Introduction
Lung cancer is one of the leading causes of
cancer-related deaths in the world. Non-small cell lung cancer
(NSCLC) accounts for nearly 85% in all lung cancer cases, with
adenocarcinoma, squamous cell carcinoma and large cell carcinoma
being the main histological subtypes (1). Current clinical treatments such as
surgery, radiotherapy and chemotherapy are still insufficient for
NSCLC-treatment (2,3) and new therapeutics need to be
developed.
Traditional Chinese Medicine is efficient in
improving clinical symptoms, regulating tumor growth and preventing
the recurrence of lung cancer in patients. In our previous study,
we found that the “Ke Jinyan Priscription”, given by the State
Medical Master Zhou Zhongying, inhibited the proliferation of
NCI-H157 or A549 transplanted tumors, respectively, in nude mice,
and the physical status of the animals was significantly improved
(4). Some ingredients extracted
from the formula showed significant inhibitory effects on NSCLC
cell lines under screening on the high-throughput platform
(5,6). In particular, one of the derived
compounds, ophiopogonin B (OP-B), showed especially potent
inhibition of a series of NSCLC cell lines, and in H157 and H460
cell lines it mainly induced autophagic cell death which was
caspase-independent (7).
Besides autophagy, the caspase-independent
programmed cell death (PCD) includes paraptosis, mitotic
catastrophe, and the descriptive model of apoptosis-like and
necrosis-like PCD. Once caspase-mediated routes fail in
vivo, caspase-independent cell death pathways are important for
cleaning unwanted or harmful cells, which can also be triggered by
cytotoxic agents or other death stimuli (8). As potentially new cancer therapies
could be developed, the growing knowledge of the
caspase-independent cell death pathways is very important for
oncology research (8).
To establish whether OP-B triggered different types
of caspase-independent cell death in NSCLC cell lines, herein, we
tested its effects on human lung adenocarcinoma A549 cells. The
results revealed that OP-B induced mitotic catastrophe in A549
cells in vitro, and the mechanism may due to the inhibition
of Myt1 and p-Histone H3 (Ser10) and the specific regulation of the
cell cycle, combined with induction of autophagy, it resulted in
caspase-independent apoptosis in this cell line. Moreover, OP-B
inhibited expression of XIAP, survivin and the phosphorylation of
Histone H3 (Ser10), thereby induced autophagy and apoptosis in
vivo. Compared with its effects on H157 and H460 cells, the
investigation demonstrated that OP-B may be more efficient in
treating A549 cells.
Materials and methods
Reagents
Ophiopogonin B (OP-B) (MW: 722.9, HPLC ≥98%) was
purchased from Nanjing Zelang Medical Technology Co., Ltd.
(Nanjing, China). The chemicals used were staurosporine,
chloroquine (CQ), propidium iodide (PI),
(3-(4,5-dimethyl-thiazol-2-yl)-2,5 diphenyltetrazolium bromide
(MTT), DAPI and Hoechst 33258 (Sigma-Aldrich, St. Louis, MO, USA).
The Alexa Fluor 488 Annexin V/Dead Cell apoptosis kit (Invitrogen,
Waltham, MA, USA) and the In Situ Cell Death Detection kit (Roche,
Indianapolis, IN, USA) were also obtained commercially.
Cell culture and transient
transfection
The human NSCLC cell line (A549) was obtained from
the Institute of Biochemistry and Cell Biology (Shanghai, China).
The A549 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) and F12 medium (HyClone Laboratories,
Logan, UT, USA), respectively, supplemented with 10% fetal bovine
serum (FBS; Gibco, Waltham, MA, USA), 100 U/ml
penicillin-streptomycin mixed antibiotics at 37ºC in a humidified
5% CO2 incubator.
For transient transfection, A549 cells were seeded
in 6-cm culture dishes at a density of 106 cells/dish
and then transfected with rat Beclin1 siRNAs (C-300506-03-0005;
Dharmacon, Lafayette, CO, USA) using Lipofectamine 2000 reagent
(13778-075; Invitrogen) according to the manufacturer's
protocol.
Transmission electron microscopy
(TEM)
After being exposed to 10 μM of OP-B for 48 h, the
cells were trypsinized, washed with phosphate-buffered saline (PBS)
and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2)
overnight at 4ºC. The next day, cells were washed three times with
0.1 mol/l phosphate buffer. Thereafter, the cells were fixed in 1%
aqueous osmium, dehydrated with increasing concentrations of
ethanol (30, 50, 70, 80, 90 and 100%) and embedded in araldite. The
ultrathin sections were prepared with a microtome (Leica
Microsystems, Wetzlar, Germany) and mounted on copper grids. The
samples were stained with 2% aqueous uranyl acetate and lead
citrate and observed by TEM (JEOL, Ltd., Tokyo, Japan).
