Introduction
Angiogenesis is an important physiological process
during promoting tumor growth or in metastatic tumors (1). Suppression of endothelial cell
proliferation or induction of cell apoptosis is a good strategy for
blocking tumor angiogenesis (2).
The human umbilical vein endothelial cells (HUVECs) are the most
widely used endothelial cell model, which can be examined through
many processes for anti-angiogenic actions (3). Moreover, the induction of endothelial
cell apoptosis is one of the central antiangiogenic mechanisms
(3,4). Two major important pathways
contribute to the apoptotic processes, including the intrinsic
mitochondria-mediated pathway and the extrinsic death receptor
signaling (5). Mitochondrial
permeability can be regulated to release various apoptotic factors
such as cytochrome c, Apaf-1 and pro-caspase-9 to cytosol to
form apoptosome and to activate the downstream of caspase-9
(6,7). The membrane death receptors
(extrinsic apoptotic pathway) located in the membrane include
Fas/CD95, death receptor 4 (DR4) and DR5 that can influence the
distal executioner caspases (6).
In addition, reactive oxygen species (ROS) production and DNA
damage caused by anticancer drugs lead to an increase of
phosphorylation of ataxia-telangiectasia-mutated kinase (ATM) and
p53 to trigger human cancer cell apoptosis (8). p53 phosphorylation on the residue of
Ser15 has been linked to apoptosis and shown to be a transcription
factor to modulate apoptotic target genes such as Fas and DR5
(9). p53 gene expression has been
shown to upregulate both the extrinsic and the intrinsic apoptotic
signaling pathways (6,10).
Kaempferol is a dietary flavonoid and is found in
fruits and vegetables and in traditional Chinese medicines
(11,12). The pharmacological activities of
kaempferol were reported to exhibit anti-inflammatory, antioxidant,
cardio-protective and antitumor activities (12). Our previous study demonstrated that
kaempferol-induced apoptosis in human osteosarcoma cells is
mediated through endoplasmic reticulum stress and
mitochondria-dependent signaling (13). Kaempferol also induces autophagy by
AMPK and AKT signaling and causes G2/M phase arrest via
downregulation of CDK1/cyclin B in human hepatocarcinoma cells
(14). However, there is no report
addressing the possible anti-angiogenetic mechanism of kaempferol.
The objective of the current study was to explore apoptotic
evidence and its underlying molecular mechanism induced by
kaempferol in HUVECs. Kaempferol might induce both the extrinsic
and the intrinsic apoptotic pathways in HUVEC cells through
ROS-mediated p53/ATM/death receptor signaling.
Materials and methods
Chemicals and reagents
Caffeine, 4,6 -diamidino-2-phenylindole
dihydrochloride (DAPI), kaempferol,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
N-acetylcysteine (NAC) and propidium iodide (PI) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Medium 200, Low Serum
Growth Supplement (LSGS), Trypsin-EDTA,
2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) and
Fluo-4/AM were purchased from Thermo Fisher Scientific (Carlsbad,
CA, USA). Caspase-3, Caspase-8 and Caspase-9 Colorimetric Assay
kits and caspase-8 inhibitor Z-IETD-FMK were bought from R&D
Systems Inc. (Minneapolis, MN, USA). Primary antibodies [Fas/CD95,
DR4, DR5, p-ATM (Ser1981), ATM and β-actin], horseradish peroxidase
(HRP)-conjugated secondary antibodies against rabbit or mouse
immunoglobulin and p53 siRNA were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Anti-caspase-3, -8 and -9
antibodies were obtained from Cell Signaling Technology (Danvers,
MA, USA). Antibodies against p53 and p-p53 (Ser15) were obtained
from Abcam (Cambridge, Cambridgeshire, UK).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from the Bioresource Collection and Research Center
(BCRC, Hsinchu, Taiwan) and cultured in Medium 200 plus LSGS at
37°C in a humidified atmosphere with 5% CO2. The cells
were used between the second to fifth passages.
Cell viability
HUVECs were plated onto 96-well microplates at a
density of 5×103 cells/100 μl per well and then
incubated with kaempferol at the concentrations of 0, 50, 100, 150
and 200 μM for 24, 48 and 72-h treatment. Cell viability was
determined by MTT assay as previously described (15), and the optical density ratio of the
treatment to the control (% of control) was calculated.
