Introduction
Gastric cancer is one of the most common malignant
tumors worldwide. In less developed countries, stomach cancers are
also leading causes of cancer death, which is generally about twice
as high in men as in women (1).
The incidence and mortality rates vary widely across countries, the
highest in high-income Asia Pacific, east Asia, and Andean Latin
America (2), which was related to
dietary patterns, food storage, and the availability of fresh
produce.
Chemotherapy is widely used in cancer treatment, it
shows better therapy effect, but toxic and side effects cause
serious harm to cancer patients. Recently, research interest has
turned to the traditional medicine, and investigations of new
anticancer drugs with low toxicity. Rabdosia rubescens, a
medical plant, has been used to treat cancer in China for a long
time (3), and has been reported to
show better effects in the treatment of urinary bladder carcinoma
(4), esophageal carcinoma
(5,6), prostate cancer (7), and oridonin is one of the most
important antitumor active ingredient of Rabdosia rubescens
(8,9).
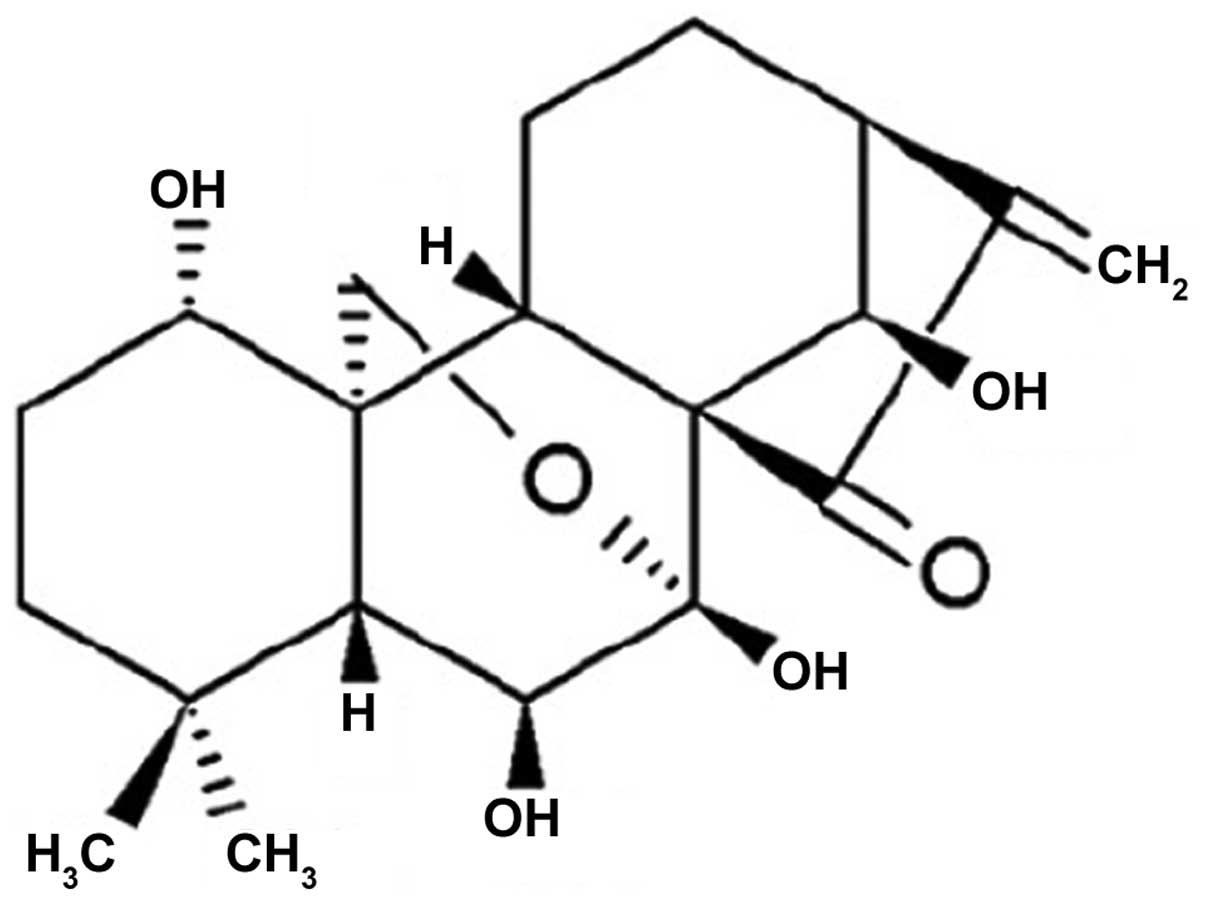
Oridonin, molecular formula
C20H28O6 (Fig. 1), is a diterpenoid compound
(10). Previous studies have shown
that oridonin has antitumor activities in vivo and in
vitro (11–13), and oridonin inhibited proliferation
of cancer cells by inducing autophagic pathways (14–18),
arresting the cell cycle on G0/G1 phase
(19) or G2/M phase
(20–24), inducing apoptosis of human
laryngeal cancer cells (25),
esophageal cancer (26),
colorectal carcinoma (27),
pancreatic cancer (28),
hepatocellular carcinoma (29,30).
However, few reports exist on oridonin-induced apoptosis on gastric
cancer. Therefore, this study explored apoptosis and related
protein expression induced by oridonin on human gastric cancer
SGC-7901 cells.
Materials and methods
Chemicals and other reagents
Oridonin (>98%) was purchased from National
Institutes for Food and Drug Control (Beijing, China). Doxorubicin
was obtained from Pharmacia Italia S.p.A., Gaggiano, Italy.
Hydroxycamptothecin (HCPT) was provided by Shanghai Longxiang
Biological Medicine Development Co. Ltd. (Shanghai, China).
Dimethyl sulfoxide (DMSO), trypsin, Tris, glycine, acrylamide,
methylene diacrylamide and Tween-20 were purchased from
Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 cell culture medium
was purchased from Gibco (Grand Island, NY, USA). Fetal calf serum
(FCS) was purchased from Sijiqing Hangzhou Bio Engineering Co.,
Ltd. (Hangzhou, China). Hoechst33342, Annexin V-FITC apoptosis
detection kit, DAB Horseradish Peroxidase Color Development kit
were obtained from the Beyotime Institute of Biotechnology
(Jiangsu, China). Antibodies for cytochrome c, Bcl-2, Bax,
caspase-3, cleaved-caspase-3, β-actin, and the secondary antibodies
were purchased from ZSGB-BIO (Beijing, China). All other chemicals
and solvents used were the highest purity grade.
