Introduction
Irinotecan has been used for around two decades in
chemotherapies against several advanced cancers. Especially in the
first-line treatment of metastatic colorectal cancers, irinotecan
has been one of the mainstay drugs, with longer progression-free
survival and overall survival (1–3).
However, no clear predictive marker of irinotecan efficacy has been
identified, despite its widespread use.
Irinotecan directly inhibits topoisomerase I (Top I)
to prevent re-ligation of the nicked DNA strand during DNA
replication (4,5), and then it has been suggested that a
high expression level of Top I correlates with its efficacy
(6). For instance, in a large
prospective study, the UK MRC FOCUS trial for advanced colorectal
cancer patients, moderate or high Top I expression was associated
with longer survival in patients treated with irinotecan in
addition to 5-fluorouracil (7).
Conversely, in the CAIRO study, in which advanced colorectal cancer
patients were also prospectively interrogated, no association was
found between the response to irinotecan and Top I expression
(8,9). Additionally, another report mentioned
that the cells that acquired resistance to SN38 (an active
metabolite of irinotecan) maintained Top I expression levels
(10). Therefore, Top I expression
is supposed to be insufficient for the prediction of irinotecan
efficacy clinically.
On the contrary, it has been supposed that cytotoxic
chemotherapies show susceptibility in highly proliferative cells.
Actually, a recent meta-analysis study suggested that the response
to Top I inhibitors is associated with the cell growth rate
(11). One of the critical points
in the regulation of cellular proliferation is the G1/S transition,
which is controlled by the phosphorylation status of RB protein. In
malignant tumor cells, RB inactivation with its phosphorylation
leads to cellular proliferation.
Considering the above, we investigated whether the
efficacy of SN38 is related to the cell proliferation rate and the
phosphorylation status of RB in colorectal cancer cells. We then
validated the relationship between the levels of phosphorylated RB
and the responses to irinotecan in clinical samples of patients
suffering from advanced colorectal cancers. The present study
suggests a feasible predictive marker of irinotecan efficacy for
colorectal cancer in a clinical setting.
Materials and methods
Cell culture and reagents
HCT116, SW480 and SW620 cells were obtained from the
American Type Culture Collection. CCK-81, CoCM-1 and SW837 cells
were obtained from the Health Science Research Resources Bank.
LIM1215 cells were obtained from the European Collection of Cell
Cultures. Caco-2 cells were obtained from RIKEN BioResource Center.
The authenticity of each cell line was confirmed by short tandem
repeat profiling at each cell bank. All the cells purchased from
each cell bank were immediately expanded after receipt, and stocks
of each cell line were prepared within 3 passages and stored in
liquid nitrogen. For experiments, cells were used for fewer than 3
months after resuscitation. All cells were cultured in Dulbecco's
modified Eagle's medium (DMEM). Medium was supplemented with 10%
fetal bovine serum (FBS), 4 mM glutamine, 50 U/ml penicillin and
100 μg/ml streptomycin. All cells were incubated at 37°C in a
humidified atmosphere of 5% CO2. SN38 was obtained from
Sigma (St. Louis, MO, USA). It was dissolved in the solvent
dimethyl sulfoxide (DMSO) as stock and stored at −20°C.
Cell viability assay
The cell viability was measured by a Cell Counting
Kit-8 assay according to the manufacturer's instructions (Dojindo
Laboratories, Kumamoto, Japan) to determine the IC50
values of each cell line. After the incubation of cells for 72 h
with the indicated concentrations of SN38, kit reagent WST-8 was
added to the medium and incubated for a further 4 h. The absorbance
of samples (450 nm) was measured using a multi-plate reader (DS
Pharma Biomedical Co., Ltd., Osaka, Japan). Cell numbers were
measured using the ViaCount Assay according to the manufacturer's
instructions (EMD Millipore, Billerica, MA, USA) to determine the
doubling time of each cell line.
Protein isolation and western
blotting
Cells were lysed with a buffer containing 50 mM
Tris-HCl, 1% SDS, 1 mM DTT, 2 μg/ml leupeptin, 2 μg/ml aprotinin,
0.5 mM phenylmethylsulfonyl fluoride and phosphatase inhibitor
cocktail (Nacalai Tesque, Kyoto, Japan). The lysate was sonicated
and centrifuged at 20,400 × g for 20 min at 4°C and the supernatant
was collected. Equal amounts of lysate were boiled for 5 min and
loaded onto a 12% (for CDK2, CDK4, CDK6 and GAPDH detection) or 7%
(for phospho-RB, pRB, topoisomerase-I, BCRP and α-tubulin
detection) polyacrylamide gel, subjected to electrophoresis and
transferred to PVDF membranes (EMD Millipore). The following
primary antibodies were used: mouse anti-human pRB (BD Biosciences,
San Jose, CA, USA), mouse anti-human α-tubulin (EMD Millipore),
mouse anti-human CDK6, rabbit anti-human phospho-RB (Ser780),
rabbit anti-human phospho-RB (Ser807/811) (Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-human CDK2, rabbit
anti-human CDK4 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
rabbit anti-human topoisomerase-I, mouse anti-human BCRP (Abcam,
Cambridge, UK), and mouse anti-human GAPDH (HyTest Ltd., Turku,
Finland). The blots were incubated with the appropriate
HRP-conjugated secondary antibody (GE Healthcare, Piscataway, NJ,
USA), and the signals were detected with Chemi-Lumi One (Nacalai
Tesque) or Western Chemiluminescent HRP Substrate (EMD
Millipore).
Small interfering RNA transfection
Small interfering RNAs (siRNA) were obtained from
Ambion (Carlsbad, CA, USA) for CDK2 (UAAGUACGAACAGGGACUCca), CDK4
(UGUGGGUUAAAAGUCAGCAtt), CDK6 (UUCUACGAAACAUUUCUGCaa), and
Silencer® Select Negative Control #2 siRNA. Cells were
transfected with 50 nM of each siRNA using Lipofectamine RNAiMAX
Reagent (Invitrogen, Carlsbad, CA, USA). At 24 h after the
transfection, cells were incubated with 5 nM SN38, 0.1% DMSO or
DMEM for 72 h, and harvested for cell cycle analysis and western
blotting.