Cell morphological detection and DNA
content analysis by flow cytometry
HUVECs were treated with 100 μM kaempferol for 24
and 48 h. The cells were examined and photographed using a
phase-contrast microscope. Then the cells were collected and fixed
in 75% ethanol overnight at −20°C before being stained with 0.1 M
phosphate/citric acid buffer (0.2 M NaHPO4 and 0.1 M
citric acid, pH 7.8) and 40 μg/ml PI for 30 min at room temperature
in the dark. The cells were determined with BD FACSCalibur (BD
Biosciences, Franklin Lakes, NJ, USA) as previously described
(6).
DAPI staining and comet assay
HUVECs were treated with 100 μM kaempferol for 48 h.
The cells were fixed and incubated with 1 μg/ml DAPI following a
previously reported method (6,16).
After being harvested, cells were combined with molten low-melting
agarose (Sigma-Aldrich) at a density of 1×105 cells/ml.
The agarose-cell mixture (50 μl) was immediately pipetted onto
comet slides. The slides were then immersed in pre-chilled lysis
solution for 30 min at 4°C as previously described (10). After lysis, horizontal
electrophoresis was performed for 30 min at 300 mA. The slides were
fixed by 70% ethanol for 5 min before being stained with 50 μl
nuclear counterstain DAPI solution (final concentration: 1 μg/ml)
and viewed under a fluorescence microscope.
Immunofluorescence staining
HUVECs (5×104 cells/well) on 4-well
chamber slides were treated with 100 μM kaempferol for 24 h. Cells
were fixed in 3% formaldehyde (Sigma-Aldrich) for 15 min,
permeabilized with 0.1% Triton-X 100 in PBS for 1 h with blocking
of non-specific binding sites using 2% bovine serum albumin (BSA)
as previously described (6,17).
These fixed cells were stained with cleaved caspase-3 antibody
(1:100 dilution, Cell Signaling Technology) overnight before being
detected using a goat anti-mouse IgG secondary antibody conjugated
fluorescein isothiocyanate (FITC) (1:500 dilution, green
fluorescence) (Merck Millipore, Billerica, MA, USA), followed by
nuclei counterstaining using and PI (red fluorescence). Images were
collected with a Leica TCS SP2 Confocal Spectral Microscope (Leica
Microsystems, Heidelberg, Mannheim, Germany)
Determination of caspase-3/-8/-9
activities and effects of their specific inhibitors
HUVECs (5×106 cells) were pretreated with
or without 10 μM Z-IETD-FMK (a specific caspase-8 inhibitor) for 1
h and incubated in 75-T flasks and treated with kaempferol for 24
and 48 h. After treatment, cells were harvested and lysed, and cell
lysates (50 μg proteins) were incubated to check relative caspase
activity using Caspase-3, Caspase-8 and Caspase-9 Colorimetric
Assay Kits (R&D Systems Inc.) following the manufacturer's
instructions.
Measurements of intracellular
Ca2+ levels and mitochondrial membrane potential
(ΔΨm)
HUVECs were treated with 100 μM kaempferol for 6, 12
and 24 h. Cells were then harvested and labeled with 2 μM Fluo-4/AM
(a specific intracellular Ca2+ fluorescence probe) and
500 nM DiOC6(3),
respectively, at 37°C for 30 min. Consequently, intracellular
Ca2+ and ΔΨm were individually analyzed for fluorescence
intensity by flow cytometry as previously described (17).
Western blot analysis
HUVECs (5×106 cells) were incubated in
100 μM kaempferol for 0, 12 or 24 h. After being harvested and
lysed, the 10% SDS-polyacrylamide electrophoresis (SDS-PAGE) gels
were used to separate equal amount of protein extract from cell
lysate as detailed by Yang et al (18). The appropriate the primary
antibodies were hybridized to observe the specific protein signals.
Then the HRP-conjugated secondary antibodies were applied before
using Immobilon Western HRP substrate kit (Merck Millipore). The
densito-metric quantification of each band was performed utilizing
NIH ImageJ 1.47 software.