Cell line and culture conditions
The human gastric cancinoma SGC-7901 cell line was
obtained from American Type Culture Collection (Manassas, VA, USA).
The cells were cultured in RPMI-1640 supplement with 10% (v/v)
fetal bovine serum (FBS) and antibiotics (100 IU/ml of penicillin
and 100 μg/ml of streptomycin) at 37°C in a humidified atmosphere
containing 5% CO2.
Cell viability and cytotoxicity
The cultured cells at the exponential growth phase
were harvested from the culture flasks by trypsin and then
re-suspended in fresh RPMI-1640 medium. The cell suspensions were
dispensed into a 96-well microplate at 100 μl/well and placed in an
incubator with 5% CO2 at 37°C. After 24 h, 100 μl
various concentrations of oridonin were added and incubated for 72
h. Then the medium was discarded and 100 μl of MTT stock solution
(1 mg/ml) was added. After incubation for 4 h, DMSO (150 μl) was
added to each well to solubilize the water-insoluble purple
formazan crystals. The amount of MTT-formazan is directly
proportional to the number of living cells and was determined by
measuring the optical density (OD) at 570 nm using microplate
reader (model 680; Bio-Rad Laboratories, Hercules, CA, USA). The
percentage of cytotoxic activity compared to the untreated cells
was determined, and the IC50 was calculated by the Logit
method.
Cell nuclear morphology observation
(Hoechst 33342)
Morphology of apoptotic cell was observed by Hoechst
33342 staining assay. The cells were washed in phosphate-buffered
saline (PBS) and fixed in formaldehyde solution (4%, w/v) for 30
min. Then the fixed cells were stained with 10 mg/ml Hoechst 33342
for 10 min, and nuclear morphology was observed under a
fluorescence microscopy (Leica, Wetzlar, Germany) equipped with a
digital camera.
Confocal laser scanning microscopy
assay
Qualitative experiment of apoptosis was observed by
confocal laser scanning microscopy after staining cells with the
Annexin V-FITC apoptosis detection kit (MultiSciences Biotech Co.,
Ltd., Hangzhou, China). SGC-7901 cells (1.5×105
cells/well) were placed on 6-well plates and incubated with
oridonin for 24 h. The cells were stained by Annexin V-FITC (green
fluorescence) in the dark for phosphatidylserine (PS) examination.
Then cells were stained with PI (red fluorescence) in the dark for
nucleus examination. Stained cells were visualized by confocal
laser scanning microscopy (Leica, SP2, Wetzlar, Germany) equipped
with 488 nm Argon lasers (31).
Flow cytometric analysis of
apoptosis
Early apoptosis rate were measured using the Annexin
V-FITC apoptosis detection kit (MultiSciences Biotech Co. Ltd.,
China) as described in the supplier instructions. After exposure to
oridinin (0, 20, 40 and 80 μM) for 24 h, cells were harvested by
centrifugation, washed twice with PBS, and resuspended in Binding
Buffer, 5 μl of Annexin V-FITC and 5 μl of propidium iodide (PI, 50
mg/ml) was added and incubated at room temperature in the dark. The
data acquisition and analysis were performed using MultiCycle
software flow cytometry (Beckman Coulter, XL, USA).
Total protein extraction and western blot
assay
SGC-7901 cells were treated with different
concentration of oridonin. For isolation of total protein
fractions, cells were collected, washed twice with cold PBS, and
lysed with cell lysis buffer (50 mM Tris-Cl, pH 8.0, 120 mM NaCl,
50 mM NaF, 200 μM sodium vanadate, 0.5% NP-40, 10 mM
phenylmethylsulfonyl fluoride (PMSF), 2 μg/ml aprotinin 0.2 μl, 10
μg/ml leupeptin 10 μl). The lysates were centrifuged at 12000 × g
for 10 min at 4°C, the supernatant was saved at −20°C. Protein
concentrations of cell lysates were detected by Bradford assay
(32). Total protein samples were
separated by SDS-PAGE. The separated proteins were transferred to
NC membranes. After being blocked with blocking solution (5% skim
milk in TBS, 10 mM Tris-HCl, 150 mM NaCl, pH 7.5 plus 0.1%
Tween-20) at room temperature for 2 h. Each membrane was incubated
with primary antibodies overnight at 4°C. Afterwards, the membranes
were probed with the appropriate horseradish-peroxidase conjugated
secondary antibody for 2 h at room temperature. Detection was
performed by the DAB Horseradish Peroxidase Color Development kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Bands were recorded and relative
density units of the bands were analyzed by Gel Imaging System
(Tanon, GIS-2019, Beijing, China). Densitometrical data of multiple
experiments are shown.
Statistical analysis
The data are presented as the mean ± SD. Statistical
significance was calculated using Student's t-test. P-values of ≤5%
were considered to indicate statistically significant
differences.
Results
Effect of oridonin on SGC-7901 cell
viability
In order to evaluate the effect of oridonin on
proliferation of the SGC-7901 cells, the cells were treated with
different concentrations of oridonin for 72 h, the cell viability
was quantitated by MTT assay. The results showed that oridonin
inhibited the proliferation of SGC-7901 cells, and the
IC50 was 22.74 μM. The results are shown in Table I.
 | Table IDoses inducing 50% cell growth
inhibition (IC50) of oridonin against human gastric
cancer SGC-7901 cells. |
Table I
Doses inducing 50% cell growth
inhibition (IC50) of oridonin against human gastric
cancer SGC-7901 cells.
