Introduction
Ovarian cancer is the fourth most lethal cancer
among women and the leading cause of gynecological cancer deaths
worldwide. Currently, the standard first-line therapy for this
malignancy includes aggressive cytoreductive surgery followed by
chemotherapy with paclitaxel, platinum-based agents, or a
combination of these treatments. Ovarian cancer patients are
commonly initially sensitive to these therapies. However, the
5-year-survival has not improved over the past few decades
(1) and most survivors eventually
relapse and develop resistance to cisplatin therapy. Progression of
a cisplatin-resistant tumor ultimately leads to significant
morbidity and mortality. The molecular mechanism of
cisplatin-resistance remains largely unknown and is a major
impediment to more effective cancer treatment. Traditionally, most
cytotoxic agents are selectively targeted to cancers by exploiting
differential tumor cell characteristics, such as high proliferation
rates, hypoxia and genome instability, resulting in a favorable
therapeutic index (2). However,
platinum-based therapies also affect stromal cells in the tumor
microenvironment such as cancer-associated fibroblasts (CAFs),
which can disrupt the normal function and physiology of tissues and
organs and contribute to tumor drug-resistance (2). The underlying mechanism of cisplatin
as a stimulating factor in the stromal cells resulting in cisplatin
resistance has not been clarified in ovarian cancer. The present
study was undertaken to illuminate this issue.
CCL5 (also known as RANTES), is a member of the
CC-chemokine family and plays a crucial role in chemotherapy
resistance, relapse, metastasis and migration in human malignancy
(3). Many studies have
demonstrated that altered CCL5 expression in patients with breast
tumors, melanoma, lung, prostate, cervical and pancreatic cancer is
significantly correlated with disease progression, poor prognosis
and tumor cell chemotherapy resistance (4–7).
CCL5 can be expressed and secreted either by tumors themselves or
by the tumor microenvironment stromal cells and substantially
promotes the resistance of ovarian cancer cells to cisplatin
(8,9). However, the role of CCL5 and the
mechanisms underlying cisplatin resistance in ovarian cancers have
not yet been fully clarified.
The STAT3 is a member of family of transcription
factors that mediates the response to a variety of cytokines,
chemokines and growth factors and modulates the transcription of
genes involved in the regulation of a large variety of vital
functions, primarily including cell survival, metastasis,
migration, angiogenesis, chemotherapy resistance and the immune
response (10–14). STAT3 contributes to oncogenesis in
many human cancers, including prostate, breast, naso-pharyngeal
carcinomas and ovarian cancers (15,16).
STAT3 pathway activity has been associated with cisplatin
resistance in human ovarian carcinomas (17). Furthermore, a previous study
demonstrated that using small molecule inhibitors or interference
RNA could rescue the inherent and acquired chemoresistance of
ovarian cancer cells (18).
Moreover, the STAT3-CCL5 signaling pathway has been shown to play
an important role in tamoxifen resistance in human breast cancer
cells (3). The PI3K/Akt signaling
pathway also plays a lead role in tumor progression and
chemotherapy resistance and this signaling pathway can be activated
by CCL5 (19).
In the present study, we observed a significant
correlation between CCL5, STAT3 and PI3K/Akt signaling pathways in
regulating cisplatin-induced cisplatin resistance in ovarian cancer
cells. We suggest that cisplatin-induced CCL5 secretion derived
from CAFs promotes cisplatin resistance in ovarian cancer.
Materials and methods
Cell culture
The human ovarian cancer cell line SKOV3 was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). A cisplatin-resistant ovarian cancer cell line
(C13*) was a gift from Professor Benjamin K. Tsang, Ottawa Health
Research Institute, Ottawa, Canada (20). Cells were cultured in Macoy'5A
medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco), penicillin (100 units/ml) and
streptomycin (100 μg/ml) at 37°C in a humidified atmosphere
containing 5% CO2.
Reagents
Recombinant human CCL5 (RANTES) was purchased from
PeproTech, Inc., (Rocky Hill, NJ, USA) and anti-CCL5 neutralizing
antibody was purchased from R&D Systems (Minneapolis, MN, USA).
Cisplatin (DDP) was purchased from Sigma-Aldrich (St. Louis, MO,
USA). LY294002 was obtained from Selleck Chemicals (Houston, TX,
USA).
Patient samples
Sixty-two patients with serous ovarian cancer staged
as III–IV (FIGO) at Tongji Hospital (Wuhan, Hubei, China) provided
informed consent for the collection of solid tumor specimens during
the years 2010 to 2013. All patients underwent debulking and
subsequent platinum-centered chemotherapy. The protocol was
approved by the Ethics Committee of Tongji Hospital. Platinum
resistance or platinum sensitivity was defined as relapsed or
progression within six months or after six months from the last
platinum-based chemotherapy, respectively.
Isolation and primary culture of
fibroblasts and immunocytochemistry (ICC) on cultured CAFs
Fresh tumor tissue samples were obtained from
patients who underwent initial cytoreductive surgery diagnosed as
high-grade serous ovarian cancer through intraoperative fast
biopsy. The detailed procedure was performed as previously
described (21). Primary
antibodies included α-smooth muscle actin (α-SMA) monoclonal
antibody (1:100 dilution; Abcam, Cambridge UK); mouse monoclonal
antibody vimentin (1:100 dilution; Wuhan Boster Biological
Technology, Ltd., Wuhan, China); mouse monoclonal antibody
cytokeratin 8 (1:100 dilution; Wuhan Boster Biological
Technology).
Conditioned medium
DDP was administered to CAFs at proper
concentrations for 12 h, and CAFs without DPP administration were
used as control. The medium was replaced with serum-free medium for
culturing for another 24 h. The treated supernatant was referred to
as CM*, and the untreated supernatant as the control medium.
siRNA transfection assay
The STAT3 siRNA transfection experiment was
performed according to the manufacturer's protocol using
Lipofectamine™ 3000 (Invitrogen). The siRNA sequences for STAT3
were 5′-GGA GCA GCA CCU UCA GGA UTT-3′ as previously described
(18).