Measurements of ROS production after
N-acetylcysteine and caffeine pre-treatment for cell viability
HUVECs were treated with 100 μM kaempferol for 6, 12
and 48 h. Cells were then harvested and labeled with 20 μM
H2DCFDA (a specific ROS fluorescent probe) at 37°C for
30 min. Consequently, ROS was analyzed for fluorescence intensity
by flow cytometry as previously described (19). Cells were incubated with 100 μM
kaempferol for 48 h before individual pretreatment with or without
the 10 mM N-acetylcysteine (NAC, an antioxidant) or 1 mM caffeine
(an ATM kinase inhibitor) for 1 h. After that, cells were
determined for cell viability by MTT assay as described above.
Small interference RNA transfection
HUVEC cells were grown to 70% confluence in 6-well
culture plates, and control siRNA (100 nM) or p53 siRNA (100 nM)
was transfected using Lipofectamine 2000 (Thermo Fisher Scientific)
according to the manufacturer's instructions. After transfection,
cells were seeded and thereafter exposed to 100 μM kaempferol for
48 h before analyses using western blot and MTT assay,
respectively.
Statistical analysis
The data represent the mean ± standard deviation
(SD) from at least three separate experiments. Statistical analysis
was carried out using Student's t-test, and P<0.05 was
considered statistically significant.
Results
Kaempferol induces growth inhibition in
HUVECs
At first, our study focused on the growth inhibition
effects of kaempferol on HUVECs. Cells were treated with 0, 50,
100, 150 and 200 μM of kaempferol, and cell number was counted at
24, 48 and 72 h. Our results showed that kaempferol decreased
viable HUVECs in a concentration- and time-dependent manner
(Fig. 1). The IC50 of
kaempferol was 103.25±4.15 μM after 24-h treatment.
Kaempferol triggers morphological changes
and apoptosis in HUVECs
To understand whether apoptotic mechanisms are
involved in kaempferol-treated HUVECs, the morphological changes
and DNA content using flow cytometric analysis were investigated.
Our results showed that HUVECs were detached from the surface of
the plate and showed shrinkage in kaempferol-treated cells
(Fig. 2A, right), and the control
group showed normal morphology (Fig.
2A, left). The results from the DNA content demonstrated that
kaempferol induced an increase of hypodiploid apoptotic cell
population (sub-G1 phase) at 24 and 48 h treatments (Fig. 2B). These effects are
time-dependent. Our results indicated that kaempferol provoked
apoptotic cell death in HUVECs.
Kaempferol induces DNA condensation, DNA
damage and caspase-3 protein expression in HUVECs
To confirm the apoptotic evidence in
kaempferol-treated HUVECs, DAPI stain for DNA condensation and
comet assay for DNA damage were monitored. Our results showed that
kaempferol induced DNA condensation (Fig. 3A) and DNA damage (Fig. 3B) in HUVECs cells. It is well known
that caspase-3 is a key mediator of cell apoptosis (13,14).
Next, we used caspase-3 immunofluorescence staining and confocal
laser scanning microscopy to observe the caspase-3 protein
expression. The caspase-3 protein expression (green color) was
observed in the cytosol of kaempferol-treated HUVECs (Fig. 3C). Our results demonstrated that
kaempferol provoked apoptotic cell death through DNA damage and
caspase-3 activation in HUVECs.
Kaempferol stimulates intracellular
Ca2+ levels and loss of ΔΨm in HUVECs
To determine the roles of intracellular
Ca2+ levels and ΔΨm levels of apoptotic death induced by
kaempferol, we detected the intracellular Ca2+ by
Fluo-4/AM dye and ΔΨm by DiOC6(3) dye at 6, 12 and 24 h, respectively.
Kaempferol increased intracellular Ca2+ levels (Fig. 4A) and depletion of ΔΨm (Fig. 4B) in HUVECs. The data indicated
that kaempferol-provoked apoptotic death in HUVECs might be
mediated through Ca2+ signal and mitochondrial
pathway.