| Groups | IC50
(μM) |
|---|
| Oridonin | 22.74 |
|
Hydroxycamptothecin | 17.46 |
Effect of oridonin on SGC-7901 cell
nuclei morphology
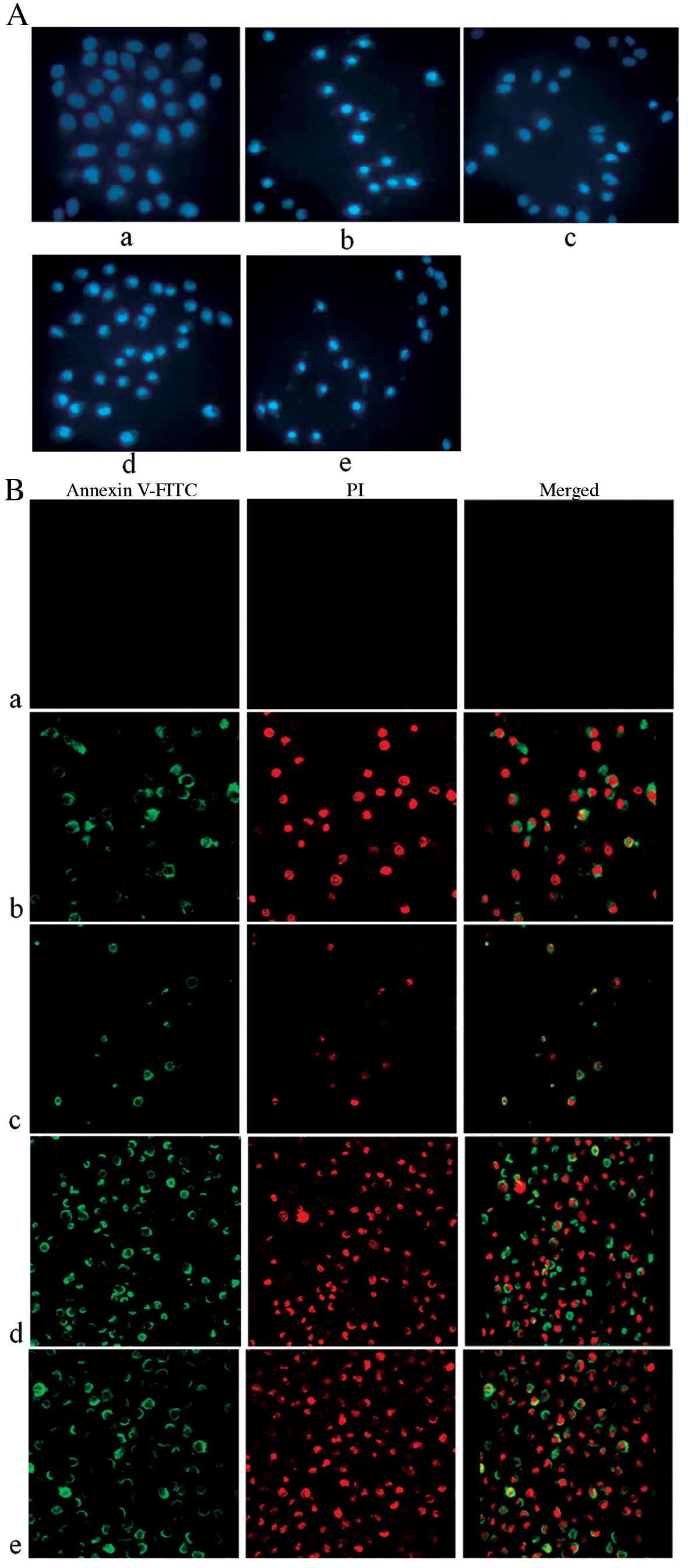
The results above can significantly demonstrate that
oridonin possessed notable antitumor activity on human gastric
cancer SGC-7901 cells. To determine whether the antitumor activity
of oridonin was due to induction of apoptosis, SGC-7901 cells were
stained with Hoechst 33342 to examine the nuclear morphological
changes. The results showed that cells of the control group had
normal nuclear morphology and the dye of Hoechst 33342 was evenly
distributed under fluorescent microscope, which indicated that the
chromatin was equivalently distributed in the nucleus. However,
after treatment with different concentrations of oridonin for 24 h,
the characteristic features of apoptosis (including marked nuclear
fragmentation, nuclear blebbing, condensation of chromatin, and
emitting brighter fluorescence) was clearly detected in the
SGC-7901 cells under the inverted fluorescence microscope (Fig. 2Ac–e). These results indicated that
oridonin induced cell apoptosis in human gastric carcinoma.
Effect of oridonin on SGC-7901 cell
membrance morphology
In order to confirm whether oridonin induced cell
apoptosis, we applied the assay of Annexin V-FITC stain for
detection of phosphatidylserine (PS), the biochemical marker of
apoptosis. PS is normally located in the inner plasma membrane,
however, in the early apoptosis the PS is transferred to its outer
surface. Annexin V-FITC combined with PS of the outer surface of
the membrance and emit green fluorescent. After treatment with
different concentrations of oridonin for 24 h, SGC-7901 cells were
stained by Annexin V-FITC (green fluorescence) and PI (red
fluorescence), and observed and photographed by laser scanning
confocal microscopy. The results showed that a large number of
cells treated with oridonin were positively stained by Annexin
V-FITC (Fig. 2Bc–e). Which showed
that oridonin was able to induce SGC-7901 cell apoptosis.
