Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer in the world and the third cause of cancer mortality
(1,2). HCC is associated with infection with
hepatitis B and C virus. Most HCC patients are diagnosed at the
late stage and lose the opportunity for surgical operation
(3). Conventional chemotherapy
with oxaliplatin, doxorubicin, and fluorouracil as monotherapy or
in combination for patients with advanced HCC is usually
ineffective with a low response rate and severe side effects
(4–6). Sorafenib, a multitargeted tyrosine
kinase inhibitor which has been shown to improve by 2.8 months the
median overall survival (OS) and by 2.7 months the median
progression-free survival (PFS) for unresectable HCC (7). Currently, the prognosis of HCC is
poor even with multidisciplinary comprehensive treatment.
Therefore, identification and development of novel and safe
treatment strategies are urgently needed to improve treatment
outcomes.
In recent years, Chinese medicine is emerging as an
attractive new generation of anticancer drugs due to their ability
to effectively eliminate cancer cells with low toxicity (8–10).
Salvia miltiorrhiza (Danshen), a popular Chinese herb, has
been widely used for treating angina pectoris, myocardial
infarction (MI) and stroke (11,12).
Salvianolic acid B (Sal B) is one of the major water-soluble
compounds and active ingredients of Danshen (13,14).
Recently, the anticancer effects of Sal B have been demonstrated in
human cancer cell lines including prostate, breast, liver, and head
and neck squamous cell cancers (15–17).
However, the effects of Sal B on HCC and the antitumor mechanisms
have not been adequately studied.
Additionally, studies on the antitumor activity of
Sal B have focused on inhibition of proliferation and induction of
apoptosis (15). Sal B has also
been reported to induce autophagy (18). Understanding the interplay between
apoptosis and autophagy induced by Sal B in HCC may identify new
targets for cancer therapy and improve the therapeutic efficiency
of HCC. Currently, there are no reports on the effects of Sal B on
the interplay between autophagy and apoptosis in HCC cells.
In this study, we demonstrated that Sal B inhibited
the growth and induced cell death of human HCC cells in
vitro. We also found that autophagy together with apoptosis is
involved in Sal B-induced cell death in HCC cells. Inhibition of
autophagy attenuated Sal B-induced cell death by reducing
apoptosis. Moreover, Sal B-induced cell death was associated with
AKT/mTOR signaling inhibition. These results suggest that Sal B
could be a potential anticancer agent for the treatment of HCC.
Materials and methods
Chemicals and antibodies
Sal B was purchased from Sigma (Sigma-Aldrich Corp.,
St. Louis, MO, USA). 3-MA and CQ were purchased from J&K
Chemical Ltd. (J&K Chemical Ltd., Beijing, China). JC-1 and
LysoTracker Red were obtained from Invitrogen (Guangzhou, China).
The primary antibodies against LC-3, p62, Beclin-1, cleaved PARP,
cleaved caspase-3, cytochrome c, total or phospho-AKT
(Ser473), total or phospho-mTOR (Ser2448), phospho-4EBP1 (Thr70),
and phospho-P70S6K (Thr389) were purchased from Cell Signaling
Technology (Boston, MA, USA). The secondary antibodies were
HRP-conjugated anti-rabbit IgG purchased from Cell Signaling
Technology. The FITC-conjugated anti-rabbit IgG was purchased from
Beyotime (Beyotime, Nantong, China).
Cells lines and cultures
SK-Hep-1 and Bel-7404 cells were obtained from the
American Type Culture Collection (ATCC, Rockville, MD, USA). The
cell lines were frozen in liquid nitrogen soon after arrival. The
experiments with these cells were carried out within 6 generations
after resuscitation. SK-Hep-1 and Bel-7404 cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal
bovine serum (FBS), 100 U penicillin and 100 U streptomycin at 37°C
in a humidified incubator of 5% CO2 and 95% air.
Cell viability assay
Cell proliferation was determined by MTS assay.
Cells were seeded into 96-well plates and treated with Sal B, CQ,
3-MA or a combination. After treatment, 10 μl MTS (Promega) was
added into each well for 2-h incubation. The absorbance was
measured using a model ELX800 Microplate Reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA) at 490 nm to calculate the
proliferation. Three independent experiments were performed to
determine the half maximal inhibitory concentration values
(IC50).
Colony forming assay
Cells were incubated at a density of 1,000 cells per
well in 6-well plates and treated with a determined dose of Sal B
or vehicle control for 2 weeks. After fixation with 4%
paraformaldehyde, the colonies formed were counterstained with
crystal violet staining solution.
Apoptosis analysis
The cell apoptotic rate was determined by flow
cytometry analysis using a fluorescein isothiocyanate (FITC)
Annexin V Apoptosis Detection kit (KeyGen Biotech, Nanjing, China).
Cells were collected by trypsinization, washed twice and
resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. Then, 100 μl of cells were mixed with 5
μl of FITC Annexin V and 5 μl PI and incubated for 15 min. The
samples were sent out for analysis by flow cytometry. The results
were analyzed with the BD FACSCalibur™ system.
Immunofluorescence analysis
Cells, seeded at 3×105 into 6-well
culture plates, were treated with a determined dose of Sal B for
indicated intervals and were incubated with Lyso Tracker for 60
min. Thereafter, cells were washed twice with PBS, followed by
fixation in 4% paraformaldehyde and permeabilized with 1% CHAPS
buffer (150 mM NaCl, 10 mM HEPES, 1.0% CHAPS) at room temperature
for 15 min. Hereafter, cells were incubated with anti-LC3
antibodies for 2 h at 37°C, and incubated with FITC-conjugated
anti-rabbit IgG for 1 h at 37°C, and the cell nuclei were stained
by DAPI (Invitrogen) for 15 min. Samples were examined under a
Zeiss LSM 710 fluorescence microscopy system (Carl Zeiss Inc,
Dublin, CA, USA). Images were processed with ZEN LE software. For
quantification of LC3-positive cells, 150–200 cells were randomly
selected from the acquired images and counted. Cells with more than
five dots of specific green or yellow signals were considered to be
LC3-positive.
Transmission electron microscope
Cells, seeded at 3×105 into 6-well
culture plates, were treated with a dose of 200 μM Sal B for 24 h.
Then, the cells were washed and fixed for 30 min in 2.5%
glutaraldehyde. The samples were treated with 1.5% osmium
tetroxide, dehydrated with acetone and embedded in Durcupan resin.
Thin sections were stained with lead citrate and examined by TECNAI
10 electron microscopy (Philips, Eindhoven, The Netherlands) at 60
kV.
RNA interference
For ATG6 (Beclin-1) interference, two siRNA
oligonucleotides targeting ATG6 were synthesized by GenePharma
(Shanghai, China). The sequences of the sense strands of the RNAs
targeting ATG6 used in this study were as follows: ATG6 siRNA-1
(si1): 5′-GUGAGAAGCAAGCCCU UAUTT-3′, ATG6 siRNA-2 (si2):
5′-CUCCAGUGCUAAGCUACAU-TT-3′. A non-specific oligo that is not
complementary to any human genes was used as a negative control.