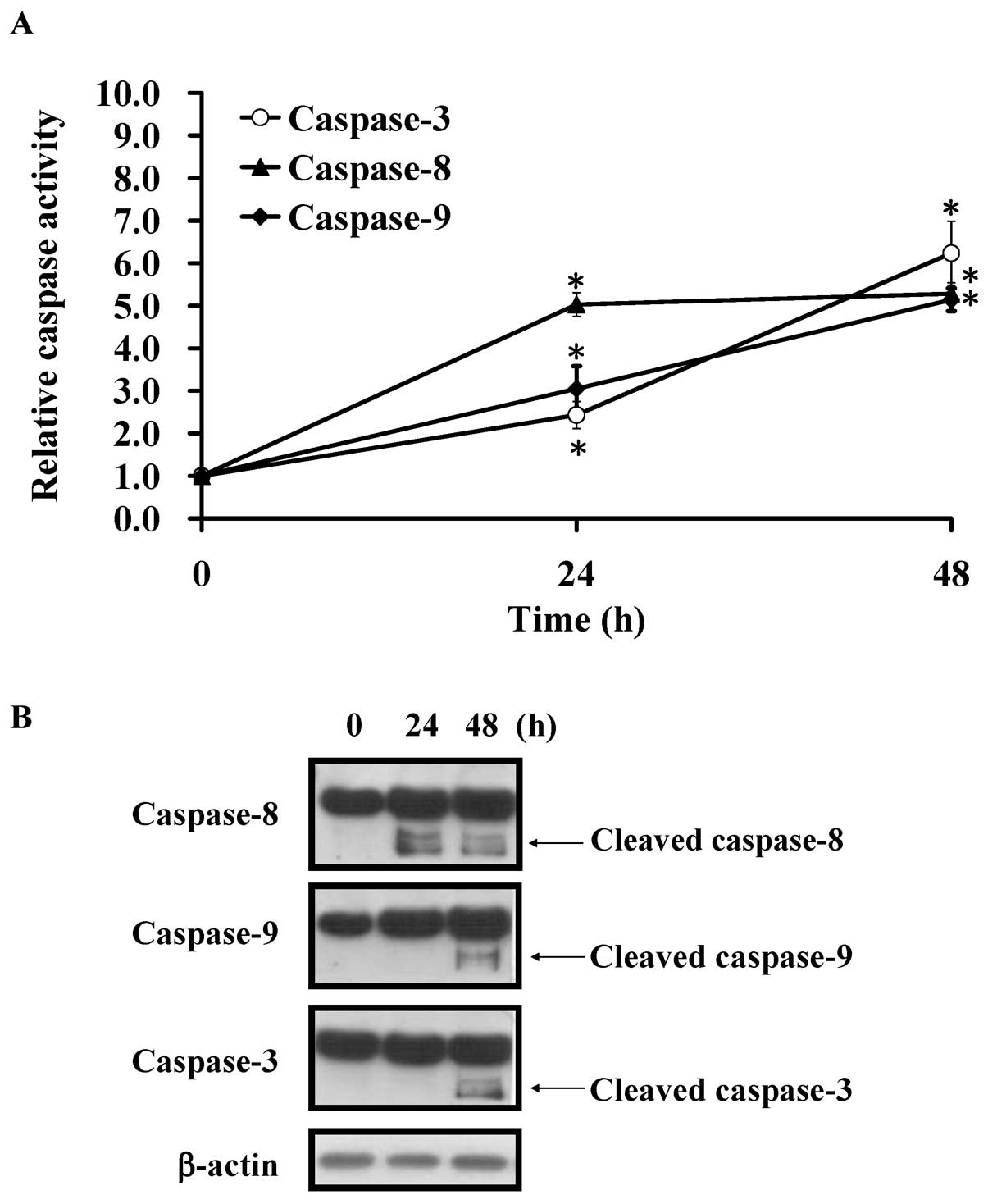
Kaempferol induces the activity of
caspase-8, -9 and -3 in HUVECs
To determine the major caspase pathway of apoptotic
death induced by kaempferol, we further detected the activity of
caspase-8, -9 and -3 at 24 and 48 h, respectively. Our data
inducted that the activities of caspase-8, -9 and -3 were
significantly increased in kaempferol-treated HUVECs in a
time-dependent manner (Fig. 5A).