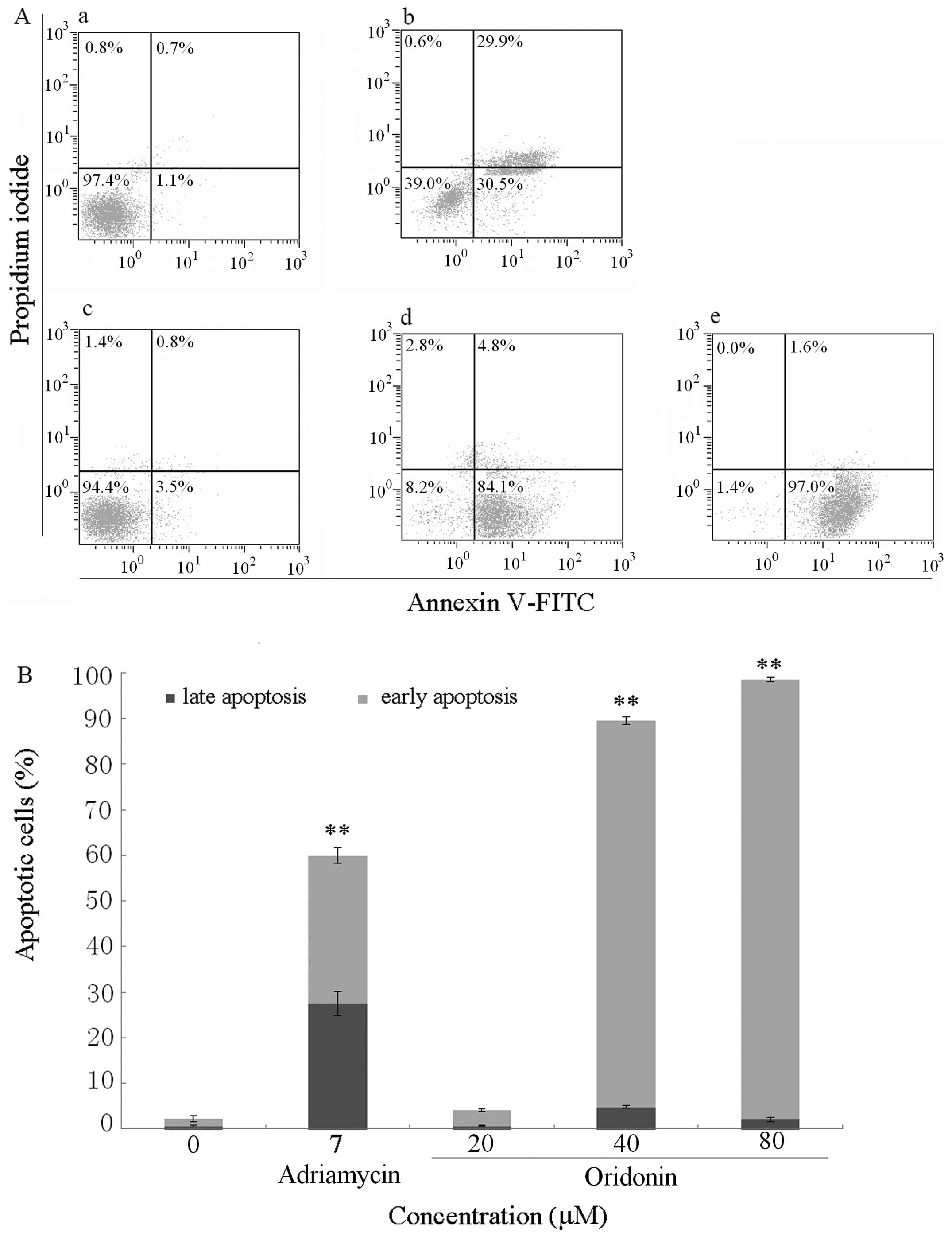
Oridonin induces early apoptosis rate of
SGC-7901 cells
To quantify the apoptotic rate of oridonin on
SGC-7901 cells, the Annexin V-FITC/PI staining and flow cytometry
was adopted. The data obtained showed that oridonin induced early
apoptosis of SGC-7901 cells in a dose-dependent manner (Fig. 3 and Table II). When the cells were treated
with 0, 20, 40, 80 μmol/l oridonin for 24 h, the average proportion
of Annexin V-staining positive cells and PI-staining negative cells
(early apoptotic cells) significantly increased from 1.53%±0.67% in
control to 3.33%±0.29, 84.80%±0.82 and 96.43%±0.51%, respectively
(Table I). Fig. 3B shows the graphic representation
of the increase in the early apoptotic cells with increase in the
dose of oridonin.
 | Table IIOridonin-induced cell apoptotic rate
on SGC-7901 cells. |
Table II
Oridonin-induced cell apoptotic rate
on SGC-7901 cells.
| Group | Dosage (μM) | Early apoptotic
rate (%) | Late apoptotic rate
(%) |
|---|
| Control | - | 1.53±0.67 | 0.70±0.20 |
| Doxorubicin | 7 | 32.33±1.68b | 27.60±2.65b |
| Oridonin | 20 | 3.33±0.29 | 0.73±0.12 |
| Oridonin | 40 | 84.80±0.82b | 4.83±0.25 |
| Oridonin | 80 | 96.43±0.51b | 2.13±0.47 |
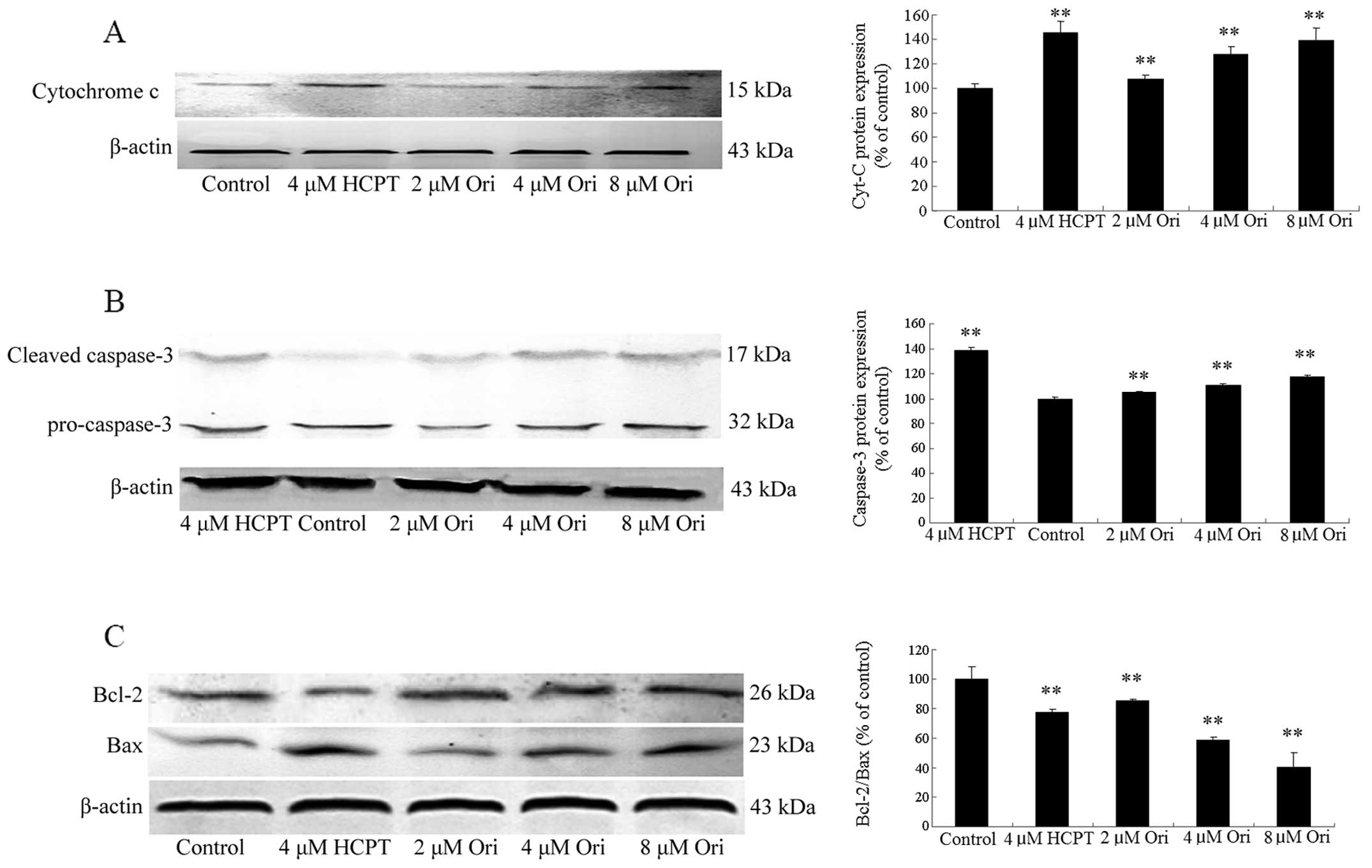
Oridonin affects apoptosis-associated
protein expression in SGC-7901 cells
Whether or not oridonin induced apoptosis in
SGC-7901 cell through the effects of apoptosis-associated protein,
western blots were adopted to examined the protein expression of
mitochondrial pathway of SGC-7901 cell treated with 2, 4, 8 μM
oridonin. The analysis results showed that all concentrations of
oridonin (2, 4, 8 μM) resulted in a significant increase of
cytochrome c (Fig. 4A) and
remarkable cleavage of caspases-3 were detected compared with the
control group (Fig. 4B).
Mitochondrial dysfunction is regulated by Bcl-2 family proteins,
thus the Bcl-2 family proteins were examined in the study. Oridonin
caused a significant reduction in Bcl-2 expression whereas the
expression of Bax was significantly increased, which led to
decrease of Bcl-2/Bax in a concentration-dependent manner (Fig. 4C). Thus, oridonin could induce
apoptosis in SGC-7901 cells with mitochondrial pathway
involved.
Discussion
Rabdosia rubescens are used in Chinese folk
medicine for treatment of esophageal cancer in Taihang Mountains
area of China for a long time. Research showed that oridonin was
one of the most important antitumor active ingredient of
Rabdosia rubescens (33–36).
Our previous studies showed that oridonin could arrest the cell
cycle in G2/M phase in human gastric cancer SGC-7901
cells (3). G2/M phase
cell cycle arrest induced by oridonin would cause cell apoptosis,
in order to confirm this hypothesis, we observed apoptotic effect
of oridonin on SGC-7901 cells and the expression of apoptosis
related protein, which has scarely been reported in SGC-7901
gastric cancer cells.
In the Hoechst 33342 assay, cells stained by
Hoechst33342 were observed by fluorescence microscopy, a classic