The mixture of si1 and si2 was used to increase the inhibitory
activity. Cells were transfected with siRNA using HiPerFect
(Qiagen) according to the manufacturer’s protocol.
Plasmids transfection
The pcDNA3-AKT-T7 plasmid was a gift from William
Sellers (Addgene plasmid 9003). Cells were seeded into 6-well
plates the day before transfection. Attractene (Qiagen) was used
for transfection according to the manufacturer’s protocol. Ater 24
h of incubation, the cells were subjected to different treatments.
pcDNA3 empty vectors were used as controls for transfection
experiments.
Isolation of cytosolic protein
fractions
Cells were seeded in 6-well culture plates at 40–50%
confluence. The next day, the cells were incubated with a
determined dose of Sal B. Then, the cells were trypsinized, washed
twice with ice-cold PBS, and the cytosolic protein fractions were
isolated using the Cell Mitochondria Isolation kit (Beyotime)
according to the manufacturer’s protocol.
Western blot analysis
Western blot analysis was performed as described
previously (19). Briely, proteins
(40 μg) were resolved by 6–15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, transferred to
polyvinylidene diluoride membranes (Millipore, Billerica, MA, USA).
The membranes were then blocked in 5% (w/v) skimmed-milk for 1 h,
followed by incubation with appropriate primary antibodies (diluted
1:1,000–1:5,000) overnight at 4°C. Bound antibodies were detected
with peroxidase-conjugated goat anti-rabbit or goat anti-mouse
secondary antibodies and the blots developed using enhanced
chemiluminescence (Millipore). Western blot analysis data were
quantified by ImageJ densitometric analysis and normalized by
GAPDH.
Statistical analysis
All experiments were performed at least three times,
and the data were presented as the mean ± standard deviation.
GraphPad Prism 5.0 was used for statistical analysis. A difference
was considered significant at P<0.05.
Results
Sal B inhibits growth of HCC cells
We first examined the inhibitory effect of Sal B on
viability in HCC cell lines. As shown in Fig. 1A, Sal B significantly inhibited the
growth of cancer cells in a dose-dependent manner, with a half
maximal inhibitory concentration (IC50) of 143.82 μM for
SK-Hep-1 and 240.11 μM for Bel-7404 after 48 h of exposure.
Colony-formation experiments also indicated that Sal B markedly
inhibited the growth of HCC cells (Fig. 1B and C). We also investigated the
effects of Sal B on the growth of HL-7702 cells (a normal human
liver cell line). The results showed that the cytotoxic effect of
Sal B on HL-7702 cells (IC50 758.63 μM) appeared much
lower than that observed in SK-Hep-1 and Bel-7404 cells (Fig. 1D).
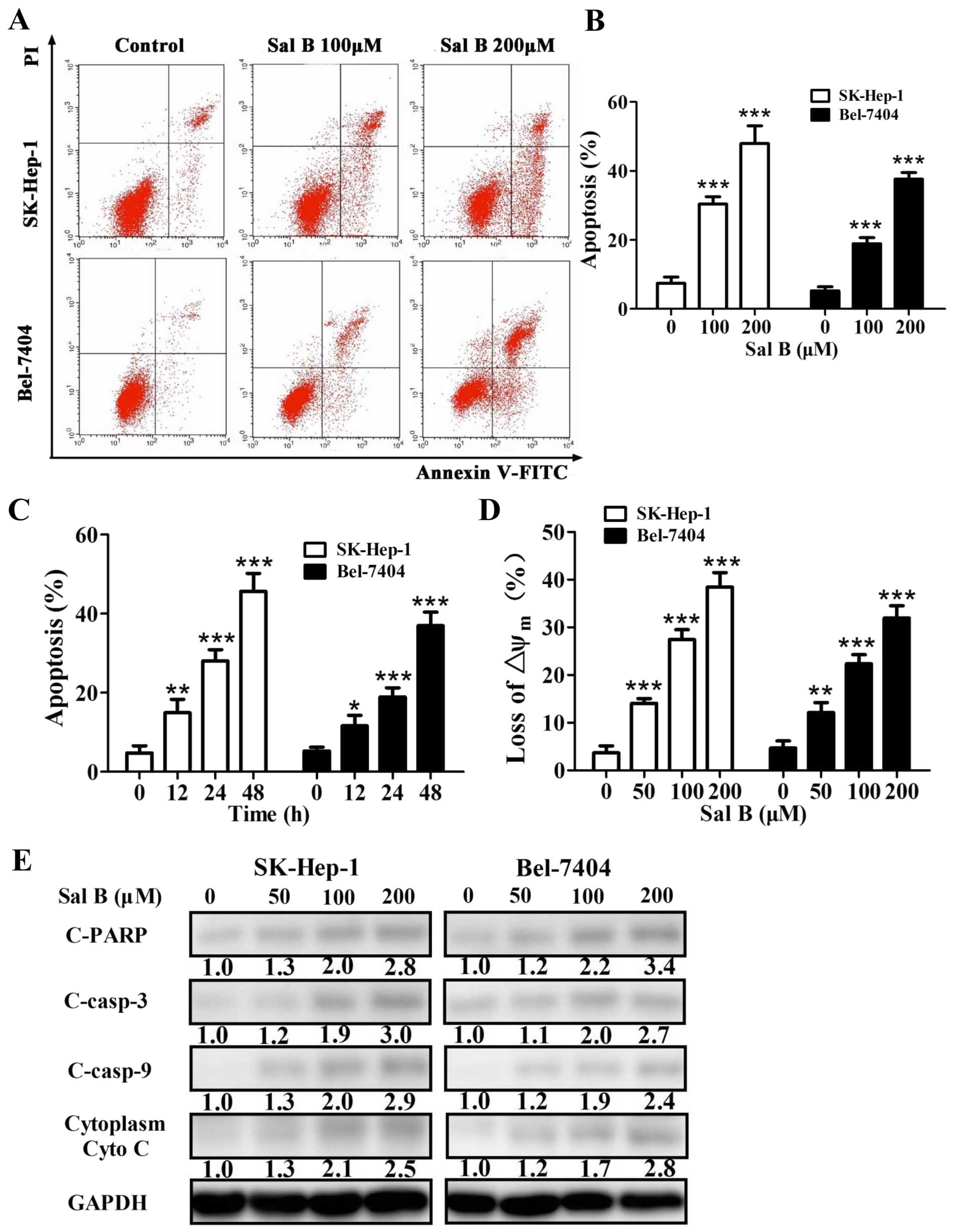
Sal B induces apoptotic cell death in HCC
cells
To examine the cell growth inhibition induced by Sal
B related to apoptosis, Sal B-treated cells were stained with
propidium iodide (PI)/Annexin V-FITC and quantified by flow
cytometry. The quantification shown for apoptosis reflected the
values for total apoptosis. We also determined whether apoptosis
was dose- or time-dependent altered in cells treated with Sal B.
Sal B induced a dose- and time-dependent increase of apoptosis in
HCC cells (Fig. 2A–C).