These results suggested that both the intrinsic
mitochondria-mediated pathway and the extrinsic death receptor
signaling are involved in kaempferol-induced apoptosis in HUVECs.
To confirm our suggestion, we used western blotting to detect the
cleaved form of caspase-8, -9 and -3. Our results showed that the
cleaved caspase-8, -9 and -3 protein level were significantly
increased in HUVECs prior to kaempferol challenge at 48 h (Fig. 5B). Strikingly, caspase-8 activity
was significantly increased at 24-h treatment in treated HUVECs.
The data indicated that extrinsic death receptor pathway is a key
signal in kaempferol-induced apoptosis of HUVECs.
Z-IETD-FMK blocks the activity of
caspase-8, -9 and -3 in kaempferol-treated HUVECs
Our hypothesis that kaempferol-provoked apoptosis is
mediated mainly through extrinsic death receptor pathway. To prove
our hypothesis, Z-IETD-FMK (a specific caspase-8 inhibitor) was
used to block caspase-8, -3, and -9 activities. Our results
demonstrated that pre-incubation with the specific inhibitor of
Z-IETD-FMK strongly decreased the activity of caspase-8 (Fig. 6A), caspase-3 (Fig. 6B), and caspase-9 (Fig. 6C) compared with kaempferol
treatment alone. Overall, these data demonstrated that extrinsic
death receptor pathway is a crucial element in kaempferol-triggered
apoptosis of HUVECs.
Kaempferol increases ROS generation and
N-acetylcysteine reduces kaempferol-induced growth inhibition
effect in HUVECs
Our findings showed that kaempferol increased
intracellular ROS production at 6, 12 and 24 h in HUVECs by using
flow cytometry and H2DCFDA (a specific fluorescent
probe) (Fig. 7A). Cells showed a
significant inhibitory effect on kaempferol-induced growth
inhibition after pre-treatment with NAC (Fig. 7B). These data indicated that ROS
production is important in kaempferol-triggered apoptosis of
HUVECs.
ATM-p53-mediated death receptor pathway
is involved in kaempferol-induced apoptosis
It was reported that ROS can modulate death receptor
pathway in cancer cells (8,10).
Our hypothesis showed that extrinsic death receptor pathway is a
central component in kaempferol-triggered apoptosis of HUVECs. The
results revealed that kaempferol stimulated the death
receptor-associated protein levels, including Fas/CD95, DR4 and DR5
in HUVECs (Fig. 8A). It is well
documented that p53 gene and its phosphorylation at the Ser15
interact Fas/CD95 activation during cell apoptosis (6,20).
To elucidate the possible signaling pathway in kaempferol-provoked
apoptosis, the levels of associated proteins were evaluated.
Kaempferol increased the protein level of ATM, p53, phosphorylation
of ATM and p53, followed by increasing levels of Fas, DR4 and DR5
based on the exposure time (Fig.
8B). Our results indicated that kaempferol increased the
protein level of Fas/CD95, DR4 and DR5 through the
ATM-p53-dependent regulation of transcription levels. We also
re-checked the kaempferol-caused ATM-p53-dependent signal in
HUVECs, and caffeine (an ATM kinase inhibitor) and p53 siRNA were
used to block ATM and p53 function. Pre-treatment with caffeine
reversed the inhibition of cell viability in treated group
(Fig. 9A). Moreover, p53 siRNA
also had a similar effect in kaempferol-treatment group (Fig. 9B). The results from our
experimental approaches indicate that kaempferol-induced apoptosis
of HUVECs is mediated through ATM-p53-mediated pathway.
Discussion
Kaempferol is a flavonol present in fruits and
vegetables, including onions, kale, broccoli, apples, cherries,
berries, tea and red wine (11,12).
Kaempferol has many biological properties, including anti-cancer,
antioxidant activity, anti-inflammatory effects (12,21).
