Introduction
Breast cancer is one of the most common malignancies
in women, with ~1.05 million new cases annually and 3.1% increasing
rate (1,2). Currently, surgery, chemotherapy,
radio-therapy and endocrine therapy are the mainstream clinical
strategies for breast cancer, but 25% mortality rate still remains
(3). The deterioration and poor
prognosis of breast cancer are attributed to tumor invasion and
metastasis (1).
Epithelial-mesenchymal transition (EMT) and EMT-induced acquisition
of cancer stem cell (CSC)-like phenotypes are crucial for invasion
and metastasis of tumor cells (4–7).
Hence, finding the key molecules that regulate formation of EMT and
CSCs is warranted to reveal the pathological progression of breast
cancer and develop novel therapeutic approaches.
The stromal cell-derived factor-1 (SDF-1, CXCL12) is
a highly conserved chemoattractant cytokine responsible for
regulating diverse biological processes ranging from embryonic
development, stem cell movement, angiogenesis and tumor generation
(8–10). Clinical research reported that the
expression of SDF-1 was elevated significantly in various
carcinomas associated with tumor grade, lymph node metastasis, TNM
stage and prognosis (11–13). Moreover, the organs with
constitutive SDF-1 secretion, such as liver, lung, lymph nodes, and
bone marrow, are also the most common sites for secondary
metastasis of breast cancer (14–16).
On the contrary, as a potent leukocytic chemokines, CXCL12 also has
a potential to promote anticancer immunity by inducing
CD8+ T cell activity, enhancing cytotoxicity, increasing
the number of CD11c+ cells in the tumor-draining lymph
nodes and reducing the accumulation of myeloid-derived suppressor
cells in the spleen (17). In
vitro studies revealed that SDF-1 was secreted by cancer
associated fibroblasts (CAFs) or myofibroblasts and bound to its
receptors (CXCR4 and CXCR7) on cancer cell surface to activate
downstream intracellular signal pathways that regulate the
metastasis, angiogenesis, and drug-resistant of cancer cells
(18). Gao et al and Jiang
et al both demonstrated that the proliferation, migration
and invasion of pancreatic cancer cells and epithelial ovarian
cancer cells were enhanced through SDF-1/CXCR4 axis after treating
with certain concentrations of SDF-1 (19,20).
Kang et al overexpressed SDF-1 in MDA-MB-231 cells to create
an autocrine loop of SDF-1/CXCR4 and found that SDF-1 boosted the
invasiveness and migration of breast cancer cells (21). Although SDF-1 is crucial for the
migration and invasion of cancer cells, little is known about the
roles of SDF-1 in EMT or CSC-like phenotype formation in breast
cancer and the detailed mechanisms.
In the present study, we established SDF-1
overexpressing MCF-7 cells to investigate the effect of SDF-1 on
the proliferation, migration, invasion, EMT, and CSC-like phenotype
formation in breast cancer cells and explore the underlying
mechanism. The results showed that overexpression of SDF-1 induced
EMT of MCF-7 cells through the NF-κB pathway to obtain the CSC-like
phenotypes, ultimately facilitating metastasis of breast cancer
cells.
Materials and methods
Cell lines and mammosphere culture
MDA-MB-231, MDA-MB-435, and MCF-7 cell lines were
purchased from Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences. All cells were cultured in Dulbecco's modified
Eagle's medium (DMEM, Gibco, Grand Island, NY, USA) containing 10%
fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and
streptomycin/penicillin (100 U/ml) at 37°C in a 5% CO2
incubator. When cells reached 80–90% confluence, western blot
analysis was employed to select a cell line with the lowest SDF-1
expression for subsequent experiments.
MCF-7 cell mammospheres were cultured according to
previously published methods by Wang et al (22). Briefly, MCF-7 cells were suspended
in serum-free DMEM/F12 (1:1) medium containing 20 ng/ml epidermal
growth factor (EGF, PeproTech, St. Louis, MO, USA), 10 ng/ml basic
fibroblast growth factor (b-FGF, PeproTech), B27 (Gibco), and ITS
(insulin, transferrin and selenium, Sigma-Aldrich, St. Louis, MO,
USA), and seeded into ultralow attachment plates (Corning, NY, USA)
at a density of 1×105 cells/ml. Fresh mammosphere media
(2 ml) was added into the well every 2–3 days without removing the
old media. Mammospheres were collected every seven days for
subsequent experiment.
Conduction of SDF-1 overexpressing vector
and transfection
Full length SDF-1 coding sequences were amplified by
PCR, and cloned into the EcoRI-BamHI fragment of
pEGFP-N1 expression vector. The primers of SDF-1 were designed as
follows: forward, 5′-TCAGAATTCATGAACGCCAAGGT CGTGG-3′
(the underline represents EcoRI site); reverse,
5′-CCTCGGATCCTCACATCTTGAACCTC-TTG-3′
(the underline represents BamHI site). Subsequently, the
plasmid pEGFP-N1-SDF-1 was transfected into MCF-7 cells using
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) strictly
following the manufacturer's instructions. The non-transfected
control cells and the cells with the empty vector transfected
(pEGFP-N1) were evaluated in parallel as control. The stably
transfected cells were screened by G418 (400 μg/ml, Invitrogen)
after 24 h of transfection. As pEGFP-N1 plasmid contains green
fluorescent protein, we employed a fluorescence microscope to
investigate and calculate the efficiency after 24 h of transfection
which was 25–35%. Then the expression of SDF-1 was examined at 7–14
days of transfection.
Real-time (RT)-PCR
Total RNA from MCF-7 cells was extracted by High
Purity Total RNA Fast Extraction kit (BioTeke, Beijing, China)
following the manufacturer instructions and then
reverse-transcribed into cDNA. RT-PCR was carried out by Exicycler™
96 (Bioneer, Daejeon, Korea) using SYBR Green mastermix (Solarbio,
Beijing, China) with the following protocol: initial denaturation
at 95°C for 10 min, 40 cycles consisting of 95°C for 10 sec, 60°C
for 20 sec and 72°C for 30 sec. Primer sequences were: OCT4,
5′-AGCGATCAAG CAGCGACTA-3′ (forward) and 5′-GGAAAGGGACCGAGG AGTA-3′
(reverse); Nanog, 5′-GCAGGCAACTCACTTTA TCC-3′ (forward) and
5′-CCCACAAATCACAGGCATAG-3′ (reverse); SDF-1,
5′-GTGCCCTTCAGATTGTAGCC-3′ (forward) and
5′-CCTTCCCTAACACTGGTTTCA-3′ (reverse); SOX2,
5′-CATCACCCACAGCAAATGAC-3′ (forward) and
5′-CAAAGCTCCTACCGTACCACT-3′ (reverser); β-actin,
5′-CTTAGTTGCGTTACACCCTTTC TTG-3′ (forward) and
5′-CTGTCACCTTCACCGTTCCAG TTT-3′ (reverse). Relative expression was
obtained by 2−ΔΔCT method. β-actin served as an internal
control.
