Introduction
Pancreatic cancer is a one of the most malignant
digestive system cancers with a high morbidity and mortality in
both developed and developing countries. Pancreatic cancer is
characterized by no early detectable biomarkers, strong resistance
to chemotherapy and radiation therapy, highly metastatic potential,
worst prognosis and only 5% of 5-year survival rate (1,2).
Therefore, searching for new safer treatments for pancreatic cancer
is essential.
Recently, traditional Chinese medicines have become
a 'hot spot' for its ability to simultaneously address multiple
targets, no side-effects to patients, improving the sensitivity of
chemotherapy and radiation therapy and enhancing immunity.
Oridonin, extracted from Rabdosia rubescens, is a natural
ent-kaurane diterpenoid compound, which has many physiological and
pharmacological effects, including heat-clearing, detoxifying,
anti-inflammatory and anti-tumorigenicity (3). Previous studies have reported that
oridonin can inhibit proliferation, induce cell cycle arrest and
apoptosis and metastasis in many cancers (4–7), but
the definite molecular action mechanisms of inhibition of
pancreatic cancer have not yet been clarified.
MicroRNAs (miRNAs) are highly conserved, small
non-coding RNAs with lengths of 17–25 nucleotides (nt), which play
important gene-regulatory roles in animals and plants by pairing
with target protein coding genes at the post-transcriptional level
(8). An increasing number of
studies indicate that miRNAs play crucial roles in many important
biological processes, including development, proliferation,
apoptosis, immune response and even tumorigenesis (9). There are more than 15,000 miRNAs
found in over 140 species (10),
while most of their functions have not yet been defined and
validated. The miR-200 family is known to be involved in the
epithelial-to-mesenchymal transition (EMT), a complex biological
process, in which polarized epithelial cells convert into a
mesenchymal phenotype, epithelial cells lose their epithelial
characteristics and acquire motile mesenchymal properties: loss of
cell-cell adhesion; increased motility and invasiveness (11,12).
During the acquisition of EMT characteristics, cancer cells lose
the epithelial markers, such as E-cadherin, and gain the
mesenchymal markers, such as vimentin, fibronectin and N-cadherin
(13). EMT plays key roles in many
physiological and pathological process, such as embryonic
development, wound healing and carcinogenesis (14). EMT was recently classified as type
III EMT: type I EMT is during implantation, embryogenesis and organ
development; type II EMT is associated with tissue regeneration and
organ fibrosis; type III EMT is associated with cancer progression
and metastasis (15). The
miR-200b-3p is a member of the miR-200 family, which has been
demonstrated to play an important role in migration and invasion in
many types of cancer, such as cervical (16), breast cancer (17) and esophageal squamous cell
carcinoma (18). However, the
specific role and molecular mechanism of miR-200b-3p in human
pancreatic cancer remain poorly understood.
In the present study, the relationship among
oridonin, miR-200b-3p and pancreatic cancer on EMT were
investigated to look for the molecular mechanism or signaling
pathways on the migration in pancreatic cancer in order to find new
safer treatments for the pancreatic cancer.
Materials and methods
Cell culture
The BxPC-3 and PANC-1 human pancreatic cancer cell
lines were provided by the Institute of Biochemistry and Cell
Biology, Shanghai Institute of Biological Sciences, Chinese Academy
of Sciences. The cells were respectively cultured in RPMI-1640
(BxPC-3) (Gibco, Grand Island, NY, USA) or Dulbecco's modified
Eagle's medium (DMEM; Gibco) culture medium containing 10% fetal
bovine serum (FBS; Gibco) in a humidified incubator with 5%
CO2 at 37°C. Exponentially growing cells were used for
the experiments.
Cell viability assay
Cell viability was quantified using Cell Counting
kit-8 (CCK-8; Beyotime Institute of Biotechnology, Shanghai, China)
according to the manufacturer's instructions. BxPC-3 cells
(1×104 cells/well) and PANC-1 cells (5×103
cells/well) were respectively seeded into 96-well plates in a final
volume of 100 µl and cultured for 24 h. Then, the medium was
replaced by 100 µl culture medium containing different
concentrations of oridonin (0, 20, 40, 60, 80, 100, 120, 140 or 160
µM) (Gracia Chemical Technology Company, Ltd., Chengdu,
China; 98% purity, HPLC), and the cells were incubated for 24 h.
The negative control cells were treated with medium containing 0.1%
dimethyl sulfoxide (DMSO) only. The same DMSO concentration was
used for drug delivery. After oridonin treatment, 10 µl of
CCK-8 solution was added into each well and the cells were
incubated for 4 h at 37°C. The absorbance was measured at 450 nm
using a microplate reader (Model 680; Bio-Rad Laboratories,
Hercules, CA, USA) and the percentage of cell viability was
calculated as follows: Viability ratio % =
A450(oridonin)/A450(control) × 100%. The IC50 value was
calculated as the concentration of oridonin that inhibited cell
growth by 50%. At least 3 independent experiments were
performed.