High-content screening (HCS)
Apoptosis and dead cells induced by OP-B were
assayed using the KineticScan Reader (Thermo Fisher Scientific,
Waltham, MA, USA). The principle of the assay is that cells are
labeled with a cocktail of fluorescent dyes (including Hoechst
33258 and Alexa Fluor 488 Annexin V) that indicate the cellular
properties of interest, including nuclear structure, cell membrane
permeability, as well as early and late stages of apoptosis. All
procedures were performed according to the manufacturer's
instructions. The cells were plated at a density of
8×103 cells/well in each well of a 96-well plate. After
culturing for 24 h, cells were incubated with 10 μM OP-B for
another 24 h. Thirty minutes before the completion of incubation, a
cocktail of fluorescent dyes was added to each well. The cells were
then fixed with pre-warmed fixation solution and washed twice with
PBS. Plates were then sealed and processed on an HCS Reader to
acquire images. Images were analyzed with HCS software, and
fluorescence intensities of the Hoechst 33258 and Annexin V/PI dyes
were calculated. As for DAPI staining, the cells were cultured and
treated and detected by HCS as above.
Western blot analysis
After treatment with different concentrations of
OP-B, the cells were lysed in RIPA buffer containing 50 mmol/l
Tris-HCl (pH 8.0), 150 mmol/l NaCl, 1% Nonidet-P40, 1% sodium
deoxycholate, 0.1% SDS, 0.1 mmol/l DTT, 0.05 mmol/l PMSF, 0.002
mg/ml aprotinin, 0.002 mg/ml leupeptin and 1 mmol/l
NaVO3. The protein concentrations of supernatants were
determined by the BCA protein assay. Equal amounts of protein were
loaded and separated by 10 or 12% SDS-PAGE and then transferred
onto polyvinylidene difluoride membranes. The membranes were
incubated overnight with appropriate primary antibodies against LC3
I/II, Beclin-1, Atg3, Atg-5/12, p-Histone H3 (Ser10), caspase-3,
Bcl-2, Bax, cyclin D1, cyclin D3, CDK4, CDK6, cyclin A2, CDK2,
cyclin B1, Myt1, p-cdc2, p21, p27, survivin, XIAP or β-actin
overnight at 4ºC, and then with HRP-conjugated secondary antibodies
(anti-rabbit or mouse immunoglobulin G) for an additional hour at
room temperature. Immunoreactivity was detected by enhanced
chemiluminescence (ECL; Bio-Rad Laboratories, Hercules, CA, USA).
β-actin was used as a loading control. Immunoblot experiments were
performed at least three times. Image acquisition was performed
using Image Lab™ software.
Immunohistochemistry assay
The immunohistochemistry for LC3 localization in the
tumor was performed as previously described (9).
Terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
assay
TUNEL staining was performed by using the ‘In Situ
Cell Death Detection kit’ from Roche following the
manufacturer's instructions.
Cell cycle analysis and apoptosis
detection
After cells were treated with or without OP-B for 24
h, they were harvested by centrifugation, washed with ice-cold PBS
and fixed in ice-cold 70% ethanol overnight. The cells were then
treated with 40 μg/ml RNase at 37ºC and stained with 40 μg/ml PI
for 30 min. The percentage of cells in each phase (sub G1, G0/G1, S
and G2/M) was calculated (Becton-Dickinson, Franklin Lakes, NJ,
USA).
MTT assay
Cell viability was assessed by the MTT reduction
assay as described by Mosmann (10). Viability of non-treated cells was
taken as 100% and IC50 values were determined from three
independent experiments.
Nude mouse xenografts
Five-week-old athymic BALB/c mice were maintained
under specific pathogen-free conditions and manipulated according
to protocols approved by the Shanghai Medical Experimental Animal
Care Commission. Exponentially growing A549 cells (2×106
in 0.2 ml medium) were inoculated subcutaneously into the flank of
the mice. After 7 days, tumor-bearing mice were randomly divided
into three groups, which were treated with OP-B (15 or 75 mg/kg
p.o. daily; n=6) or corn oil (control, 100 μl, p.o. daily; n=6) for
21 consecutive days. Tumor growth was determined by measuring the
size of the tumors every 2 days. The mice were euthanized at day
21, and the tumors were isolated, photographed and embedded in
paraffin or frozen at −80ºC for subsequent western blot detection
of p-Histone H3 (Ser10), survivin and XIAP, immunohistochemical
analysis of LC3 expression as well as for the TUNEL assay.