Cell cycle analysis
Cells were exposed to SN38 at the indicated
concentrations for 72 h and then harvested. They were permeabilized
with 0.1% Triton X-100 and the nuclei were stained with propidium
iodide. The DNA contents were measured using a FACSCalibur
(Becton-Dickinson, Franklin Lakes, NJ, USA) and analyzed with
ModFit LT (Verity Software House, Topsham, ME, USA).
Human tissue samples
Primary tumor samples were obtained from 23 patients
with clinical Stage IV colorectal cancer, who underwent a
colectomy/rectectomy at Kyoto Prefectural University of Medicine
between 2008 and 2013. The samples were embedded in paraffin after
24 h of formalin fixation. The patient eligibility criteria were:
no synchronous tumors, and not having received a preoperative
chemotherapy or a radiation therapy. All the patients gave their
written informed consent. Relevant clinicopathological and survival
data were obtained from the hospital database. After operations, 22
patients underwent second- or third-line chemotherapies with
irinotecan, which targeted metastasis or recurrence, and only 1
patient underwent treatment with irinotecan as the first-line
therapy. The following treatments with irinotecan were applied:
FOLFIRI (5-FU+leucovorin+irinotecan), FOLFIRI+BEV
(FOLFIRI+bevacizumab), FOLFIRI +C-mab (FOLFIRI+cetuximab),
FOLFIRI+P-mab (FOLFIRI +panitumumab), CPT-11+C-mab
(irinotecan+cetuximab), IRIS (irinotecan+S-1), and IRIS+BEV
(IRIS+bevacizumab). Computed tomography (CT) was performed after
the treatment with irinotecan for approximately 5 courses. The
curative effects were evaluated by RECIST v1.1 (12), referring to the pictures of CT. A
total of 5 patients were classified with partial response (PR), 10
with stable disease (SD) and 8 with progressive disease (PD). We
classified PR patients into a responder group, and SD and PD
patients into a non-responder group. Disease staging was
principally based on The Union for International Cancer Control/TNM
Classification of Malignant Tumours (7th edition) (13).
Immunohistochemistry
Paraffin sections (3 mm thick) of tumor tissue were
subjected to immunohistochemical staining for phosphorylated RB
(serine 780) and Ki-67 (MIB-1) using the avidin-biotin-peroxidase
method. Briefly, paraffin sections were dewaxed in xylene and
hydrated through a graded series of alcohol. Endogenous peroxidase
activity was quenched by incubating the sections for 30 min in 0.3%
H2O2. The sections were then treated with a
protein blocker and incubated with antibodies. Sections were
incubated for 1 h at 37°C with a mouse anti-RB (phospho S780)
antibody (Abcam), and were incubated for 1 h at room temperature
with Ki-67 (Santa Cruz Biotechnology). The avidin-biotin-peroxidase
complex system (Vectastain ABC Elite kit; Vector Laboratories,
Burlingame, CA, USA) was used for color development with