RNA isolation, quantitative real-time PCR
and target prediction
BxPC-3/PANC-1 cells in logarithmic growth phase were
seeded in 60-mm dishes at a density of 2×105 cell/well
and incubated overnight. One group of these cells was subsequently
treated with 87.8 µM (BxPC-3)/55.8 µM (PANC-1)
oridonin and another was used as a blank control group cultured in
medium containing 0.1% DMSO for 24 h. Total RNA (containing small
RNAs) was extracted using the TRIzol LS reagent (Invitrogen/Life
Technologies) following the manufacturer's protocol. cDNA was
synthesized in a MyCycler™ Thermal Cycler (Bio-Rad Laboratories),
and miR-200b-3p levels were quantified by quantitative real-time
polymerase chain reaction (qPCR) in a real-time PCR detector
(Bio-Rad Laboratories) using the PrimeScript™ miRNA qPCR Starter
kit ver. 2.0 (Takara, Dalian, China) following the manufacturer's
protocol, and calculated using RNU6B as the internal control by the
2−ΔΔCT method. The 5′–3′ sequence of the primer
miR-200b-3p is TAATACTGCCTGGTAATGATGA. All of the reactions were
run in triplicate. In order to find the functional explanation of
miR-200b-3p, we explored the most potential targets of miR-200b-3p.
The prediction of miR-200b-3p targets was performed using online
software TargetScan (http://www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de/cgi-bin/new_PicTar_vertebrate.cgi)
and miRanda (http://www.microrna.org/microrna/home.do). The
intersection of the results from these three types of software was
taken as the final target genes.
Oligonucleotide transfection
The miR-200b-3p mimic, the mimics negative control
precursor (NC), inhibitor and an inhibitor negative control
precursor (NC) were obtained from Shanghai GenePharma, Co., Ltd.
(Shanghai, China). These transfections were performed using
Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) following the
manufacturer's protocol. Cells were grown in 6-well culture plates
at a density of 2×105 (BxPC-3)/3×105 (PANC-1)
cells/well for 24 h. For each well, 5 µl human miR-200b-3p
mimics/inhibitor or mimics/inhibitor inhibitor NC was added to 250
µl culture medium. Next, 5 µl of Lipofectamine 2000
was added to 250 µl culture medium and then mixed. The
mixture was added to the cells, incubated for 6 h and then was
replaced by 2000 µl of new culture medium. For western blot
assays, cells were collected 48 h after the transfection.
Western blot analysis
Total protein was extracted from cells or tissues.
BxPC-3 or PANC-1 cells treated with oridonin or transfected by
miR-200b-3p were rinsed twice with phosphate-buffered saline (PBS)
and lysed in RIPA lysis buffer (Beyotime Institute of
Biotechnology) along with PMSF. The lysates were centrifuged, and
the supernatants were collected to be quantitated using a
bicinchoninic acid assay kit (PQ0011; Multi Sciences Biotech, Co.,
Ltd., Hangzhou, China). A total of 40 µg of proteins was
loaded onto an 8% SDS-polyacrylamide gel for electrophoresis
followed by transfer to polyvinylidene difluoride (PVDF) membranes.
Skim milk (5%) in Tris-buffered saline and Tween-20 was used to
block the membrane for 2 h at room temperature. Then, the membrane
was incubated overnight at 4°C with the following primary antibody:
TCF8/ZEB1 (D80D3) rabbit mAb (1:1,000, #3396; Cell
SignalingTechnology, Danvers, MA, USA), E-cadherin (24E10) rabbit
mAb (1:1,000, #3195; Cell Signaling Technology), anti-fibronectin
antibody (1:1,000, ab2413; Abcam), anti-N cadherin antibody [5D5]
(1:1,000, ab98952; Abcam), anti-β-actin monoclonal antibody
(1:1,000, Mab1445; Multi Sciences Biotech). The membranes were then
incubated with secondary antibodies conjugated with horseradish
peroxidase (HRP): goat anti-mouse IgG, (GAM0072; Multi Sciences
Biotech) or goat anti-rabbit IgG (GAR0072 Multi Sciences Biotech)
for 2 h at room temperature. The membranes were then visualized
using an ECL substrate kit (P1425; Multi Sciences Biotech) on the
Omega Lum G Imaging System. β-actin levels were used to standardize
protein loading. ImageJ software was used to quantify band
intensities. All analyses were conducted in triplicate.
Lentivirus transduction
The lentiviral LV-miR-200b-3p and LV-NC
(miR-200b-3p-expressing or vector-control lentivirus) was purchased
from Shanghai GenePharma. Lentivirus transfections were performed
according to the manufacturer's instructions to establish
anti-miR-200b-3p-expressing stable clones in BxPC-3 and PANC-1
cells (LV-miR-200b-3p). The control clones (LV-NC) were produced
with a similar method. Briefly, the BxPC-3 cells (2×105)
or PANC-1 cells (1×105) were seeded in each 24-well
culture plate for 24 h and then were infected with LV-miR-200b-3p
or LV-NC in the presence of 5 µg/ml polybrene and incubated
for 24 h. After replaced by 2000 µl of new culture medium,
the cells were cultured for another 48 h. The cells were selected
using 2 µg/ml (BxPC-3) and 1 µg/ml (PANC-1) puromycin
to establish stable cell lines.