CCL5 neutralization assay
Anti-CCL5 antibody neutralization was added to the
culture cell using 10 μg/ml with or without recombinant human
CCL5.
Drug sensitivity assay
A cell counting kit was used to analyze cell
viability. Cells were cultured in 96-well plates overnight
(1×104 cells/well). After 24 h, cells were treated with
DDP at indicated concentrations in CM* or control medium for 48 h.
After addition of CCK-8 for 2 h, the number of survival cells was
detected at the absorbance of 450 nm by a microplate reader
(Bio-Rad Laboratories). Each experiment was performed three times.
The results were analyzed using GraphPad Prism 5 (GraphPad Software
Inc., La Jolla, CA, USA).
Apoptosis assay
Apoptosis assay was performed as previously
described (22).
Western blot analysis
Western blot analysis was performed as previously
described (22). Primary mouse
monoclonal antibody against human MRP1 and MRP2 (1:1,000 dilution;
Wuhan Boster Biological Technology), rabbit polyclonal antibody
against human Bcl-2 (1:1,000 dilution; Epitomics, Burlingame, CA,
USA), rabbit polyclonal antibody against human cleaved-PARP
(1:1,000 dilution; Epitomics), rabbit monoclonal antibody against
human activated caspase-3 (1:1,000 dilution; Cell Signaling
Technology, Inc., Beverly, MA, USA) and p-Aktser473
(1:1,000 dilution; Cell Signaling Technology), and goat monoclonal
antibody against human CCL5 (1:1,000 dilution; R&D Systems,
Inc., Minneapolis, MN, USA), and rabbit monoclonal antibody against
human p-STAT3 (Tyr705, 1:1,000 dilution; Abcam).
Cytokine/chemokine array
CAFs were first pretreated with DDP at a
concentration of 10 μM or without DDP for 12 h, and then replaced
with serum-free medium for another 24 h. Next, the conditioned
medium (defined as CM*) and control medium were centrifuged and the
supernatant was passed through a 0.22 μm filter. The CM* and
control medium were tested using the RayBio Human Cytokine Antibody
Array G Series 5 according to manufacturer's instructions. After
blocking the array chip, 100 ml of sample was added per sub-array
for incubation. Subsequent washes and biotin-conjugated antibody
and fluorescent dye-conjugated streptavidin incubations followed,
and fluorescence detection was achieved using a 4000A Axon GenePix
laser scanner. Background-deducted signal values were used and
normalized against positive controls within the chip. Data are
expressed as a ratio of CM* to control media.
RNA extraction and real-time RT-PCR
Total RNA was extracted from CAFs or cell lines
using the PrimeScript RT reagent kit (Takara) and SYBR Premix Ex
Taq (Takara) according to the manufacturer's instructions. Primer
sequences for the endogenous reference genes,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the specific
primer sequences used are shown in Table I. The comparative Ct method was
used to calculate the relative changes in gene expression.
 | Table IPrimers for real-time RT-PCR. |
Table I
Primers for real-time RT-PCR.
| Gene | Sense | Antisense |
|---|
| GAPDH |
5′-GAAATCCCATCACCATCTTCCAGG-3′ |
5′-GAGCCCCAGCCTTCTCCATG-3′ |
| CCL5 |
5′-CTTGACCTGTGGACGACTGC-3′ |
5′-ATCCAGTGAGAAAAGCCCGT-3′ |
| MIF |
5′-CCGGACAGGGTCTACATCAA-3′ |
5′-GCGAAGGTGGAGTTGTTCCA-3′ |
| MIP-1β |
5′-AGCACCAATGGGCTCAGAC-3′ |
5′-TCACTGGGATCAGCACAGAC-3′ |
| MCP-3 |
5′-TGCTCAGCCAGTTGGGATTA-3′ |
5′-GTGGCTACTGGTGGTCCTTC-3′ |
| IL-6 |
5′-ACCCCCAATAAATATAGGACTGGA-3′ |
5′-AAGGCGCTTGTGGAGAAGG-3′ |
| IL-8 |
5′-TGTGAAGGTGCAGTTTTGCCA-3′ |
5′-ACCCAGTTTTCCTTGGGGTC-3′ |
| OPG |
5′-CATGTTCGTGGCCCTCCTG-3′ |
5′-GGATCCATCTGCGCTCTGAA-3′ |
| STAT3 |
5′-ACCTGCAGCAA-TACCATTGAC-3′ |
5′-AAGGTGAGGGACTCAAACTGC-3′ |
Colony formation assay
Chemosensitivity was also determined by a standard
colony formation assay. Briefly, cells seeded in 6-well plates were
treated with or without continuous CCL5 (100 ng/ml) at indicated
doses and then exposed to DDP (50 μmol/l). Plates were incubated at
37°C for 14 days then stained with crystal violet and counted. Each
assay was performed in triplicate.
Paraffin section immunohistochemistry and
immunofluorescence
Immunohistochemistry and immunofluoresence staining
were done as previously described (23). The results for CCL5 staining were
scored on the basis of the cell cytoplasm staining (score 0, no
cytoplasm staining; score 1, weak staining; score 2, moderate
staining; score 3, strong staining). For further statistical
analysis, scores 0 and 1 were categorized as low expression, and
scores 2 and 3 were categorized as high expression as previously
described.
In vivo xenograft studies
Twelve female nude BALB/c mice (Beijing HFK
Bioscience Co., Ltd., Beijing, China) were subcutaneously injected
5×06 C13* cells resuspended in 100 μl phosphate-buffered
saline into the left flank and 5×106 C13* cells mixed
with 2.5×106 CAF cells injected into the right flank.