diaminobenzidine tetrahydrochloride. The sections were
counterstained with hematoxylin. Finally, the sections were
dehydrated through a graded series of alcohol, cleared in xylene
and mounted. Tumor cells with immunohistochemical expression in the
cytoplasm were counted as phosphorylated RB-positive. For scoring
the phosphorylated RB expression, the percentage of the total cell
population that expressed phosphorylated RB (serine 780) was
evaluated for each case. The labeling index (LI) of
immunohistochemistry for Ki-67 was determined by counting positive
tumor cells in the most intensely stained region. Severely
keratinized portions in the nest of colorectal cancers were
excluded.
Statistical analysis
Significance was assessed by Student's t-test or
Mann-Whitney U test for comparisons between two groups. Fisher's
exact test was performed to investigate the correlations between
clinicopathological parameters and phosphorylated RB expression
level. Differences were considered significant when the P-value was
<0.05.
Results
The sensitivity to SN38 is inversely
correlated with the doubling time of cells
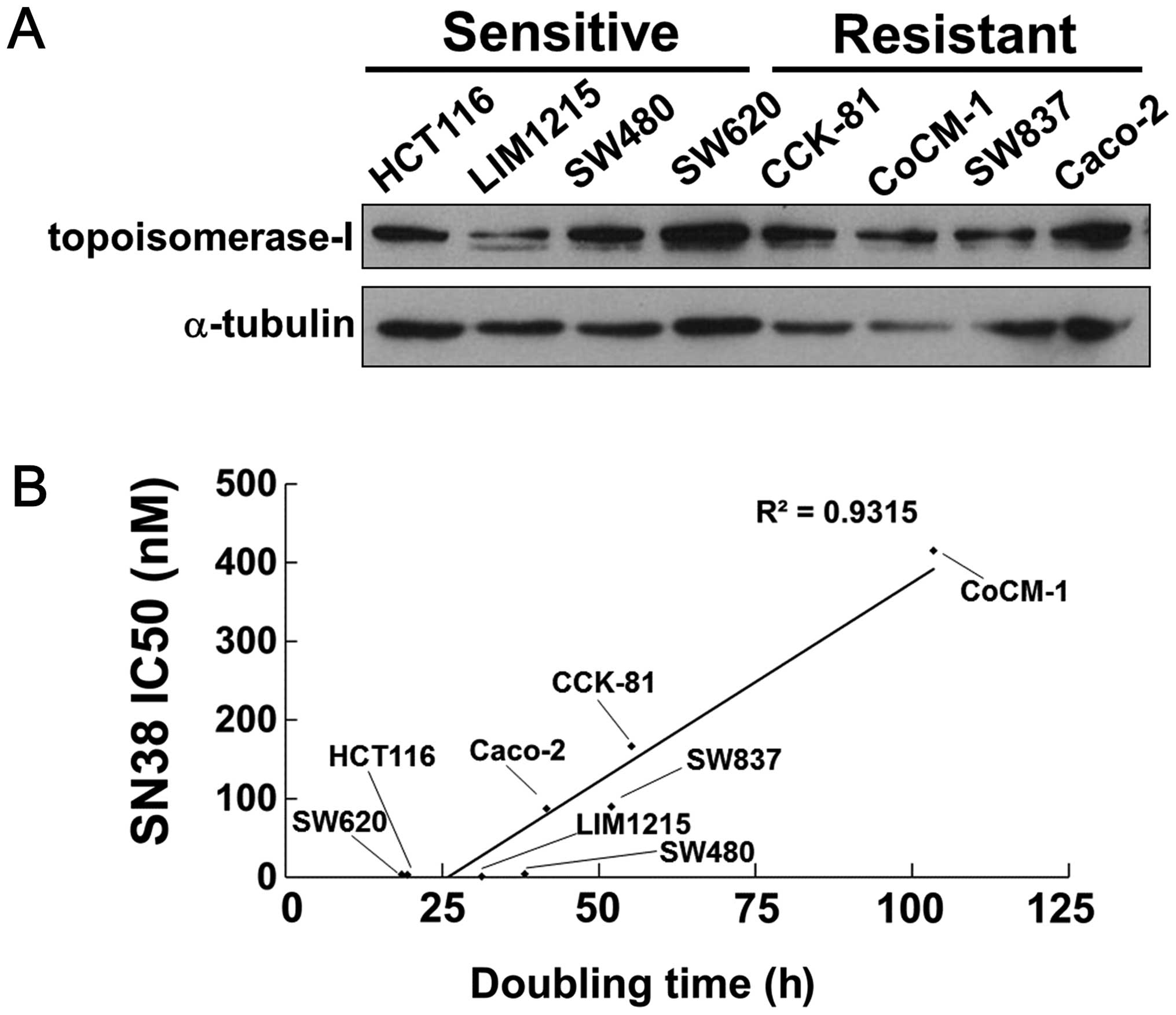
We examined each IC50 value of SN38 using
eight human colorectal cancer cells as shown in Table I. The HCT116, LIM1215, SW480 and
SW620 cells were sensitive to SN38, with IC50 values of
<4 nM. The CCK-81, CoCM-1, SW837 and Caco-2 cells were resistant
to SN38, with IC50 values of >80 nM. We found no
association between the sensitivity to SN38 and the expression
level of Top I in these cell lines (Fig. 1A).
 | Table IThe IC50 values and the
doubling times of eight colorectal cancer cell lines. |
Table I
The IC50 values and the
doubling times of eight colorectal cancer cell lines.
| SN38 IC50
(nM) | |
|---|
|
| |
|---|
| Cell name | Ave. | SD | Doubling time
(h) |
|---|
| Sensitive |
| HCT116 | 3.54 | 2.74 | 18.55 |
| LIM1215 | 1.22 | 0.55 | 31.27 |
| SW480 | 3.97 | 3.30 | 38.16 |
| SW620 | 3.19 | 0.77 | 19.47 |
| Resistant |
| CCK-81 | 166.46 | 51.23 | 55.18 |
| CoCM-1 | 415.10 | 48.03 | 103.41 |
| SW837 | 89.64 | 28.69 | 52.01 |
| Caco-2 | 86.99 | 19.56 | 41.65 |
We next measured the doubling times of the cell
lines (Table I) and plotted them
against the IC50 values of SN38. As shown in Fig. 1B, the doubling times of the cell
lines and the IC50 values of SN38 showed an appreciable
positive correlation (R2=0.9315) (Fig. 1B). These results indicate that
highly proliferative cells are more sensitive to SN38.
Phosphorylation status of RB is relevant
to the sensitivity to SN38 of cell lines
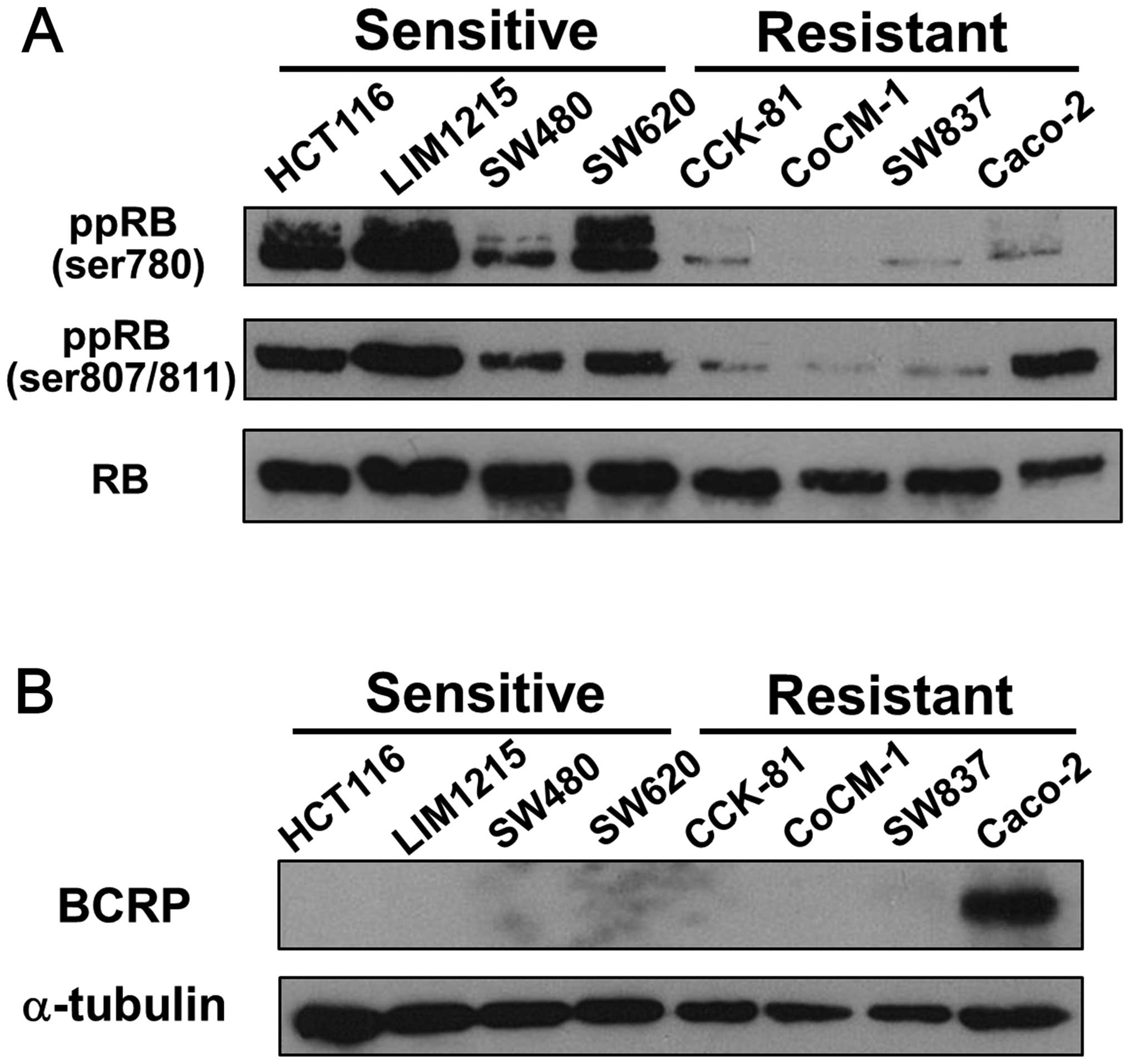
We then performed western blotting to detect the
phosphorylated RB of each cell line, which is unable to bind to the
transcription factor E2F, resulting in proliferation of the cells.