Statistical analysis
Unless otherwise stated, data are expressed as the
mean ± standard deviation (SD) and analyzed by the Student's
t-test. A P-value <0.05 was considered statistically
significant.
Results
OP-B treatment induces
caspase-independent apoptosis in A549 cells
Cocktail-staining by Annexin V/PI/Hoechst 33258
combined with fluorescence density analysis by HCS KineticScan
Reader showed that double-positive Annexin V/PI labeling
significantly occurred in A549 cells treated with 10 or 20 μM of
OP-B (Fig. 1A and B).
TUNEL staining assay is another effective method for
marking clipped nucleic acids and nucleus-morphology observation of
apoptotic cells. Compared with staurosporine (STS), a positive
inducer of apoptosis which significantly induced cell chromatin
condensation and TUNEL-detectable fragmentation, 10 μM of OP-B
treatment resulted in DNA-fragmentation moderately in A549 cells
(Fig. 1D).
Further detection of apoptosis-related protein
showed that OP-B-treatment did not induce activation of caspase-3
or change the levels of Bcl-2 and Bax (Fig. 1E).
Taken together, OP-B induced DNA-fragmentation and
cell death in A549 cells caspase-independently.
OP-B causes cell cycle arrest and mitotic
catastrophe in A549 cells
Next, flow cytometry (FCM) was used to analyze the
effect of OP-B on cell cycle distribution in A549 cells. As shown
in Fig. 2A, after treatment with
5, 10 or 20 μM of OP-B, the number of cells in the sub G1 phase
increased to 6.74±0.11, 11.30±0.88 or 19.41±0.59%, respectively,
which in the vehicle-treated group was only 1.07±1.30%, the results
were in accordance with that detected by HCS. Meanwhile, the cells
in the S phase accumulated from 7.95±3.01 (vehicle treated) to
14.92±1.41 (under 10 μM of OP-B) or 32.89±0.94% (under 20 μM of
OP-B), and the cells in the G2/M phase increased from 4.49±1.18
(vehicle treated) to 37.22±2.23 or 28.02±0.13% under 10 or 20 μM of
OP-B treatment, respectively. The results suggested that OP-B
induced cell death and cell cycle arrested in S and G2/M phase.
 | Figure 2OP-B induces cell cycle arrest in S
and G2/M phase and mitotic catastrophe in A549 cells. (A) A549
cells were treated with 5–20 μM of OP-B for 24 h, then fixed in
ethanol and stained with PI. The DNA content and percentage of
cells in each cell cycle phase was determined by flow cytometry.
Data are presented as the mean ± standard error. (B) A549 cells
were treated with or without 10 μM of OP-B for 24 h, and then
expression levels of cyclin D1, cyclin D3, CDK4, CDK6, cyclin A2,
CDK2, cyclin B1, Myt1, p-cdc2, p-Histone H3, p21 and p27 were
detected by western blotting, β-actin was used as a loading
control. (C) A549 cells treated with or without 10 μM of OP-B for
24 h were then stained by DAPI and the nucleus morphology was
observed by HCS (200) (multiploid of nucleus are pointed out with
red arrows). The experiment was repeated three times and yielded
similar results. |
The regulation of cell cycle transition is known to
be governed by the concerted action of cyclin-dependent kinases
(CDKs) and their regulatory cyclin subunits (11,12).
Among them, cyclin A, cyclin E and CDK2 regulate normal S-phase
progression, while cyclin B1 is involved in G2-phase progression.
During progression into mitosis, the critical regulatory step is
dephosphorylation of cdc2 at Tyr15 and Thr14, while phosphorylation
of cdc2 by the protein kinases Wee1 and Myt1 results in inhibition
of cdc2 (13). Moreover,
phosphorylation of Histone H3 (Ser10) can drive mitotic chromosomal
condensation during M phase entry (14).