Mice were housed under specific pathogen-free conditions and all
animal experiments were carried out in accordance with the Guide
for the Care and Use of Laboratory Animals of Tongji Hospital in
Wuhan, China. When tumors reached a mean size of 50 mm3,
the mice were randomly assigned into two groups with 6 mice per
group. The experiment group was treated with DDP 5 mg/kg
intraperitoneally once a week for four weeks. The control group
mice were injected with sterile saline. Tumor volumes were
calculated as length x width2/2. The tumors were weighed
at sacrifice following cervical dislocation under anesthesia.
Statistical analysis
All data are expressed as mean ± SD. The means were
calculated at least from three independent experiments. Statistical
significance of differences was analyzed by two-tailed Student's
t-test or ANOVA. P-value <0.05 was considered statistically
significant. The statistical analyses were done using the SPSS 17.0
(SPSS Inc., Chicago, IL, USA).
Results
DDP-induced cytokine secretion derived
from CAFs promotes ovarian cancer cell resistance to DDP in
vitro
A previous study indicated that chemotherapy-induced
cytokine or chemokine secretion from the tumor microenvironment
promoted prostate cancer therapy resistance (2). We first obtained primary human CAFs
isolated from patients diagnosed with high-grade serous ovarian
cancer undergoing initial cytoreductive surgery. Morphological and
immunocytochemical evaluation showed that CAFs displayed no
cytokeratin 8 staining, but strongly expressed vimentin and α-SMA,
the makers of mesenchymal cells (Fig.
1A). To verify whether DDP contributes to the cytokine
secretion of the tumor microenvironment while killing tumor cells,
which results in the cisplatin resistance of the ovarian cancer, we
administered DDP to C13* and SKOV3 cells at the proper
concentration for 48 h in CM* or control conditional medium, and
CCK8 was used to detect C13* or SKOV3 cell viability. Ovarian
cancer cells were more resistant to DDP in CM* than control medium
(Fig. 1B and C). The apoptosis
rates of C13* or SKOV3 cells were also calculated by flow
cytometric analysis (Fig. 1D and
E). Western blotting assay indicated that the anti-apoptosis
protein Bcl-2 was increased and that the apoptosis proteins
cleaved-PARP and cleaved caspase-3 were markedly decreased. MRP1/2
(multidrug-resistance proteins 1/2) is a major protein involved in
MDR (multidrug resistance) (24–26).
MDR has a profound effect on cancer chemotherapy. In contrast, MRP1
and MRP2 were significantly increased compared with control
(Fig. 1F and G).
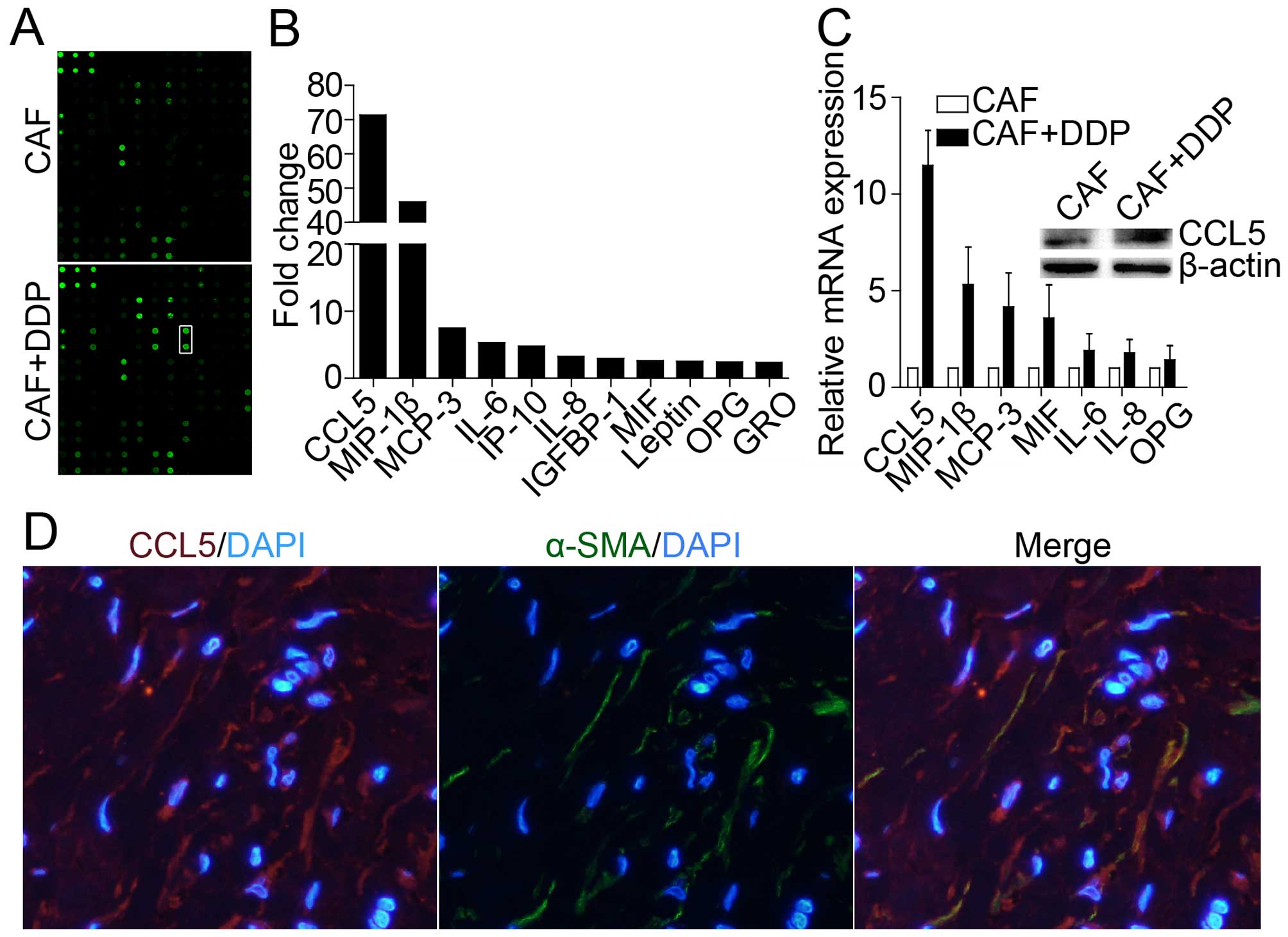
CCL5 is strongly secreted from CAFs after
cisplatin treatment
To determine which factors influence acquired
cisplatin resistance after cisplatin treatment, cytokine/chemokine
assay was performed according to the manufacturer's protocol. As
shown in Fig. 2A and B, a series
of factors were secreted and increased in CM* compared with the
control medium. Many of these factors were associated with tumor
cell malignant phenotype, including chemotherapy resistance and
tumor progression, migration and invasion (27–29).