Kaempferol induces apoptosis and cell cycle arrest in various
cancer cell lines, including colon cancer (22), liver cancer (23), gastric cancer (24), and bladder cancer (25) cells. Kim et al (26) demonstrated that kaempferol can
modulate angiogenesis and immune-endothelial cell adhesion. Zhao
et al (27) showed that
kaempferol from Pu-erh tea has anti-cancer and anti-angiogenesis
effects. Currently, the mechanism involved in the kaempferol
anti-angiogenesis effects is unknown. In this study, we are the
first to report that kaempferol induced growth inhibition (Fig. 1) and apoptosis (Figs. 2 and 3) in HUVEC cells. Our results also showed
that kaempferol induced the activity of caspase-8, -9 and -3
(Fig. 4) and Fas/CD95, DR4 and DR5
at protein levels (Fig. 8) in
HUVEC cells. Moreover, major cell signaling involved in
kaempferol-treated HUVECs were investigated, we focused on the
ROS-ATM-p53 signaling. Our results demonstrated that kaempferol
induced ROS production (Fig. 7),
ATM, p53, phosphorylation of ATM and phosphorylation of p53 protein
levels (Fig. 9). We used the
specific inhibitors that include Z-IETD-FMK (a specific caspase-8
inhibitor), N-acetylcysteine (NAC, an antioxidant), caffeine (an
ATM kinase inhibitor) and p53 siRNA to confirm this pathway. We
found that kaempferol triggered HUVEC apoptosis through the
ROS-mediated ATM/p53 signaling.
The p53 tumor suppressor protein is an essential
regulator in controlling cell growth and cell death (6,20).
In response to intracellular and extracellular stress, p53 is
activated and serves as a transcription factor that orchestrates
various targets, which in turn modulate multitude of cellular
processes such as DNA repair, cell cycle arrest and apoptosis
(28,29). It is reported that p53-inducible
pro-apoptotic genes trigger apoptosis through both the extrinsic
and the intrinsic apoptotic molecular pathways (30). Our results showed that kaempferol
significantly increased ROS production (Fig. 7A) and the protein levels of
Fas/CD95, DR4, DR5, ATM, p-ATM (Ser1981), p53, and p-p53 (Ser15) in
HUVECs (Fig. 8). In addition,
knockdown of p53 expression by p53 siRNA significantly inhibited
the cell growth inhibitory effects (Fig. 9A) after treatment with kaempferol
in HUVECs. Based on our results, we suggest that p53 might be
involved in kaempferol-upregulated death receptor signaling.
In addition to the death receptor pathway, our
results suggest that kaempferol induced apoptosis through
mitochondria-dependent pathway. The elevation of
DiOC6(3) fluorescence
indicated the loss of ΔΨm in kaempferol-treated HUVECs (Fig. 4B). The dissipation of ΔΨm is
attributed to the opening of mitochondrial permeability transition
(MTP) pore. Hence, we suggest that kaempferol led to the persistent
opening of the MTP pore, which resulted in mitochondrial swelling
and the rupture of mitochondrial outer membrane, ultimately the
release of intermembrane proteins such as cytochrome c,
Apaf-1, pro-caspase-9, AIF and Endo G that trigger cell apoptosis
(6,19).
Oxidative stress is closely related to cancer and
often associated with cancer prevention and cancer therapy agents
(15,31). It was report that reactive oxygen
species (ROS) not only function as a regulator of subcellular
events but are also able to induce cell apoptosis (8). Yang et al (32) demonstrated that kaempferol reduced
the glutamate-induced oxidative stress in mouse-derived hippocampal
neuronal HT22 cells. Ondricek et al (33) showed that kaempferol rescued RGC-5
cells from iodoacetic acid-induced cell death, as well as reduced
caspase activation and ROS generation. However, Jeong et al
(34) demonstrated that kaempferol
caused an increase in generation of reactive oxygen species (ROS),
and induced cell death in human glioma cells. Kim et al
(35) also showed that kaempferol
induced the generation of fluorescent DCF in the MCF-7 cells, and
treatment with N-acetylcysteine suppressed kaempferol-induced PARP
cleavage. Sharma et al (36) also showed kaempferol in
glioblastoma cells induced apoptosis through oxidative stress. In
this study, kaempferol was found to be less cytotoxic towards
HUVECs after pre-treatment with N-acetylcysteine, suggesting that
kaempferol induced oxidative stress in HUVECs (Fig. 7B). Based on the result from
H2DCFDA assay, surprisingly kaempferol was found to
stimulate the ROS formation in HUVECs.