After treated with 0, 5 and 10 μM of OP-B, the
correlated proteins in the cells was detected by western blot
assay. The results showed that OP-B did not affect the expression
of cyclin D, CDK4, CDK6, cyclin A2, CDK2 and cyclin B1, while
significantly inhibited the expression of Myt1 and the
phosphorylation of cdc2 (Tyr15) and Histone H3 (Ser10). Whereas,
detection of CDK inhibitors p21Waf1/Cip1 and
p27Kip1 showed that OP-B markedly increased the level of
p27Kip1 (Fig. 2B).
Cell cycle arrest in G2/M phase was apt to induce
mitotic catastrophe which was characterized as large cells with
multiple micronuclei and decondensed charomatin. After 24-h
treatment with 10 μM of OP-B, A549 cells were stained with DAPI and
detected by HCS, the results showed that OP-B induced formation of
micronuclei in the nucleus (Fig.
2C).
Thus, we speculated that inhibition of OP-B on Myt1
and p-Histone H3 (Ser10) and enhancement of p27Kip1
induced cell cycle arrest in S and G2/M phase and resulted in
mitotic catastrophe.
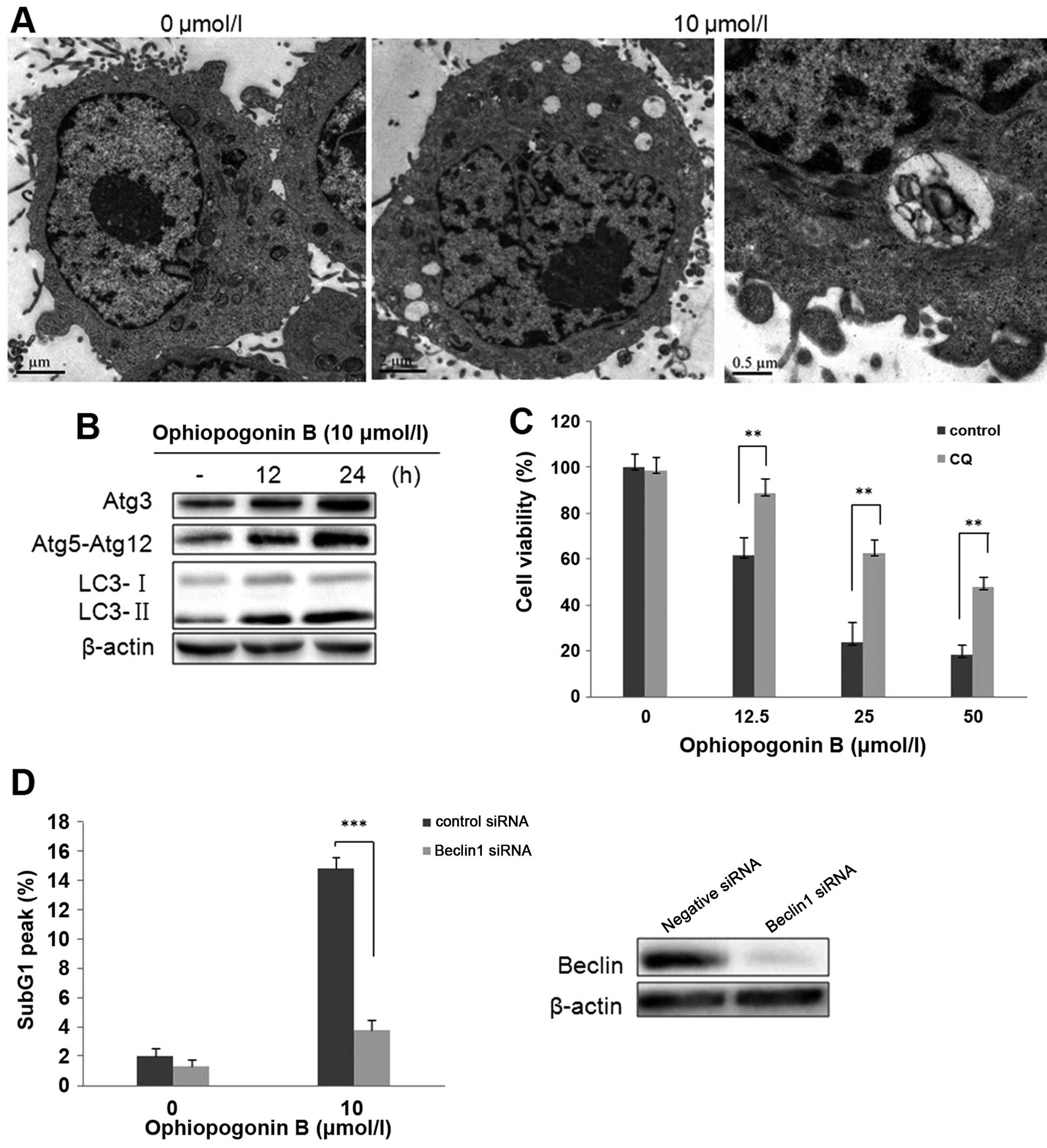
Autophagy is involved in cell death
induced by OP-B in A549 cells
We have reported that OP-B induced autophagy in H157
and H460 cells (7). To investigate
whether this type of cell death also occurs in OP-B treated A549
cells, we detected autophagy in this cell line. First, the
micro-morphological change of OP-B treated A549 cells was observed
by TEM. The results showed that OP-B treatment also resulted in
numerous vacuoles in the cytoplasm (Fig. 3A). Then, detection of
microtubule-associated protein 1 light chain 3 (LC3), the specific
marker of autophagy showed that 12 or 24 h of treatment with OP-B
significantly increased the transition of LC3-I to LC3-II (Fig. 3B). Atg proteins participate in the
processes of autophagosome-formation (15). Synchronism detection showed that
OP-B also upregulated the expression of Atg3, Atg5-Atg12 in the
cells (Fig. 1B). Taken together,
the results verified that OP-B also induced autophagy in A549
cells.
As is known, at the last phage of autophagy,
lysosome fuses with autophagosome, then formats autophagolyso-some.