CCL5 was significantly increased up to ~72-fold compared with the
control medium. We also detected these cytokines with qRT-PCR and
found that CCL5 expression was increased up to 12-fold compared
with control (Fig. 2C). A previous
study demonstrated that CCL5 could be secreted or expressed by
tumor cells or by extracellular matrix (ECM) (4,6,7,9). We
found that CCL5 was derived from the tumor microenvironment of
CAFs, which was further confirmed through immunofluorescence double
staining co-localization, α-SMA-positive stromal cells co-expressed
CCL5, suggesting that CAF cells may produce CCL5 (Fig. 2D).
CCL5 stimulation enhanced ovarian cancer
cell resistance to cisplatin
To verify the function of CCL5 in ovarian cancer
cells, Annexin V/PI double staining assay was used to assess C13*
or SKOV3 cell apoptosis. CCL5 stimulation significantly decreased
tumor cell apoptosis rates compared with cisplatin treatment alone
(Fig. 3A and C). In addition, C13*
and SKOV3 cells were selected to determine cell viability via CCK-8
after treatment with or without CCL5 stimulation for 48 h. C13* and
SKOV3 cells were more resistant to cisplatin after CCL5 treatment
compared with C13* and SKOV3 cells not undergoing CCL5 treatment
(Fig. 3D and E). Moreover, C13*
cells treated with CCL5 (100 ng/ml) exhibited ~20% increased colony
formation compared with control after exposure to DDP (50 μmol/l)
(Fig. 3F). These results support
that CCL5 stimulation enhances cisplatin resistance.
CCL5 stimulation promotes cisplatin
resistance of ovarian cancer cells via the p-STAT3 and PI3K-Akt
signaling pathways in vitro and in vivo
Previous studies have demonstrated that
constitutively activated phosphorylation of STAT3 contributed to
the development of tumor growth, drug resistance and angiogenesis
(30). The PI3K/Akt signaling
pathway plays a vital role in the regulation of numerous cellular
functions, including tumor progression, angiogenesis, adhesion,
migration, survival and drug resistance, in many human cancers
(31) and is involved in
cisplatin-based chemotherapy resistance in epithelial ovarian
cancer (32). In addition, the Akt
signaling pathway can be activated via chemokines or cytokines,
such as CCL5 (19,33,34).
We hypothesized that these two pathways were involved in the
regulation of cisplatin resistance by CCL5. We treated C13* and
SKOV3 cells with a range of CCL5 concentrations for different
durations. Phosphorylation of STAT3 (Tyr705) and Akt (ser473) was
increased in a time- and dose-dependent manner, as indicated by
western blot assay (Fig. 4A and
B). Then, 100 ng/ml CCL5 with or without DDP was administered
for 48 h because this concentration and duration resulted in the
highest STAT3 and Akt phosphorylation. Western blot assay
demonstrated that p-STAT3 and p-Akt expression was strongly
enhanced when cells were treated with DDP combined with CCL5
compared with that with DDP alone (Fig. 4C and D). Expression of the
anti-apoptosis protein Bcl-2 was increased, and expression of the
apoptosis proteins cleaved PARP and cleaved caspase-3 was decreased
(Fig. 4C and D). We next examined
the effect of anti-CCL5 antibody on the apoptosis rate of C13* and
SKOV3 cells. Flow cytometry indicated that the apoptosis rate was
increased via concomitant administration of anti-CCL5
neutralization antibody (Fig. 4E and
F), and p-STAT3 and p-Akt levels were decreased, as indicated
by western blot assay (data not shown). To further confirm whether
the STAT3 and Akt signaling pathways are involved in the regulation
of cisplatin resistance via CCL5, small interfering RNA for STAT3
and small molecular inhibitor LY294002 for inhibiting Akt were
used. CCL5-induced anti-apoptosis was abolished by STAT3 siRNA and
the PI3K-Akt inhibitor LY294002, as indicated using flow cytometry
Annexin V/PI (Fig. 4G and H). In
contrast, CCL5-induced phosphorylation levels of STAT3 and Akt were
decreased by STAT3 siRNA and LY294002 treatment (data not shown).
These results suggest that CCL5 stimulation enhanced cisplatin
resistance via the STAT3 and PI3K/Akt signaling pathways.
Expression and clinicopathological
characteristics of CCL5 in ovarian cancer patients
Previous studies have demonstrated that high tissue
and plasma CCL5 levels are correlated with advanced breast cancer
and could be used as a prognostic indicator for breast, cervical
and gastric cancers (6,35,36).
