl-2 protein
family members: Versatile regulators of calcium signaling in cell
survival and apoptosis. Annu Rev Physiol. 70:73–91. 2008.
View Article : Google Scholar
|
|
21
|
Park HJ, Jeon YK, You DH and Nam MJ:
Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2
family in human hepatic cancer cells. Food Chem Toxicol.
60:542–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fennell DA: Caspase regulation in
non-small cell lung cancer and its potential for therapeutic
exploitation. Clin Cancer Res. 11:2097–2105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balzer EM and Konstantopoulos K:
Intercellular adhesion: Mechanisms for growth and metastasis of
epithelial cancers. Wiley Interdiscip Rev Syst Biol Med. 4:171–181.
2012. View
Article : Google Scholar
|
|
24
|
Guruvayoorappan C and Kuttan G:
Amentoflavone inhibits experimental tumor metastasis through a
regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase,
lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in
lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol.
30:711–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen RX, Xia YH, Xue TC, Zhang H and Ye
SL: Down-regulation of osteopontin inhibits metastasis of
hepatocellular carcinoma cells via a mechanism involving MMP-2 and
uPA. Oncol Rep. 25:803–808. 2011.
|
|
26
|
Borkham-Kamphorst E, Alexi P, Tihaa L,
Haas U and Weiskirchen R: Platelet-derived growth factor-D
modulates extracellular matrix homeostasis and remodeling through
TIMP-1 induction and attenuation of MMP-2 and MMP-9 gelatinase
activities. Biochem Biophys Res Commun. 457:307–313. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davis ME, Gumucio JP, Sugg KB, Bedi A and
Mendias CL: MMP inhibition as a potential method to augment the
healing of skeletal muscle and tendon extracellular matrix. J Appl
Physiol. 115:884–891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang R, Ke ZF, Wang F, Zhang WH, Wang YF,
Li SH and Wang LT: GOLPH3 overexpression is closely correlated with
poor prognosis in human non-small cell lung cancer and mediates its
metastasis through upregulating MMP-2 and MMP-9. Cell Physiol
Biochem. 35:969–982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu X, Chen L, Xu B, Xie Q, Sun M, Deng X,
Wu C and Jiang J: Increased MT2-MMP expression in gastric cancer
patients is associated with poor prognosis. Int J Clin Exp Pathol.
8:1985–1990. 2015.PubMed/NCBI
|
|
30
|
El-Badrawy MK, Yousef AM, Shaalan D and
Elsamanoudy AZ: Matrix metalloproteinase-9 expression in lung
cancer patients and its relation to serum MMP-9 activity,
pathologic type, and prognosis. J Bronchol Interv Pulmonol.
21:327–334. 2014. View Article : Google Scholar
|