Then we measured the mitochondrial membrane
potential (ΔΨm) by flow cytometry and found that Sal B treatment
led to depolarization of ΔΨm in a dose-dependent manner (Fig. 2D). Western blot analyses showed
that cleaved caspase-9, cleaved caspase-3, cleaved poly(ADP-ribose)
polymerase (PARP), and cytosolic Cyto c were increased after
treatment with Sal B for 24 h (Fig.
2E). The level of cleaved PARP, cleaved caspase-3, cleaved
caspase-9, and cytosolic Cyto c increased 2.8-, 3.0-, 2.9- and
2.5-fold, respectively, in SK-Hep-1 cells treated with 200 μM Sal B
for 24 h (Fig. 2E). The level of
cleaved PARP, cleaved caspase-3, cleaved caspase-9, and cytosolic
Cyto c increased 3.4-, 2.7-, 2.4- and 2.8-fold, respectively, in
Bel-7404 cells treated with 200 μM Sal B for 24 h (Fig. 2E). These results demonstrated that
Sal B induced apoptosis in the HCC cells.
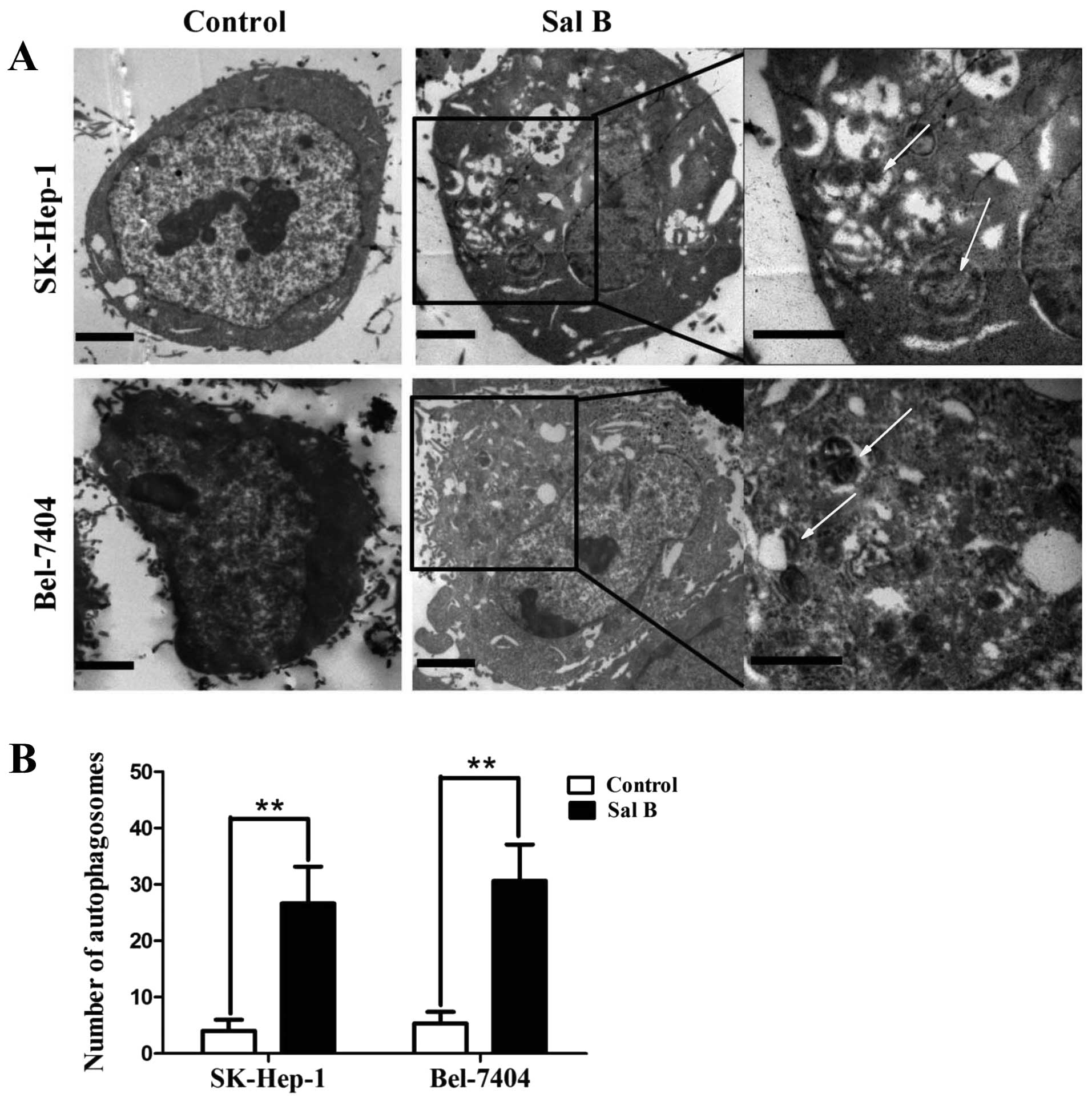
Sal B induces autophagy in HCC cells
Apoptosis and autophagy are highly interactive. To
examine whether Sal B could induce autophagy, the treated cells
were analyzed by western blot analysis and electron microscopy. The
characteristics of autophagosomes are described as double-layer
structure with cytoplasmic components. After treatment with Sal B
for 24 h, most of the HCC cells displayed an extensive accumulation
of double structures with a broad range of morphologies, indicating
the formation of autophagosomes (Fig.