Serum CCL5 may be useful in differentiating benign ovarian tumors
from malignancy (37). We examined
ovarian cancer tissue excised from a patient prior to chemotherapy
and relapsed tissues after four courses of standard chemotherapy,
CCL5 expression was significantly increased after exposure to
chemotherapy, as demonstrated by IHC (Fig. 5A). Moreover, we assessed CCL5
expression in 62 serous ovarian cancer patient tissue sections
using IHC. CCL5 was strongly expressed and secreted both by tumor
cells and the tumor stromal cells. Clinic data demonstrated that
platinum-resistant patients had higher CCL5 expression than
platinum-sensitive patients (Fig. 5B
and C) (P<0.0001). Moreover, CCL5 expression was also
positively associated with cancer stage (Table II) (P<0.0001). No significant
associations were observed between CCL5 expression and clinical
variables, such as age, differentiation and lymph node metastasis.
These data suggest that CCL5 is significantly associated with
platinum-based chemotherapy response.
 | Table IICorrelation of CCL5 expression with
clinicopathological characteristics of late-stage ovarian cancer
patients (N=62). |
Table II
Correlation of CCL5 expression with
clinicopathological characteristics of late-stage ovarian cancer
patients (N=62).
| Variables | N | CCL5 |
|---|
|
|---|
| Low (n=24) n
(%) | High (n=38) n
(%) | P-value |
|---|
| Age (years) median
(range) | 52 (38–72) | | | |
|
Differentiation |
| Well | 3 | 2 (8.3) | 1 (2.6) | 0.568 |
| Moderate | 20 | 8 (33.3) | 12 (31.6) | |
| Poor | 39 | 14 (58.4) | 25 (65.8) | |
| Lymph node
metastasis |
| Positive | 15 | 4 (16.7) | 11 (28.9) | 0.366 |
| Negative | 47 | 20 (83.3) | 27 (71.1) | |
| FIGO stage |
| IIIa–IIIc | 51 | 21 (87.5) | 30 (78.9) | <0.0001a |
| IV | 11 | 3 (12.5) | 8 (21.1) | |
| Chemotherapy |
| Sensitive | 37 | 21 (87.5) | 16 (42.1) | <0.0001a |
| Resistant | 25 | 3 (12.5) | 22 (57.9) | |
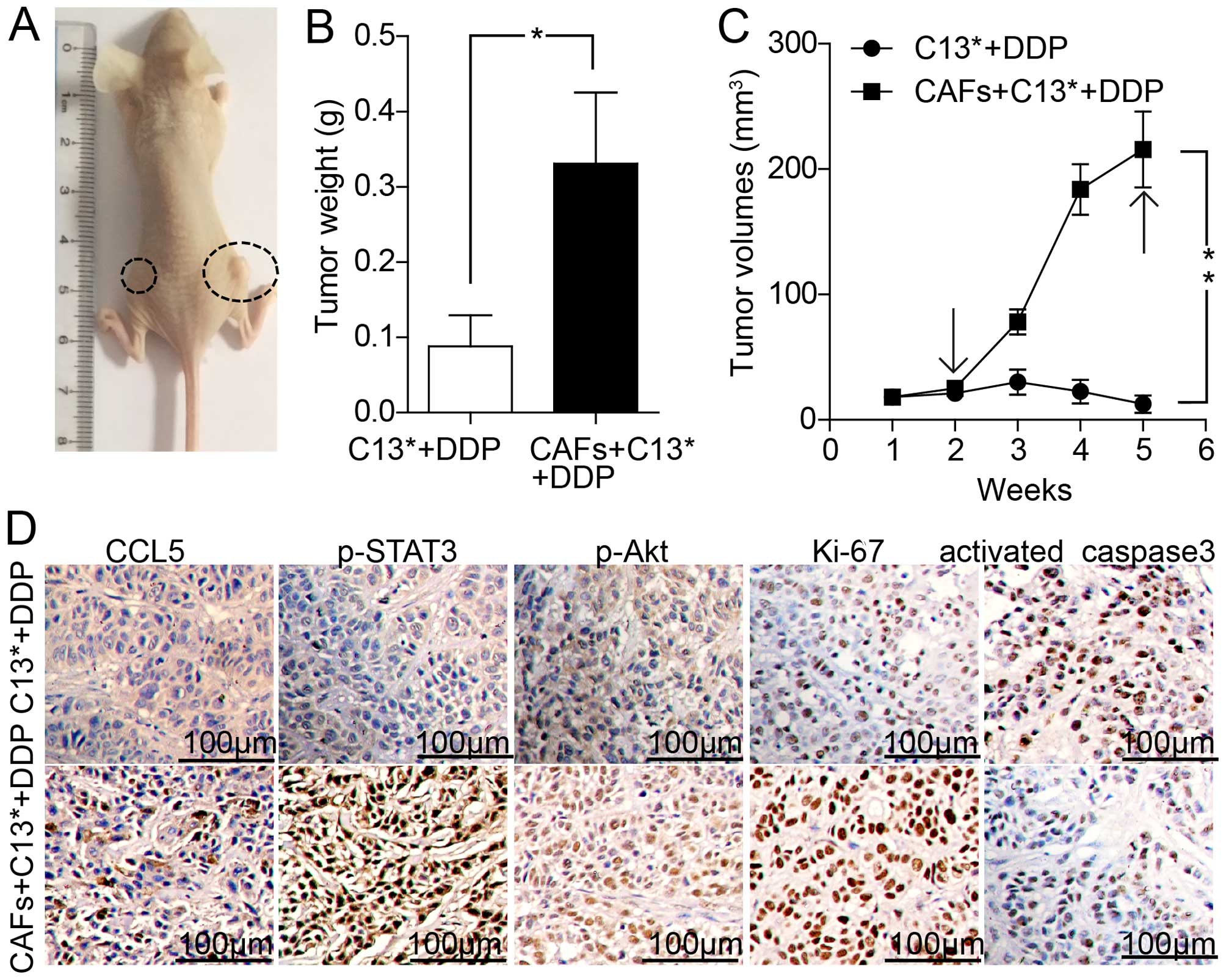
CCL5 derived from CAFs promotes ovarian
cancer cell resistance to DDP in vivo
To further investigate the above effects, we
injected the human ovarian cancer C13* cells and primary human
ovarian cancer-associated fibroblasts (CAFs) into athymic BALB/c
nude mice. The tumor weight and tumor growth were assessed after
DDP treatment for four weeks. As shown in Fig. 6A–C, the tumor weight and tumor
volume of C13* cells with CAFs in the right flank increased rapidly
compared with C13* cells alone in the left flank. IHC assay
indicated that the right tumors displayed an increased
proliferation percentage of Ki-67 positive tumor cells and
decreased apoptosis rates of activated caspase-3 positive tumor
cells. Tumors in the right flank exhibited increased CCL5
expression compared with left flank tumors. Moreover, expression of
p-STAT3, and p-Akt were strongly increased in the group injected
with C13* mixed with CAFs compared with the group injected with
C13* alone after DDP treatment for four weeks (Fig. 6D). Taken together, these results
demonstrated that CCL5 derived from CAFs promoted not only ovarian
cancer cell resistance to DDP in vitro, but also ovarian
cancer cell proliferation, growth and resistance to DDP in
vivo.