Migration assays
The migration assays was performed using 24-well
Transwell chambers (8 µm; Millpore Corp., Billerica, MA,
USA). (BxPC-3) or 3×104 (PANC-1) cells
(4×104) or treated with oridonin, miR-200b-3p or NC in
200 µl medium without FBS were seeded in the upper chamber
of Transwell migration chambers without Matrigel (8 µm;
Millpore Corp.). The lower chamber was filled with 500 µl
culture medium containing 10% FBS. After 24 h, the upper chamber
was washed twice with 1X PBS and fixed by 4% polyformaldehyde for
30 min. The cells remaining on the upper membrane were removed with
cotton wool, whereas the cells that had migrated through the
membrane were stained with 0.1% crystal violet for 30 min, or DAPI
(1 µg/ml) for 15 min. After three washes with PBS, the
samples were photographed and counted in five independent ×400
fields for each well using an microscope (Olympus, Tokyo, Japan).
The mean values of the data were obtained from three separate
chambers.
Detection of ICAM-1 and VCAM-1
production
The levels of ICAM-1 and VCAM-1 in response to
treatment of cells with oridonin (0, or 87.8 µM/55.8
µM) was determined using a human enzyme linked immunosorbent
assay (ELISA) kits. Briefly, culture supernatants of cells were
collected at 24 h post-treatment and cleared of cell debris by
brief centrifugation. ICAM-1 and VCAM-1 levels were measured using
human intercellular adhesion molecule 1, ICAM-1 ELISA kit
(CSB-E04574h; Cusabio Biotech, Co., Ltd., Wuhan, China) and human
vascular cell adhesion molecule 1, VCAM-1 ELISA kit (CSB-E04753h;
Cusabio Biotech) according to the manufacturer's instructions. The
absorbance was measured at 490 nm using a microplate reader.
Immunofluorescence and confocal
microscopy
For immunofluorescence experiments, PANC-1 cells
were plated onto 35-mm glass-based dishes (801002; NEST
Biotechnology, New Orleans, LA, USA) glass one day before the
treatment with oridonin (0 or 55.8 µM) for 24 h. Cells were
fixed in 4% polyformaldehyde for 30 min and permeabilized with 0.2%
Triton X-100 for 15 min. Then, they were blocked with 5% BSA for 1
h. Primary antibody incubations with the antibody β-tubulin (1:200,
ab009; Multi Sciences Biotech) in PBS containing 2% FBS were
performed overnight at 4°C followed by incubation with the goat
anti-mouse IgG H&L (DyLight 488) (1:400, ab96871; Abcam) in PBS
containing 2% FBS as a secondary antibody for 2 h at room
temperature. Finally, cell nuclei were stained with DAPI (0.5
µg/ml) for 15 min, and images were obtained using a confocal
fluorescence microscope. PBS was used for all washing steps.
Tumor formation assay in nude mice
The 16 BALB/C nude mice (5-week-old, 20–25 g body
weight) were provided from the Animal Experimental Center of the
Zhejiang Chinese Medical University in accordance with the
Institutional policies. The mice were randomly divided into four
groups (n=4/group): the control group, the oridonin group, the
miR-200b-3p group and the NC group. The control group and the
oridonin group were injected with 5×106 BxPC-3 cells;
the miR-200b-3p group and the NC group were injected with
5×106 BxPC-3 cells infected with miR-200b-3p or mock
vector, respectively. These cells were all suspended in 200
µl RPMI-1640 medium and injected subcutaneously into the
flank of each nude mice. When the volume of tumor reached 150
mm3, oridonin was injected into the mice of the oridonin
group by intraperitoneal injection at a concentration of 10 mg/kg
and the other groups were injected with the same amount of saline
every day. Tumor sizes and body weights were measured two or three
times per week. The tumor volume was calculated as follows: Tumor
volume (mm3) = 1/2× length × width2. After 7
weeks, the mice were sacrificed and the xenograft tumors were
excised, weighed, harvested and fixed for the next experiment.
H&E staining and
immunohistochemistry
For histopathologic analysis, tumor sections from
the control group and the normal pancreas from the nude mice were
fixed in 10% paraformaldehyde and embedded in paraffin for
hematoxylin and eosin staining. Immunohistochemistry analysis (IHC)
was performed as per the manufacturer's protocol of
Histostain™-Plus kits (Bioss, Beijing, China). In brief, mouse
tumor tissues were fixed in 10% paraformaldehyde and embedded in
paraffin, and antigen retrieval was carried out using a target
retrieval solution. The sections were incubated with a rabbit
antibody to E-cadherin (#3195; 1:100; Cell Signaling Technology) or
vimentin (#5741; 1:100; Cell Signaling Technology) overnight at
4°C, followed by another incubation with a horseradish
peroxidase-conjugated goat-anti-rabbit secondary antibody for 2 h
at 37°C. Finally, the sections were counterstained with hematoxylin
and images (×20) were acquired with a microscope.
Statistical analysis
Statistical analysis was performed using Statistical
Program for Social Sciences (SPSS) software 19.0. The results from
at least three independent experiments are presented as means ± SD.
Two different groups were analyzed using the two-tailed Student's
t-test. All tests performed were two-sided. P<0.05 and
P<0.001 were considered statistically significant.
Results
Oridonin affects cell viability and
inhibits cell proliferation
To investigate the effect of oridonin on viability
in BxPC-3 and PANC-1 cells, cells were treated with various
concentrations of oridonin for 24 h. As shown in Fig. 1A and B, oridonin inhibited BxPC-3
and PANC-1 cell proliferation in a dose-dependent manner. At very
low doses (20 µM), oridonin showed no significant difference
with the control cells in BxPC-3 and PANC-1 cells. The cell
proliferation was inhibited in BxPC-3 and PANC-1 cells following
treatment with 40 µM oridonin compared to the control
(P<0.05). The 50% inhibitory concentration of oridonin
(IC50) was 87.8 µM in BxPC-3 cells and 55.8
µM in PANC-1 cells by using SPSS 19.0 software. Then, BxPC-3
and PANC-1 cells were treated with oridonin at its IC50
value.