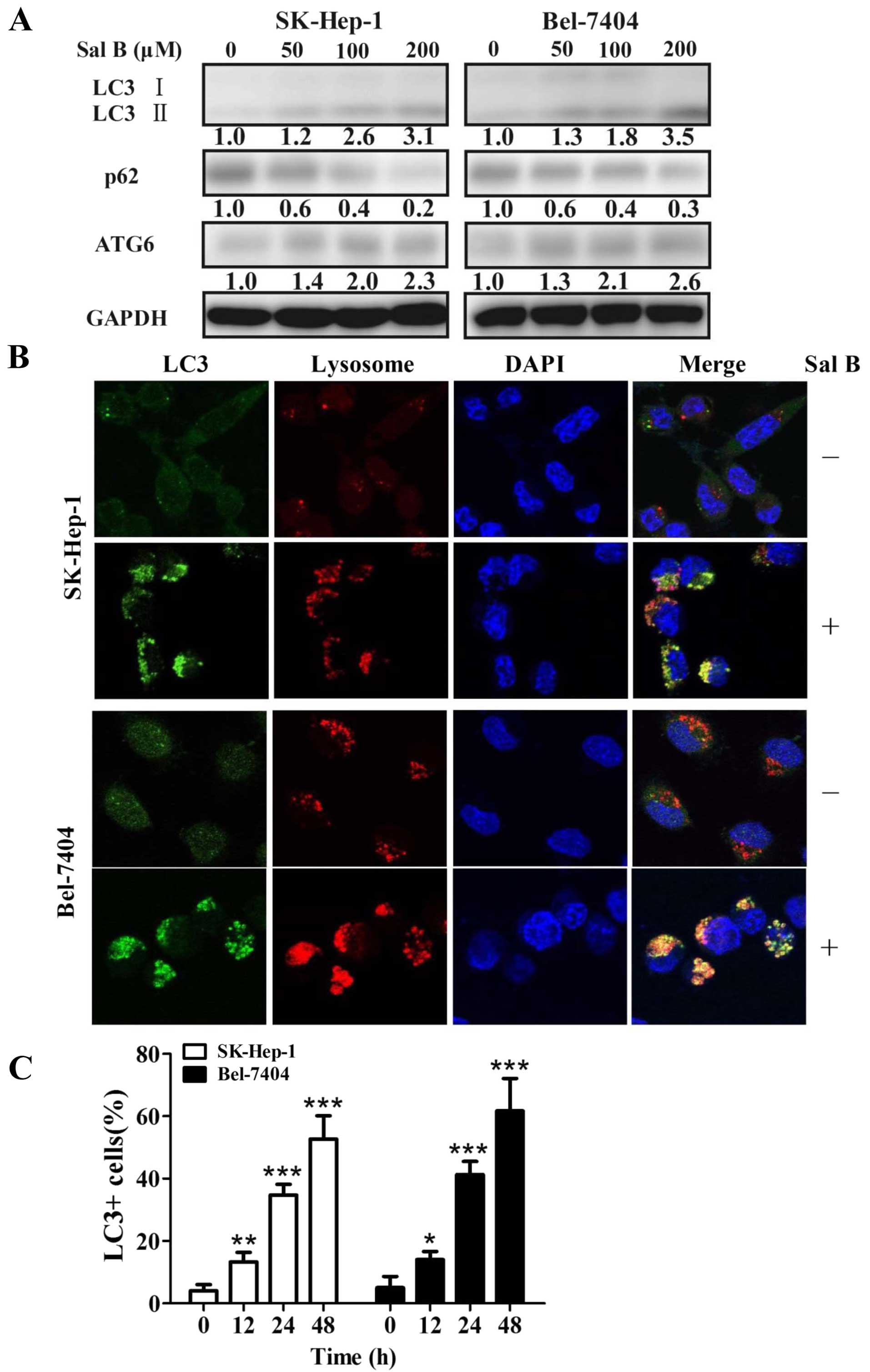
3). The significantly increased expression of LC3-II, Beclin-1
and the attenuated expression of p62/SQSTM1 were observed in cells
treat with Sal B for 24 h (Fig.
4A). The level of LC3-II and Beclin-1 increased 3.1- and
2.3-fold, while the level of p62 decreased 80%, in SK-Hep-1 cells
treated with 200 μM Sal B for 24 h (Fig. 4A). The level of LC3-II and Beclin-1
increased 3.5- and 2.6-fold, respectively, while the level of p62
decreased 70%, in Bel-7404 cells treated with 200 μM Sal B for 24 h
(Fig. 4A).
For further confirmation, the cells were incubated
with an antibody against microtubule-associated protein 1 light
chain 3 (LC3). The punctate LC3 II-labeled autophagolysosome
vacuoles were frequently observed in cells treated with Sal B for
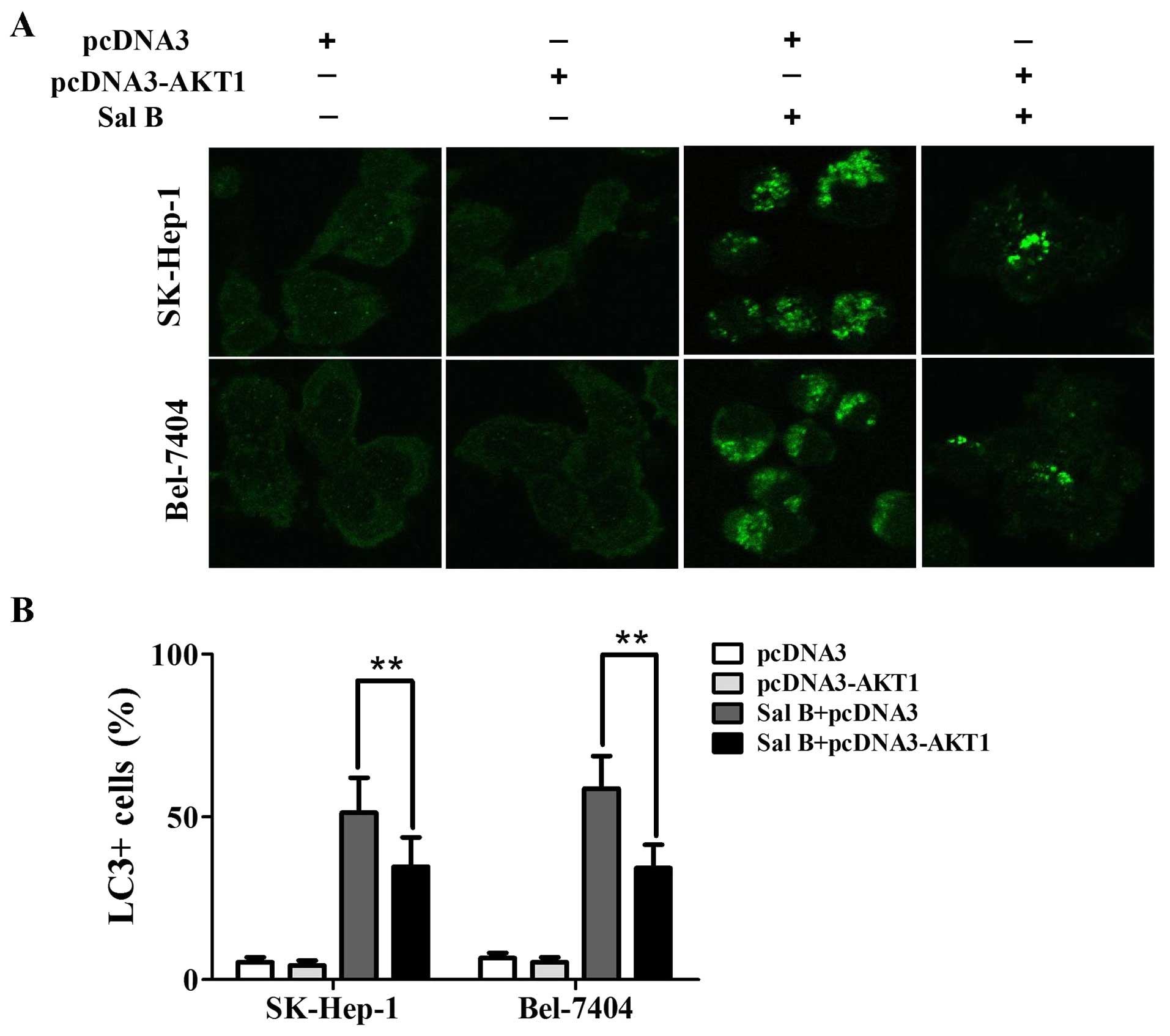
48 h compared with their controls (Fig. 4B). Next, we determined whether
autophagy was time-dependently altered in cells treated with Sal B.
A time-dependent increase of punctate LC3-II dots was observed from
12-, 24- and 48-h in cells treated with Sal B (Fig. 4C).