The SN38-sensitive cell lines showed hyperphosphorylation of RB
without alteration of the total amount of RB protein, while the
resistant cell lines showed hypophosphorylation of RB, with the
exception of the phosphorylated RB at serines 807/811 in Caco-2
cells (Fig. 2A). Caco-2 cells are
known to express BCRP (breast cancer resistance protein) (14), which transports SN38 (15). Indeed, we confirmed that only
Caco-2 cells expressed BCRP among these cell lines (Fig. 2B), suggesting that these cells show
resistance to SN38 despite hyperphosphorylation of RB at serines
807/ 811.
Taken together, these results suggest that the
phosphorylation status of RB could predict the efficacy of
irinotecan.
Phosphorylation status of RB protein
detected by immunohistochemistry may predict the response to
irinotecan in patient specimens
We then examined whether the expression levels of
phosphorylated RB could predict the response to irinotecan in the
patient specimens. The 23 patients with colorectal cancers were
classified into responder and non-responder groups, according to
the responses to the treatments including irinotecan, as described
in Materials and methods (Table
II). The patient characteristics of the irinotecan responders
and non-responders are shown in Table III. There was no significant
difference in the overall survival between the responders and
non-responders (data not shown). Next, we analyzed the
relationships between various clinicopathological parameters and
the phosphorylated RB expression levels determined by
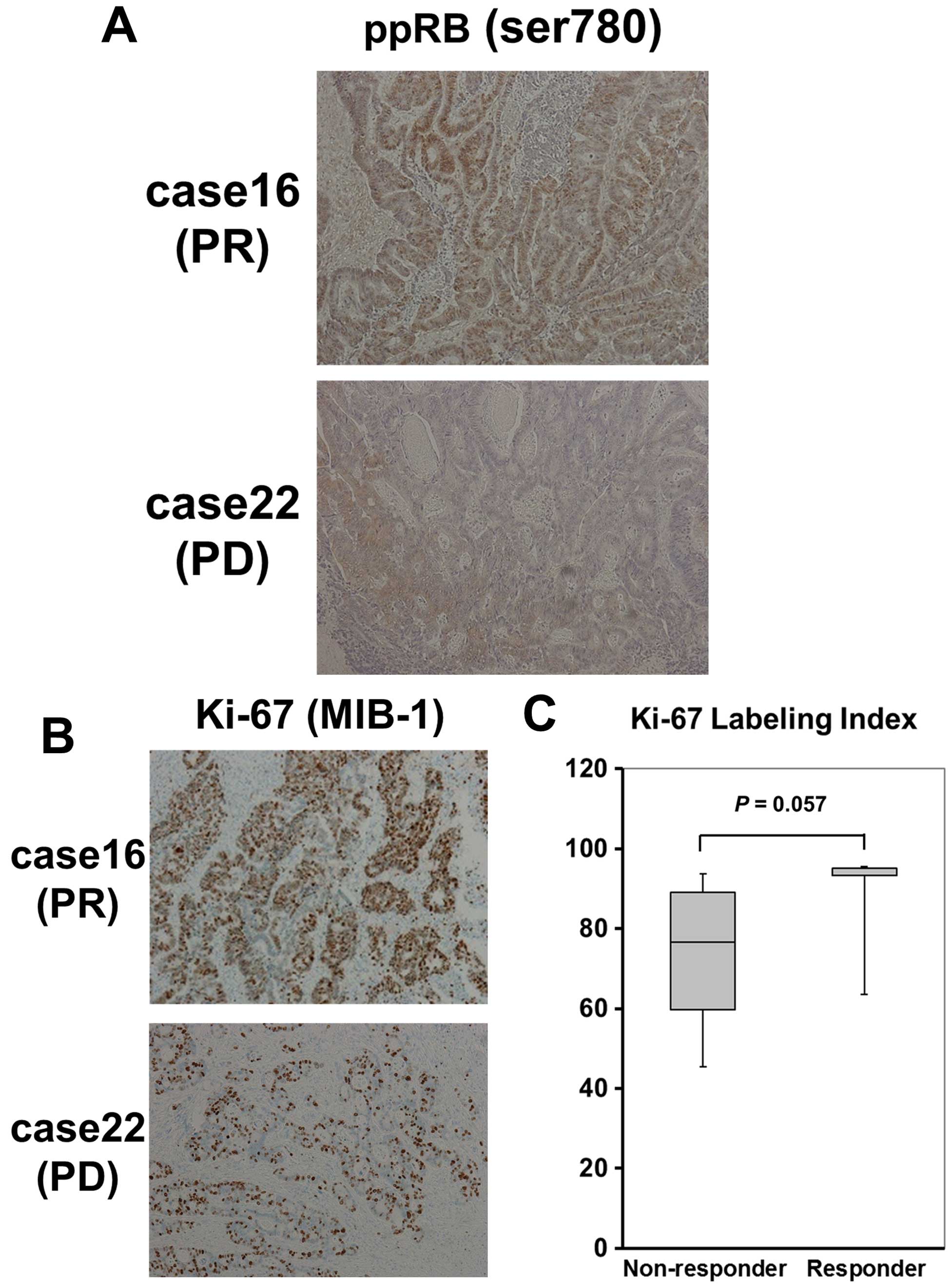
immunohistochemistry (IHC). Representative phosphorylated RB
expression levels of the tumors are shown in Fig. 3A. We then evaluated the
phosphorylated RB expression levels in 23 samples, as described in
Materials and methods, and defined the appropriate IHC cut-off
values of stained tumor cells. As a consequence, we found that the
colorectal cancer tissues of irinotecan responders showed a
significantly higher positivity rate of the phosphorylated RB
(serine 780) at a cut-off value of 25% (P=0.0006; Table IV), while the labeling index (LI)
of Ki-67 (MIB-1), which is clinically used as a diagnostic marker
of tumor proliferation, showed no statistically significant
difference between responders and non-responders (Fig. 3B and C).