Oridonin treatment alters miR-200b-3p
expression in BxPC-3 and PANC-1 cells
A previous microarray study has shown that 105
miRNAs are differentially expressed in BxPC-3 cells treated with
oridonin at concentrations of 0 and 87.8 µM (19). As shown in Fig. 1C, the qPCR verified results showed
that miR-200b-3p was downregulated 2.22-fold (P<0.05).
Furthermore, we have validated the results in another human
pancreatic cancer cell line PANC-1. In PANC-1 cells, oridonin
treatment also downregulated the expression levels of miR-200b-3p
(P<0.01). A miRNA usually exerts its function by suppressing the
expression of target genes. Therefore, our next aim was to
investigate the targets of miR-200b-3p using three different types
of online software: TargetScan, PicTar and miRanda. The
intersection of the three software predictions was taken as the
final potential target genes. Based on the bioinformatics
prediction that there are two potential positions, 369–375 and
463–469, in the 3′UTR of ZEB1 mRNA targeted by miR-200b-3p
(Fig. 1D). These results suggested
that miR-200b-3p is downregulated in human pancreatic cancer BxPC-3
and PANC-1 cells with oridonin treatment, and may be a potential
therapeutic target of oridonin for pancreatic cancer, although
further studies are required to explore this possibility.
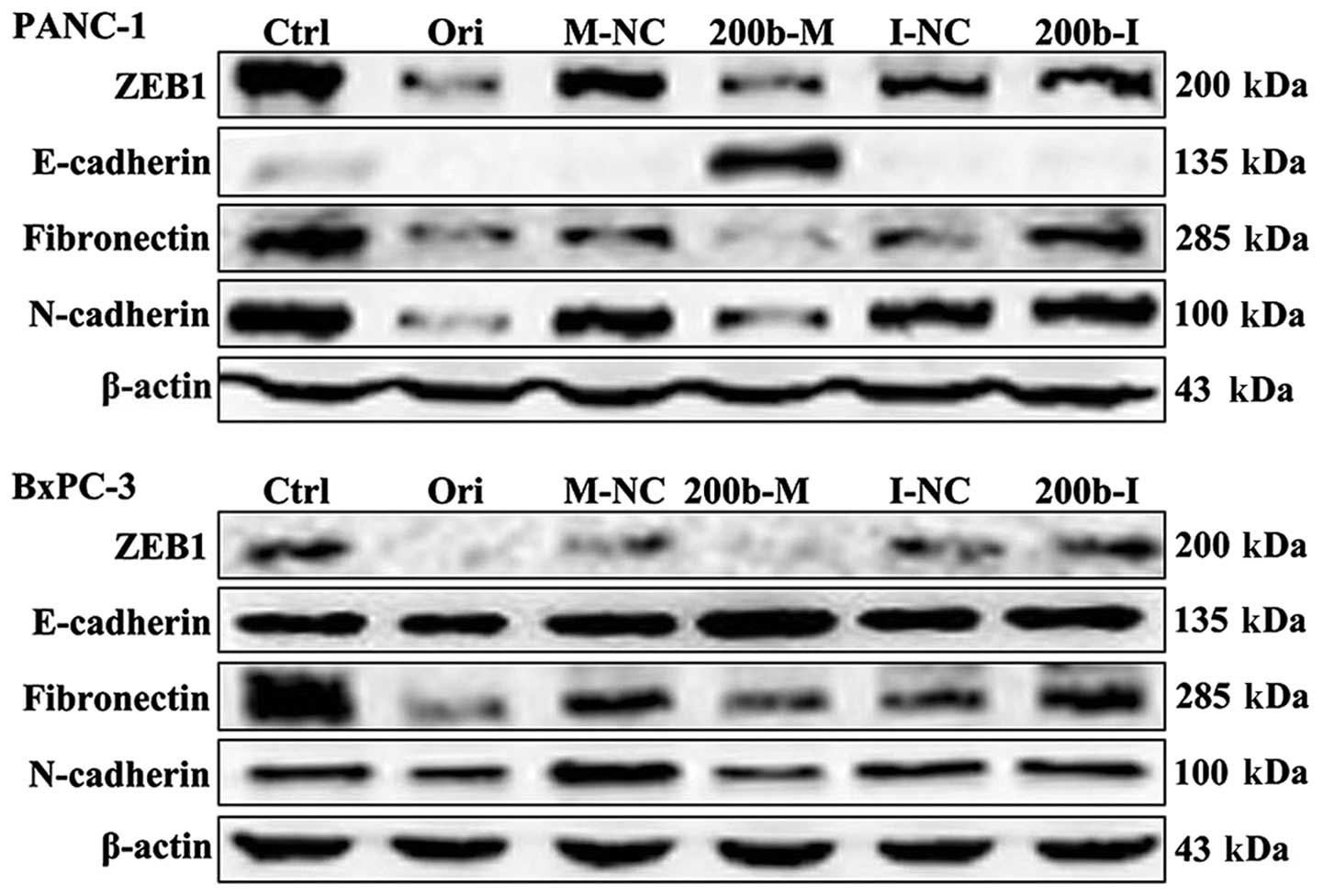
Oridonin/miR-200b-3p regulate expression
of proteins on EMT in BxPC-3 and PANC-1 cells
To verify the candidate genes regulated by
miR-200b-3p, we subsequently compared ZEB1 protein levels in cells
transfected with miR-200b-3p and the control by western blot
analysis. As shown in Fig. 2, ZEB1
protein levels were significantly downregulated in BxPC-3 and
PANC-1 cells transfected with miR-200b-3p mimics, but not in those
transfected with mimics NC. These results demonstrated that ZEB1 is
a target of miR-200b-3p. We next examined the effect of
oridonin/miR-200b-3p on EMT in BxPC-3 and PANC-1 cells. BxPC-3 and
PANC-1 cells were treated with oridonin at its IC50 or
transfected with miR-200b-3p for western blot analysis. The results
showed that the protein expression levels of ZEB1, E-cadherin,
N-cadherin and fibronectin decreased in the BxPC-3 and PANC-1 cells
treated with oridonin, comparing to the control. miR-200b-3p
overexpression significantly suppressed ZEB1, N-cadherin and
fibronectin expression and increased the expression of E-cadherin
(Fig. 2).
