Introduction
Thyroid cancer is among the most common endocrine
malignancies with a rising incidence over the last decades
(1). The most common variants of
primary malignant thyroid neoplasms comprise papillary thyroid
carcinoma (PTC) and follicular thyroid carcinoma (FTC) that are
both derived from the follicular epithelium. In contrast, medullary
thyroid cancer (MTC) that arises from parafollicular C-cells
accounts only for a minor proportion of thyroid malignancies
(1). Among frequently activated
oncogenic signaling pathways in thyroid cancer is the
RAS/RAF/MEK/ERK (MAPK) pathway (1). The MAPK pathway transduces signals
from the extracellular environment through the cytoplasm to the
nucleus and activates a variety of biochemical processes such as
cell proliferation, differentiation, and apoptosis (2,3).
Extracellular stimuli include growth factors, hormones, and
cytokines interacting with their respective receptors which (via
adaptor proteins) activate small guanine nucleotide-binding
proteins belonging to the Ras superfamily of small GTPases. Ras in
turn recruits and activates RAF proteins such as the kinase BRAF
(V-raf murine sarcoma viral oncogene homolog B1) to initiate
subsequent phosphorylation events including MEK1/2 and ERK1/2 to
activate downstream transcription factors mediating the
aforementioned cellular outcomes (2,3).
Frequent genetic alterations in thyroid cancer that result in
aberrant activation of the RAS/RAF/MEK/ERK pathway include the RAS
genes HRAS, KRAS and NRAS as well as
BRAF, found in 61.7% of PTCs mostly represented by the
BRAFV600E mutation (4) leading to a single amino acid
substitution from valine to glutamic acid at codon 600 (5). Oncogenic
BRAFV600E was reported to be associated
with a poor clinical outcome, recurrence and treatment failure in
PTC (6–8). Vemurafenib is a potent kinase
inhibitor of BRAFV600E which is successfully used in
patients with malignant melanoma (5,9) and
may also have beneficial effects in patients with metastatic PTC
(10).
The nuclear transport machinery is essential to
eukaryotic cell function by providing the selective exchange of
macromolecules between the nucleus and the cytoplasm (11). Nuclear transport receptors
represent an important part of the nuclear transport system and
belong mainly to the karyopherin superfamily (11). This includes importins, exportins,
and transportins shuttling cargos between the nuclear and
cytoplasmic compartment through the nuclear pore complex in the
respective direction (12,13). The exportin cellular apoptosis
susceptibility (CAS) is a pivotal transport factor by re-shuttling
importin-α (imp-α) from the nucleus to the cytoplasm for subsequent
importin-β1 (imp-β1)/imp-α-dependent import of protein cargos
(12,14). As indicated by its name CAS was
first described and characterized as an apoptosis susceptibility
protein by Brinkmann et al (15,16).
Moreover, CAS was reported to be overexpressed in a variety of
solid tumors [breast (17), liver
(18,19) and ovarian (20) cancer] suggesting also
pro-tumorigenic properties. However, the expression profile and
functional role of CAS in thyroid carcinoma also in the context of
the frequently occurring driver mutation
BRAFV600E has not been reported so
far.
Here, we demonstrate that CAS expression depends on
the histological subtype, the disease stage and on the presence or
absence of the BRAFV600E mutation. Our
data also suggest the requirement of CAS to maintain cell growth
and survival in a BRAFV600E expressing PTC
cell line as well as additive effects of CAS siRNA and vemurafenib
treatment.
Material and methods
Tissue microarray (TMA)
The TMA of thyroid carcinomas contained 49 tumor
samples and 17 non-tumor (NT) thyroid tissue samples from patients
who underwent surgery at the University Hospital Heidelberg or at
other local hospitals between 2002 and 2011. It includes 17 NT, 14
FTC, 20 PTC and 15 MTC largely represented by two cores per patient
except for small sized tumors due to limited tumor extent. The
tumors were diagnosed based on the latest classification of
endocrine tumors of the World Health Organization (2004). Tissue
samples were provided by the tissue bank of the National Center for
Tumor Diseases (NCT, Heidelberg, Germany) in accordance with the
regulations of the tissue bank and the approval of the Ethics
Committee of Heidelberg University. Tumor tissues were formalin
fixed, paraffin-embedded and slides were stained with hematoxylin
and eosin (H&E). These full-section H&E slides were
re-evaluated by two independent pathologists assigning
representative and viable tumor areas for coring. The TMA block was
cut with a microtome into 5-μm sections, which were mounted on
glass slides and processed for immunohistochemical staining.
The TMA of lymph node and soft tissue metastasis of
different thyroid carcinomas contained 5 lymph node metastases of
PTCs, 6 soft tissue metastases and 1 lung metastasis of FTCs and 4
lymph node metastases and 2 lung metastases of MTCs. With a tissue
microarray instrument (Beecher MTA-1; Beecher Instruments, Inc.,
Sun Prairie, WI, USA) 2 mm-diameter cores were punched from the
assigned areas of the donor blocks and arrayed on the recipient
(TMA) block for further processing as described above.
Immunohistochemical staining (IHC)
procedure and evaluation
Immunohistochemical staining was performed with a
commercially available monoclonal anti-CAS mouse anti-human
antibody (Ab54674; Abcam, Cambridge, UK) in a 1:50 dilution (Dako
REAL Antibody Diluent; Dako, Hamburg, Germany) based on a
well-established protocol as previously described (19).