In conclusion, the molecular signaling pathway in
HUVECs caused by kaempferol is summarized in Fig. 10. Our study discovered that
kaempferol reduced HUVEC viability and induced DNA damage and DNA
fragmentation through activating the levels of caspase-3, -8, and
-9 signaling, which were upregulated by ROS-mediated p53/ATM
molecules following stimulations of p53 downstream protein levels
of Fas/CD95, DR4, and DR5. Our results suggest that kaempferol
warrants further development as an anti-angiogenetic agent.
Acknowledgements
This study was financially supported by research
grants from Kaohsiung Veterans General Hospital Pingtung Branch,
Pingtung, Taiwan.
References
|
1
|
Padma VV: An overview of targeted cancer
therapy. Biomedicine (Taipei). 5:192015. View Article : Google Scholar
|
|
2
|
Wang J, Li G, Wang Y, Tang S, Sun X, Feng
X, Li Y, Bao G, Li P, Mao X, et al: Suppression of tumor
angiogenesis by metformin treatment via a mechanism linked to
targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget.
6:44579–44592. 2015.PubMed/NCBI
|
|
3
|
He MF, Gao XP, Li SC, He ZH, Chen N, Wang
YB and She JX: Anti-angiogenic effect of auranofin on HUVECs in
vitro and zebrafish in vivo. Eur J Pharmacol. 740:240–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beedie SL, Peer CJ, Pisle S, Gardner ER,
Mahony C, Barnett S, Ambrozak A, Gütschow M, Chau CH, Vargesson N,
et al: Anticancer properties of a novel class of tetrafluorinated
thalidomide analogues. Mol Cancer Ther. 14:2228–2237. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang CH, Wang GH, Chou TH, Wang SH, Lin
RJ, Chan LP, So EC and Sheu JH: 5-epi-Sinuleptolide induces cell
cycle arrest and apoptosis through tumor necrosis
factor/mitochondria-mediated caspase signaling pathway in human
skin cancer cells. Biochim Biophys Acta. 1820:1149–1157. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai AC, Pan SL, Sun HL, Wang CY, Peng CY,
Wang SW, Chang YL, Kuo SC, Lee KH and Teng CM: CHM-1, a new
vascular targeting agent, induces apoptosis of human umbilical vein
endothelial cells via p53-mediated death receptor 5 up-regulation.
J Biol Chem. 285:5497–5506. 2010. View Article : Google Scholar :
|
|
10
|
Chiu YJ, Hour MJ, Lu CC, Chung JG, Kuo SC,
Huang WW, Chen HJ, Jin YA and Yang JS: Novel quinazoline HMJ-30
induces U-2 OS human osteogenic sarcoma cell apoptosis through
induction of oxidative stress and up-regulation of ATM/p53
signaling pathway. J Orthop Res. 29:1448–1456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang WW, Tsai SC, Peng SF, Lin MW, Chiang
JH, Chiu YJ, Fushiya S, Tseng MT and Yang JS: Kaempferol induces
autophagy through AMPK and AKT signaling molecules and causes G2/M
arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human
hepatic cancer cells. Int J Oncol. 42:2069–2077. 2013.PubMed/NCBI
|
|
12
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen HJ, Lin CM, Lee CY, Shih NC, Peng SF,
Tsuzuki M, Amagaya S, Huang WW and Yang JS: Kaempferol suppresses
cell metastasis via inhibition of the ERK-p38-JNK and AP-1
signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep.
30:925–932. 2013.PubMed/NCBI
|
|
15
|
Lu CC, Chen HP, Chiang JH, Jin YA, Kuo SC,
Wu TS, Hour MJ, Yang JS and Chiu YJ: Quinazoline analog HMJ-30
inhibits angiogenesis: Involvement of endothelial cell apoptosis
through ROS-JNK-mediated death receptor 5 signaling. Oncol Rep.