Colony formation assay
Cells were resuspended in DMEM complete media and
seeded in 35-mm plates at a density of 102 cells/plate.
All plates were incubated at 37°C in an atmosphere of 5%
CO2 for ~14 days. The suspension was decanted and
replaced with fresh medium every 3 days. Then cells were fixed in
4% formaldehyde for 20 min. After being washed twice with
phosphate-buffered salines (PBS, pH 7.4), cells were stained with
Wright-Giemsa dye composite for 5 min. The number of colonies was
calculated with an inverted microscope. Cells containing >50
cells were counted as a colony.
MTT analysis
Cells were plated in 96-well plates at a density of
2×103 per well with five replicates for each testing
point and cultured in a 37°C, 5% CO2 incubator for 24,
48, 72 and 96 h, respectively. Thereafter, cells in each well were
exposed to MTT (0.2 mg/ml, Sigma-Aldrich) for 4 h followed by
incubation with 200 μl DMSO (Sigma-Aldrich) to dissolve the dark
blue crystals before reading the optical density (OD) at 490 nm in
a microplate reader (BioTek, VT, USA).
Wound healing assay
Cells were inoculated in 6-well plates until 80–90%
confluence. A wound was gently created with a 200-μl pipette tip on
each cell monolayer, and each well was rinsed with a serum-free
culture medium to remove detached cells. The migrating cells were
imaged under an inverted microscope at 0, 12 and 24 h of culturing.
The result was the ratio of the migrated distance to the initial
distance.
Matrigel-based invasion analysis
The Matrigel-based invasion analysis was performed
in a 24-well Transwell system (Corning, Tewksbury, MA, USA) with a
Matrigel (BD Biosciences, San Jose, CA, USA) pre-coated
polycarbonate membrane in the top chamber. Cells were harvested and
resuspended in FBS-free DMEM, then plated in the top chamber at a
density of 2×104 per well. DMEM (800 μl) supplemented
with 30% FBS was added into the lower chamber as a chemoattractant.
After 24 h of incubation, the non-invading cells on the
upper-surface of membrane were removed with cotton swabs, and the
invading cells on undersurface of the membrane were fixed with 4%
paraformaldehyde for 20 min and stained with crystal violet for 5
min. Invasion was analyzed in five randomly selected areas under an
inverted microscope in a blinded manner.
Flow cytometry assay
Cells in each group were trypsinized, fixed with 70%
ethanol, and washed with fresh medium. Thereafter, the collected
MCF-7 cells were stained with FITC-conjugated CD44 antibody and
PE-conjugated CD24 antibody (BD Biosciences). Then the mixture was
incubated for 30 min at room temperature in the dark. The labeled
cells were washed and analyzed immediately on a FACS (fluorescence
activated cell sorting) Vantage (BD Biosciences).
Aldehyde dehydrogenase (ALDH) activity
assay
The Aldehyde Dehydrogenase Activity Colorimetric
Assay kit (Sigma-Aldrich) was employed to analyze ALDH enzymatic
activity. In brief, mammospheres were resuspended in ALDH binding
buffer (200 μl per 1×106 cells) then centrifuged at
13,000 g for 10 min. Fifty microliters supernatant from each sample
was mixed with 43 μl ALDH binding buffer, 2 μl ALDH substrate, and
5 μl acetaldehyde in the dark. The absorbance values at OD 450 nm
were detected at 2–3-min intervals from 5 min (T initial) up to the
standard value, the last but one absorbance value was defined as T
final, and ALDH activity was calculated based on T initial and T
final.
Immunofluorescence staining
Cells grown on coverslips were fixed with 4%
formaldehyde for 15 min and permeabilized with 0.1% Triton X-100
(Amresco, Cochran Road Solon, OH, USA) for 30 min, cells were
washed with PBS for three times at the end of each step.
Subsequently, the non-specific bindings were blocked by goat serum
(Solarbio) at room temperate for 15 min. Thereafter, cells were
incubated overnight at 4°C with primary antibody against E-cadherin
(1:200 diluted, Boster, Wuhan, China) followed by incubated with
Cy3-labeled goat anti-rabbit IgG secondary antibody (1:200,
Beyotime, Haimen, China) for 1 h at room temperature. The unbound
antibodies in each step were washed with PBS three times. After
counterstaining with 4′, 6-diamidino-2-phenylindole (DAPI) and
finally rinsed with PBS, each coverslip was mounted inversely onto
a slide with anti-fluorescent mounting media (Solarbio) added.
Images were captured by a laser scanning confocal microscope.
NF-κB p65-siRNA interference
siRNA for NF-κB p65 and the control siRNA were
designed and synthesized by GenePharma Co., Ltd. (Shanghai, China).
The sequences were: NF-κB p65-siRNA: 5′-AGGACAUAUGAGACCUUCA-3′,
control siRNA: 5′-UUCUCCGAACGUGUCACGU-3′. NF-κB p65-siRNA (75 pmol)
and control siRNA were transfected into the indicated MCF-7 cells,
respectively, using the Lipofectamine 2000 reagent (Invitrogen)
according to the manufacturer's instructions. Cells were harvested
at 24 h after transfection for western blot analysis.
Western blot analysis
Cells in each sample were lysed with RIPA lysate
(Beyotime) including 1% phenylmethanesulfonyl fluoride (PMSF,
Beyotime). For NF-κB p65 detection, nuclear and cytoplasmic
fraction proteins were extracted using Nuclear and Cytoplasmic
Protein Extraction kit (Beyotime) following the manufacturer's
instructions. All protein concentrations were detected using a
bicinchoninic acid (BCA) protein assay kit (Beyotime). Then equal
amounts of different proteins were loaded and separated using
SDS-polyacrylamide gel electrophoresis (PAGE) and
electrotransferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). After being blocked with 5% non-fat
milk for 1 h, the membranes were probed with primary antibodies
against SDF-1, Nanog, SOX2 (all 1:200 diluted, Santa Cruz
Biotechnology, Santa Cruz, CA, USA), E-cadherin, fibronectin, NF-κB
p65 (all 1:400 diluted, Boster), CXCR4, CXCR7 (both 1:500 diluted,
Biosharp), OCT4, Vimentin, Slug, p-IκB, IκB (all 1:500 diluted,
Bioss, Beijing, China) at 4°C overnight and subsequently incubated
with their corresponding secondary antibodies (1:5,000 dilution,
Beyotime) for 45 min at 37°C. Unbound antibodies in each step were
washed with TBST four times. The positive bands were visualized
through enhanced chemiluminescence (ECL) solution (Qihai Biotec,
Shanghai, China) and measured by Gel-Pro-analyzer software
(Bethesda, MD, USA). Histone H3 and β-actin both served as internal
controls.