BRAFV600E immunohistochemical staining
was performed with a commercially available ready-to-use monoclonal
mouse anti-human antibody (790-4855, 12,0 μg/ml; F. Hoffmann-La
Roche AG, Basel, Switzerland) by using an automated immunostaining
instrument (BenchMark Ultra IHC/ISH staining module; Ventana
Medical Systems, Tucson, AZ, USA). Based on the manufacturer's
protocol the OptiView DAB IHC Detection kit (OptiView; Ventana
Medical Systems) was used. The procedure included the following
steps: 4 min deparaffination at 62ºC, rinsing with EZ Prep,
incubation with Cell Conditioner No. 1 (both from Ventana Medical
Systems) for 64 min at 90ºC, 24 min treatment with the primary
antibody at 36ºC, 4 min exposure to OptiView Peroxidase Inhibitor,
12 min incubation with OptiView HQ Universal Linker, 12 min
treatment in OptiView HRP Multimer, 8 min incubation with a mixture
of OptiView H2O2 and DAB, 4 min exposure to
OptiView copper, counterstaining with haematoxylin for 12 min, 4
min incubation with bluing reagent. Each incubation was followed by
multiple rinsing steps in reaction buffer (Ventana Medical
Systems). Dehydration of each TMA slide was performed as follows:
1×5 min 70% ethanol, 1×5 min 96% ethanol, 2×5 min 100% ethanol, 1×5
min Xylene by using the Leica Autostainer XL. Finally the slides
were mounted with cover slips (Leica CV5030).
The immunohistochemical staining of cytoplasmic and
nuclear CAS was semi-quantitatively evaluated using a score
calculated by multiplying staining intensity and quantity.
Intensity range: 0, negative; 1, weak; 2, moderate; and 3, strong.
Quantity range: 0, no staining; 1, staining in <1%; 2, staining
in <10%; 3, staining in 10–50%; and 4, staining in >50% of
tumor cells. In case of 2 representative areas of tumor samples the
mean of the two resulting products was used.
Cell culture
The papillary thyroid cancer cell line B-CPAP was
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium with
10% fetal calf serum and 1% penicillin/streptomycin. RPMI media and
the antibiotics were purchased from Sigma (Munich, Germany).
Transfection of siRNAs
CAS-specific siRNAs were purchased from Eurofins MWG
Operon (Ebersberg, Germany), transfected with Oligofectamine
(Invitrogen, Karlsruhe, Germany) according to the manufacturer's
instructions and used at a final concentration of 50 nM. Sequences
were as follows CAS#1, 5′-CUGACGGUAUCAAAUAUAUUA-3′, CAS#2, 5′-GGAA
CUCAGCGAUGCAAAU-3′. The Qiagen All-Stars duplex served as the
negative control for all knockdown experiments.
Cell growth assays
Cells were seeded into 96-well plates 24 h after
siRNA transfection. The cell number of each condition was assessed
at different time-points after transfection by using crystal-violet
(Serva, Heidelberg, Germany) as a staining reagent. Dried cells
were solubilized in 50% ethanolic 1 M Natriumcitrate solution and
colorimetric measurement was performed at 550 nm using an ELISA
reader (FLUOstar Omega; BMG Labtech, Ortenberg, Germany) and
normalized to the control siRNA condition. Cell proliferation was
examined using a bromodeoxyuridine enzyme-linked immunosorbent
assay (Cell Proliferation Biotrak ELISA system, version 2; GE
Healthcare/Amersham, Freiburg, Germany) according to the