Oridonin/miR-200b-3p inhibit the
migration of BxPC-3 and PANC-1 cells
To study the role of oridonin/miR-200b-3p in cell
migration, we assessed the capacity of migration in BxPC-3 and
PANC-1 cells after treatment with oridonin or transfection with a
miR-200b-3p lentivirus by using crystal violet stain and DAPI. As
shown in Fig. 3, much fewer cells
were found to infiltrate the membranes without Matrigel under the
treatment of oridonin or miR-200b-3p, compared with untreated
groups (P<0.01) in BxPC-3 and PANC-1 cells. These results
indicate that oridonin or miR-200b-3p inhibits the migration of
BxPC-3 and PANC-1 cells in vitro.
Oridonin reduces the expression of ICAM-1
and VCAM-1 in BxPC-3 and PANC-1 cells
We next investigated the effect of oridonin on
intercellular adhesion molecules, including the human intercellular
adhesion molecule 1 (ICAM-1) and human vascular cell adhesion
molecule 1 (VCAM-1). Cells were treated with 87.8 µM
oridonin (BxPC-3) or 55.8 µM oridonin (PANC-1) for 24 h. The
ELISA results showed that treatment of cells with oridonin reduced
the expression of ICAM-1 and VCAM-1 (Fig. 4). ICAM-1 expression was
significantly reduced in the oridonin treatment groups compared
with the negative control group in BxPC-3 cell (94.51±2.02 vs.
152.09±2.11 ng/l; P<0.01) and PANC-1 cell (112.66±3.21 vs.
153.92±2.38 ng/l; P<0.01). Similar trends were observed for
VCAM-1 expression, which was significantly reduced in the oridonin
treatment groups compared with the negative control group in BxPC-3
cells (432.14±8.37 vs. 475.06±2.09 µg/l; P<0.01) and
PANC-1 cells (471.22±4.37 vs. 368.64±5.23 µg/l;
P<0.05).
Oridonin alters the structure of the
cytoskeleton in PANC-1 cells
To research the effect of oridonin on cytoskeleton
beta microtubule protein (β-tubulin) in PANC-1 cells, we observed
the β-tubulin in PANC-1 cells after treatment with oridonin by
immunofluorescence and confocal microscopy. As shown in the
Fig. 5, PANC-1 cells are fusiform,
β-tubulin are dense filiform and distributed uniformly,
parallelling to the longitudinal axis of the cells. Compared with
the control group, the PANC-1 cells treated with oridonin are
deformed and the edges are irregular, with β-tubulin arranged
disorderly, significant changes had taken place. The result
illustrated that the structure of cytoskeleton in PANC-1 cells can
be changed by oridonin and then influence the cell morphology and
function.