method to distinguish apoptotic cells, normal cells and necrotic
cells (37,38). A small amount of Hoechst 33342
could penetrate the normal cell membranes and emit equivalent dark
blue fluorescence after combination with DNA. However, lighter blue
fluorescence was emitted in the apoptotic cells, because of
membrane permeability enhancement, a large amount of Hoechst 33342
penetration, DNA breakage and function inactivation of
P-glycoprotein. The fluorescence was darker in the necrotic cells
than that in apoptotic cells because the structure of DNA of
necrotic cells is unbroken. Thus, the fluorescence of apoptotic
cells were lighter than that in the normal cells and the necrotic
cells. As shown in Fig. 2Aa, cells
of the control group had normal nuclear morphology under
fluorescent microscope after Hoechst 33342 staining, indicating
that the chromatin was equivalently distributed in the nucleus. The
test group cells marked with irregular nuclei, crescent-shaped
nuclei, condensation of chromatin and the morphological
characteristics of apoptosis, which include emitting brighter
fluorescence (Fig. 2Ac–e), were
detected after treatment with different concentrations of oridonin
for 24 h. These results indicated that oridonin is capable of
inducing apoptosis in SGC-7901 cells.
In order to further evaluate whether oridonin could
induce apoptosis in SGC-7901 cells, the cells were treated with the
stain of Annexin V-FITC/PI and detected by confocal microscopy. The
results showed that a large number of cells treated by oridonin
were positively stained by Annexin V-FITC (Fig. 2Bc–e), which showed that oridonin
could induce SGC-7901 cell apoptosis. The conclusion was consistent
with that detected by Hoechst 33342 assay.
After qualitative research of apoptosis induced by
oridonin, cells stained with Annexin V-FITC/PI were detected by
flow cytometer to quantify the early apoptotic rate induced by
oridonin. The percentage of live, early apoptotic, late apoptotic
and dead cells were calculated. The results showed that oridonin
was able to induce apoptosis of SGC-7901 cells (Fig. 3 and Table II), which was visible from the
percentage increase in mean fluorescence intensity in the early
apoptotic stages of the treated cells when compared to the
control.
It is well known that apoptosis can be regulated by
apoptotic related protein. Bcl-2 family members and caspase family
members play important roles in inducing cell apoptosis. The Bcl-2
family proteins, such as the anti-apoptotic protein bcl-2 and the
pro-apoptotic protein bax, could enhance the membrane permeability
of the mitochondria, which results in cytochrome c release from
mitochondria to the cytoplasm (39). Cytochrome c is combined with
apoptosis protease activating factor-1, recruits and cleaves
procaspase-9, and activates caspase-3, which is responsible for
apoptosis (40). In order to
examine the underlying mechanism of apoptosis of oridonin, the
respective expression of Bcl-2, Bax, cytochrome c and cleaved
caspase-3 was examined.