manufacturer's instructions and the optical density was measured at
450 nm using a microplate photometer (Multiskan Ascent; Thermo
Electron Corporation, Waltham, MA, USA).
Gel electrophoresis and
immunoblotting
Cells were harvested in cell lysis buffer (Cell
Signaling/New England Biolabs, Frankfurt, Germany) supplemented
with a protease inhibitor cocktail (Serva, Heidelberg, Germany).
The concentration of whole cell protein lysates was determined by
Bradford assays (Bio-Rad Protein assay; Bio-Rad, Hercules, CA,
USA). After boiling the samples for 5 min at 90ºC, proteins were
separated by SDS/PAGE and transferred to a nitrocellulose membrane
(Whatman, Dassel, Germany). The membrane was blocked with 5%
milk/TBST (Milchpulver; Roth, Karlsruhe, Germany) for 1 h and
incubated overnight with anti-CAS antibody (Ab54674; Abcam,
Cambridge, UK) diluted 1:500, with anti-β-actin antibody (MP
Biomedicals, Illkirch, France) diluted 1:10,000, anti-pERK antibody
(9101S) diluted 1:500 and anti-PARP antibody (9542L; both from Cell
Signaling Technology) in a 1:500 dilution. After rinsing with TBST
the membranes where incubated for 1 h with the corresponding
fluorescently-labeled secondary antibodies (LI-COR Bioscience, Bad
Homburg, Germany). After washing with TBST signal detection was
performed using Odysee Sa Infrared Imaging system (LI-COR
Bioscience).
Results
We first set out to analyze the expression and
intracellular localization of CAS in different subtypes of thyroid
carcinoma compared to NT thyroid tissue. To do so, we performed
immunohistochemical CAS staining of a TMA consisting of 20 PTC, 14
FTCs, 15 MTC, and 17 NT. The patient characteristics are listed in
Table I. The median IHC score
(calculated by multiplying staining intensity and quantity) was
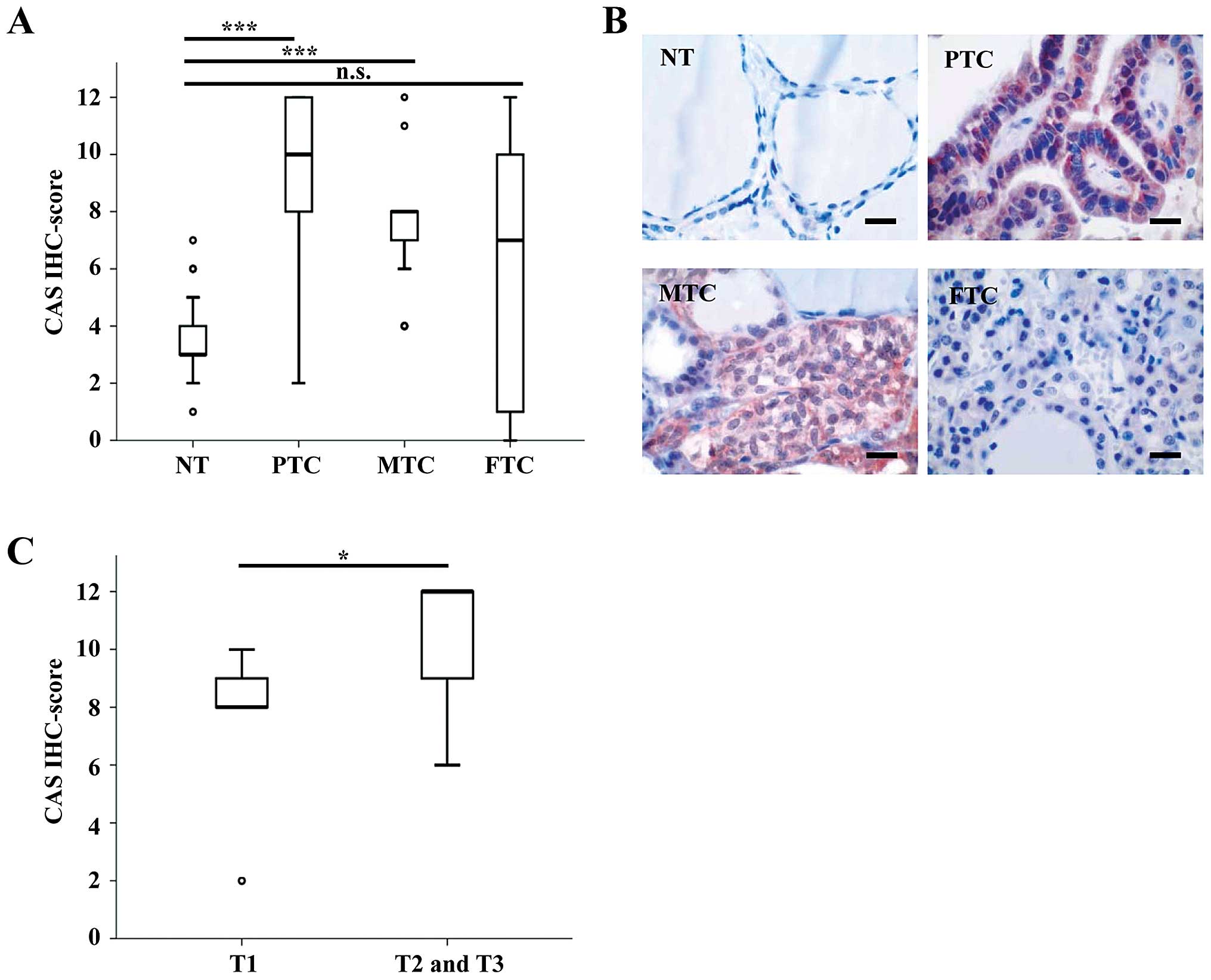
significantly higher in PTCs and MTCs compared to NT (Fig. 1A). In contrast, CAS immunostaining
was not significantly different between FTCs and NT. Representative
pictures of each tumor subtype are displayed in Fig. 1B and illustrate that CAS was
predominantly localized in the cytoplasm. These data suggest that
CAS is overexpressed in primary PTCs and MTCs, but not in primary
FTCs compared to NT. In addition, among primary PTCs we found
significantly higher IHC scores of CAS in advanced T-stages (pT2
and pT3) compared to the early T-stage (pT1), as demonstrated in
Fig. 1C.
 | Table IPatient characteristics including
age, gender, pT-stage, and metastases. |
Table I
Patient characteristics including
age, gender, pT-stage, and metastases.
| Patient
characteristics | Non-tumor
(n=17) | PTC (n=20) | MTC (n=15) | FTC (n=14) |
|---|
| Age (years) |
| Mean ± SD | 50±17 | 50±15 | 58±16 | 62±20 |
| Range | 15–87 | 25–83 | 15–82 | 29–87 |
| Gender |
| Female | 10 | 16 | 9 | 9 |
| Male | 7 | 4 | 6 | 5 |
| Stage |
| T1 | - | 5 | 10 | 2 |
| T2 | - | 6 | 2 | 2 |
| T3 | - | 9 | 3 | 9 |
| T4 | - | - | - | 1 |
| Metastases |
| Lymph node | - | 5 | 4 | 1 |
| Distant | - | - | 2 | 7 |
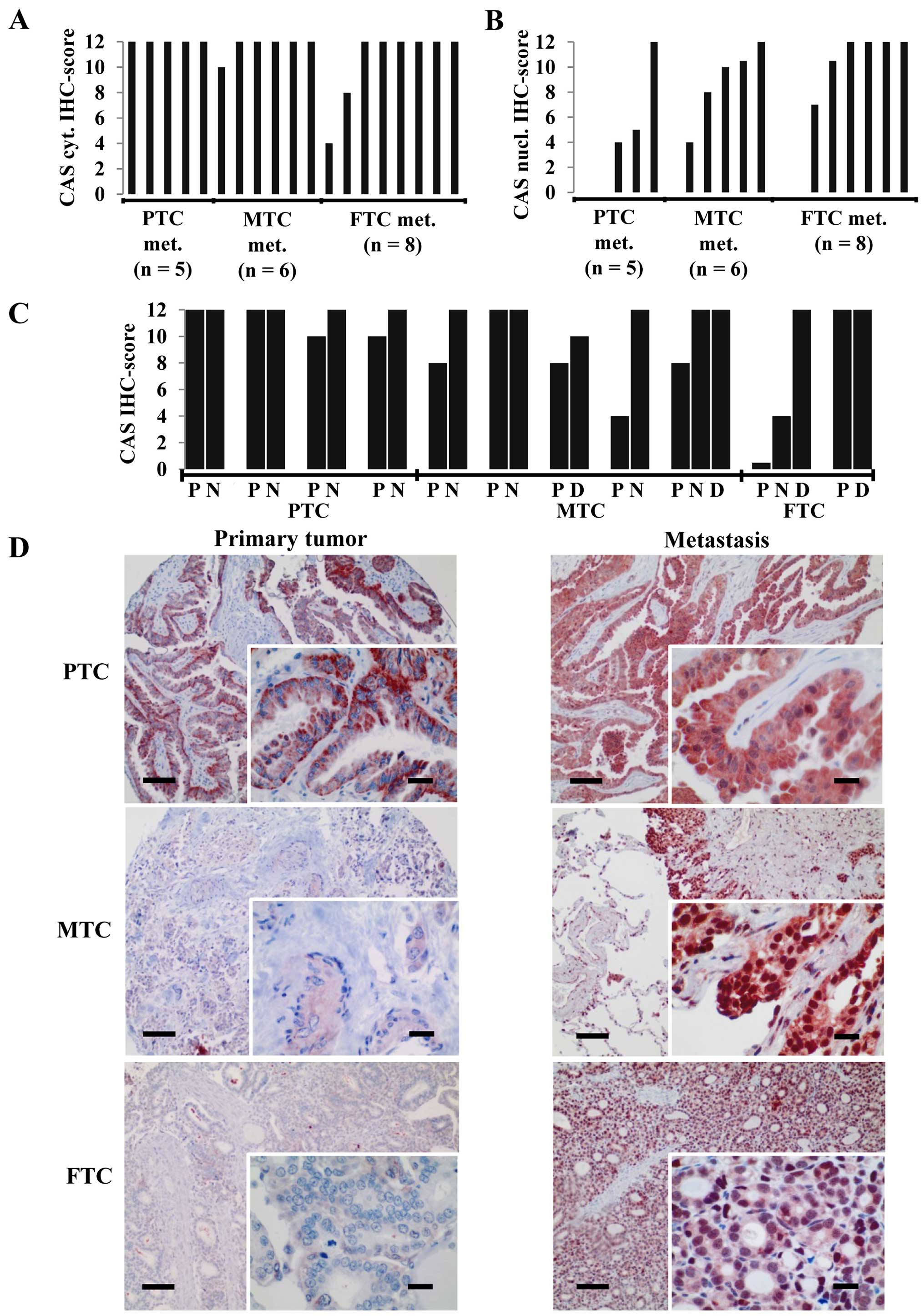
While the aforementioned analyses were performed on
primary tumors we investigated CAS expression in lymph node (N) or
hematogenous [distant (D)] metastases of PTCs, MTCs and FTCs.