Statistical analysis
All values are reported as mean ± standard deviation
(SD). Kolmogorov-Smirnov (K-S) test and homogeneity of variance
test were employed in each experiment and one-way analysis of
variance (ANOVA) was used followed by the Bonferroni post hoc test
to compare differences between groups. All statistical analysis was
performed by GraphPad Prism 5.0 software. P<0.05 was considered
statistically significant.
Results
Stable overexpression of SDF-1 in breast
cancer cells
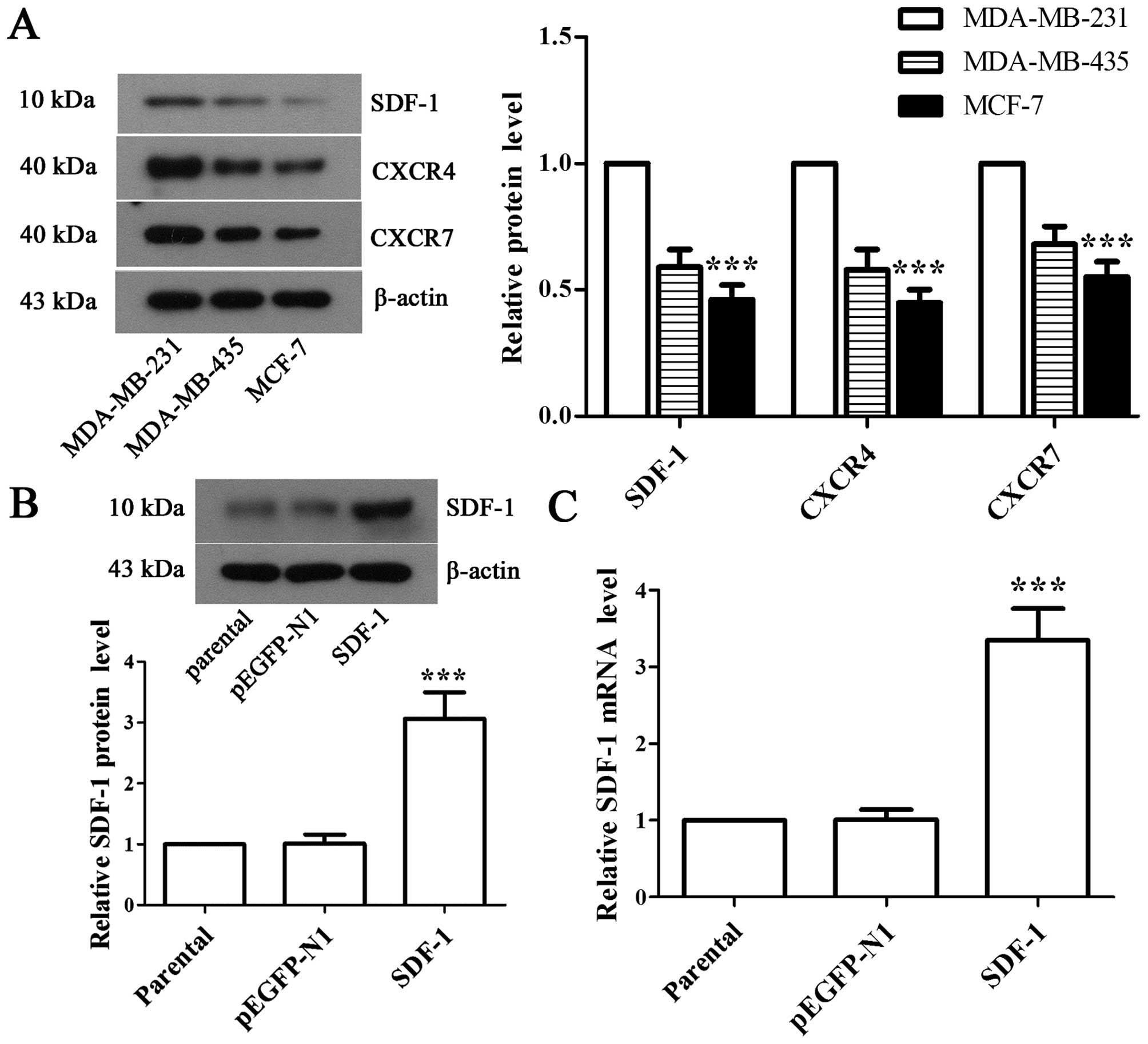
To explore the potential effects of SDF-1, we first
detected the expressions of SDF-1, CXCR4 and CXCR7 in MDA-MB-231,
MDA-MB-435, and MCF-7 cells, respectively. Western blot analysis
showed that MCF-7 cell line had the lowest expression of SDF-1 and
its receptors (Fig. 1A). Hence we
selected MCF-7 cell line for detailed mechanistic studies. Next,
the recombinant pEGFP-N1-SDF-1 plasmid was transfected into MCF-7
cells, and western blot analysis and RT-PCR were performed to
validate the expression of SDF-1 in positive monoclonal cells. The
results showed that the expression of SDF-1 was increased by
3.03-fold (Fig. 1B, P<0.001)
and 3.32-fold (Fig. 1B,
P<0.001) at protein and mRNA levels, respectively, in
SDF-1-transfected MCF-7 cells compared with pEGFP-N1-transfected
MCF-7 cells, which suggested stable overexpression of SDF-1 in
MCF-7 cell line.
Overexpression of SDF-1 promotes the
proliferation, migration and invasion of MCF-7 cells
Colony formation and MTT assay were employed to
visualize the effect of SDF-1 on the proliferation of MCF-7 cells.
We found that the colony formation rate in overexpressing SDF-1
MCF-7 cells was enhanced by 1.54-fold compared with parental
(Fig. 2A, P<0.01). At the same
time, the proliferation capability of MCF-7 cells with SDF-1
transfection was increased significantly from 48 to 96 h as
compared to parental (Fig. 2B,
P<0.05). The above results indicated that SDF-1 was able to
enhance the proliferation of MCF-7 cells.