Discussion
Cisplatin-based treatment is the first-line
chemotherapy for ovarian cancer patients after tumor debulking
surgery. However, acquired cisplatin resistance commonly results in
treatment failure and high incidence of tumor relapse and
mortality. Therefore, better understanding of the mechanisms
involved in the regulation of cisplatin resistance is crucial for
improving ovarian cancer treatment. Many studies have focused on
cell-autonomous mechanisms of cisplatin resistance. In contrast, we
propose that cisplatin-induced CCL5 secretion derived from the
tumor microenvironment, whose dominant components are CAFs, confers
acquired resistance to cisplatin. Recent studies have demonstrated
that cells in the tumor microenvironment could modulate the
response of cancer cells to chemotherapy through the production of
secreted factors. Sun et al (2) found that treatment-induced damage to
the tumor microenvironment promoted prostate cancer therapy
resistance through WNT16B. Nuclear factor-κB (NF-κB) is a key
component in mediating WNT16B upregulation upon DNA damage caused
by chemotherapy (38). Bruchard
et al (39) argued that
chemotherapy-triggered cathepsin B released in myeloid-derived
suppressor cells activates the Nlrp3 inflammasome and promotes
tumor growth, indicating a new mechanism of chemotherapy
resistance. Two other studies have shown that paracrine signaling
from tumor microenvironment cells can affect cancer cell drug
sensitivity (40,41).
In the tumor microenvironment, CAFs not only play
crucial roles in tumor growth, progression, and metastasis, but are
also involved in drug resistance. Here, we investigated the role of
cisplatin-induced CCL5 secretion from CAFs in ovarian cancer cell
resistance to cisplatin. CCL5, also known as RANTES, is a member of
the CC-chemokine family and plays a critical role in tumor
progression and prognosis (3). A
previous study demonstrated that CCL5 could be used as a prognostic
indicator for breast and cervical cancer (6). High plasma CCL5 levels were
positively associated with advanced breast cancer, and tumor-cell
derived CCL5 might promote breast cancer progression and metastasis
(6). To the best of our knowledge,
this is the first study to investigate whether CCL5 promotes
ovarian cancer cell resistance to cisplatin in vitro and
in vivo. Human recombination CCL5 neutralization antibody
was used to verify whether CCL5 substantially promoted cisplatin
resistance, and acquired cisplatin resistance was abolished in
vitro. These results partly explain CCL5-induced cisplatin
resistance. We focused on the STAT3 signaling pathway, a member of
STAT transcription factor family, given its significant role in
tumorigenesis and regulation of chemotherapy resistance in cancer
cells (17,18,42).
Previous studies have demonstrated that STAT3 and CCL5 contribute
to the maintenance of tamoxifen resistance in breast cancer, and
STAT3 phosphorylation is constitutively activated and retained via
CCL5 stimulation (3,9,43).
Based on these reports, we examined the signaling pathways
activated by CCL5 in vitro and in vivo and found that
STAT3 phosphorylation was enhanced in a time- and dose-dependent
manner. We also found that AKT phosphorylation levels, which play a
vital role in tumor malignancy phenotype, were significantly
activated by CCL5 stimulation, consistent with previous reports
(19,33,34).
Next, we used small molecular interfering RNA to knock down STAT3
gene expression or the small molecular inhibitor LY294002 to
inhibit the PI3K-Akt signaling pathway. Acquired cisplatin
resistance was significantly reversed via CCL5 stimulation, similar
to administration of the CCL5-neutralizing antibody. Expression
levels of the anti-apoptotic gene Bcl-2 appeared to be positively
associated with p-STAT3 levels, in agreement with a previous study,
indicating that STAT3 activation plays a critical role in
inhibiting apoptosis and promoting proliferation by regulating the
Bcl-2 family (44–46).
Previous studies have demonstrated that tumor cells
are the main driver of lymphangiogenesis and angiogenesis promotion
by secreting specific factors (47,48).
Here, we focused on elements in the tumor microenvironments and
preliminarily explored the mechanisms of cisplatin-induced CCL5
secretion derived from CAFs, which results in acquired resistance
to cisplatin. The present study has several disadvantages. We
confirmed cisplatin-induced CCL5 secretion may promote cisplatin
resistance in ovarian cancer in vivo and in vitro but
did not confirm whether other chemotherapy drugs or small
molecular-targeted therapies have similar effects. Thus, further
research in other tumor types investigating proper combination
therapies in vivo should be conducted.