Interestingly, regardless of the subtype the large majority of
metastatic tumors exhibited a strong CAS cytoplasmic
immunoreactivity reflected by a maximum IHC score of 12 (Fig. 2A). In addition, most of the
metastatic neoplasms also displayed a moderate to strong nuclear
CAS positivity (Fig. 2B) that was
rarely observed in the primary tumors. The comparison of those
carcinomas for which the primary tumor and the metastases of the
same patient were available indicates that CAS immunostaining was
either increased in the metastasis or maintained on a high level
(Fig. 2C and D), but never
decreased. We concluded that upon metastatic spread thyroid
carcinomas including FTC are characterized by a strong CAS
expression with cytoplasmic and nuclear localization.
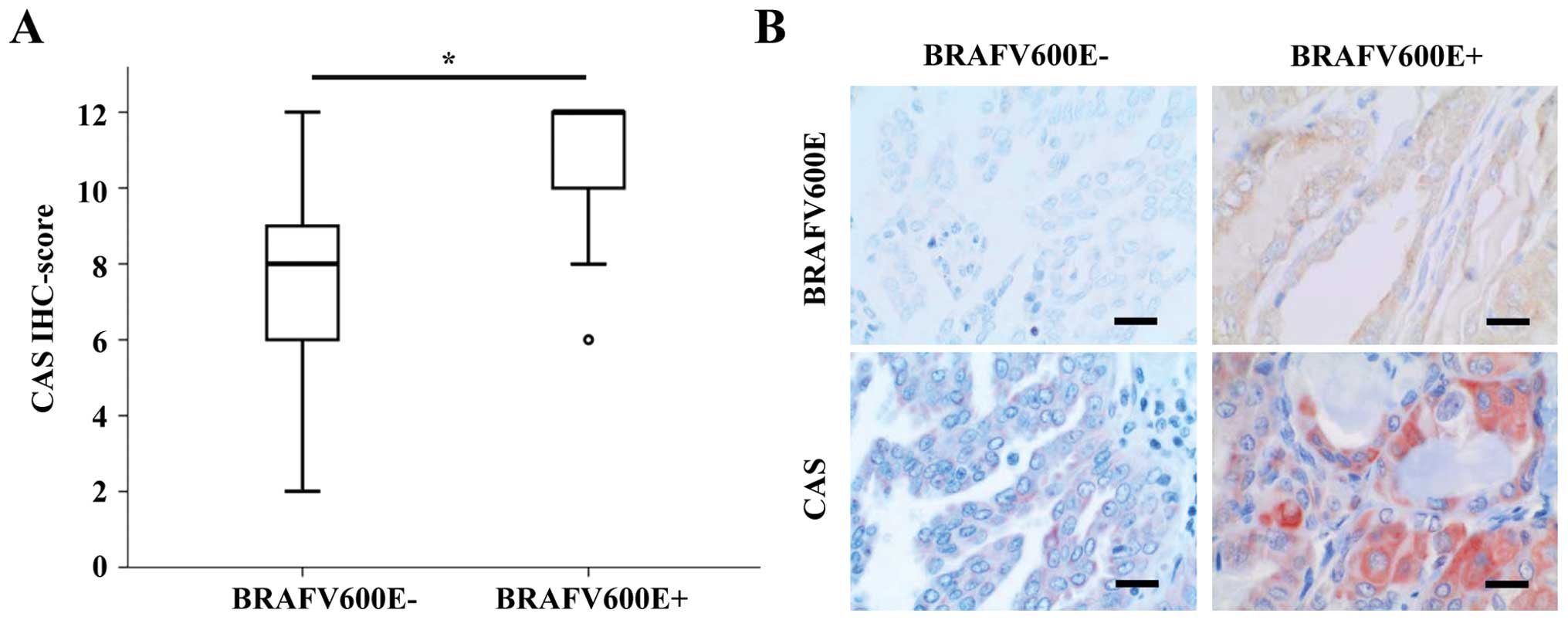
Among the most frequent driver mutations in PTC is
the BRAFV600E mutation (4). Thus, we tested if CAS IHC scores
differ between BRAFV600E positive and negative tumors as
classified by a specific BRAFV600E antibody. In fact,
CAS immunoreactivity was significantly higher in
BRAFV600E positive PTCs (median IHC score of 11 vs. 7)
(Fig. 3A). Representative pictures
of CAS and BRAFV600E IHCs of the respective PTC groups
are shown in Fig. 3B.