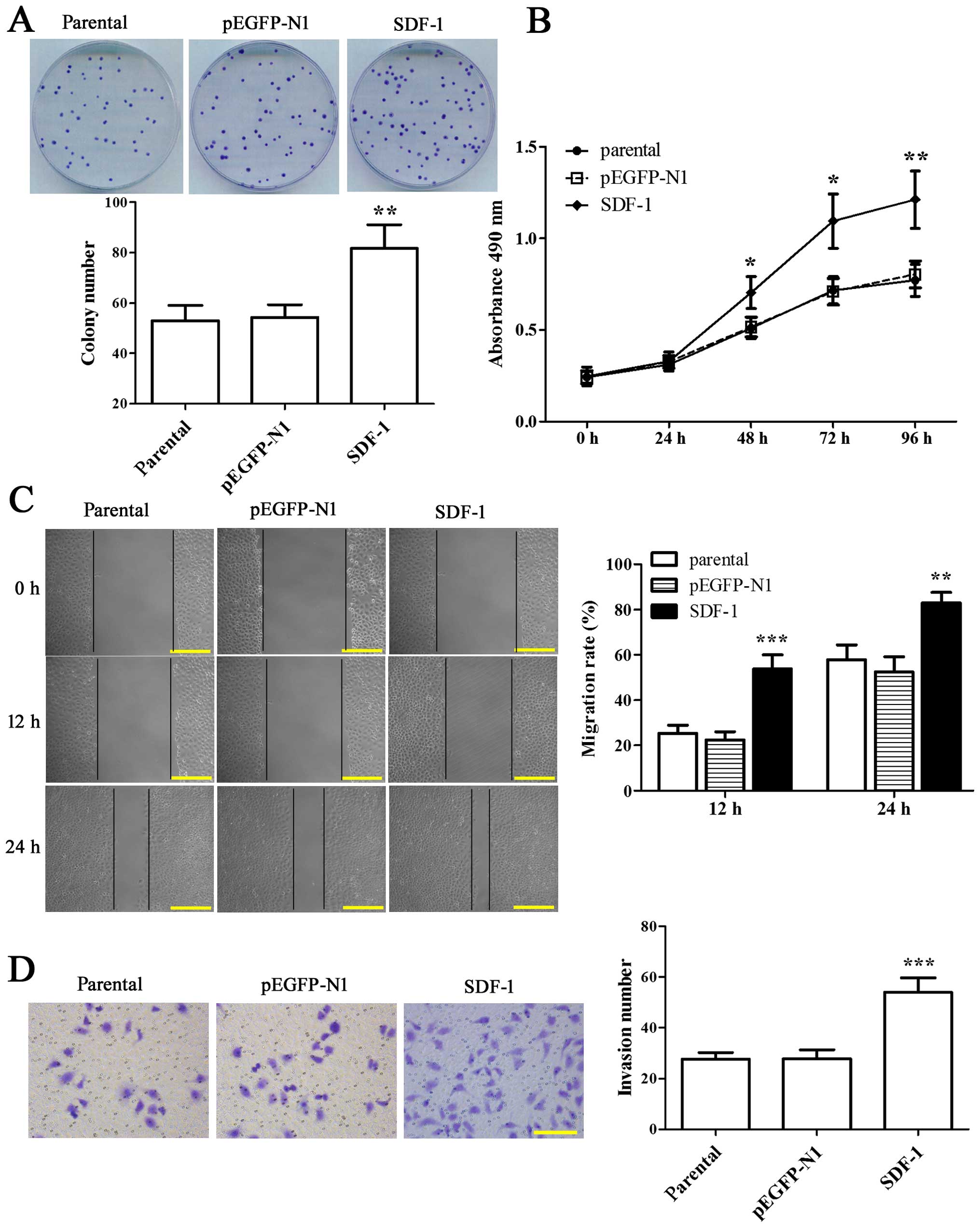 | Figure 2Overexpression of SDF-1 promotes the
proliferation, migration and invasion of MCF-7 cells. (A) Cells
were seeded in 35-mm plates and incubated for 14 days before being
fixed with 4% formaldehyde. Then the cells were stained with
Wright-Giemsa dye composite to calculate the number of colony
formation, and the representative images are shown. (B) Cells were
plated in 96-well plates with five replicates for each testing
point and cultured for 24, 48, 72 and 96 h, respectively. MTT assay
was used to evaluate cell viability at OD 490 nm. (C) A wound was
created on cell monolayer in each well, cells were cultured for 12
or 24 h, and the distance migrated was measured. Representative
photomicrographs are shown on the left. Scale bars indicate 200 μm
at ×200 magnification. (D) The Matrigel-based invasion assay was
carried out in a Transwell system. After 24 h of incubation, the
invading cells on undersurface of the membrane were fixed with 4%
paraformaldehyde, stained with crystal violet and counted with five
randomly selected microscopic fields. Representative examples of
photographs are shown on the left. Scale bar indicates 200 μm at
×200 magnification. Experiments were all done in triplicates for
statistical significance, and the results are expressed as mean ±
SD. *P<0.05, **P<0.01,
***P<0.001 vs parental. |
We further evaluated the effect of SDF-1 on the
migratory and invasive potential of MCF-7 cells through wound
healing and Transwell assay. The results at 12 and 24 h after