This study found, for the first time, that
cisplatin-induced CCL5 secretion derived from CAFs was able to
promote tumor progression and drug-resistance through the p-STAT3
and p-Akt signal pathways. Taken together, our results support
several conclusions. First, we observed that cisplatin resistance
occurs via the tumor microenvironment, not only the tumor cells,
which more closely reflects the real body environment, providing a
novel foundation for the mechanism of cisplatin resistance. Second,
our results suggested that components of the tumor microenvironment
are important contributors to acquired cisplatin resistance. Third,
clinical data confirmed that CCL5 expression is strongly correlated
with chemotherapy sensitivity, which may provide a reference for
clinical drug use. Lastly, we demonstrated that combining
approaches that target constituents of the tumor microenvironment
might enhance conventional cancer chemotherapy. Therefore, STAT3
knockdown, small molecule inhibitors, or CCL5 blockade using
neutralizing antibody in combination with conventional cisplatin
treatment may provide a new therapeutic approach for ovarian cancer
patients with acquired cisplatin resistance.
Acknowledgements
The present study was supported by the National
Basic Research Program of China (973 Program, 2015CB553903); the
Chinese 863 Program (2012AA02A507); the National Nature and Science
Foundation of China (81230038; 81272859; 81025011; 81090414;
81000979; 81101962).
References
|
1
|
Bast RC Jr: Molecular approaches to
personalizing management of ovarian cancer. Ann Oncol. 2(Suppl 8):
viii5–viii1. 2011.
|
|
2
|
Sun Y, Campisi J, Higano C, Beer TM,
Porter P, Coleman I, True L and Nelson PS: Treatment-induced damage
to the tumor microenvironment promotes prostate cancer therapy
resistance through WNT16B. Nat Med. 18:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yi EH, Lee CS, Lee JK, Lee YJ, Shin MK,
Cho CH, Kang KW, Lee JW, Han W, Noh DY, et al: STAT3-RANTES
autocrine signaling is essential for tamoxifen resistance in human
breast cancer cells. Mol Cancer Res. 11:31–42. 2013. View Article : Google Scholar
|
|
4
|
Velasco-Velázquez M, Jiao X, De La Fuente
M, Pestell TG, Ertel A, Lisanti MP and Pestell RG: CCR5 antagonist
blocks metastasis of basal breast cancer cells. Cancer Res.
72:3839–3850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luboshits G, Shina S, Kaplan O, Engelberg
S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I and Ben-Baruch
A: Elevated expression of the CC chemokine regulated on activation,
normal T cell expressed and secreted (RANTES) in advanced breast
carcinoma. Cancer Res. 59:4681–4687. 1999.PubMed/NCBI
|
|
6
|
Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki
Y and Abe A: Correlation of tissue and plasma RANTES levels with
disease course in patients with breast or cervical cancer. Clin
Cancer Res. 7:285–289. 2001.PubMed/NCBI
|
|
7
|
Zhang Y, Yao F, Yao X, Yi C, Tan C, Wei L
and Sun S: Role of CCL5 in invasion, proliferation and proportion
of CD44+/ CD24− phenotype of MCF-7 cells and
correlation of CCL5 and CCR5 expression with breast cancer
progression. Oncol Rep. 21:1113–1121. 2009.PubMed/NCBI
|
|
8
|
Jiao X, Katiyar S, Willmarth NE, Liu M, Ma
X, Flomenberg N, Lisanti MP and Pestell RG: c-Jun induces mammary
epithelial cellular invasion and breast cancer stem cell expansion.
J Biol Chem. 285:8218–8226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diaz N, Minton S, Cox C, Bowman T, Gritsko
T, Garcia R, Eweis I, Wloch M, Livingston S, Seijo E, et al:
Activation of stat3 in primary tumors from high-risk breast cancer
patients is associated with elevated levels of activated SRC and
survivin expression. Clin Cancer Res. 12:20–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnston PA and Grandis JR: STAT3
signaling: Anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: A
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukada T, Hibi M, Yamanaka Y,
Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K and Hirano
T: Two signals are necessary for cell proliferation induced by a
cytokine receptor gp130: Involvement of STAT3 in anti-apoptosis.
Immunity. 5:449–460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kube D, Holtick U, Vockerodt M, Ahmadi T,
Haier B, Behrmann I, Heinrich PC, Diehl V and Tesch H: STAT3 is
constitutively activated in Hodgkin cell lines. Blood. 98:762–770.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sheng WJ, Jiang H, Wu DL and Zheng JH:
Early responses of the STAT3 pathway to platinum drugs are
associated with cisplatin resistance in epithelial ovarian cancer.
Braz J Med Biol Res. 46:650–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han Z, Feng J, Hong Z, Chen L, Li W, Liao
S, Wang X, Ji T, Wang S, Ma D, et al: Silencing of the STAT3
signaling pathway reverses the inherent and induced chemoresistance
of human ovarian cancer cells. Biochem Biophys Res Commun.
435:188–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang CY, Fong YC, Lee CY, Chen MY, Tsai
HC, Hsu HC and Tang CH: CCL5 increases lung cancer migration via
PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 77:794–803.
2009. View Article : Google Scholar
|
|
20
|
Asselin E, Mills GB and Tsang BK: XIAP
regulates Akt activity and caspase-3-dependent cleavage during
cisplatin-induced apoptosis in human ovarian epithelial cancer
cells. Cancer Res. 61:1862–1868. 2001.PubMed/NCBI
|
|
21
|
Navab R, Strumpf D, Bandarchi B, Zhu CQ,
Pintilie M, Ramnarine VR, Ibrahimov E, Radulovich N, Leung L,
Barczyk M, et al: Prognostic gene-expression signature of
carcinoma-associated fibroblasts in non-small cell lung cancer.
Proc Natl Acad Sci USA. 108:7160–7165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weng D, Song X, Xing H, Ma X, Xia X, Weng
Y, Zhou J, Xu G, Meng L, Zhu T, et al: Implication of the
Akt2/survivin pathway as a critical target in paclitaxel treatment
in human ovarian cancer cells. Cancer Lett. 273:257–265. 2009.