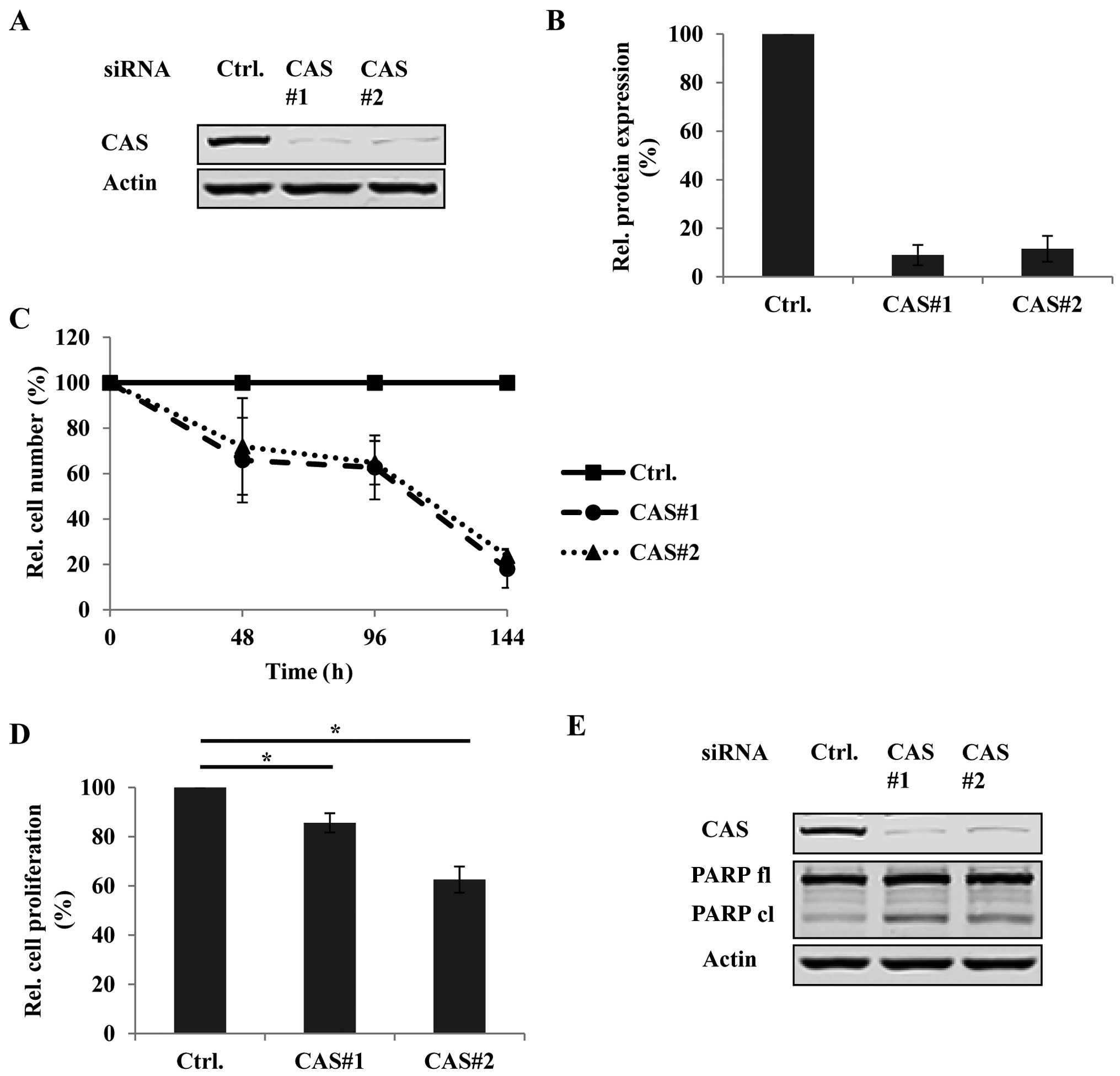
Based on these findings we chose the PTC cell line
B-CPAP reported to express the BRAFV600E mutation for
functional characterization of CAS in vitro by using
RNAi. We first tested the knockdown efficiency of two
different CAS-specific siRNAs (CAS#1 and CAS#2) by immunoblotting
(Fig. 4A) and densitometric
analyses (Fig. 4B). Fig. 4A and B demonstrate up to 90%
reduction of CAS protein compared to the control siRNA (ctrl.)
treated condition. Encouraged by this high knockdown efficiency we
then determined how CAS depletion affects tumor cell growth in
B-CPAP by monitoring the cell number of each knockdown condition
48, 96 and 144 h after transfection using crystal violet assays.
Fig. 4C shows that the number of
CAS siRNA-treated cells was strikingly lower with up to 70% less
cells after 144 h of transfection compared to the controls. We
further investigated if this decrease in tumor cell number was
caused by reduced proliferation and/or increased cell death. The
proliferation activity in the presence or absence of CAS was
measured by BrdU assays. Indeed, upon CAS depletion BrdU
incorporation was significantly reduced compared to the control
siRNA treated cells (Fig. 4D)
indicating less proliferative capacity. Furthermore, CAS knockdown
was also associated with increased apoptosis as indicated by
elevated PARP cleavage (Fig.
4E).
Taken together, these data suggest that CAS is
essential for tumor cell growth in PTC by maintaining cell
proliferation and preventing cell death.