Chloroquine (CQ) is the inhibitor of lysosome which inhibits
lysosomal degradation (16). In
order to determine the role of autophagy in OP-B-induced cell death
in A549 cells, the effect of CQ on OP-B-induced cell viability was
initially evaluated. As shown in Fig.
3C, the decreased cell viability that resulted from 24 h of
exposure to OP-B was effectively rescued by pretreatment with CQ,
suggesting that OP-B-mediated autophagy promotes cell death in A549
cells.
Importantly, genetic inhibition of OP-B-mediated
autophagy by transfection with Beclin1 siRNA also significantly
decreased the cell distribution in Sub G1 phase exerted by OP-B in
A549 cells (Fig. 3D). Together,
the data indicated that OP-B-induced autophagy was the vital reason
which resulted in apoptosis in A549 cells.
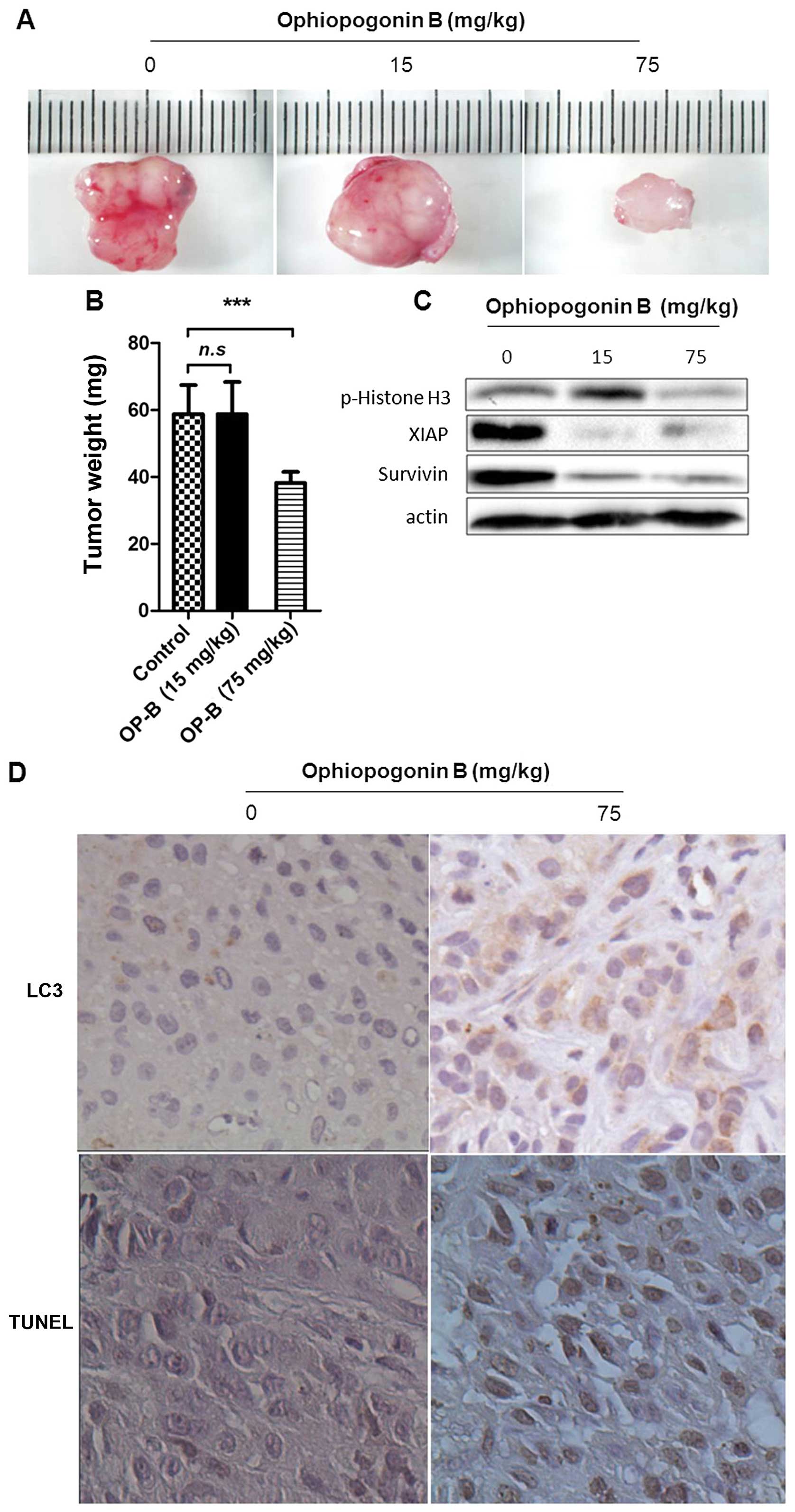
OP-B inhibits NSCLC xenograft growth in
association with induced autophagy and apoptosis
To further authenticate the effects of OP-B
described above, animal experiments were designed to test whether
OP-B could induce autophagic and apoptotic cell death in
vivo, and NSCLC xenografts in nude mice were established using
A549 cells. The results showed that the growth of NSCLC tumors was
significantly inhibited by treatment with OP-B at 75 mg/kg compared
with the vehicle controls (Fig. 4A and
B). Results of western blot assay showed that the high dose of
OP-B significantly inhibited the phosphorylation of Histone H3
(Ser10), and at both of the doses of OP-B the levels of survivin
and XIAP were inhibited (Fig. 4C).
The tumors from OP-B-treated mice exhibited significantly increased
expression of LC3; moreover, treatment with OP-B significantly
induced cellular apoptosis in tumor tissues as evidenced by the
TUNEL assay (Fig. 4D). These
results indicated that OP-B could induce autophagic and apoptotic
cell death in A549 cells in vivo, which may be an important
mechanism underlying the antitumor activities of OP-B.
Discussion
In our previous study, we reported that OP-B
inhibited proliferation of a panel of NSCLC cell lines;
importantly, it showed a relatively low IC50 in A549
cells (2.63 μM) (7). As