View Article : Google Scholar
|
|
23
|
Sun C, Li N, Yang Z, Zhou B, He Y, Weng D,
Fang Y, Wu P, Chen P, Yang X, et al: miR-9 regulation of BRCA1 and
ovarian cancer sensitivity to cisplatin and PARP inhibition. J Natl
Cancer Inst. 105:1750–1758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cole SP, Bhardwaj G, Gerlach JH, Mackie
JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM and
Deeley RG: Overexpression of a transporter gene in a
multidrug-resistant human lung cancer cell line. Science.
258:1650–1654. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kruh GD and Belinsky MG: The MRP family of
drug efflux pumps. Oncogene. 22:7537–7552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seike T, Fujita K, Yamakawa Y, Kido MA,
Takiguchi S, Teramoto N, Iguchi H and Noda M: Interaction between
lung cancer cells and astrocytes via specific inflammatory
cytokines in the microenvironment of brain metastasis. Clin Exp
Metastasis. 28:13–25. 2011. View Article : Google Scholar :
|
|
28
|
Wang D, Yamamoto S, Hijiya N, Benveniste
EN and Gladson CL: Transcriptional regulation of the human
osteopontin promoter: Functional analysis and DNA-protein
interactions. Oncogene. 19:5801–5809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rath BH, Fair JM, Jamal M, Camphausen K
and Tofilon PJ: Astrocytes enhance the invasion potential of
glioblastoma stem-like cells. PLoS One. 8:e547522013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu L, Nam S, Tian Y, Yang F, Wu J, Wang
Y, Scuto A, Polychronopoulos P, Magiatis P, Skaltsounis L, et al:
6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces
apoptosis of human melanoma cells. Cancer Res. 71:3972–3979. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang HY, Zhang PN and Sun H: Aberration
of the PI3K/AKT/ mTOR signaling in epithelial ovarian cancer and
its implication in cisplatin-based chemotherapy. Eur J Obstet
Gynecol Reprod Biol. 146:81–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang CH, Yamamoto A, Lin YT, Fong YC and
Tan TW: Involvement of matrix metalloproteinase-3 in CCL5/CCR5
pathway of chondrosarcomas metastasis. Biochem Pharmacol.
79:209–217. 2010. View Article : Google Scholar
|
|
34
|
Wang SW, Wu HH, Liu SC, Wang PC, Ou WC,
Chou WY, Shen YS and Tang CH: CCL5 and CCR5 interaction promotes
cell motility in human osteosarcoma. PLoS One. 7:e351012012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eissa SA, Zaki SA, El-Maghraby SM and
Kadry DY: Importance of serum IL-18 and RANTES as markers for
breast carcinoma progression. J Egypt Natl Canc Inst. 17:51–55.
2005.PubMed/NCBI
|
|
36
|
Kim HK, Song KS, Park YS, Kang YH, Lee YJ,
Lee KR, Kim HK, Ryu KW, Bae JM and Kim S: Elevated levels of
circulating platelet microparticles, VEGF, IL-6 and RANTES in
patients with gastric cancer: possible role of a metastasis
predictor. Eur J Cancer. 39:184–191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsukishiro S, Suzumori N, Nishikawa H,
Arakawa A and Suzumori K: Elevated serum RANTES levels in patients
with ovarian cancer correlate with the extent of the disorder.
Gynecol Oncol. 102:542–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ostman A: The tumor microenvironment
controls drug sensitivity. Nat Med. 18:1332–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bruchard M, Mignot G, Derangère V, Chalmin
F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL,
et al: Chemotherapy-triggered cathepsin B release in
myeloid-derived suppressor cells activates the Nlrp3 inflammasome
and promotes tumor growth. Nat Med. 19:57–64. 2013. View Article : Google Scholar
|
|
40
|
Wilson TR, Fridlyand J, Yan Y, Penuel E,
Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al:
Widespread potential for growth-factor-driven resistance to
anticancer kinase inhibitors. Nature. 487:505–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Straussman R, Morikawa T, Shee K,
Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J,
Frederick DT, et al: Tumour micro-environment elicits innate
resistance to RAF inhibitors through HGF secretion. Nature.
487:500–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Benabbou N, Mirshahi P, Cadillon M, Soria
J, Therwath A and Mirshahi M: Hospicells promote upregulation of
the ATP-binding cassette genes by insulin-like growth factor-I via
the JAK2/STAT3 signaling pathway in an ovarian cancer cell line.
Int J Oncol. 43:685–694. 2013.PubMed/NCBI
|
|
43
|
Kim JE, Kim HS, Shin YJ, Lee CS, Won C,
Lee SA, Lee JW, Kim Y, Kang JS, Ye SK, et al: LYR71, a derivative
of trimeric resveratrol, inhibits tumorigenesis by blocking
STAT3-mediated matrix metalloproteinase 9 expression. Exp Mol Med.
40:514–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ji T, Gong D, Han Z, Wei X, Yan Y, Ye F,
Ding W, Wang J, Xia X, Li F, et al: Abrogation of constitutive
Stat3 activity circumvents cisplatin resistant ovarian cancer.
Cancer Lett. 341:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aoki Y, Feldman GM and Tosato G:
Inhibition of STAT3 signaling induces apoptosis and decreases
survivin expression in primary effusion lymphoma. Blood.
101:1535–1542. 2003. View Article : Google Scholar
|
|
46
|
Real PJ, Sierra A, De Juan A, Segovia JC,
Lopez-Vega JM and Fernandez-Luna JL: Resistance to chemotherapy via
Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer
cells. Oncogene. 21:7611–7618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cao Y: Opinion: Emerging mechanisms of
tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer.
5:735–743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rasila KK, Burger RA, Smith H, Lee FC and
Verschraegen C: Angiogenesis in gynecological oncology-mechanism of
tumor progression and therapeutic targets. Int J Gynecol Cancer.
15:710–726. 2005. View Article : Google Scholar : PubMed/NCBI
|