wounding both showed that the wound healing rate in SDF-1-
overexpressed MCF-7 cells was notably elevated compared with
parental (Fig. 2C, P<0.01).
Moreover, the number of invading cells was 54±5.7 in SDF-1
overexpressed-MCF-7 cells in the present of FBS, which was
increased compared with parental (27.6±2.7) (Fig. 2D, P<0.001). Thus, the results
strongly supported that overexpression of SDF-1 could promote
migration and invasion of MCF-7 cells.
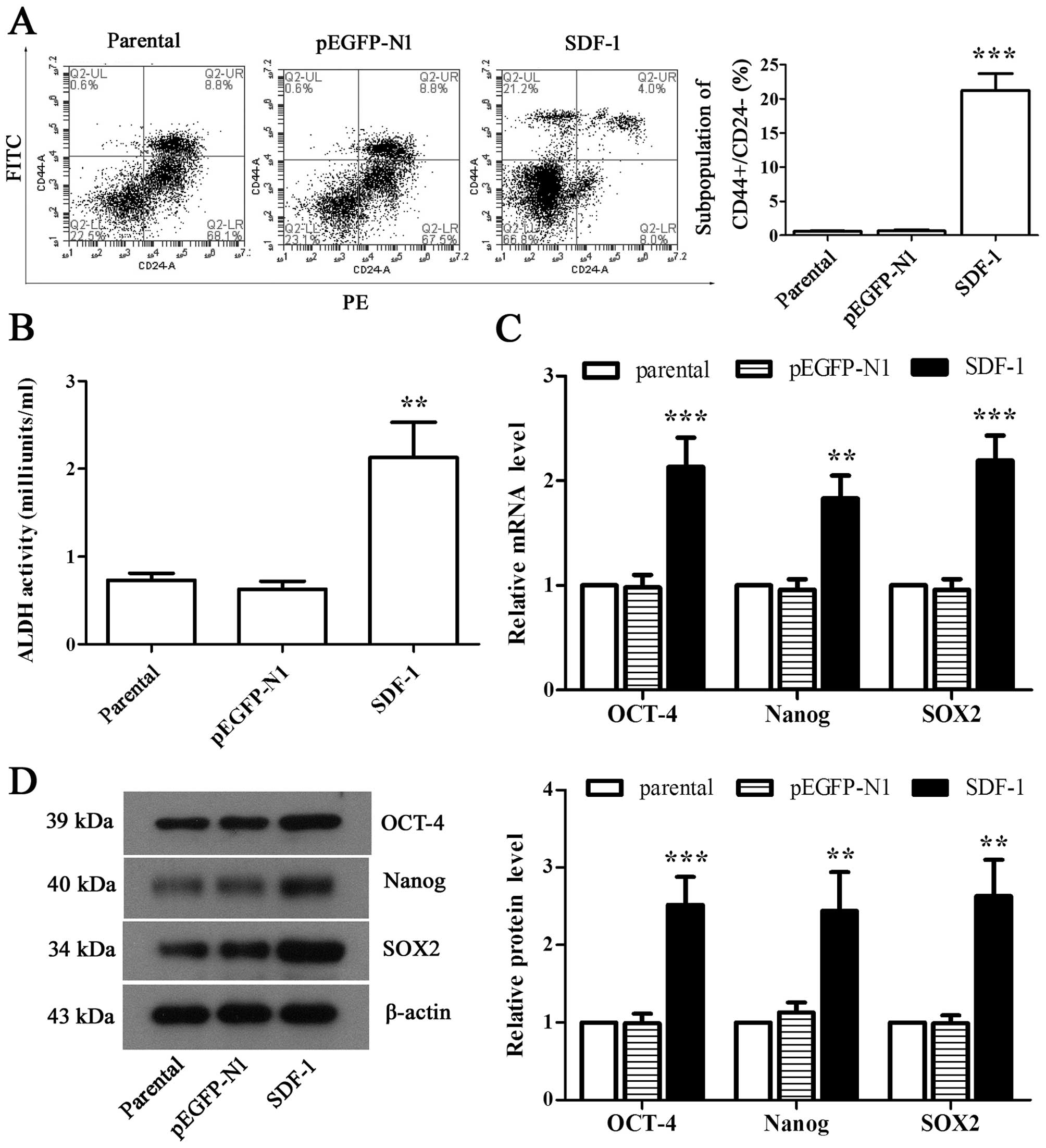
Overexpression of SDF-1 induces CSC-like
phenotype formation from MCF-7 cells
To observe whether overexpression of SDF-1
contributes to the formation of CSC-like phenotypes in MCF-7 cells,
we detected the proportion of CD44+/CD24−
cells through flow cytometry. Our analysis showed that SDF-1 caused
an increased accumulation of cell population with
CD44+/CD24− phenotype (Fig. 3A, P<0.01). Similarly, ALDH
activity was also dramatically elevated by overexpressing SDF-1 in
MCF-1 cells (Fig. 3B, P<0.01).
It was further observed that the expression of OCT-4, Nanog, and
SOX2 in both mRNA and protein levels was elevated significantly in
SDF-1-overexpressed MCF-7 cells compared with parental as observed
by RT-PCR and western blot analysis (Fig. 3C and D, P<0.01). These results
suggested that MCF-7 cells obtained the CSC-like phenotype by
overexpressing SDF-1.
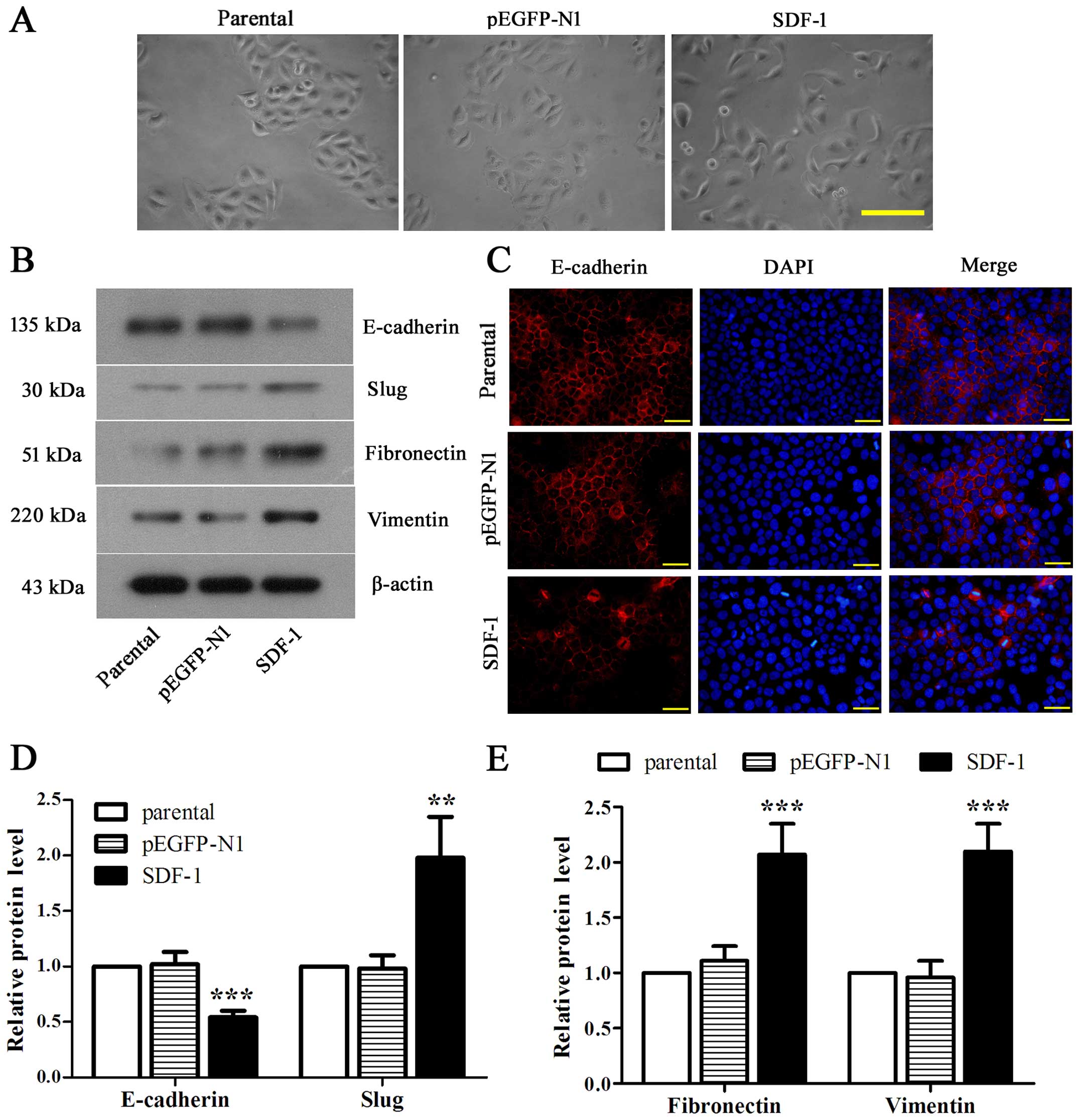
Overexpression of SDF-1 boosts EMT of
MCF-7 cells
To address the effect of SDF-1 on EMT of MCF-7
cells, we first observed the loose connections between cells with
SDF-1 overexpression (Fig. 4A).