approximately 40% of lung cancers are adenocarcinomas in clinic, in
the present study we focused on the effect of OP-B on A549 cells
in vitro and in vivo to further elaborate the
underlying mechanisms of OP-B in NSCLC.
Firstly, positive labeling of Annexin V/PI and TUNEL
staining both demonstrated that OP-B caused apoptosis in A549 cells
(Fig. 1A–C), while detection of
caspase-3, Bcl-2 and Bax showed that the apoptosis was caspase and
mitochondrial independent (Fig.
1D).
Cell cycle arrest is another key factor preventing
tumor growth. In the previous study, we reported that OP-B induced
cell cycle arrest in the G0/G1 phase in H157 and H460 cell lines
(7). However, our current flow
cytometric analysis in A549 cells showed that OP-B markedly
suppressed the cell mitotic progression through arresting cells in
the S and G2/M phase; whereas, it dose-dependently induced the
increase of cells in the Sub-G1 phase (Fig. 2A). It is well known that the
eukaryotic cell cycle is regulated by the coordinated activity of
CDK kinase complexes which form and are activated at specific cell
cycle stage (17). These
cyclin-CDK complexes often bind to the endogenous inhibitor
p21WAF1/CIP1 or p27KIP1, which inhibits their
kinase activities and prevent cell cycle progression. Detection of
these proteins showed that OP-B upregulated p27KIP1,
while downregulated Myt1, p-cdc2 and p-Histone H3 (Ser10) in A549
cells (Fig. 2B). Phosphorylation
at Ser10, Ser28 and Thr11 of Histone H3 is tightly correlated with
chromosome condensation during both mitosis and meiosis (18), and singular phosphorylation of
Histone H3 (Ser10) can act as part of a molecular mechanism driving
mitotic chromosomal condensation during M phase entry (14). Further observation of the nucleus
morphology by DAPI staining showed that OP-B treatment resulted in
micronuclei formation in the cells, being the typical
characteristic of mitotic catastrophe (Fig. 2C). Taken together, upregulation of
p27KIP1 and suppression of p-Histone H3 (Ser10) resulted
in cell cycle arrest in S and G2/M phase, and further induced
mitotic catastrophe in A549 cells.