Considering the immunohistochemical profile and the
function of CAS we hypothesized that CAS could be downstream of the
MAPK signaling cascade. We therefore determined how treatment of
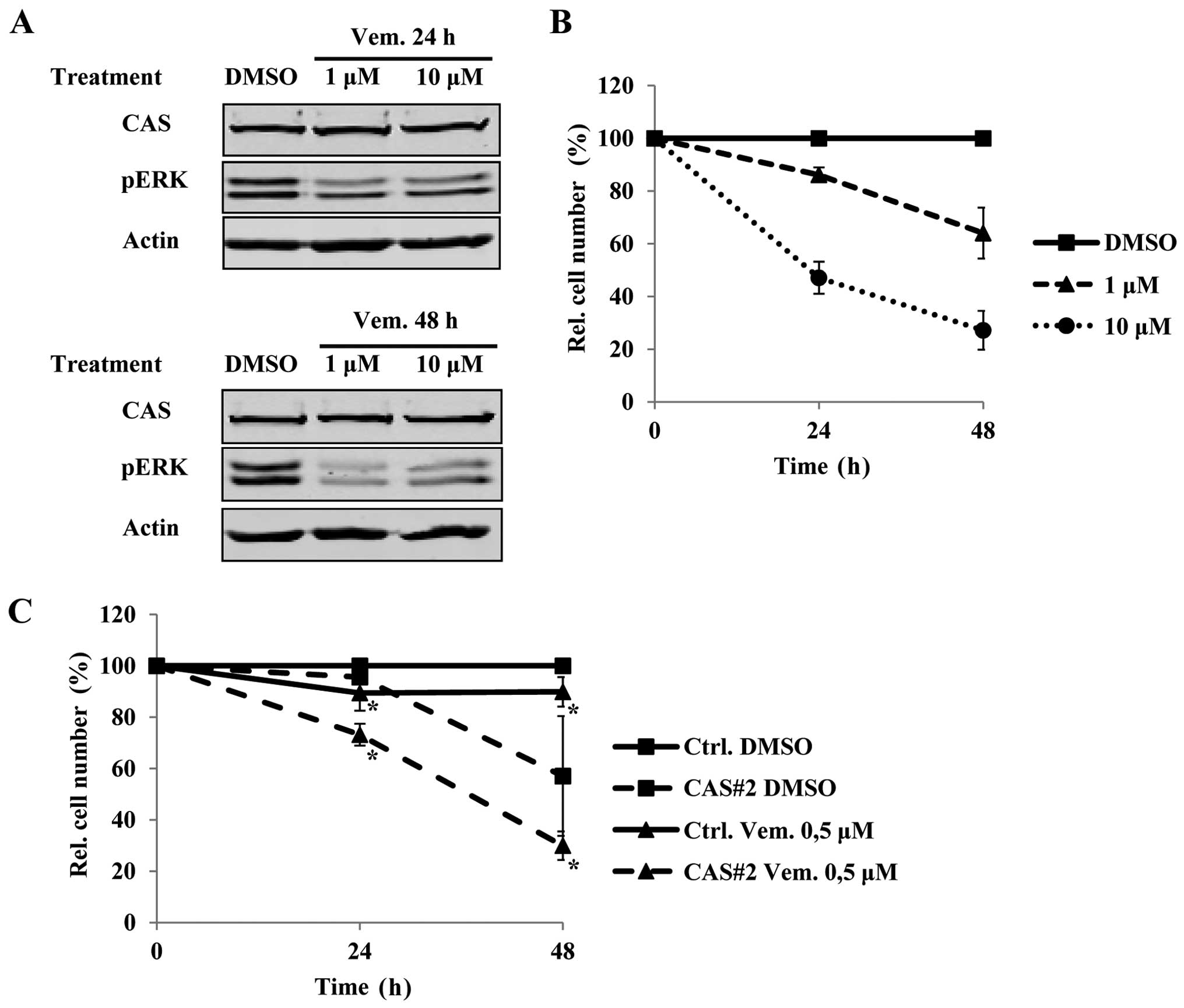
B-CPAP cells with the BRAF inhibitor vemurafenib affects CAS
protein levels. Interestingly, vemurafenib treatment for 24 and 48
h at a concentration of 1 and 10 μM did not affect CAS protein
(Fig. 5A), while it was sufficient
to downregulate pERK (Fig. 5A) and
to reduce cell growth (Fig. 5B).
This suggested that vemurafenib and CAS siRNA treatment reduce cell
growth independently. Therefore, we tested if combining both
treatments leads to additive effects. Indeed, even a low-dose
treatment of vemurafenib of 0.5 μM that itself had only a mild
effect on tumor cell viability, resulted in a further reduction of
tumor cell number in the combined treatment compared to the
CAS-siRNA treatment alone (Fig.
5C). Collectively, these data suggest that targeting CAS and
BRAF in BRAFV600E positive PTCs could be a promising
therapeutic approach.
Discussion
In the present study we analyzed the
immunohistochemical profile of the nuclear transport factor CAS in
primary and metastatic thyroid carcinomas. Including different
carcinoma subtypes such as PTC, FTC and MTC in the analyses
revealed that overexpression of CAS appears to be subtype-dependent
at least in the primary tumors. However, the fact that CAS
immunoreactivity was significantly higher only in primary PTC and
MTC, but not in primary FTC needs further validation in larger
patient cohorts before firm conclusions can be drawn. Nevertheless,
increased CAS expression in PTC and MTC is in principle consistent
with previous studies describing high expression levels of CAS in
other carcinomas such as liver (18,19),
breast (17), ovarian (20), endometrial (21) and prostate (22) cancer, among others. The
immunohistological CAS data are also consistent with the functional
experiments upon CAS depletion indicating that CAS is required to
maintain cell growth of thyroid cancer cells. The BrdU assays in
B-CPAP indicate that the reduced cell number after CAS knockdown
compared to the control siRNA treated condition is at least in part
the result of reduced cell proliferation. Several studies reported
that high expression levels of CAS can be found in high
proliferating tissues and tumors (17,18,23,24).
Nevertheless, considering CAS as a proliferation-associated protein
remains a matter of debate in the field (25–27).
Exogenous CAS overexpression from a cDNA-construct in HT-29 (colon
carcinoma) and MCF-7 (breast cancer) failed to increase, and
instead reduced cell proliferation (26,27).
Therefore, the authors concluded that the role of CAS in cancer
progression is not stimulating proliferation. Further studies
predominantly from the same group rather emphasize a role of CAS in
migration, invasion, and metastatic spread (25,26,28,29).