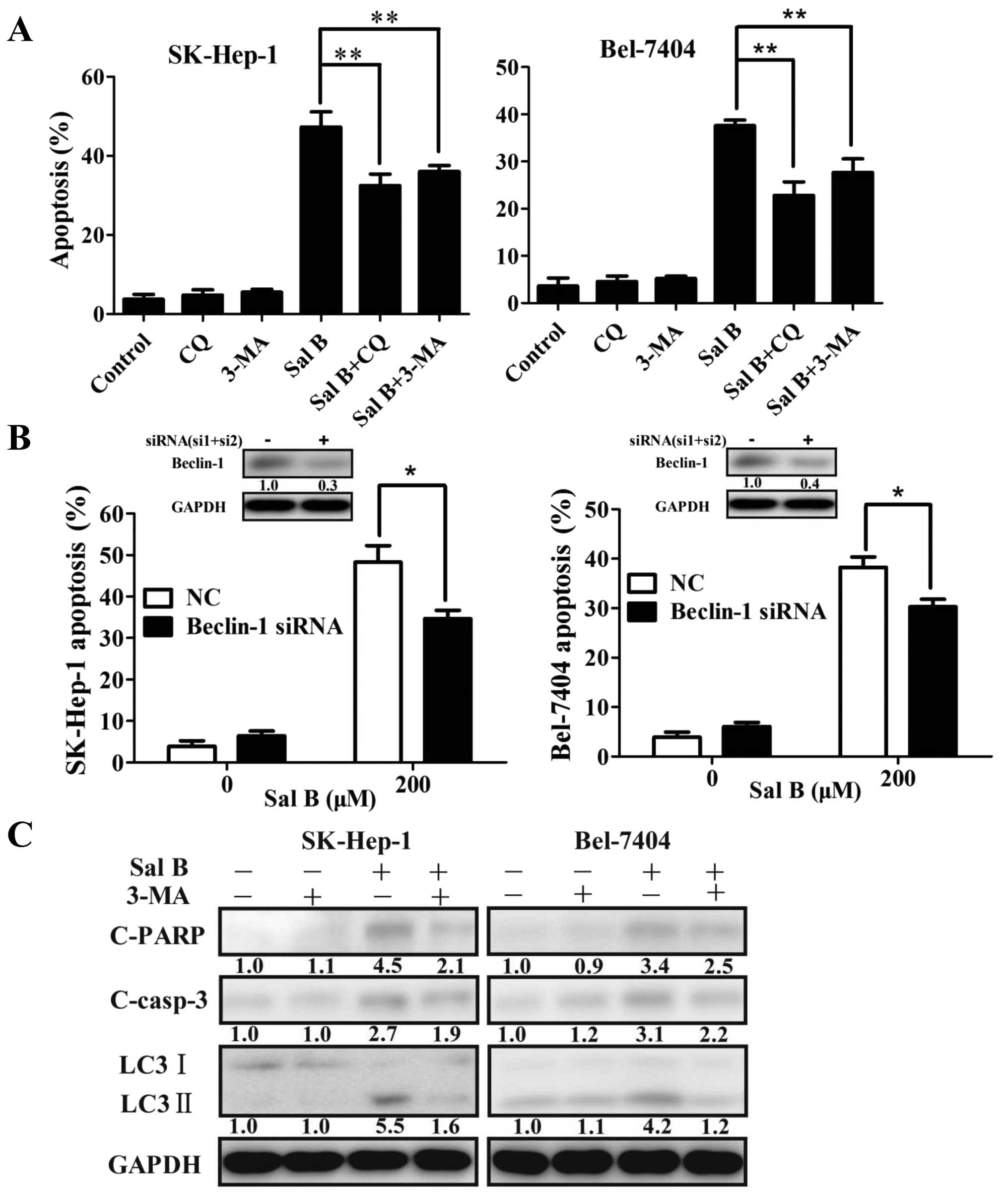
Interactions between Sal B-induced
autophagy and apoptotic cell death in HCC cells
The relationships between autophagy and apoptosis
are complicated. There is consensus that the relationships between
autophagy and apoptosis are highly depended on the tumor types and
stimulus characteristic. To confirm the role of Sal B-induced
autophagy in HCC cells promoted or inhibited apoptosis, we treated
the cells with the autophagy inhibitor 3-MA and CQ. Addition of 5
mM 3-MA or 5 μM CQ attenuated Sal B-induced apoptosis in HCC cells
(Fig. 5A). In addition, siRNAs
against Beclin-1 was used to block autophagy. The level of Beclin-1
decreased 70% in SK-Hep-1 cells and 60% in Bel-7404 cells treated
with siRNAs against Beclin-1 (Fig.
5B). Inhibition of autophagy attenuated Sal B-induced apoptosis
in HCC cells (Fig. 5B).
Western blot analysis was used to detect the
influence of 3-MA pretreatment on cleaved PARP and cleaved
caspase-3 expression induced by Sal B. Autophagy level was found to
significantly decrease after 3-MA pretreatment, and the expressions
of cleaved PARP and cleaved caspase-3 also decreased (Fig. 5C). The level of cleaved PARP,
cleaved caspase-3, and LC3-II decreased from 4.5- to 2.1-, 2.7- to
1.9- and 5.5- to 1.6-fold, respectively, in SK-Hep-1 cells treated
with 200 μM Sal B and the combination of Sal B and 3-MA for 24 h
(Fig. 5C). The level of cleaved
PARP, cleaved caspase-3, and LC3-II decreased from 3.4- to 2.5-,
3.1- to 2.2- and 4.2-to 1.2-fold, respectively, in Bel-7404 cells
treated with 200 μM Sal B and the combination of Sal B and 3-MA for
24 h (Fig. 5C). All the results
demonstrated that the inhibition of autophagy in HCC cells had the
potential of attenuating Sal B-induced apoptosis.
AKT/mTOR signaling pathway is involved in
Sal B-induced autophagy in HCC cells
AKT/mTOR signaling pathway is the key regulatory
molecule of both autophagy and apoptosis. Therefore, we
investigated whether the AKT/mTOR pathway played a central role in
Sal B-mediated cell death. Western blot analysis confirmed that the
levels of phosphorylated AKT, mTOR and its downstream effector
p70S6K and p-4EBP1 were significantly reduced by Sal B (Fig. 6A). The level of phosphorylated AKT,
mTOR, p70S6K and p-4EBP1 decreased 70, 70, 70 and 60%,
respectively, in SK-Hep-1 cells treated with 200 μM Sal B for 24 h
(Fig. 6A). The level of
phosphorylated AKT, mTOR, p70S6K and p-4EBP1 decreased 70, 80, 70
and 70%, respectively, in Bel-7404 cells treated with 200 μM Sal B
for 24 h (Fig. 6A).