Autophagy has a complex relationship with apoptosis,
especially in tumor cell lines (19–22).
Here, cell micromorphology observed by TEM combined with detection
of autophagy-related proteins by western blotting showed that OP-B
also induced autophagy in A549 cells (Fig. 3A and B). Moreover, lysosome
inhibitor CQ or Beclin1 siRNA knockdown were shown to both
attenuate the level of cell apoptosis (Fig. 3C and D), indicating that autophagy
promoted apoptosis in A549 cells. As known, not only caspases,
other proteases can also execute programmed cell death, and they
can be directed by several cellular organelles, including
mitochondria, lysosomes, and the ER, which can collaborate with
each other or act independently. Different death routes may overlap
and several characteristics may be displayed at the same time
(6,23–25).
For these multiple action of OP-B on A549 cells
in vitro, we used 15 and 75 mg/kg of OP-B in A549 xenografts
nude mice to detect its effect in vivo. The results showed
that OP-B exerted significant inhibition of tumor proliferation
(Fig. 4A and B). The mechanism may
be related with phosphorylation inhibition of Histone H3 (Ser10)
and suppression on survivin and XIAP (Fig. 4C), which resulted in mitotic
catastrophe and further induced autophagy and apoptosis (Fig. 4D) of the tumor cells.
Taken together, other than its action on H157 and
H460 cells, OP-B exerted overlapped types of cell death in A549
cells, including mitotic catastrophe, autophagy and
caspase-independent apoptosis (Fig.
5). The difference may due to the unique characteristics of
different cell lines. As having the distinguishing trait of type II
pulmonary epithelial cells, A549 cells usually form confluent
monolayers during growth, and they express P450 IA1 and P450 IIB6
isozymes, which facilitate delivery of macromolecules and are
important for metabolism of drugs (26). This may be the reason for A549
cells to be more sensitive to OP-B. Thus, OP-B may be developed as
an alternative agent that can be used for the classification and
treatment of NSCLC.
Acknowledgements
The present study was supported by grants from the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD), the Natural Science Foundation of Jiangsu
Province (BK20131415 to M.C.), the National Natural Science
Foundation of China (81503374 to M.C.), the Natural Science
Foundation of Jiangsu Province (BK20151003 to Y.G.), China and
Europe taking care of healthcare solutions, CHETCH Grant Agreement
Number: PIRSES-GA-2013-612589 (to X.Z.).
References
|
1
|
Dresler CM, Fratelli C, Babb J, Everley L,
Evans AA and Clapper ML: Gender differences in genetic
susceptibility for lung cancer. Lung Cancer. 30:153–160. 2000.
View Article : Google Scholar
|
|
2
|
Jacobson FL, Austin JHM, Field JK, Jett
JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R,
Strauss GM, et al: Development of The American Association for
Thoracic Surgery guidelines for low-dose computed tomography scans
to screen for lung cancer in North America: Recommendations of The
American Association for Thoracic Surgery Task Force for Lung
Cancer Screening and Surveillance. J Thorac Cardiovasc Surg.
144:25–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong F, Jiang M, Huang Z, Chen M, Chen K,
Zhou J, Yin L, Tang Y, Wang M, Ye L, et al: A novel herbal formula
induces cell cycle arrest and apoptosis in association with
suppressing the PI3K/AKT pathway in human lung cancer A549 cells.
Integr Cancer Ther. 13:152–160. 2014. View Article : Google Scholar
|
|
5
|
Zhu J, Chen M, Chen N, Ma A, Zhu C, Zhao
R, Jiang M, Zhou J, Ye L, Fu H, et al: Glycyrrhetinic acid induces
G1-phase cell cycle arrest in human non-small cell lung cancer
cells through endoplasmic reticulum stress pathway. Int J Oncol.