Then we identified the significantly downregulated E-cadherin and
notably upregulated slug, fibronectin and vimentin in
SDF-1-overexpressed MCF-7 cells compared with parental (Fig. 4B, D and E, P<0.01). Accordingly,
the fluorescence intensity of E-cadherin was appeared to be reduced
obviously in SDF-1 overexpressed-MCF-7 cells as compared with
parental (Fig. 4C). Collectively,
these data revealed that overexpression of SDF-1 could induce EMT
in MCF-7 cells.
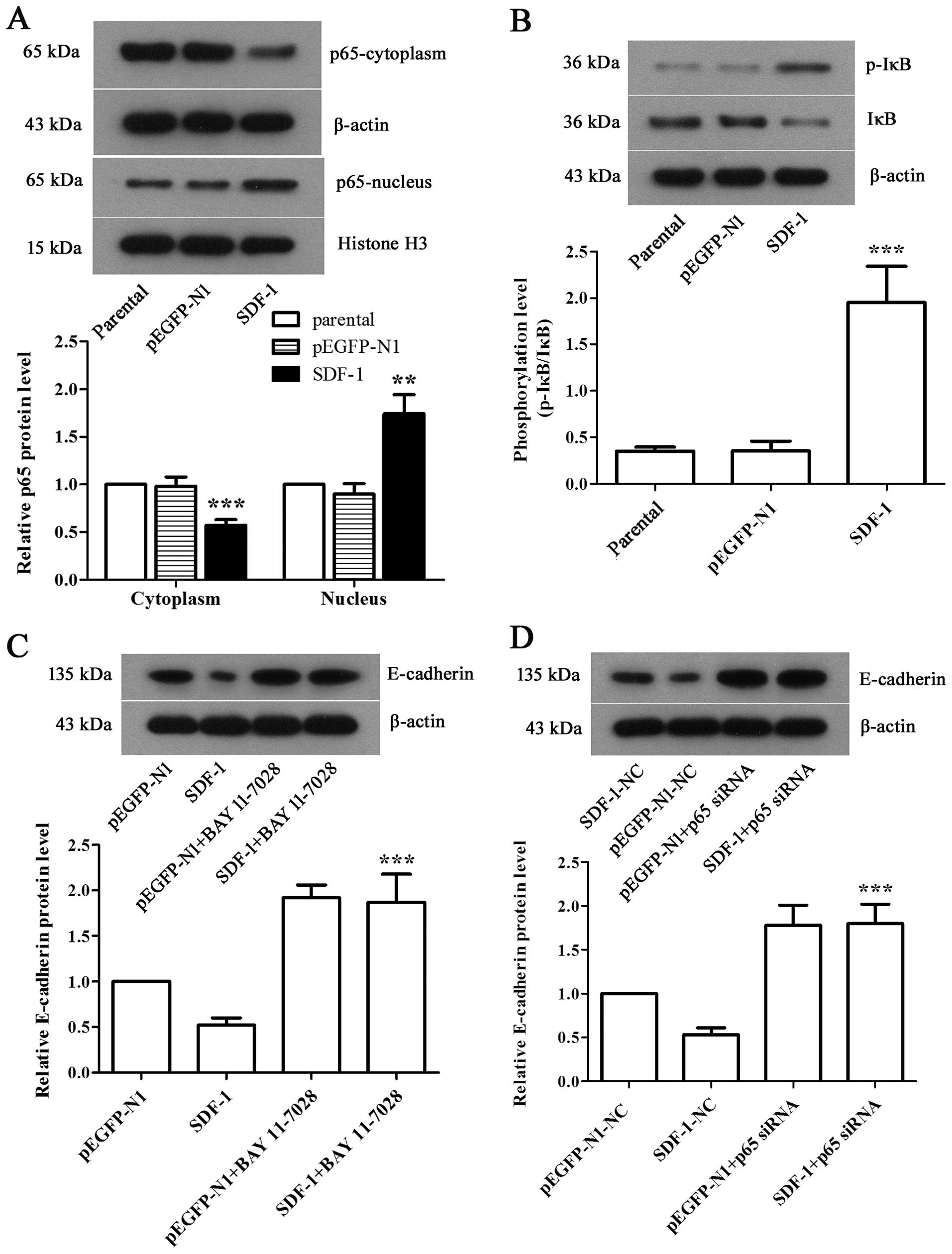
NF-κB pathway is involved in
SDF-1-mediated EMT in MCF-7 cells
To investigate the mechanism by which SDF-1 induced
EMT in MCF-7 cells, western blot analysis was applied to examine
the expression levels of related proteins in NF-κB pathway. We
discovered a apparent decrease of cytoplasmic p65 level and a
significant increase of nuclear p65 level in SDF-1 overexpressed
MCF-7 cells compared with parental (Fig. 5A, P<0.01), along with the
notably elevated phosphorylation level of cytoplasmic IκB (Fig. 5B, P<0.001), suggesting the
activation of NF-κB pathway. We further employed BAY 11–7028, an
antagonist target NF-κB pathway, to inhibit NF-κB pathway, and
performed siRNA interference to silence NF-κB p65 gene in SDF-1
overexpressed-MCF-7 cells. Both results showed the significantly
upregulated E-cadherin expression (Fig. 5C and D, P<0.001), which
indicated that NF-κB played a prominent role in the progression of
EMT in SDF-1 overexpressed breast cancer cells.
Discussion
SDF-1 is involved in a broad range of biological
procedures including cell adhesion, migration, invasion,
chemotaxis, cell cycle, proliferation, apoptosis, angiogenesis, and
cell communication. However, whether SDF-1 could induce CSC-like
phenotypes and EMT of breast cancer cells and the detailed
mechanism remain unclear. Here, we overexpressed SDF-1 in the
poorly invasive MCF-7 cells (23).
Then we found that overexpressing SDF-1 could trigger EMT in MCF-7
cells by activating NF-κB pathway and induce CSC-like phenotypes to
increase the abilities of proliferation, migration, and invasion.
Overall, these results further identified the roles of SDF-1 in the
metastases of breast cancer cells.
The interactions between SDF-1 and receptors CXCR4
and CXCR7 control multiple steps of tumor growth and metastasis in
>20 human malignancies, including breast cancer (24). In addition, overexpression of SDF-1
can recruit more macrophages. The increased recruitment of
cancer-associated macrophages (CAMs) is not contributed only to
tumor angiogenesis by releasing vascular endothelial growth factor
(VEGF) but also capable of inducing tumor cell motility and
invasion through paracrine loop signaling (25–28).
On the contrary, hypoxia has been reported as an important driving
force for the multistep process of metastasis, and the accumulated
CAMs could exacerbate the oxidant microenvironment of the tumor.
Hypoxia improves metastatic seeding of cancer cells by enhancing
CXCR4 expression to enable tumor cells to home to SDF1 highly
expressed secondary organs (29).
In our study, we created an autocrine loop of SDF-1 and its
receptors and found that overexpression of SDF-1 could increase the
growth, migration, and invasion in MCF-7 cells without recruiting
CAMs, consistent with Kang and colleagues (30).