 | Table IIClinicopathological characteristics
of 23 cases of colorectal cancer. |
Table II
Clinicopathological characteristics
of 23 cases of colorectal cancer.
| Case no. | Age (years) | Gender | Location | cStage (UICC
7) | pStage (UICC
7) |
Metastasis/Recurrence | 1st therapy | Therapeutic
response | 2nd therapy | Therapeutic
response | 3rd therapy | Therapeutic
response |
|---|
| 1 | 66 | Female | T | IVb | IVb | Peritoneum | mFOLFOX6 | PD | FOLFIRI | PD | | |
| 2 | 55 | Female | Ra | IVa | IVa | Liver | FOLFOX | SD |
FOLFIRI+BEV | SD | | |
| 3 | 60 | Female | A | IVb | IVb | Peritoneum | mFOLFOX6 | PD |
CPT-11+C-mab | PD | | |
| 4 | 54 | Male | D | IVb | IVb | Peritoneum | mFOLFOX6 | PD |
CPT-11+C-mab | PD | | |
| 5 | 53 | Female | Rs | IVa | IIIc | Lung | FOLFOX | PD | FOLFOX+BEV | PR |
FOLFIRI+BEV | PR |
| 6 | 52 | Female | Ra | IVa | IVa | Lung | FOLFOX+BEV | SD | FOLFIRI | PD | | |
| 7 | 68 | Male | Rs | IVa | IIb | Peritoneum | FOLFIRI | PD | | | | |
| 8 | 67 | Male | T | IVa | IVa | Liver | mFOLFOX6+BEV | PR |
FOLFIRI+BEV | PD | | |
| 9 | 64 | Male | Rs | IVa | IVa | Liver | mFOLFOX6 | PR | mFOLFOX6+BEV | PR |
FOLFIRI+BEV | SD |
| 10 | 67 | Male | Rs | IVb | IVb | Liver/lymph
node | mFOLFOX+BEV | PR |
FOLFIRI+BEV | PR | | |
| 11 | 74 | Male | T | IVa | IVa | Liver | FOLFOX+BEV | SD |
FOLFIRI+BEV | PD | | |
| 12 | 61 | Male | Rs | IVa | IVa | Liver | FOLFOX | SD |
FOLFIRI+BEV | SD | | |
| 13 | 64 | Male | T | IVa | IVa | Liver | FOLFOX+C-mab | SD |
FOLFIRI+C-mab | SD | | |
| 14 | 60 | Female | Rb | IVa | IVa | Liver | XELOX | SD | IRIS | SD | | |
| 15 | 39 | Male | S | IVb | IVb | Peritoneum | XELOX+BEV | PD |
FOLFIRI+P-mab | SD | | |
| 16 | 67 | Female | Ra | IVa | IVa | Lung | XELOX | SD | FOLFIRI | PR | | |
| 17 | 55 | Female | T | IVb | IVb | Peritoneum | XELOX+BEV | PD |
FOLFIRI+P-mab | SD | | |
| 18 | 64 | Female | Rb | IVa | IVa | Lymph node | XELOX | PD | FOLFIRI | SD | | |
| 19 | 72 | Female | Rb | IVa | IVa | Lung | XELOX | PD |
FOLFIRI+P-mab | SD | | |
| 20 | 62 | Female | Rs | IVa | IVa | Liver | XELOX+BEV | PD |
FOLFIRI+P-mab | PR | | |
| 21 | 55 | Female | A | IVb | IVb | Liver/lung | FOLFOX BEV | PD |
IRIS+BEV | PR | | |
| 22 | 74 | Male | T | IVb | IVb | Liver/lung | XELOX | PD | FOLFIRI | PD | | |
| 23 | 45 | Female | T | IVa | IVa | Liver | XELOX BEV | PD |
IRIS+BEV | SD | | |
 | Table IIIClinicopathological characteristics
according to the response to the treatment with irinotecan. |
Table III
Clinicopathological characteristics
according to the response to the treatment with irinotecan.
| Variables | Responder
(n=5) | Non-responder
(n=18) | P-value |
|---|
| Gender |
| Male | 1 | 9 | 0.339 |
| Female | 4 | 9 | |
| Age (years) |
| ≥65 | 2 | 6 | 1 |
| <65 | 3 | 12 | |
| Location |
| Colon | 1 | 10 | 0.317 |
| Rectum | 4 | 8 | |
| pT |
| pT0-2 | 0 | 4 | 0.539 |
| pT3-4 | 5 | 14 | |
| pN |
| pN0-1 | 1 | 13 | 0.056 |
| pN2 | 4 | 5 | |
| pM |
| M0 | 1 | 1 | 0.395 |
| M1 | 4 | 17 | |
| pStage |
| I–III | 1 | 1 | 0.395 |
| IV | 4 | 17 | |
 | Table IVAssociations between the
clinicopathological characteristics of colorectal cancer patients
and phosphorylated RB (serine 780) (cut-off value, 25%). |
Table IV
Associations between the
clinicopathological characteristics of colorectal cancer patients
and phosphorylated RB (serine 780) (cut-off value, 25%).
| ppRB (ser780) | |
|---|
|
| |
|---|
| Variables | Negative (<25)
(n=19) | Positive (≥25)
(n=4) | P-value |
|---|
| Gender |
| Male | 9 | 1 | 0.604 |
| Female | 10 | 3 | |
| Age (years) |
| ≥65 | 6 | 2 | 0.589 |
| <65 | 13 | 2 | |
| Location |
| Colon | 10 | 1 | 0.59 |
| Rectum | 9 | 3 | |
| pT |
| pT0-2 | 4 | 0 | 1 |
| pT3-4 | 15 | 4 | |
| pN |
| pN0-1 | 13 | 1 | 0.26 |
| pN2 | 6 | 3 | |
| pM |
| M0 | 2 | 0 | 1 |
| M1 | 17 | 4 | |
| pStage |
| I–III | 2 | 0 | 1 |
| IV | 17 | 4 | |
| Treatment
response |
| Responder | 1 | 4 |
0.0006a |
| Non-responder | 18 | 0 | |
Taking these findings together, IHC of the
phosphorylated RB (serine 780) in tumor might be useful to predict
the response to irinotecan, rather than Ki-67.