Based on the results from western blot analysis,
oridonin increased the protein expression of Bax, and decreased the
protein expression of Bcl-2 (Fig.
4C). The ratio of Bcl-2/Bax expression was decreased. Which led
to cytochrome c release to the cytoplasm, as shown on the results
(Fig. 4A) oridonin also increased
the expression of cytochrome c in the cytoplasm in SGC-7901 cells.
These observations suggest that oridonin induced apoptosis of
SGC-7901 cells via mitochondria-dependent pathway.
Caspase-3, one of the family members of cysteinyl
aspartate proteases, is an executioner enzyme inducing apoptosis
(41). Mitochondrial pathway
(42), death receptor-mediated
pathway (43) and endoplasmic
reticulum pathway (44) are the
major signal transduction pathways that induced apoptosis (45,46),
which ultimately induce cell apoptosis by activating caspase-3
(47). We found that oridonin
evoked caspase-3 activation, as evidenced by the appearance of 17
kDa subunits, which showed that oridonin induced SGC-7901 apoptosis
was caspase-3 dependent.
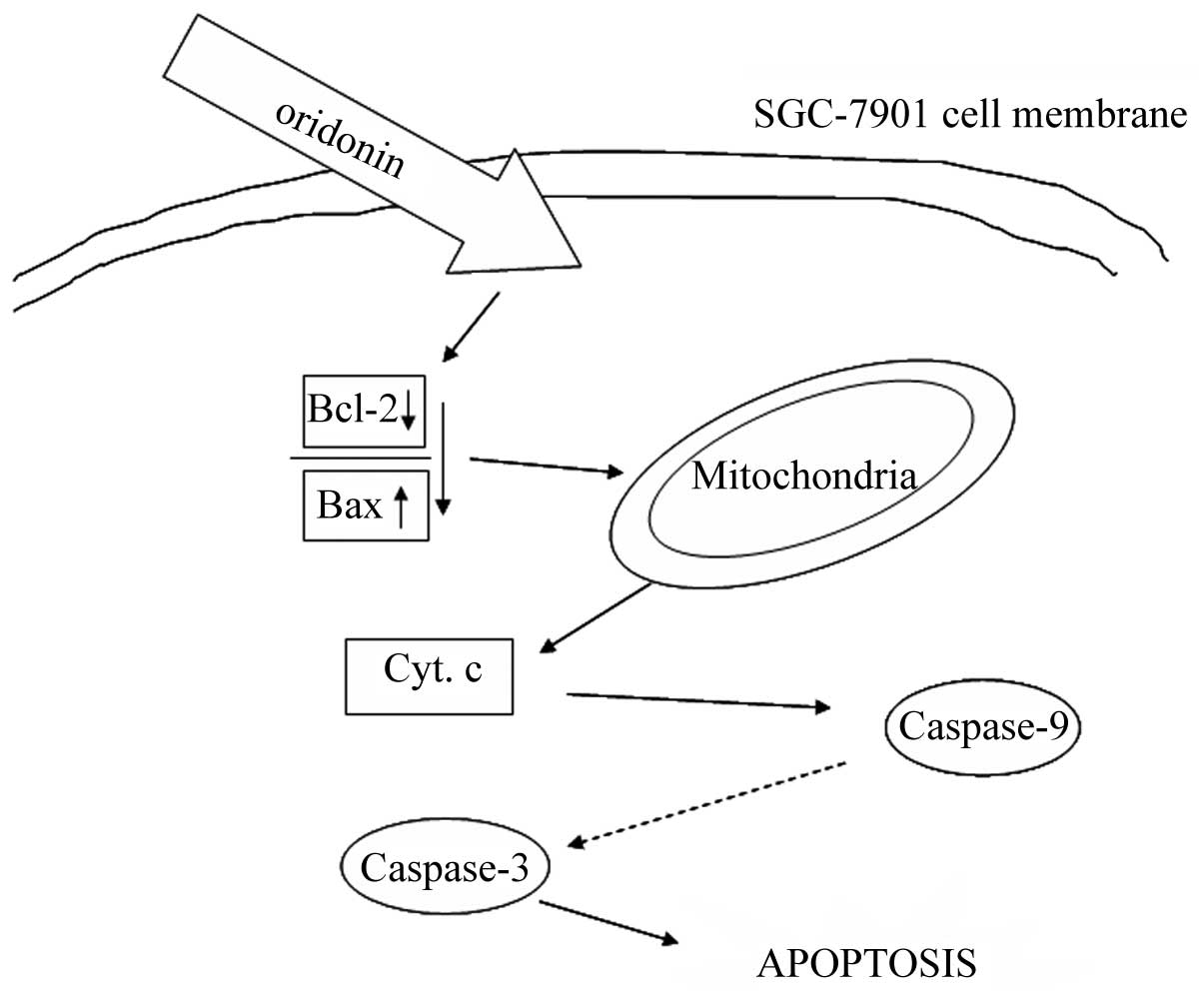
In conclusion, the possible significant molecular
signal pathways for oridonin inducing apoptosis in SGC-7901 cells
is shown in Fig. 5. Oridonin may
decrease the ratio of Bcl-2/Bax, which lead to dysfunction of
mitochondria and cause cytochrome c release, then activate the
caspase-3 leading to apoptosis. The present study demonstrated that
oridonin induced apoptosis in SGC-7901 cells via the mitochondrial
signal pathway, which may represent one of the major mechanisms of
oridonin-mediated apoptosis in SGC-7901 cells.