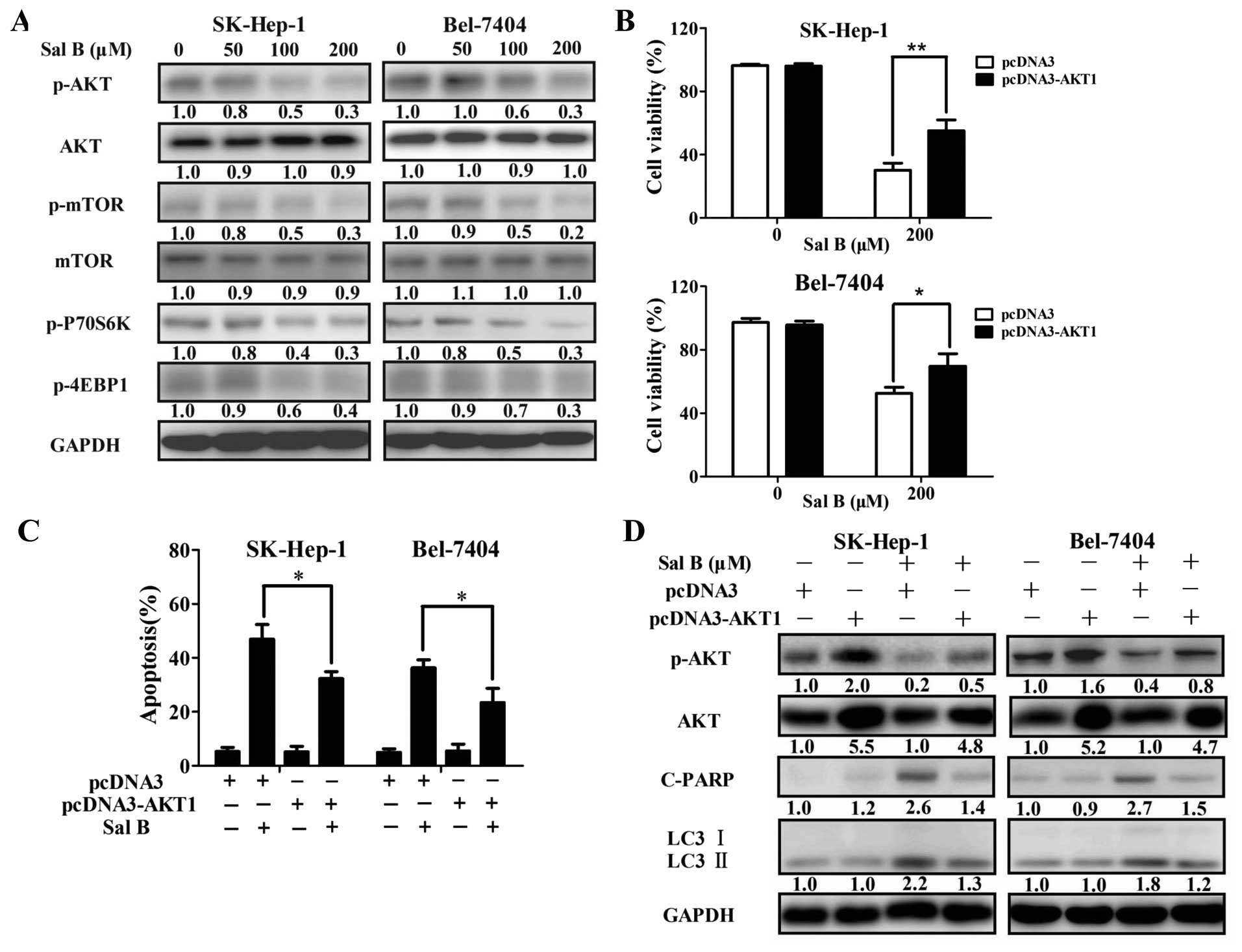 | Figure 6AKT signaling is a critical mediator
in regulating Sal B-mediated biological effects. (A) Immunoblotting
for phospho-AKT, mTOR, S6K, 4EBP1, total AKT, and mTOR in cells
treated with Sal B for 24 h. Relative quantity of proteins was
calculated by ImageJ densitometric analysis and normalized by
GAPDH. After transient overexpression of AKT, cells were treated
with Sal B, and then cell viability (B) and apoptotic rate (C) were
determined by MTS assay or flow cytometry. (D) Autophagy and
apoptosis-associated proteins, including LC3, cleaved PARP, and
total AKT were analyzed by immunoblotting. Relative quantity of
proteins were calculated by ImageJ densitometric analysis and
normalized by GAPDH. *P<0.05, **P<0.01.
The data are representative of three independent experiments. |
To further identify the role of AKT in Sal B-induced
biological effects, HCC cells were transiently transfected with
pcDNA3-AKT-T7 plasmid. The level of phosphorylated AKT and total
AKT increased 2.0- and 5.5-fold in SK-Hep-1 cells, while 1.6- and
5.2-fold in Bel-7404 cells transfected with pcDNA3-AKT-T7 plasmid
(Fig. 6D). As shown in Fig. 6B–D, enforced expression of AKT
significantly attenuated Sal B-induced growth inhibition, and
apoptosis. Furthermore, Sal B-induced autophagy was also decreased
in the transfected cells (Figs. 6D
and 7). The level of cleaved PARP
and LC3-II decreased from 2.6- to 1.4- and 2.2- to 1.3-fold,
respectively, in SK-Hep-1 cells treated with 200 μM Sal B and the
combination of Sal B and pcDNA3-AKT (Fig. 6D). The level of cleaved PARP and
LC3-II decreased from 2.7- to 1.5- and 1.8- to 1.2-fold,
respectively, in Bel-7404 cells treated with 200 μM Sal B and the
combination of Sal B and pcDNA3-AKT (Fig. 6D). These results suggested that
overexpression of AKT can override the Sal B-induced biological
effects in HCC cells. Taken together, these data demonstrate that
AKT is a critical mediator in regulating Sal B-mediated biological
effects.
Discussion
Currently, the prognosis of HCC is poor even with
multidisciplinary comprehensive treatment, and recurrence rate is
>50% (20,21). There is an urgent need for
development of efficacious therapies. Growing evidence indicates
that Chinese medicine plays a promising role in developing novel
anticancer drugs. Understanding the anti-neoplastic mechanisms of
Chinese medicine may help improve the efficacy of these agents.
In this study, we demonstrated that Sal B markedly
inhibited the proliferation of HCC cells. We also showed that Sal B
induced mitochondria-mediated apoptosis in HCC cells, accompanied
by a decrease of mitochondrial potential, and increase of cytosol
cytochrome c. Release of cytochrome c from the
mitochondria into cytosol activates intrinsic apoptosis (22). Cytosolic Cyto c initiates the
apoptotic process by activating a downstream cascade of caspases
through processing of procaspase-9 (23). In accord with our findings, some
researchers reported that Sal B inhibited the growth of cancer
cells through induction of apoptosis (24,25).
Both apoptosis and autophagy are crucial mechanisms
regulating cell survival (26). We
thus examined whether Sal B induced autophagy. Autophagy is a
tightly regulated intracellular self-digestive process involving
the lysosomal degradation of cytoplasmic organelles and proteins
(27,28). In this process, cells digest their
own cellular contents by lysosomal degradation and recycle the
ingredients to maintain cell survival (29–31).