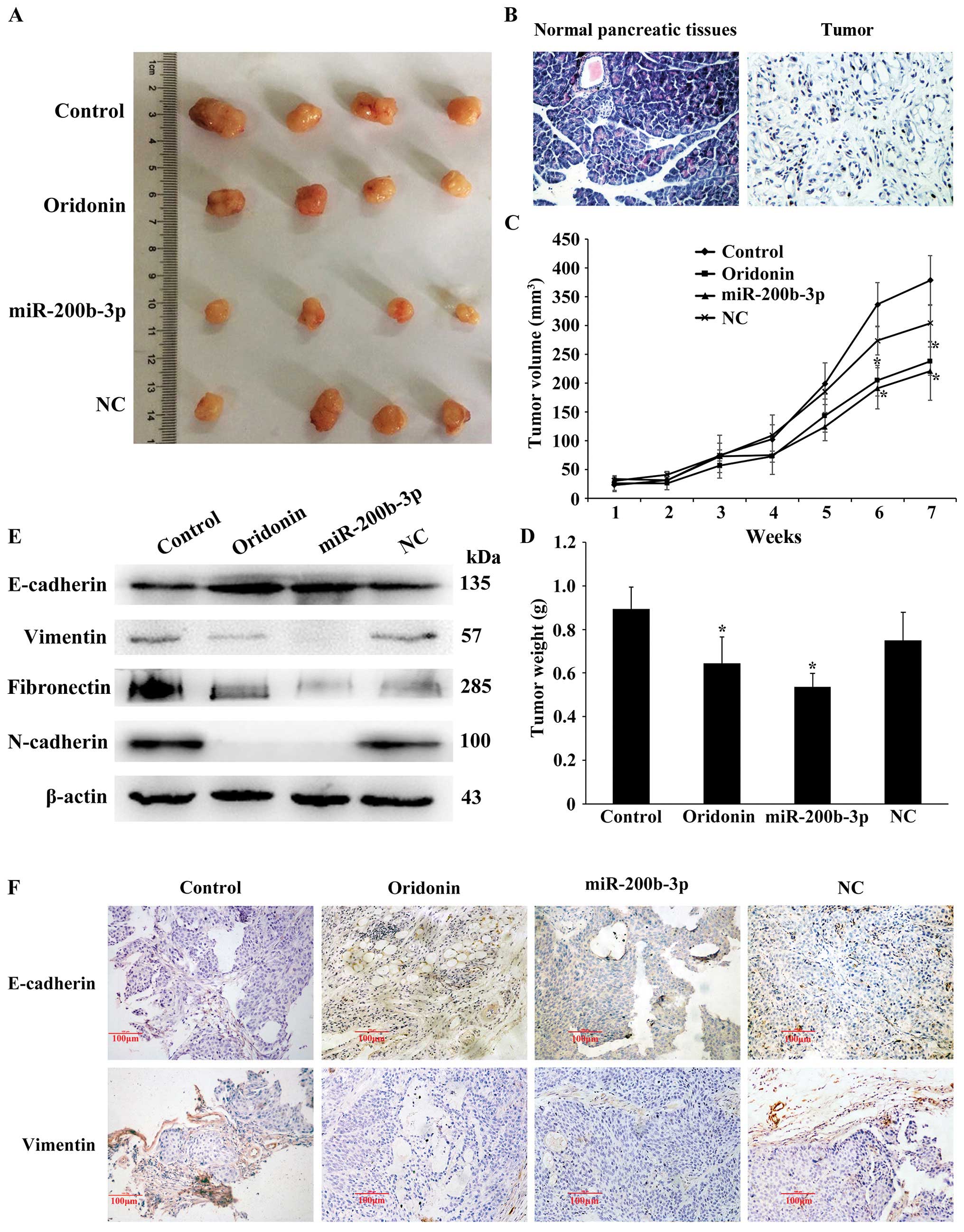
Oridonin/miR-200b-3p suppresses
tumorigenicity and EMT in nude mice
Next, we identified the effect of
oridonin/miR-200b-3p on tumorigenicity and EMT in mouse models. The
normal pancreatic tissues and transplantation tumor were stained
using hematoxylin and eosin (H&E). The result showed that the
pancreatic tissues are segmented into lobules by a small amount of
connective tissue separately. Acinar cells appear as grape-like
cell clumps, cells presenting cone-shape, nuclear is round or
ovoid, located at the base of the cells, with abundant eosinophilic
cytoplasm. The pancreatic duct arranges as arborization and the
boundaries of the blood vessels and gland are clear. A few
funicular permutations and colored shallow light islet cells
distributed in the pancreatic tissue. In the heteroplastic
pancreatic cancer tissue, the cell sizes show inconformity and the
arrangements are irregular, acinar cells decreased significantly
and cytoplasmic staining was shallow. The tissues are mainly made
up of a few different ductal glands, accompanied by abundant
fibrous stroma. The cell abnormality is high, columnar cells and
spindle cells are mixed, no bleeding or necrosis (Fig. 6B). When injected with oridonin or
BxPC-3 cells stably expressing miR-200b-3p in nude mice, the tumors
grew more slowly than that of the control, and the gap expanded
until the endpoint of 7-weeks (Fig.
6A). Consistently, the oridonin-treated tumors and the
miR-200b-3p overexpressed tumors were smaller in size and weight
compared with the control tumors (Fig.
6C and D). Together, our data indicated that
oridonin/miR-200b-3p suppresses tumorigenicity in vivo. We
next examined the effect of oridonin/miR-200b-3p on EMT in mouse
models by western blot analysis. The results showed that the
protein expression levels of vimentin, N-cadherin and fibronectin
were decreased while the expression of E-cadherin was increased in
nude mice treated with oridonin or miR-200b-3p, comparing with the
control (Fig. 6E). In addition,
immunohistochemical staining for E-cadherin and vimentin in primary
tumor tissues showed that the oridonin-treated group and the
miR-200b-3p overexpressed group exhibited an increased expression
of E-cadherin, an epithelial marker and a reduced expression of
vimentin, a mesenchymal marker (Fig.
6F). These results revealed that oridonin or upregulation of
miR-200b-3p is associated with EMT in vivo.
Discussion
With the discovery of miRNAs, it has been shown that
miRNAs are important in the regulation of gene expression networks,
and they also play crucial roles in many biological processes.
Abnormal expression of miRNAs is associated with many diseases,
such as nervous system diseases, cardiovascular disease and cancer
(20). miRNAs can function as
tumor suppressors or oncogenes in cancer. To date, studies have
demonstrated that there are 95 aberrant miRNAs (let-7 family,
miR-7, miR-92, miR-93, miR-196a, miR-190, miR-186, miR-221,
miR-222, miR-200b, miR-15b and miR-95) have been shown altered in
pancreatic cancer (21).