Acknowledgements
This study was financially supported by Science and
Technology Innovation Team Program in Higher Education Institutions
of Heilongjiang Province (2014TD009), and the Program for New
Century Excellent Talents in Heilongjiang Provincial University
(1251-NCET-019), Science and Technology Research Project of
Heilongjiang Province Department of Education (12541571).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fitzmaurice C, Dicker D, Pain A, Hamavid
H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R,
Wolfe C, et al; Global Burden of Disease Cancer Collaboration. The
global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao SY, Li J, Qu XY, Zhu N and Ji YB:
Downregulation of Cdk1 and cyclinB1 expression contributes to
oridonin-induced cell cycle arrest at G2/M phase and growth
inhibition in SGC-7901 gastric cancer cells. Asian Pac J Cancer
Prev. 15:6437–6441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu PY, Zhao GX and Chang LS: Local
thermotherapy with rabdosia liquid as prophylactic measure for
recurrence of superficial urinary bladder carcinoma: A
non-randomized contemporary controlled study. Zhongguo Zhong Xi Yi
Jie He Za Zhi. 25:1115–1117. 2005.(In Chinese).
|
|
5
|
Wang RL: A report of 40 cases of
esophageal carcinoma surviving for more than 5 years after
treatment with drugs. Zhonghua Zhong Liu Za Zhi. 15:300–302.
1993.(In Chinese). PubMed/NCBI
|
|
6
|
Wang RL, Gao BL, Xiong ML, Mei QD, Fan KS,
Zuo ZK, Lang TL, Gao GQ, Ji ZC, Wei DC, et al: Potentiation by
Rabdosia rubescens on chemotherapy of advanced esophageal
carcinoma. Zhonghua Zhong Liu Za Zhi. 8:297–299. 1986.(In Chinese).
PubMed/NCBI
|
|
7
|
de la Taille A, Hayek OR, Burchardt M,
Burchardt T and Katz AE: Role of herbal compounds (PC-SPES) in
hormone-refractory prostate cancer: Two case reports. J Altern
Complement Med. 6:449–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lou H, Gao L, Wei X, Zhang Z, Zheng D,
Zhang D, Zhang X, Li Y and Zhang Q: Oridonin nanosuspension
enhances anti-tumor efficacy in SMMC-7721 cells and H22 tumor
bearing mice. Colloids Surf B Biointerfaces. 87:319–325. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Jiang L, Wang S, Shi H, Wang J,
Wang R, Li Y, Dou Y, Liu Y, Hou G, et al: The antitumor activity of
the novel compound jesridonin on human esophageal carcinoma cells.
PLoS One. 10:e01302842015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen J, Zhang D, Zhao Z, Jia L, Zheng D,
Liu G, Hao L, Zhang Q, Tian X, Li C, et al: Synthesis,
characterization, in vitro and in vivo evaluation of PEGylated
oridonin conjugates. Int J Pharm. 456:80–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie
J, Zhang FX, Weng XQ, Shen ZX, Chen J, et al: Oridonin, a
diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion
protein and shows potent antitumor activity with low adverse
effects on t(8;21) leukemia in vitro and in vivo. Blood.
109:3441–3450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou H, Zhang X, Gao L, Feng F, Wang J, Wei
X, Yu Z, Zhang D and Zhang Q: In vitro and in vivo antitumor
activity of oridonin nanosuspension. Int J Pharm. 379:181–186.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang CJ, Zhu GJ, Yu L and Shi BH:
Preparation, in vitro, and in vivo antitumor activity of folate
receptor-targeted nanoliposomes containing oridonin. Drug Dev Res.
74:43–49. 2013. View Article : Google Scholar
|
|
14
|
Ye LH, Li WJ, Jiang XQ, Chen YL, Tao SX,
Qian WL and He JS: Study on the autophagy of prostate cancer PC-3
cells induced by oridonin. Anat Rec (Hoboken). 295:417–422. 2012.
View Article : Google Scholar
|
|
15
|
Ye YC, Wang HJ, Xu L, Liu WW, Liu BB,
Tashiro S, Onodera S and Ikejima T: Oridonin induces apoptosis and
autophagy in murine fibrosarcoma L929 cells partly via NO-ERK-p53
positive-feedback loop signaling pathway. Acta Pharmacol Sin.
33:1055–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Y, Fan SM, Song JK, Tashiro S, Onodera
S and Ikejima T: Hydroxyl radical (·OH) played a pivotal role in
oridonin-induced apoptosis and autophagy in human epidermoid
carcinoma A431 cells. Biol Pharm Bull. 35:2148–2159. 2012.
View Article : Google Scholar
|
|
17
|
Zang L, He H, Ye Y, Liu W, Fan S, Tashiro
S, Onodera S and Ikejima T: Nitric oxide augments oridonin-induced
efferocytosis by human histocytic lymphoma U937 cells via autophagy
and the NF-κB-COX-2-IL-1β pathway. Free Radic Res. 46:1207–1219.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Liu JH, Chai K, Tashiro S, Onodera
S and Ikejima T: Inhibition of c-Met promoted apoptosis, autophagy
and loss of the mitochondrial transmembrane potential in
oridonin-induced A549 lung cancer cells. J Pharm Pharmacol.
65:1622–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsieh TC, Wijeratne EK, Liang JY,
Gunatilaka AL and Wu JM: Differential control of growth, cell cycle
progression, and expression of NF-kappaB in human breast cancer
cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin,
diterpenoids from the chinese herb Rabdosia rubescens. Biochem
Biophys Res Commun. 337:224–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang T, Tan Y, Zhao R and Liu Z: DNA
damage induced by oridonin involves cell cycle arrest at G2/M phase
in human MCF-7 cells. Contemp Oncol (Pozn). 17:38–44. 2013.
|
|
21
|
Wang H, Ye Y, Chui JH, Zhu GY, Li YW, Fong
DWF and Yu ZL: Oridonin induces G2/M cell cycle arrest and
apoptosis through MAPK and p53 signaling pathways in HepG2 cells.
Oncol Rep. 24:647–651. 2010.PubMed/NCBI
|
|
22
|
Qi X, Zhang D, Xu X, Feng F, Ren G, Chu Q,
Zhang Q and Tian K: Oridonin nanosuspension was more effective than
free oridonin on G2/M cell cycle arrest and apoptosis in the human
pancreatic cancer PANC-1 cell line. Int J Nanomed. 7:1793–1804.