Knockdown of both CDK4 and CDK6 reduces
G2/M accumulation induced by SN38 with RB dephosphorylation
We next investigated whether the phosphorylation of
RB plays a crucial role in the efficacy of irinotecan. We performed
the knockdown of CDKs, which are well known to phosphorylate RB
protein, to suppress the expression of phosphorylated RB. CDK2,
CDK4, CDK6 and CDK4/6 were silenced by siRNA in the SN38-sensitive
cell lines (Fig. 4A). RB protein
was converted to the unphosphorylated form most strikingly after
the simultaneous knockdown of CDK4 and CDK6 in SW620 and SW480
cells, while the knockdown of CDK2 did not affect the
phosphorylation status of RB protein (Fig. 4B).
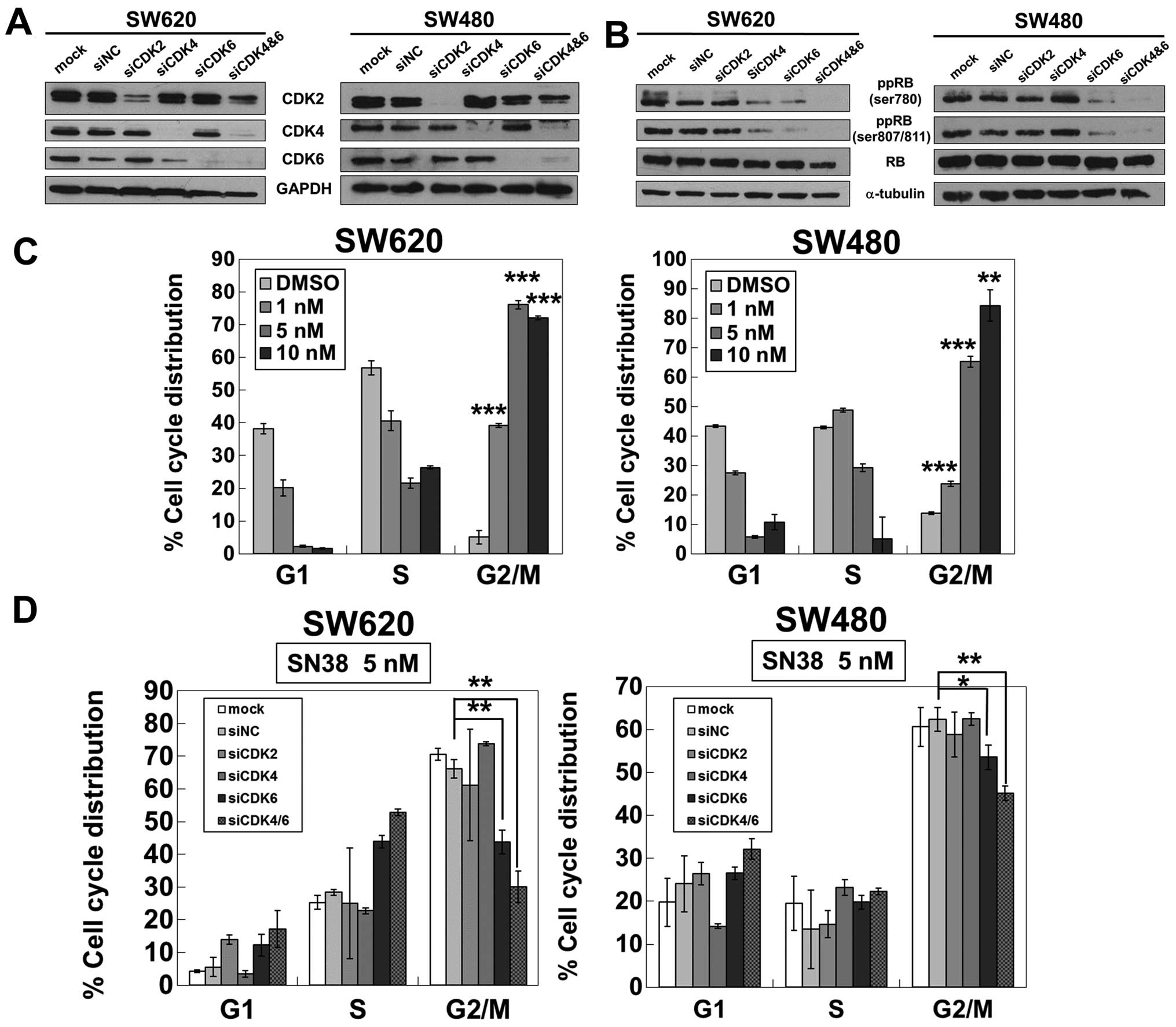 | Figure 4The simultaneous knockdown of CDK4 and
CDK6 affects the phosphorylation status of RB with a decrease of
G2/M accumulation induced by SN38. (A) The knockdown efficacies of
siCDK2, siCDK4, siCDK6, and siCDK4/6 were validated by western
blotting in SW620 and SW480 cells. GAPDH was used as a loading
control. (B) The phosphorylated RB at serine 780, serines 807/811
and total RB were analyzed by western blotting in SW620 and SW480
cells transfected with siCDK2, siCDK4, siCDK6, siCDK4/6, or
negative control (siNC). α-tubulin was used as a loading control.