32:597–606. 2014.PubMed/NCBI
|
|
16
|
Lu CC, Yang SH, Hsia SM, Wu CH and Yen GC:
Inhibitory effects of Phyllanthus emblica L. on hepatic steatosis
and liver fibrosis in vitro. J Funct Foods. 20:20–30. 2016.
View Article : Google Scholar
|
|
17
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis - the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calderón-Montaño JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho HJ and Park JH: Kaempferol induces
cell cycle arrest in HT-29 human colon cancer cells. J Cancer Prev.
18:257–263. 2013. View Article : Google Scholar
|
|
23
|
Zhang Q, Cheng G, Qiu H, Zhu L, Ren Z,
Zhao W, Zhang T and Liu L: The p53-inducible gene 3 involved in
flavonoid-induced cytotoxicity through the reactive oxygen
species-mediated mitochondrial apoptotic pathway in human hepatoma
cells. Food Funct. 6:1518–1525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song H, Bao J, Wei Y, Chen Y, Mao X, Li J,
Yang Z and Xue Y: Kaempferol inhibits gastric cancer tumor growth:
An in vitro and in vivo study. Oncol Rep. 33:868–874. 2015.
|
|
25
|
Dang Q, Song W, Xu D, Ma Y, Li F, Zeng J,
Zhu G, Wang X, Chang LS, He D, et al: Kaempferol suppresses bladder
cancer tumor growth by inhibiting cell proliferation and inducing
apoptosis. Mol Carcinog. 54:831–840. 2015. View Article : Google Scholar
|
|
26
|
Kim JD, Liu L, Guo W and Meydani M:
Chemical structure of flavonols in relation to modulation of
angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem.
17:165–176. 2006. View Article : Google Scholar
|
|
27
|
Zhao X, Song JL, Kim JD, Lee JS and Park
KY: Fermented Pu-erh tea increases in vitro anticancer activities
in HT-29 cells and has antiangiogenetic effects on HUVECs. J
Environ Pathol Toxicol Oncol. 32:275–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jänicke RU, Sohn D and Schulze-Osthoff K:
The dark side of a tumor suppressor: Anti-apoptotic p53. Cell Death
Differ. 15:959–976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tor YS, Yazan LS, Foo JB, Wibowo A, Ismail
N, Cheah YK, Abdullah R, Ismail M, Ismail IS and Yeap SK: Induction
of apoptosis in MCF-7 cells via oxidative stress generation,
mitochondria-dependent and caspase-independent pathway by ethyl
acetate extract of Dillenia suffruticosa and its chemical profile.
PLoS One. 10:e01274412015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuribayashi K, Finnberg N, Jeffers JR,
Zambetti GP and El-Deiry WS: The relative contribution of
pro-apoptotic p53-target genes in the triggering of apoptosis
following DNA damage in vitro and in vivo. Cell Cycle.
10:2380–2389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang EJ, Kim GS, Jun M and Song KS:
Kaempferol attenuates the glutamate-induced oxidative stress in
mouse-derived hippo-campal neuronal HT22 cells. Food Funct.
5:1395–1402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ondricek AJ, Kashyap AK, Thamake SI and
Vishwanatha JK: A comparative study of phytoestrogen action in
mitigating apoptosis induced by oxidative stress. In Vivo.
26:765–775. 2012.PubMed/NCBI
|
|
34
|
Jeong JC, Kim MS, Kim TH and Kim YK:
Kaempferol induces cell death through ERK and Akt-dependent
down-regulation of XIAP and survivin in human glioma cells.
Neurochem Res. 34:991–1001. 2009. View Article : Google Scholar
|
|
35
|
Kim BW, Lee ER, Min HM, Jeong HS, Ahn JY,
Kim JH, Choi HY, Choi H, Kim EY, Park SP, et al: Sustained ERK
activation is involved in the kaempferol-induced apoptosis of
breast cancer cells and is more evident under 3-D culture
condition. Cancer Biol Ther. 7:1080–1089. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharma V, Joseph C, Ghosh S, Agarwal A,
Mishra MK and Sen E: Kaempferol induces apoptosis in glioblastoma
cells through oxidative stress. Mol Cancer Ther. 6:2544–2553. 2007.
View Article : Google Scholar : PubMed/NCBI
|