Traditional Chinese medicines have become a widely
discussed topic in relation to their potential antitumor
properties. However, the mechanisms of the antitumour activity have
not been completely delineated. Recently, some studies showed that
the cancer may be inhibited by the active ingredients of
traditional Chinese medicines though regulating miRNAs, which may
be treated as targets for cancer therapies (22–24).
Oridonin is one of the most effective antitumor agents obtained
from Rabdosia rubescens. In the present study, the results
demonstrate that oridonin can inhibit the growth of human BxPC-3
and PANC-1 pancreatic cancer cells in dose-dependent manner, and
the IC50 was 87.8 µM in BxPC-3 cells and 55.8
µM in PANC-1 cells, respectively. In our previous study, we
found that oridonin alters the expression profiles of miRNAs in the
BxPC-3 human pancreatic cancer cells, 105 miRNAs were significantly
altered: 49 miRNAs were significantly downregulated, while 56 were
significantly upregulated. Among them, miR-200b-3p was
significantly downregu-lated (19), and also significantly downregulated
in the PANC-1 cells (Fig. 1C).
Next, we tried to explore the mechanism by which miR-200b-3p exerts
influence on the BxPC-3 and PANC-1 cells. Zinc finger E-box-binding
homeobox 1 (ZEB1) was predicted to be the theoretical target gene
of miR-200b-3p using three public bioinformatic algorithms in
combination (Fig. 1D).
ZEB1 is a member of the ZEB family of transcription
factors, it contains two zinc finger clusters and a homeodomain
(25). Growing evidence shows that
ZEB1 plays an important role in cell proliferation, apoptosis,
metastasis and angiogenesis (26–29).
EMT is a crucial step which cancer cells must pass before they can
undergo metastasis and ZEB1 is one of the key inducers of
epithelial-to-mesenchymal transition. In the present study, we
found that overexpression of miR-200b-3p significantly
downregulated the expression of ZEB1 protein, which demonstrated
that ZEB1 is one of the target genes of miR-200b-3p. Whereas the
expression of miR-200b-3p and ZEB1 were all downregulated by
oridonin in the BxPC-3 and PANC-1 cells. These results demonstrate
that the relationship among oridonin, miR-200b-3p and ZEB1 is not a
single cascade relationship, which may be affected by other genes
and signal pathways. It remains to be investigated in our further
study.
The miR-200 family is composed of miR-141, -200a,
-200b, -200c, -429 and miR-205. Kundu et al (30) found that the miR-200 family and the
miR-183-96-182 cluster can target Foxf2 to inhibit EMT, invasion
and metastasis in lung cancers. In anaplastic thyroid cancer,
miR-200 involves in epithelial-to-mesenchymal transition (EMT) by
regulating EGF/EGFR signaling (31). A recent study has shown that Pien
Tze Huang can inhibit metastasis of human colorectal carcinoma
cells via modulating TGF-β1/ZEB/miR-200 signaling network (32). The miR-200 family has been
demonstrated as EMT-suppressive miRNAs in many types of cancer such
as triple-negative breast cancer (33), bladder cancer cells (34) and prostate cancer (35). To date, the study of pancreatic
cancer has demonstrated that miR-200a (36) and miR-141 (37) can inhibit invasion and migration,
miR-200c is a prognostic biomarker (38), miR-429 determines poor outcome and
inhibits cells growth (39), but
there are few reports on miR-200b and EMT. In the present study, we
demonstrate that overexpression of miR-200b-3p significantly
inhibits the expression of ZEB1, N-cadherin, fibronectin and
increases the expression of E-cadherin (Fig. 2). These data demonstrate that
miR-200b-3p could inhibit EMT. To further study the effect of
oridonin/miR-200b-3p in inhibiting cell migration in the BxPC-3 and
PANC-1 cells, Transwell migration assay was carried out in 24-well
modified chambers precoated without Matrigel. The result showed
that oridonin or miR-200b-3p can inhibit the migration of BxPC-3
and PANC-1 cells in vitro (Fig.
3). But as shown in the Fig.
2, oridonin significantly inhibits the expression of ZEB1,
N-cadherin, fibronectin, but did not increase the expression of
E-cadherin. This result illustrates that oridonin could the
expression of the mesenchymal markers, but could not increase the
expression of the epithelial markers. The reason may be that the
expression of E-cadherin is regulated by many other genes and the
inhibition of migration by oridonin is not mainly through type III
EMT in the BxPC-3 and PANC-1 cells.
The type II EMT is characterized by the
differentiation of epithelial cells into new fibroblast-like cells
and associated with inflammation in wound repair, tissue
regeneration or organ fibrosis. In the process of organ fibrosis,
type II EMT can continue to respond to ongoing inflammation,
leading eventually to organ fibrosis, which is in essence an
unabated form of wound healing due to persistent inflammation
(15). Rabdosia rubescens
is characterized as heat-clearing, detoxifying, anti-inflammatory
and anti-nociceptive. The pancreatic cancer is related to
inflammation. In our previous study, we found that inflammatory
factors (IL-1β, IL-6 and IL-33) were decreased in BxPC-3 cells
treated with oridonin, and oridonin also regulated nuclear
transcription factor pathways (40). In addition, TGF-β1 induced EMT
could be enhanced by inflammatory factors (TNF-α and IL-1β) in
human bronchial epithelial cells (41,42).