46:981–988. 2015.PubMed/NCBI
|
|
6
|
Zhao R, Chen M, Jiang Z, Zhao F, Xi B,
Zhang X, Fu H and Zhou K: Platycodin-D induced autophagy in
non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK
signaling pathways. J Cancer. 6:623–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen M, Du Y, Qui M, Wang M, Chen K, Huang
Z, Jiang M, Xiong F, Chen J, Zhou J, et al: Ophiopogonin B-induced
autophagy in non-small cell lung cancer cells via inhibition of the
PI3K/Akt signaling pathway. Oncol Rep. 29:430–436. 2013.
|
|
8
|
Bröker LE, Kruyt FA and Giaccone G: Cell
death independent of caspases: A review. Clin Cancer Res.
11:3155–3162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang JJ, Zhu Y, Xie KL, Peng YP, Tao JQ,
Tang J, Li Z, Xu ZK, Dai CC, Qian ZY, et al: Yin Yang-1 suppresses
invasion and metastasis of pancreatic ductal adenocarcinoma by
down-regulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent
mechanism. Mol Cancer. 13:1302014. View Article : Google Scholar
|
|
10
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sherr CJ: The Pezcoller lecture: Cancer
cell cycles revisited. Cancer Res. 60:3689–3695. 2000.PubMed/NCBI
|
|
12
|
Malumbres M and Barbacid M: To cycle or
not to cycle: A critical decision in cancer. Nat Rev Cancer.
1:222–231. 2001. View
Article : Google Scholar
|
|
13
|
Lew DJ and Kornbluth S: Regulatory roles
of cyclin dependent kinase phosphorylation in cell cycle control.
Curr Opin Cell Biol. 8:795–804. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hendzel MJ, Wei Y, Mancini MA, Van Hooser
A, Ranalli T, Brinkley BR, Bazett-Jones DP and Allis CD:
Mitosis-specific phosphorylation of histone H3 initiates primarily
within pericentromeric heterochromatin during G2 and spreads in an
ordered fashion coincident with mitotic chromosome condensation.
Chromosoma. 106:348–360. 1997. View Article : Google Scholar
|
|
15
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwai-Kanai E, Yuan H, Huang C, Sayen MR,
Perry-Garza CN, Kim L and Gottlieb RA: A method to measure cardiac
autophagic flux in vivo. Autophagy. 4:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin YH, Choi J, Shin S, Lee KY, Park JH
and Lee SK: Panaxadiol selectively inhibits cyclin A-associated
Cdk2 activity by elevating p21WAF1/CIP1 protein levels
in mammalian cells. Carcinogenesis. 24:1767–1772. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Preuss U, Landsberg G and Scheidtmann KH:
Novel mitosis-specific phosphorylation of histone H3 at Thr11
mediated by Dlk/ZIP kinase. Nucleic Acids Res. 31:878–885. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenberg-Lerner A, Bialik S, Simon H-U
and Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar
|
|
22
|
Codogno P and Meijer AJ: Autophagy and
signaling: Their role in cell survival and cell death. Cell Death
Differ. 12(Suppl 2): 1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen TS, Wang XP, Sun L, Wang LX, Xing D
and Mok M: Taxol induces caspase-independent cytoplasmic
vacuolization and cell death through endoplasmic reticulum (ER)
swelling in ASTC-a-1 cells. Cancer Lett. 270:164–172. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bröker LE, Huisman C, Span SW, Rodriguez
JA, Kruyt FA and Giaccone G: Cathepsin B mediates
caspase-independent cell death induced by microtubule stabilizing
agents in non-small cell lung cancer cells. Cancer Res. 64:27–30.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huisman C, Ferreira CG, Bröker LE,
Rodriguez JA, Smit EF, Postmus PE, Kruyt FA and Giaccone G:
Paclitaxel triggers cell death primarily via caspase-independent
routes in the non-small cell lung cancer cell line NCI-H460. Clin
Cancer Res. 8:596–606. 2002.PubMed/NCBI
|
|
26
|
Foster KA, Oster CG, Mayer MM, Avery ML
and Audus KL: Characterization of the A549 cell line as a type II
pulmonary epithelial cell model for drug metabolism. Exp Cell Res.
243:359–366. 1998. View Article : Google Scholar : PubMed/NCBI
|