The latter would also be in line with the expression pattern of CAS
described in this study that was particularly high in metastatic
thyroid carcinomas. Besides reduced cell proliferation we also
found induced PARP-cleavage in cells depleted of CAS which
indicates an increase in apoptotic cell death. This finding is
consistent with previous analyses in HCC cells (19), where CAS depletion resulted in
higher Caspase 3-activity and increased PARP-cleavage. However,
these observations need to be discussed in light of the initially
described function of CAS as a pro-apoptotic factor (15,16).
Differences in the experimental set up and cell line variability
require consideration in this matter. Brinkmann et al
(15,16) conducted their experiments under
stress-conditions (TNFα- and exotoxin-treatment), while we studied
CAS function under regular, non-stress conditions. Here we used the
thyroid carcinoma cell line B-CPAP as opposed to MCF-7 cells
analyzed in the aforementioned study. Finally, the timeframe of the
analyses seems a decisive determinant as well. For instance, in
another study focusing on CAS in the context of p53-mediated
apoptosis Tanaka et al (30) report reduced apoptosis in MCF-7
cells after UV-treatment for 36 h in the CAS-knockdown conditions,
but subsequent cell death at later time-points. Clearly, these data
demonstrate that CAS plays a dual and context-dependent role in
apoptosis. To further explore the functions of CAS in thyroid
cancer it will be interesting to investigate how papillary
carcinoma cell lines with lower CAS expression or non-tumorigenic
thyroid cells behave upon CAS overexpression. Titration of a CAS
cDNA expression construct in time course experiments may reveal if
pro- or anti-apoptotic/-proliferative functions are determined by
dosage and timing in this particular tumor entity.
The BRAFV600E mutation has
received wide attention as an important factor correlated to an
aggressive course of PTC and as a predictor for disease recurrence
(7,8). However, some concerns have been
raised regarding its prognostic and predictive power, which may be
explained by a recent study showing that BRAFV600E PTCs
consist of at least four molecular subtypes (4). We demonstrate that
BRAFV600E-positive PTCs show significantly higher CAS
IHC scores compared to those being negative for this mutation, but
it remains to be investigated in a larger patient cohort if and how
the aforementioned molecular subtypes differ in their CAS
immunoreactivity and if CAS itself is of prognostic value.
Surprising in this context was the finding that CAS
protein remained unaltered in B-CPAP cells even after high-dose
treatment of vemurafenib, since the IHC analysis suggested that CAS
could be a downstream target of the RAS/RAF/MEK/ERK pathway. That
vemurafenib was effective in these cells could be demonstrated by
reduced ERK 1/2 phosphorylation and reduced cell number in the
treatment condition. This raises the possibility that either the
residual amount of phosphorylated ERK was still sufficient to
maintain CAS expression or that another pathway can compensate the
vemurafenib mediated BRAF blockade. Transcription factors (TFs)
possibly involved in the former scenario (as downstream targets of
ERK) are Ets and Elk1. In fact, several binding sites of these TFs
can be identified in the promoter region (as defined by 2000 bps
upstream of the transcription start site) of CAS in silico
(http://genome.ufl.edu/mapper/mapper-main). However, to
further substantiate these assumptions chromatin
immunoprecipitation experiments and luciferase assays would be
required. Moreover, it is also conceivable that CAS may not be a
direct downstream target of the MAPK signaling cascade at all, but
may be required for preferentially used transport pathways in
BRAFV600E positive tumors. Elucidating the functional
and regulatory link between BRAFV600E and CAS represents
a rewarding subject for future studies. Nonetheless, in light of
apparently independent effects of CAS and BRAFV600E on
tumor cell viability the combinatorial treatment of CAS siRNA and
vemurafenib could be the basis for a therapeutic approach. This,
however, requires validation in several thyroid cancer cell lines
and appropriate mouse models as well as testing to what extent
‘normal’, immortalized thyroid cells and non-tumorous thyroid
tissue are affected by this treatment. Once tumor-specific effects
have been established it would also be of considerable interest to
study if major effects of CAS on thyroid cancer cell growth and
survival can be linked to its role as an exporter for importin-αs,
such as imp-α1 [as previously shown for hepatocellular carcinoma
(19)]. If so, a compound
targeting the CAS/imp-α(1)
interaction may be more effective than a CAS siRNA approach since
the utility of siRNA therapeutics are limited by several factors
(e.g. unfavorable physicochemical characteristics, instability with
short plasma half-lives and lysosomal degradation upon endocytosis)
(31).
Targeting a nuclear transport factor in a
therapeutic context is not without precedence. For solid and
hematological malignancies selective inhibitors of nuclear export
(SINE), compounds inhibiting exportin-1 [chromosome region
maintenance 1 (CRM1)] like selinexor, have already entered phase
I/II of clinical trials (32).
Future studies are required to show if CAS and other associated
transport factors can serve as targets for effective molecular
treatment strategies in thyroid carcinomas resistant to or
recurrent upon conventional therapies.
Acknowledgements
We thank David Jansen and Veronika Geißler as well
as other members of Tissue Bank of the National Center for Tumor
Diseases (NCT) for their support. K.H. received a fellowship by the
Rahel-Goitein-Straus Program, Medical Faculty Heidelberg. S.S.
acknowledges funding by the German Research Foundation
(Si1487/3-1).
References
|
1
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cantwell-Dorris ER, O'Leary JJ and Sheils
OM: BRAFV600E: Implications for carcinogenesis and molecular
therapy. Mol Cancer Ther. 10:385–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network.
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luke JJ and Hodi FS: Vemurafenib and BRAF
inhibition: A new class of treatment for metastatic melanoma. Clin
Cancer Res. 18:9–14. 2012. View Article : Google Scholar
|
|
6
|
Xing M, Westra WH, Tufano RP, Cohen Y,
Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, et
al: BRAF mutation predicts a poorer clinical prognosis for
papillary thyroid cancer. J Clin Endocrinol Metab. 90:6373–6379.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK,
Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, et al: The association of
the BRAF(V600E) mutation with prognostic factors and poor clinical
outcome in papillary thyroid cancer: A meta-analysis. Cancer.