Our data revealed that Sal B could induce autophagy accompanied by
apoptosis in HCC cells. Apoptosis and autophagy could be induced by
the same stimulus, but the interaction between them was still
unclear. In our study, we found that suppression of autophagy by
pharmacological inhibitors (3-MA and CQ) or Beclin-1 siRNA
decreased Sal B-induced apoptosis in HCC cells, revealing that the
autophagy induced by Sal B promoted HCC cell apoptosis. These
results were consistent with the findings of Kim et al
(32), who reported that the
inhibition of autophagy decreased docosahexaenoic acid-induced
apoptosis in non-small cell lung cancer cells, indicating that
autophagy was a prerequisite for apoptotic cell death. Autophagy
can inhibit, delay or promote apoptosis (33–35).
The mechansims of autophagy promoting apoptosis may include
upregulation of cells susceptible to drug-induced apoptosis and
activating of caspases (36,37).
In this study, our data showed that apoptosis level was
significantly increased with upregulation of autophagy level. We
hypothesize that Sal B could trigger apoptosis and autophagy
simultaneously, whereas autophagy increased HCC cells susceptible
to Sal B-induced apoptosis. Inhibition of autophagy could
significantly reduce the extent of Sal B-induced apoptosis. The
interaction between autophagy and apoptosis is extremely complex
and needs further investigation.
The molecular mechanism mediating apoptosis and
autophagy are complicated. Increasing evidence indicates that
autophagy and apoptosis share many common regulatory molecules,
such as AKT/mTOR signaling pathway (38). It is well known that the AKT/mTOR
pathway plays an important role in cell growth, survival,
differentiation and metabolism (39). The aberrant activation of AKT/mTOR
signaling pathway contributes to a poor prognosis and plays a
critical role in carcinogenesis of HCC (40). Inhibition of AKT/mTOR signaling
pathway causes cell death associated with apoptosis and autophagy
(41). The downstream target of
Akt/mTOR pathway can potently block Bad-induced apoptosis by
phosphorylation of Bad at S136 site to disrupt Bad’s binding to
Bcl-XL and/or Bcl-2. Thus, inhibition of Akt/mTOR pathway possibly
increases apoptosis (42).
Our results show that Sal B treatment decreases
AKT/mTOR pathway activity. This inhibitory effect was correlated
with the decrease of phosphorylation of AKT, mTOR and their
downstream targets, p70S6K and 4E-BP1. Overexpression of AKT
abolished the effects of Sal B on HCC cells. These findings
indicate that Sal B induces apoptosis and autophagy in HCC cells
through inhibition of the AKT/mTOR signaling pathway. Moreover, the
appearance of apoptosis and autophagy after Sal B treatment is
closely linked to the inhibition of AKT/mTOR pathway, demonstrating
this pathway plays a pivotal role in HCC treatment.
In conclusion, our results demonstrate for the first
time that Sal B suppressed cell proliferation and induces autophagy
and apoptosis in HCC cells through the AKT/mTOR pathway. Sal B
could act as a new anticancer agent for HCC by inducing apoptosis
and autophagy. These results will expand our knowledge of the
anticancer molecular mechanisms of Sal B and the interaction
between autophagy and apoptosis.
Acknowledgements
This study was supported by the grants from Medical
Health Research Project of Zhejiang province (2015KYB301); Science
and Technology Planning Project of Hangzhou (20150733Q20); Health
Science and Technology Plan Projects of Hangzhou (2014A11); Science
and Technology Planning Project of Wenzhou (2015Y0395).
Abbreviations:
|
CQ
|
chloroquine
|
|
Cyto c
|
cytochrome c
|
|
HCC
|
hepatocellular carcinoma
|
|
LC3
|
microtubule-associated protein 1 light
chain 3
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
PI
|
propidium iodide
|
|
PVDF
|
polyvinyldifluoride
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
|
ΔΨm
|
membrane potential
|
|
3-MA
|
3-methyladenine
|
References
|
1
|
Fares N and Peron JM: Epidemiology,
natural history, and risk factors of hepatocellular carcinoma. Rev
Prat. 63:216–217. 220–212. 2013.(In French).
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karaman B, Battal B, Sari S and Verim S:
Hepatocellular carcinoma review: Current treatment, and
evidence-based medicine. World J Gastroenterol. 20:18059–18060.
2014.PubMed/NCBI
|
|
4
|
Uhm JE, Park JO, Lee J, Park YS, Park SH,
Yoo BC, Paik SW, Koh KC, Kang WK and Lim HY: A phase II study of
oxaliplatin in combination with doxorubicin as first-line systemic
chemotherapy in patients with inoperable hepatocellular carcinoma.
Cancer Chemother Pharmacol. 63:929–935. 2009. View Article : Google Scholar
|
|
5
|
Ge S and Huang D: Systemic therapies for
hepatocellular carcinoma. Drug Discov Ther. 9:352–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon EL, Yeon JE, Lee HJ, Suh SJ, Lee SJ,
Kang SH, Kang K, Yoo YJ, Kim JH, Yim HJ, et al: Systemic cytotoxic
chemotherapy of patients with advanced hepatocellular carcinoma in
the era of sorafenib nonavailability. J Clin Gastroenterol.
48:e22–e29. 2014. View Article : Google Scholar
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al; SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han LT, Fang Y, Cao Y, Wu FH, Liu E, Mo GY
and Huang F: Triterpenoid saponin flaccidoside II from Anemone
flaccida triggers apoptosis of NF1-associated malignant peripheral
nerve sheath tumors via the MAPK-HO-1 pathway. Onco Targets Ther.
9:1969–1979. 2016.PubMed/NCBI
|
|
9
|
Xu J, Song Z, Guo Q and Li J: Synergistic
effect and molecular mechanisms of traditional Chinese medicine on
regulating tumor microenvironment and cancer cells. BioMed Res Int.
2016:14907382016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng M, Zhong LX, Zhan ZY, Huang ZH and
Xiong JP: Resveratrol treatment inhibits proliferation of and
induces apoptosis in human colon cancer cells. Med Sci Monit.
22:1101–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MS, Bang JH, Lee J, Kim HW, Sung SH,
Han JS and Jeon WK: Salvia miltiorrhiza extract protects white
matter and the hippocampus from damage induced by chronic cerebral
hypoperfusion in rats. BMC Complement Altern Med. 15:4152015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Y, Wang L, Zhang L, Wang T, Zhou Y,
Ding C, Yang R, Wang X and Yu L: Optimization of extraction and
antioxidant activity of polysaccharides from Salvia miltiorrhiza
Bunge residue. Int J Biol Macromol. 79:533–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou X, Cheung CM, Yang JM, Or PM, Lee WY
and Yeung JH: Danshen (Salvia miltiorrhiza) water extract inhibits
paracetamol-induced toxicity in primary rat hepatocytes via
reducing CYP2E1 activity and oxidative stress. J Pharm Pharmacol.