(C) SW620 and SW480 cells were treated with SN38 at the indicated
concentrations for 72 h. DNA contents of the cells were analyzed by
flow cytometry. The percentages of cells in the G1, S and G2/M
phases of the cell cycle are shown. Columns, means (n=3); bars,
standard deviation (SD). **P<0.01;
***P<0.001, compared with siNC by Student's t-test.
(D) Cell cycle distribution was analyzed in SW620 and SW480 cells
transfected with siCDK2, siCDK4, siCDK6, siCDK4/6 or siNC. Cells
were exposed to 5 nM SN38 for 72 h following the treatment of each
siRNA. DNA contents of the cells were analyzed by flow cytometry.
The percentages of cells in the G1, S, and G2/M phases of the cell
cycle are shown. Columns, means (n=3); bars, standard deviation
(SD). *P<0.05; **P<0.01, compared with
siNC by Student's t-test. |
SN38 treatment for 72 h induced dose-dependent G2/M
arrest in SW620 and SW480 cells (Fig.
4C). However, the G2/M arrest induced by 5 nM SN38 for 72 h in
these cells was restored most by siCDK4/6 (Fig. 4D), which most markedly reduced the
level of the phosphorylated form of RB protein (Fig. 4B).
The sensitivity to SN38 is dampened by
the knockdown of both CDK4 and CDK6
Finally, we investigated whether CDK4/6 activities
affected the sensitivity to SN38. As shown in Fig. 5A, SW620 cells exhibited resistance
to SN38 after both CDK4 and CDK6 were silenced. The transfection of
siCDK4/6 gave an increased median IC50 value of SN38 of
58.4 nM, while that of a negative control gave a median
IC50 value of 1.4 nM (Fig.
5B). Thus, the sensitivity to irinotecan might be governed by
the activities of CDK4/6 in colorectal cancer.
Discussion
We showed for the first time that the efficacy of
irinotecan could be predicted by phosphorylated RB expression,
which is known to be crucial for G1/S progression and cell
proliferation. We proved the positive correlation between the
phosphorylated status of RB and the efficacy of SN38 in colorectal
cancer cell lines. In line with the in vitro study, our
clinical retrospective study demonstrated that the positivity rate
of the phosphorylated RB (serine 780) was significantly higher in
colorectal cancer tissues of irinotecan responders.
The phosphorylation of RB protein endows the cells
with the ability to pass through the restriction point at the late
G1 phase, enabling the cell cycle to progress (16). In most cancer cells, RB protein is
hyperphosphorylated, resulting in uncontrolled cell proliferation
(17–20). Indeed, colorectal cancer tissues
were shown to express the phosphorylated RB at a high level
compared with adenomas or normal colonic mucosa (21). Thus, RB phosphorylation is
considered to play a crucial role in colorectal cancer
progression.
Irinotecan targets proliferative cells and works
during S phase with the inhibition of Top I (4,5,22–24).
In our clinical study, colorectal cancer tissues of irinotecan
responders showed a significantly higher positivity rate of
phosphorylated RB at serine 780, rather than that of Ki-67
clinically used as a proliferative marker. The expression of Ki-67
increases in S phase but reaches a maximum in M phase (25–27),
suggesting its efficacy as an M-phase marker. On the contrary, the
phosphorylation of RB occurs at the end of G1 phase, with cells
progressing to S phase (28,29),
suggesting its efficacy as an S-phase marker. Given that irinotecan
attacks cancer cells in S phase, it is rational that the
phosphorylated RB affects the susceptibility to irinotecan, rather
than Ki-67.
RB protein is phosphorylated by CDKs, resulting in
cell cycle progression. However, there seems to be a difference in
terms of which kinds of CDKs are essential for the proliferation of
cancer cells, depending on the cellular context (30). For instance, CDK2 activity was
shown to be related to the prognosis of breast cancer (31,32)
and renal cell carcinoma (33),
while CDK4 contributed to cell proliferation in colorectal cancer
(34) and non-small cell lung
cancer (35). We performed
knockdown experiments of CDK2, CDK4, CDK6 and CDK4/6 to convert the
phosphorylated RB to the unphosphorylated form. Of particular
interest, our data showed that silencing of both CDK4 and CDK6, but
not CDK2, led RB to adopt the most hypophosphorylated form
(Fig. 4B) and rendered cells
resistant to SN38 (Fig. 5),
suggesting that CDK4/6 activities are indispensable for the
proliferation of colorectal cancer cells. Thus, the role of CDK4
and CDK6 could be crucial in not only RB phosphorylation and
proliferation of colon cancer cells but also sensitivity to
irinotecan.
In conclusion, we found that the phosphorylation
status of RB protein could be a novel and promising predictive
marker of irinotecan efficacy for colorectal cancer, based on the
rationale that cells with highly phosphorylated RB would be more
exposed to irinotecan through proceeding to S phase. In the
chemotherapies for colorectal cancer, which have been more
complicated and diverse, it has become increasingly necessary to
stratify the treatment responders. Now we are planning larger
cohort studies to examine whether this novel predictive marker of
irinotecan efficacy could improve the outcome of colorectal
cancer.
Acknowledgements
We would like to acknowledge the funding support
from the Research Funding of Kyoto Prefectural University of
Medicine.
Abbreviations:
|
RB
|
retinoblastoma gene
|
|
CDK
|
cyclin-dependent kinase
|
References
|
1
|
Fuchs C, Mitchell EP and Hoff PM:
Irinotecan in the treatment of colorectal cancer. Cancer Treat Rev.
32:491–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colucci G, Gebbia V, Paoletti G, Giuliani
F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione
L, et al: Gruppo Oncologico Dell'Italia Meridionale: Phase III
randomized trial of FOLFIRI versus FOLFOX4 in the treatment of
advanced colorectal cancer: A multicenter study of the Gruppo
Oncologico Dell'Italia Meridionale. J Clin Oncol. 23:4866–4875.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peeters M, Price TJ, Cervantes A, Sobrero
AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJA, et
al: Randomized phase III study of panitumumab with fluorouracil,
leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as
second-line treatment in patients with metastatic colorectal
cancer. J Clin Oncol. 28:4706–4713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsiang YH, Hertzberg R, Hecht S and Liu
LF: Camptothecin induces protein-linked DNA breaks via mammalian
DNA topoisomerase I. J Biol Chem. 260:14873–14878. 1985.PubMed/NCBI
|
|
5
|
Hsiang YH and Liu LF: Identification of
mammalian DNA topoisomerase I as an intracellular target of the
anticancer drug camptothecin. Cancer Res. 48:1722–1726.