CSCs are unresponsive to chemotherapeutic and
apoptotic drugs since they can resist DNA damage (30). Breast cancer stem cells (BCSCs)
have been identified as CD44+/CD24− cells.
Huang et al reported that SDF-1 boosted the proliferation of
CD44+/CD24− cells through SDF-1/CXCR4
signaling (1). We found that the
subpopulation of MCF-7 cells with CD44+/CD24−
phenotypes was elevated after overexpressing SDF-1, suggesting the
acquirement of CSC phenotypes in overexpressing SDF-1 MCF-7 cells.
ALDH1 exhibits low or absent expression in normal breast tissue,
breast cells with increased ALDH1 expression indicate stem or
progenitor properties with broadest differentiation potential and
greatest growth capacity (31).
The transcription factors OCT4, Nanog and SOX2 are all embryonic
stem cell (ESC) markers and play an important role in maintaining
the pluripotent self-renewal of ESCs, which are downregulated in
the differentiated somatic cells (32). In response to hypoxic conditions,
hypoxia-inducible factors (HIFs) reprogram non-stem cancer cells to
a stem-like phenotype by increasing the transcription of ALDH and
inducing the expression of OCT4 and Nanog (33,34).
Here, in vitro, the mammosphere cells with increased ALDH
activity was observed, along with the upregulation of OCT4, Nanog
and SOX2 in SDF-1 overespressed MCF-7 cells, suggesting that SDF-1
could induce CSC-like phenotypes of breast cancer cells. It has
been indicated that the emergence of CSCs occurs in part because of
EMT (5). Hence, further
experiments were required to identify the effect of SDF-1 on EMT in
MCF-7 cells.
E-cadherin is an epithelial cell junction marker;
vimentin and fibronectin are mesenchymal markers. During the
progression of EMT, slug could repress E-cadherin transcription at
promoter level to break down the adherence junction (7,35).
In this study, we found that overexpression of SDF-1 increased the
expression of slug leading to downregulation of E-cadherin, along
with the elevated expression of vimentin and fibronectin,
suggesting that EMT was triggered by overexpressing SDF-1 which may
subsequently induced CSC phenotypes in MCF-7 cells to facilitated
breast cancer cells metastasis.
NF-κB pathway is associated with cell proliferation,
apoptosis, and inflammation, and involved in diverse progressions
of cancer development (36,37).
Recent studies have identified that NF-κB was an important
regulator of EMT in several cell types (38–40)
governing the induction, metastasis and maintenance of EMT
(41). Jiang et al reported
that longer times for EMT would increase activation of IκB and
NF-κB, then the expression of stem cell markers were enhanced to
promote neoplastic transformation of human keratinocytes (42). It is a well established fact that
the promoter of CXCR4 contains several binding sites for NF-κB, we
therefore speculated that NF-κB pathway may be involved in
SDF-1-induced EMT of MCF-7 cells. Our results showed the activation
of NF-κB pathway in SDF-1 overexpressing MCF-7 cells, and the
expression of E-cadherin was increased remarkably after incubating
with NF-κB inhibitor drug or silencing NF-κB p65 gene in
SDF-1-overexpressed MCF-7 cells, indicating that NF-κB pathway
regulates SDF-1-induced EMT of breast cancer cells. For the
downstream pathways of SDF-1/CXCR4 in breast CSCs, Yi et al
identified SDF-1/CXCR4-PKA-MAP2K2-ERK signaling pathway and
demonstrated the feedback regulation on MEK, ERK1/2, δ-catenin, and
PPP1Cα in breast CSCs treated with 100 ng/ml SDF-1 (43). As EMT is an important inducer of
CSCs, there must be connections between NF-κB pathway and
PKA-MAP2K2-ERK signaling pathway which is one of the strategies of
our subsequent study.
In conclusion, our data indicated that
overexpression of SDF-1 could trigger EMT of MCF-7 cells through
NF-κB pathway to further gain the CSC-like phenotypes, subsequently
promoting metastasis of MCF-7 cells. Our findings preliminarily
identified the significant roles of SDF-1 in MCF-1 cells, and
suggest that SDF-1 may become a promising candidate for breast
cancer therapy.
Acknowledgements
This study was supported by a grant from the
National Nature Science Foundation of China (no. 81371564).
Abbreviations:
|
SDF-1
|
stromal cell-derived factor-1
|
|
EMT
|
epithelial to mesenchymal
transition
|
|
CSC
|
cancer stem cell
|
References
|
1
|
Huang M, Li Y, Zhang H and Nan F: Breast
cancer stromal fibroblasts promote the generation of CD44
CD24− cells through SDF-1/CXCR4 interaction. J Exp Clin
Cancer Res. 29:802010. View Article : Google Scholar
|
|
2
|
Guerrero-Preston R, Hadar T, Ostrow KL,
Soudry E, Echenique M, Ili-Gangas C, Pérez G, Perez J,
Brebi-Mieville P, Deschamps J, et al: Differential promoter
methylation of kinesin family member 1a in plasma is associated
with breast cancer and DNA repair capacity. Oncol Rep. 32:505–512.
2014.PubMed/NCBI
|
|
3
|
Pang H, Lu H, Song H, Meng Q, Zhao Y, Liu
N, Lan F, Liu Y, Yan S, Dong X, et al: Prognostic values of
osteopontin-c, E-cadherin and β-catenin in breast cancer. Cancer
Epidemiol. 37:985–992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Salnikov AV, Bauer N,
Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J,
Schemmer P, Werner J, et al: Triptolide reverses hypoxia-induced
epithelial-mesenchymal transition and stem-like features in
pancreatic cancer by NF-kappaB downregulation. Int J Cancer.
134:2489–2503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jethwa P, Naqvi M, Hardy RG, Hotchin NA,
Roberts S, Spychal R and Tselepis C: Overexpression of Slug is
associated with malignant progression of esophageal adenocarcinoma.
World J Gastroenterol. 14:1044–1052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez-Martín L, Sánchez-Mateos P and
Cabañas C: CXCR7 impact on CXCL12 biology and disease. Trends Mol
Med. 19:12–22. 2013. View Article : Google Scholar
|
|
9
|
Luker KE and Luker GD: Functions of CXCL12
and CXCR4 in breast cancer. Cancer Lett. 238:30–41. 2006.
View Article : Google Scholar
|
|
10
|
Ray P, Lewin SA, Mihalko LA, Lesher-Perez
SC, Takayama S, Luker KE and Luker GD: Secreted CXCL12 (SDF-1)
forms dimers under physiological conditions. Biochem J.