67:980–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang M, Wang P, Xu S, Xu W, Xu W, Chu K
and Lu J: Biological activities of salvianolic acid B from Salvia
miltiorrhiza on type 2 diabetes induced by high-fat diet and
streptozotocin. Pharm Biol. 53:1058–1065. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang ZS, Luo P, Dai SH, Liu ZB, Zheng XR
and Chen T: Salvianolic acid B induces apoptosis in human glioma
U87 cells through p38-mediated ROS generation. Cell Mol Neurobiol.
33:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Ge PJ, Jiang L, Li FL and Zhu QY:
Modulation of growth and angiogenic potential of oral squamous
carcinoma cells in vitro using salvianolic acid B. BMC Complement
Altern Med. 11:542011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang QL, Wu Q, Tao YY, Liu CH and
El-Nezami H: Salvianolic acid B modulates the expression of
drug-metabolizing enzymes in HepG2 cells. Hepatobiliary Pancreat
Dis Int. 10:502–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin C, Liu Z, Lu Y, Yao Y, Zhang Y, Ma Z,
Kuai M, Sun X, Sun S, Jing Y, et al: Cardioprotective effect of
salvianolic acid B on acute myocardial infarction by promoting
autophagy and neovascularization and inhibiting apoptosis. J Pharm
Pharmacol. 68:941–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JY, Sun J, Huang MY, Wang YS, Hou MF,
Sun Y, He H, Krishna N, Chiu SJ, Lin S, et al: STIM1 overexpression
promotes colorectal cancer progression, cell motility and COX-2
expression. Oncogene. 34:4358–4367. 2015. View Article : Google Scholar :
|
|
20
|
Tagliamonte M, Petrizzo A, Tornesello ML,
Ciliberto G, Buonaguro FM and Buonaguro L: Combinatorial
immunotherapy strategies for hepatocellular carcinoma. Curr Opin
Immunol. 39:103–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Lee Y, Lee M, Heo MK, Song JS, Kim
KH, Lee H, Yi NJ, Lee KW, Suh KS, et al: A phase I/IIa study of
adjuvant immunotherapy with tumour antigen-pulsed dendritic cells
in patients with hepatocellular carcinoma. Br J Cancer.
113:1666–1676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Babbitt SE, Sutherland MC, San Francisco
B, Mendez DL and Kranz RG: Mitochondrial cytochrome c biogenesis:
No longer an enigma. Trends Biochem Sci. 40:446–455. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kulikov AV, Shilov ES, Mufazalov IA,
Gogvadze V, Nedospasov SA and Zhivotovsky B: Cytochrome c: The
Achilles’ heel in apoptosis. Cell Mol Life Sci. 69:1787–1797. 2012.
View Article : Google Scholar
|
|
24
|
Hao Y, Xie T, Korotcov A, Zhou Y, Pang X,
Shan L, Ji H, Sridhar R, Wang P, Califano J, et al: Salvianolic
acid B inhibits growth of head and neck squamous cell carcinoma in
vitro and in vivo via cyclooxygenase-2 and apoptotic pathways. Int
J Cancer. 124:2200–2209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y, Guo Y and Gu X: Salvianolic acid
B, a potential chemopreventive agent, for head and neck squamous
cell cancer. J Oncol. 2011:5345482011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryter SW, Mizumura K and Choi AM: The
impact of autophagy on cell death modalities. Int J Cell Biol.
2014:5026762014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levine B and Kroemer G: Autophagy in
aging, disease and death: The true identity of a cell death
impostor. Cell Death Differ. 16:1–2. 2009. View Article : Google Scholar :
|
|
29
|
Eskelinen EL and Saftig P: Autophagy: A
lysosomal degradation pathway with a central role in health and
disease. Biochim Biophys Acta. 1793:664–673. 2009. View Article : Google Scholar
|
|
30
|
Yang Z and Klionsky DJ: Eaten alive: A
history of macroautophagy. Nat Cell Biol. 12:814–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim N, Jeong S, Jing K, Shin S, Kim S, Heo
JY, Kweon GR, Park SK, Wu T, Park JI, et al: Docosahexaenoic acid
induces cell death in human non-small cell lung cancer cells by
repressing mTOR via AMPK activation and PI3K/Akt inhibition. BioMed
Res Int. 2015:2397642015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan WR, Chen YL, Hsu HC and Chen WJ:
Antimicrobial peptide GW-H1-induced apoptosis of human gastric
cancer AGS cell line is enhanced by suppression of autophagy. Mol
Cell Biochem. 400:77–86. 2015. View Article : Google Scholar
|
|
34
|
Jing Z, Sui X, Yao J, Xie J, Jiang L, Zhou
Y, Pan H and Han W: SKF-96365 activates cytoprotective autophagy to
delay apoptosis in colorectal cancer cells through inhibition of
the calcium/CaMKIIγ/AKT-mediated pathway. Cancer Lett. 372:226–238.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsin IL, Ou CC, Wu MF, Jan MS, Hsiao YM,
Lin CH and Ko JL: GMI, an immunomodulatory protein from Ganoderma
microsporum, potentiates cisplatin-induced apoptosis via autophagy
in lung cancer cells. Mol Pharm. 12:1534–1543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen L, Meng Y, Sun Q, Zhang Z, Guo X,
Sheng X, Tai G, Cheng H and Zhou Y: Ginsenoside compound K
sensitizes human colon cancer cells to TRAIL-induced apoptosis via
autophagy-dependent and -independent DR5 upregulation. Cell Death
Dis. 7:e23342016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Young MM, Takahashi Y, Khan O, Park S,
Hori T, Yun J, Sharma AK, Amin S, Hu CD, Zhang J, et al:
Autophagosomal membrane serves as platform for intracellular
death-inducing signaling complex (iDISC)-mediated caspase-8
activation and apoptosis. J Biol Chem. 287:12455–12468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Wang K, Lei Y, Li Q, Nice EC and
Huang C: Redox signaling: Potential arbitrator of autophagy and
apoptosis in therapeutic response. Free Radic Biol Med. 89:452–465.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JJ, Loh K and Yap YS: PI3K/Akt/mTOR
inhibitors in breast cancer. Cancer Biol Med. 12:342–354. 2015.
|
|
40
|
Janku F, Kaseb AO, Tsimberidou AM, Wolff
RA and Kurzrock R: Identification of novel therapeutic targets in
the PI3K/AKT/mTOR pathway in hepatocellular carcinoma using
targeted next generation sequencing. Oncotarget. 5:3012–3022. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang F, Mao Y, You Q, Hua D and Cai D:
Piperlongumine induces apoptosis and autophagy in human lung cancer
cells through inhibition of PI3K/Akt/mTOR pathway. Int J
Immunopathol Pharmacol. 28:362–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saito Y, Tanaka Y, Aita Y, Ishii KA, Ikeda
T, Isobe K, Kawakami Y, Shimano H, Hara H and Takekoshi K:
Sunitinib induces apoptosis in pheochromocytoma tumor cells by
inhibiting VEGFR2/Akt/mTOR/S6K1 pathways through modulation of
Bcl-2 and BAD. Am J Physiol Endocrinol Metab. 302:E615–E625. 2012.
View Article : Google Scholar
|