2012.
|
|
23
|
Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera
S and Ikejima T: Oridonin induces G2/M arrest and apoptosis via
activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK
survival pathway in murine fibrosarcoma L929 cells. Arch Biochem
Biophys. 490:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang N, Zhang JH, Qiu F, Chen S, Tashiro
S, Onodera S and Ikejima T: Induction of G(2)/M phase arrest and
apoptosis by oridonin in human laryngeal carcinoma cells. J Nat
Prod. 73:1058–1063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang N, Cao SJ, Zhou Y, He H, Tashiro S,
Onodera S, Qiu F and Ikejima T: Inhibition of caspase-9 by
oridonin, a diterpenoid isolated from Rabdosia rubescens, augments
apoptosis in human laryngeal cancer cells. Int J Oncol.
47:2045–2056. 2015.PubMed/NCBI
|
|
26
|
Pi J, Cai H, Jin H, Yang F, Jiang J, Wu A,
Zhu H, Liu J, Su X, Yang P, et al: Qualitative and quantitative
analysis of ROS-mediated oridonin-induced oesophageal cancer
KYSE-150 cell apoptosis by atomic force microscopy. PLoS One.
10:e01409352015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Jiang H, Wang C, Yang B, Zhao L,
Hu D, Qiu G, Dong X and Xiao B: Oridonin triggers apoptosis in
colorectal carcinoma cells and suppression of microRNA-32
expression augments oridonin-mediated apoptotic effects. Biomed
Pharmacother. 72:125–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bu HQ, Liu DL, Wei WT, Chen L, Huang H, Li
Y and Cui JH: Oridonin induces apoptosis in SW1990 pancreatic
cancer cells via p53- and caspase-dependent induction of p38 MAPK.
Oncol Rep. 31:975–982. 2014.
|
|
29
|
Cai DT, Jin H, Xiong QX, Liu WG, Gao ZG,
Gu GX and Qiu YH: ER stress and ASK1-JNK activation contribute to
oridonin-induced apoptosis and growth inhibition in cultured human
hepatoblastoma HuH-6 cells. Mol Cell Biochem. 379:161–169. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu M, Hong D, Bao Y, Wang C and Pan W:
Oridonin induces the apoptosis of metastatic hepatocellular
carcinoma cells via a mitochondrial pathway. Oncol Lett.
6:1502–1506. 2013.PubMed/NCBI
|
|
31
|
Hsia TC, Yu CC, Hsu SC, Tang NY, Lu HF,
Huang YP, Wu SH, Lin JG and Chung JG: Cantharidin induces apoptosis
of H460 human lung cancer cells through mitochondria-dependent
pathways. Int J Oncol. 45:245–254. 2014.PubMed/NCBI
|
|
32
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meade-Tollin LC, Wijeratne EMK, Cooper D,
Guild M, Jon E, Fritz A, Zhou GX, Whitesell L, Liang JY and
Gunatilaka AAL: Ponicidin and oridonin are responsible for the
antiangiogenic activity of Rabdosia rubescens, a constituent of the
herbal supplement PC SPES. J Nat Prod. 67:2–4. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong AM, Zhang Y, Kesler K, Deng M,
Burhenn L, Wang D, Moro A, Li Z and Heber D: Genomic and in vivo
evidence of synergy of a herbal extract compared to its most active
ingredient: Rabdosia rubescens vs. oridonin Exp Ther Med.
1:1013–1017. 2010.
|
|
35
|
Ikezoe T, Yang Y, Bandobashi K, Saito T,
Takemoto S, Machida H, Togitani K, Koeffler HP and Taguchi H:
Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits
the proliferation of cells from lymphoid malignancies in
association with blockade of the NF-kappa B signal pathways. Mol
Cancer Ther. 4:578–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen S, Gao J, Halicka HD, Huang X,
Traganos F and Darzynkiewicz Z: The cytostatic and cytotoxic
effects of oridonin (Rubescenin), a diterpenoid from Rabdosia
rubescens, on tumor cells of different lineage. Int J Oncol.
26:579–588. 2005.PubMed/NCBI
|
|
37
|
Kim KH, Kim JY, Kwak JH and Pyo S:
Different anticancer effects of Saxifragifolin A on estrogen
receptor-positive and estrogen receptor-negative breast cancer
cells. Phytomedicine. 22:820–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krajarng A, Imoto M, Tashiro E, Fujimaki
T, Shinjo S and Watanapokasin R: Apoptosis induction associated
with the ER stress response through up-regulation of JNK in HeLa
cells by gambogic acid. BMC Complement Altern Med. 15:262015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
An T, Zhang Y, Huang Y, Zhang R, Yin S,
Guo X, Wang Y, Zou C, Wei B, Lv R, et al: Neuregulin-1 protects
against doxorubicin-induced apoptosis in cardiomyocytes through an
Akt-dependent pathway. Physiol Res. 62:379–385. 2013.PubMed/NCBI
|
|
40
|
Nishida K, Yamaguchi O and Otsu K:
Crosstalk between autophagy and apoptosis in heart disease. Circ
Res. 103:343–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mazur AJ, Nowak D, Mannherz HG and
Malicka-Błaszkiewicz M: Methotrexate induces apoptosis in CaSki and
NRK cells and influences the organization of their actin
cytoskeleton. Eur J Pharmacol. 613:24–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kumar S, Yedjou CG and Tchounwou PB:
Arsenic trioxide induces oxidative stress, DNA damage, and
mitochondrial pathway of apoptosis in human leukemia (HL-60) cells.
J Exp Clin Cancer Res. 33:422014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tian CL, Wen Q and Fan TJ: Cytotoxicity of
atropine to human corneal epithelial cells by inducing cell cycle
arrest and mitochondrion-dependent apoptosis. Exp Toxicol Pathol.
67:517–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Broecker-Preuss M, Viehof J, Jastrow H,
Becher-Boveleth N, Fuhrer D and Mann K: Cell death induction by the
BH3 mimetic GX15-070 in thyroid carcinoma cells. J Exp Clin Cancer
Res. 34:692015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fulda S: Caspase-8 in cancer biology and
therapy. Cancer Lett. 281:128–133. 2009. View Article : Google Scholar
|
|
46
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun Y, Gao C, Luo M, Wang W, Gu C, Zu Y,
Li J, Efferth T and Fu Y: Aspidin PB, a phloroglucinol derivative,
induces apoptosis in human hepatocarcinoma HepG2 cells by
modulating PI3K/Akt/GSK3β pathway. Chem Biol Interact. 201:1–8.
2013. View Article : Google Scholar
|