442:433–442. 2012. View Article : Google Scholar
|
|
11
|
Papatheodorou H, Papanastasiou AD,
Sirinian C, Scopa C, Kalofonos HP, Leotsinidis M and Papadaki H:
Expression patterns of SDF1/CXCR4 in human invasive breast
carcinoma and adjacent normal stroma: Correlation with tumor
clinicopathological parameters and patient survival. Pathol Res
Pract. 210:662–667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hinton CV, Avraham S and Avraham HK: Role
of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to
the brain. Clin Exp Metastasis. 27:97–105. 2010. View Article : Google Scholar
|
|
13
|
Sun Y, Mao X, Fan C, Liu C, Guo A, Guan S,
Jin Q, Li B, Yao F and Jin F: CXCL12-CXCR4 axis promotes the
natural selection of breast cancer cell metastasis. Tumour Biol.
35:7765–7773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagasawa T, Kikutani H and Kishimoto T:
Molecular cloning and structure of a pre-B-cell growth-stimulating
factor. Proc Natl Acad Sci USA. 91:2305–2309. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips RJ, Burdick MD, Lutz M, Belperio
JA, Keane MP and Strieter RM: The stromal derived
factor-1/CXCL12-CXC chemokine receptor 4 biological axis in
non-small cell lung cancer metastases. Am J Respir Crit Care Med.
167:1676–1686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams SA, Harata-Lee Y, Comerford I,
Anderson RL, Smyth MJ and McColl SR: Multiple functions of CXCL12
in a syngeneic model of breast cancer. Mol Cancer. 9:2502010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hattermann K, Holzenburg E, Hans F, Lucius
R, Held-Feindt J and Mentlein R: Effects of the chemokine CXCL12
and combined internalization of its receptors CXCR4 and CXCR7 in
human MCF-7 breast cancer cells. Cell Tissue Res. 357:253–266.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Z, Wang X, Wu K, Zhao Y and Hu G:
Pancreatic stellate cells increase the invasion of human pancreatic
cancer cells through the stromal cell-derived factor-1/CXCR4 axis.
Pancreatology. 10:186–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang YP, Wu XH, Xing HY and Du XY: Effect
of chemokine CXCL12 and its receptor CXCR4 on proliferation,
migration and invasion of epithelial ovarian cancer cells. Zhonghua
Fu Chan Ke Za Zhi. 42:403–407. 2007.(In Chinese). PubMed/NCBI
|
|
21
|
Kang H, Watkins G, Parr C, Douglas-Jones
A, Mansel RE and Jiang WG: Stromal cell derived factor-1: Its
influence on invasiveness and migration of breast cancer cells in
vitro, and its association with prognosis and survival in human
breast cancer. Breast Cancer Res. 7:R402–R410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang R, Lv Q, Meng W, Tan Q, Zhang S, Mo X
and Yang X: Comparison of mammosphere formation from breast cancer
cell lines and primary breast tumors. J Thorac Dis. 6:829–837.
2014.PubMed/NCBI
|
|
23
|
Bae SN, Arand G, Azzam H, Pavasant P,
Torri J, Frandsen TL and Thompson EW: Molecular and cellular
analysis of basement membrane invasion by human breast cancer cells
in Matrigel-based in vitro assays. Breast Cancer Res Treat.
24:241–255. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luker KE, Lewin SA, Mihalko LA, Schmidt
BT, Winkler JS, Coggins NL, Thomas DG and Luker GD: Scavenging of
CXCL12 by CXCR7 promotes tumor growth and metastasis of
CXCR4-positive breast cancer cells. Oncogene. 31:4750–4758. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boimel PJ, Smirnova T, Zhou ZN, Wyckoff J,
Park H, Coniglio SJ, Qian BZ, Stanley ER, Cox D, Pollard JW, et al:
Contribution of CXCL12 secretion to invasion of breast cancer
cells. Breast Cancer Res. 14:R232012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goswami S, Sahai E, Wyckoff JB, Cammer M,
Cox D, Pixley FJ, Stanley ER, Segall JE and Condeelis JS:
Macrophages promote the invasion of breast carcinoma cells via a
colony-stimulating factor-1/epidermal growth factor paracrine loop.
Cancer Res. 65:5278–5283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porcile C, Bajetto A, Barbieri F, Barbero
S, Bonavia R, Biglieri M, Pirani P, Florio T and Schettini G:
Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates
ovarian cancer cell growth through the EGF receptor
transactivation. Exp Cell Res. 308:241–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porta C, Subhra Kumar B, Larghi P, Rubino
L, Mancino A and Sica A: Tumor promotion by tumor-associated
macrophages. Adv Exp Med Biol. 604:67–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kucia M, Ratajczak J and Ratajczak MZ:
Bone marrow as a source of circulating CXCR4+
tissue-committed stem cells. Biol Cell. 97:133–146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mukherjee D and Zhao J: The role of
chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer
Res. 3:46–57. 2013.PubMed/NCBI
|
|
31
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
32
|
Ogony JW, Malahias E, Vadigepalli R and
Anni H: Ethanol alters the balance of Sox2, Oct4, and Nanog
expression in distinct subpopulations during differentiation of
embryonic stem cells. Stem Cells Dev. 22:2196–2210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brahimi-Horn MC, Chiche J and Pouysségur
J: Hypoxia and cancer. J Mol Med Berl. 85:1301–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heddleston JM, Li Z, McLendon RE,
Hjelmeland AB and Rich JN: The hypoxic microenvironment maintains
glioblastoma stem cells and promotes reprogramming towards a cancer
stem cell phenotype. Cell Cycle. 8:3274–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maier HJ, Schmidt-Strassburger U, Huber
MA, Wiedemann EM, Beug H and Wirth T: NF-kappaB promotes
epithelial-mesenchymal transition, migration and invasion of
pancreatic carcinoma cells. Cancer Lett. 295:214–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar
|
|
39
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shin SR, Sánchez-Velar N, Sherr DH and
Sonenshein GE: 7,12-dimethylbenz(a)anthracene treatment of a c-rel
mouse mammary tumor cell line induces epithelial to mesenchymal
transition via activation of nuclear factor-kappaB. Cancer Res.
66:2570–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huber MA, Beug H and Wirth T:
Epithelial-mesenchymal transition: NF-kappaB takes center stage.
Cell Cycle. 3:1477–1480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang R, Li Y, Xu Y, Zhou Y, Pang Y, Shen
L, Zhao Y, Zhang J, Zhou J, Wang X, et al: EMT and CSC-like
properties mediated by the IKKβ/IκBα/RelA signal pathway via the
transcriptional regulator, Snail, are involved in the
arsenite-induced neoplastic transformation of human keratinocytes.
Arch Toxicol. 87:991–1000. 2013. View Article : Google Scholar
|
|
43
|
Yi T, Zhai B, Yu Y, Kiyotsugu Y, Raschle
T, Etzkorn M, Seo HC, Nagiec M, Luna RE, Reinherz EL, et al:
Quantitative phosphoproteomic analysis reveals system-wide
signaling pathways downstream of SDF-1/CXCR4 in breast cancer stem
cells. Proc Natl Acad Sci USA. 111:E2182–E2190. 2014. View Article : Google Scholar : PubMed/NCBI
|