1988.PubMed/NCBI
|
|
6
|
Gilbert DC, Chalmers AJ and El-Khamisy SF:
Topoisomerase I inhibition in colorectal cancer: Biomarkers and
therapeutic targets. Br J Cancer. 106:18–24. 2012. View Article : Google Scholar :
|
|
7
|
Braun MS, Richman SD, Quirke P, Daly C,
Adlard JW, Elliott F, Barrett JH, Selby P, Meade AM, Stephens RJ,
et al: Predictive biomarkers of chemotherapy efficacy in colorectal
cancer: Results from the UK MRC FOCUS trial. J Clin Oncol.
26:2690–2698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koopman M, Antonini NF, Douma J, Wals J,
Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G,
Loosveld OJJ, et al: Sequential versus combination chemotherapy
with capecitabine, irinotecan, and oxaliplatin in advanced
colorectal cancer (CAIRO): A phase III randomised controlled trial.
Lancet. 370:135–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koopman M, Knijn N, Richman S, Seymour M,
Quirke P, van Tinteren H, van Krieken JHJM, Punt CJA and Nagtegaal
ID: The correlation between Topoisomerase-I (Topo1) expression and
outcome of treatment with capecitabine and irinotecan in advanced
colorectal cancer (ACC) patients (pts) treated in the CAIRO study
of the Dutch Colorectal Cancer Group (DCCG). Eur J Cancer (Suppl).
7:321–322. 2009. View Article : Google Scholar
|
|
10
|
Gongora C, Candeil L, Vezzio N, Copois V,
Denis V, Breil C, Molina F, Fraslon C, Conseiller E, Pau B, et al:
Altered expression of cell proliferation-related and
interferon-stimulated genes in colon cancer cells resistant to
SN38. Cancer Biol Ther. 7:822–832. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K, Shrestha R, Wyatt AW, Reddy A,
Lehár J, Wang Y, Lapuk A and Collins CCA: A meta-analysis approach
for characterizing pan-cancer mechanisms of drug sensitivity in
cell lines. PLoS One. 9:e1030502014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
13
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of malignant tumours. 7th edition.
Wiley-Blackwell; Hoboken: 2009
|
|
14
|
Xia CQ, Liu N, Yang D, Miwa G and Gan LS:
Expression, localization, and functional characteristics of breast
cancer resistance protein in Caco-2 cells. Drug Metab Dispos.
33:637–643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sostelly A, Payen L, Guitton J, Di Pietro
A, Falson P, Honorat M, Valdameri G, Geze A, Boumendjel A, Freyer
G, et al: A template model for studying anticancer drug efflux
transporter inhibitors in vitro. Fundam Clin Pharmacol. 27:544–556.
2013. View Article : Google Scholar
|
|
16
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sellers WR and Kaelin WG Jr: Role of the
retinoblastoma protein in the pathogenesis of human cancer. J Clin
Oncol. 15:3301–3312. 1997.PubMed/NCBI
|
|
18
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ortega S, Malumbres M and Barbacid M:
Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim
Biophys Acta. 1602:73–87. 2002.PubMed/NCBI
|
|
20
|
Hahn WC and Weinberg RA: Modelling the
molecular circuitry of cancer. Nat Rev Cancer. 2:331–341. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gope R and Gope ML: Abundance and state of
phosphorylation of the retinoblastoma susceptibility gene product
in human colon cancer. Mol Cell Biochem. 110:123–133. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kessel D, Bosmann HB and Lohr K:
Camptothecin effects on DNA synthesis in murine leukemia cells.
Biochim Biophys Acta. 269:210–216. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li LH, Fraser TJ, Olin EJ and Bhuyan BK:
Action of camptothecin on mammalian cells in culture. Cancer Res.
32:2643–2650. 1972.PubMed/NCBI
|
|
24
|
Horwitz SB and Horwitz MS: Effects of
camptothecin on the breakage and repair of DNA during the cell
cycle. Cancer Res. 33:2834–2836. 1973.PubMed/NCBI
|
|
25
|
Brown DC and Gatter KC: Monoclonal
antibody Ki-67: Its use in histopathology. Histopathology.
17:489–503. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scholzen T and Gerdes J: The Ki-67
protein: From the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yerushalmi R, Woods R, Ravdin PM, Hayes MM
and Gelmon KA: Ki67 in breast cancer: Prognostic and predictive
potential. Lancet Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sherr CJ and McCormick F: The RB and p53
pathways in cancer. Cancer Cell. 2:103–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SJ, Nakayama S, Miyoshi Y, Taguchi T,
Tamaki Y, Matsushima T, Torikoshi Y, Tanaka S, Yoshida T, Ishihara
H, et al: Determination of the specific activity of CDK1 and CDK2
as a novel prognostic indicator for early breast cancer. Ann Oncol.
19:68–72. 2008. View Article : Google Scholar
|
|
32
|
Kim SJ, Masuda N, Tsukamoto F, Inaji H,
Akiyama F, Sonoo H, Kurebayashi J, Yoshidome K, Tsujimoto M, Takei
H, et al: The cell cycle profiling-risk score based on CDK1 and 2
predicts early recurrence in node-negative, hormone
receptor-positive breast cancer treated with endocrine therapy.
Cancer Lett. 355:217–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hongo F, Takaha N, Oishi M, Ueda T,
Nakamura T, Naitoh Y, Naya Y, Kamoi K, Okihara K, Matsushima T, et
al: CDK1 and CDK2 activity is a strong predictor of renal cell
carcinoma recurrence. Urol Oncol. 32:1240–1246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tetsu O and McCormick F: Proliferation of
cancer cells despite CDK2 inhibition. Cancer Cell. 3:233–245. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Puyol M, Martín A, Dubus P, Mulero F,
Pizcueta P, Khan G, Guerra C, Santamaría D and Barbacid M: A
synthetic lethal interaction between K-Ras oncogenes and Cdk4
unveils a therapeutic strategy for non-small cell lung carcinoma.
Cancer Cell. 18:63–73. 2010. View Article : Google Scholar : PubMed/NCBI
|