118:1764–1773. 2012. View Article : Google Scholar
|
|
8
|
Xing M, Alzahrani AS, Carson KA, Shong YK,
Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, et al:
Association between BRAF V600E mutation and recurrence of papillary
thyroid cancer. J Clin Oncol. 33:42–50. 2015. View Article : Google Scholar :
|
|
9
|
Flaherty KT, Puzanov I, Kim KB, Ribas A,
McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, et
al: Inhibition of mutated, activated BRAF in metastatic melanoma. N
Engl J Med. 363:809–819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KB, Cabanillas ME, Lazar AJ, Williams
MD, Sanders DL, Ilagan JL, Nolop K, Lee RJ and Sherman SI: Clinical
responses to vemurafenib in patients with metastatic papillary
thyroid cancer harboring BRAF(V600E) mutation. Thyroid.
23:1277–1283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chook YM and Süel KE: Nuclear import by
karyopherin-βs: Recognition and inhibition. Biochim Biophys Acta.
1813:1593–1606. 2011. View Article : Google Scholar :
|
|
12
|
Stewart M: Molecular mechanism of the
nuclear protein import cycle. Nat Rev Mol Cell Biol. 8:195–208.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
D'Angelo MA and Hetzer MW: Structure,
dynamics and function of nuclear pore complexes. Trends Cell Biol.
18:456–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kutay U, Bischoff FR, Kostka S, Kraft R
and Görlich D: Export of importin alpha from the nucleus is
mediated by a specific nuclear transport factor. Cell.
90:1061–1071. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brinkmann U, Brinkmann E, Gallo M and
Pastan I: Cloning and characterization of a cellular apoptosis
susceptibility gene, the human homologue to the yeast chromosome
segregation gene CSE1. Proc Natl Acad Sci USA. 92:10427–10431.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brinkmann U, Brinkmann E, Gallo M, Scherf
U and Pastan I: Role of CAS, a human homologue to the yeast
chromosome segregation gene CSE1, in toxin and tumor necrosis
factor mediated apoptosis. Biochemistry. 35:6891–6899. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Behrens P, Brinkmann U, Fogt F, Wernert N
and Wellmann A: Implication of the proliferation and apoptosis
associated CSE1L/CAS gene for breast cancer development. Anticancer
Res. 21:2413–2417. 2001.PubMed/NCBI
|
|
18
|
Wellmann A, Flemming P, Behrens P,
Wuppermann K, Lang H, Oldhafer K, Pastan I and Brinkmann U: High
expression of the proliferation and apoptosis associated CSE1L/CAS
gene in hepatitis and liver neoplasms: Correlation with tumor
progression. Int J Mol Med. 7:489–494. 2001.PubMed/NCBI
|
|
19
|
Winkler J, Ori A, Holzer K, Sticht C,
Dauch D, Eiteneuer EM, Pinna F, Geffers R, Ehemann V, Andres-Pons
A, et al: Prosurvival function of the cellular apoptosis
susceptibility/importin-α1 transport cycle is repressed by p53 in
liver cancer. Hepatology. 60:884–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brustmann H: Expression of cellular
apoptosis susceptibility protein in serous ovarian carcinoma: A
clinicopathologic and immunohistochemical study. Gynecol Oncol.
92:268–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peiró G, Diebold J, Baretton GB, Kimmig R
and Löhrs U: Cellular apoptosis susceptibility gene expression in
endometrial carcinoma: Correlation with Bcl-2, Bax, and caspase-3
expression and outcome. Int J Gynecol Pathol. 20:359–367. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bar-Shira A, Pinthus JH, Rozovsky U,
Goldstein M, Sellers WR, Yaron Y, Eshhar Z and Orr-Urtreger A:
Multiple genes in human 20q13 chromosomal region are involved in an
advanced prostate cancer xenograft. Cancer Res. 62:6803–6807.
2002.PubMed/NCBI
|
|
23
|
Wellmann A, Krenacs L, Fest T, Scherf U,
Pastan I, Raffeld M and Brinkmann U: Localization of the cell
proliferation and apoptosis-associated CAS protein in lymphoid
neoplasms. Am J Pathol. 150:25–30. 1997.PubMed/NCBI
|
|
24
|
Böni R, Wellmann A, Man YG, Hofbauer G and
Brinkmann U: Expression of the proliferation and
apoptosis-associated CAS protein in benign and malignant cutaneous
melanocytic lesions. Am J Dermatopathol. 21:125–128. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tai CJ, Hsu CH, Shen SC, Lee WR and Jiang
MC: Cellular apoptosis susceptibility (CSE1L/CAS) protein in cancer
metastasis and chemotherapeutic drug-induced apoptosis. J Exp Clin
Cancer Res. 29:1102010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao CF, Luo SF, Li LT, Lin CY, Chen YC
and Jiang MC: CSE1L/CAS, the cellular apoptosis susceptibility
protein, enhances invasion and metastasis but not proliferation of
cancer cells. J Exp Clin Cancer Res. 27:152008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang MC and Liao CF: CSE1/CAS
overexpression inhibits the tumorigenicity of HT-29 colon cancer
cells. J Exp Clin Cancer Res. 23:325–332. 2004.PubMed/NCBI
|
|
28
|
Yoshiura K, Nishishita T, Nakaoka T and
Yamashita N and Yamashita N: Inhibition of B16 melanoma growth and
metastasis in C57BL mice by vaccination with a syngeneic
endothelial cell line. J Exp Clin Cancer Res. 28:132009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tai CJ, Shen SC, Lee WR, Liao CF, Deng WP,
Chiou HY, Hsieh CI, Tung JN, Chen CS, Chiou JF, et al: Increased
cellular apoptosis susceptibility (CSE1L/CAS) protein expression
promotes protrusion extension and enhances migration of MCF-7
breast cancer cells. Exp Cell Res. 316:2969–2981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka T, Ohkubo S, Tatsuno I and Prives
C: hCAS/CSE1L associates with chromatin and regulates expression of
select p53 target genes. Cell. 130:638–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Lu Z, Wientjes MG and Au JL:
Delivery of siRNA therapeutics: Barriers and carriers. AAPS J.
12:492–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Senapedis WT, Baloglu E and Landesman Y:
Clinical translation of nuclear export inhibitors in cancer. Semin
Cancer Biol. 27:74–86. 2014. View Article : Google Scholar : PubMed/NCBI
|