Therefore, oridonin may inhibit the type II EMT through its
anti-inflammatory effects.
Fibronectin (FN), a glycoprotein with various
biological activity, is one of the important extracellular matrix
proteins in the type II EMT, which are involved in cellular
adhesion and migration processes (43). FN could drive angiogenesis,
metastasis and chemoresistance in pancreatic ductal adenocarcinoma
(44). The metastasis of tumors is
dependent on angiogenesis and connected with the vascular
endothelial growth factor (VEGF). In our previous study, we found
that oridonin specifically inhibits the expression of VEGF in a
dose-dependent manner. In addition, this study has shown that
oridonin also specifically inhibits the expression of fibronectin
(Fig. 2). Thus, it presumed that
the migration of pancreatic cancer may be inhibited by oridonin
though inhibiting angiogenesis and fibrosis to inhibit the type II
EMT.
Cell adhesion molecule is a variety of biological
activities of transmembrane glycoprotein, produced by cells to
mediate the interaction of cell-cell and cell-matrix, including
immunoglobulin superfamily. Intercellular adhesion molecule-1
(ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are the
members of the immunoglobulin superfamily. ICAM-1 is a cell surface
glycoprotein and plays an important role in the tumor cell
expansion or metastasis. A previous report showed that silencing of
ICAM-1 can inhibit the metastatic ability in human breast cancer
cell lines significantly (45).
Amphiregulin can enhance the expression of ICAM-1 and promote tumor
metastasis in human osteosarcoma (46). Osteoblast-derived WISP-1 promotes
migration and VCAM-1 expression in human prostate cancer cells by
downregulating miR-126 expression via αvβ1 integrin, FAK and p38
signaling pathways (47). Our
results indicate that ICAM-1 and VCAM-1 were decreased in the
BxPC-3 and PANC-1 cells by oridonin treatment which may be the
mechanism of inhibition of migration (Fig. 4).
Cytoskeleton is mainly represented by
microfilaments, intermediate filaments and microtubules and are
also associated with cellular shape and various cellular functions
(48). The actin proteins are the
structural component of microfilament to constitute the
cytoskeleton of cells. Previous studies have shown that F-actin
filaments contributed to cell migration in MDA-MB-231 cells
(49). Prolactin could promote the
migration of breast cancer cells through remodeling the actin
cytoskeleton (50). The
microtubules are composed of α-tubulin and β-tubulin, which have
important functions including maintenance of cell morphology,
cellular signaling, cell migration and formation of cell polarity
(51). The results suggest that
cytoskeleton is closely related to tumor migration. To assess
whether oridonin have impact on the cytoskeleton in PANC-1 cell, we
observed β-tubulin in PANC-1 cells after treatment with oridonin by
immunofluorescence and confocal microscopy. As shown in Fig. 5, oridonin can significantly change
the cytoskeleton of PANC-1 cells, which may be related to its
inhibition of migration in pancreatic cancer.
To extend the in vitro observations, in
vivo experiments were performed by tumor formation assay in
nude mice. Our results demonstrated that oridonin/miR-200b-3p
suppressed tumorigenicity and EMT in vivo. In this study, we
found that oridonin significantly inhibited the expression of
E-cadherin in the BxPC-3 cells and PANC-1 cells (Fig. 2), but increased the expression of
E-cadherin in nude mice (Fig. 6E).
The mechanisms are not clear. Recently, some studies showed that
the expression of E-cadherin could be regulated by stromal cells,
which is an important part of tumor microenvironment. Thus, this
may be the reason that the expression of E-cadherin was inhibited
in the BxPC-3 cells and PANC-1 cells, but increased in nude mice.
Taken together, it indicated that EMT might be an ideal target for
cancer therapy not only in epithelial cancer cells, but also in
pancreatic cancer.
In conclusion, our data suggest that oridonin was
able to inhibit the proliferation and migration of BxPC-3 and
PANC-1 cells. miR-200b-3p is downregulated in BxPC-3 and PANC-1
cells by oridonin, and functions as a novel tumor suppressor to
regulate pancreatic cancer cell migration through inhibiting the
EMT. The novel mechanisms of oridonin mediated by decreasing cancer
migration is not through III-EMT by miR-200-3p/ZEB1 axis, and may
be related to other miRNAs, II-EMT, or altering the cytoskeleton.
In addition, oridonin/miR-200b-3p inhibited pancreatic cancer
growth and EMT in nude mice. Thus, oridonin and miR-200b-3p may be
useful in the therapy of pancreatic cancer.
Acknowledgments
The present study was supported by the Zhejiang
Province Project of Science Technology Department (no. 2014C33265),
the Natural Science Foundation of Zhejiang Province (no.
LY14H160037) and the Zhejiang Medical Technology and Education (no.
2015KYB255).
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yokoyama Y, Nimura Y and Nagino M:
Advances in the treatment of pancreatic cancer: Limitations of
surgery and evaluation of new therapeutic strategies. Surg Today.
39:466–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun HD, Huang SX and Han QB: Diterpenoids
from Isodon species and their biological activities. Nat Prod Rep.
23:673–698. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu QX, Yuan SX, Ren CM, Yu Y, Sun WJ, He
BC and Wu K: Oridonin upregulates PTEN through activating p38 MAPK
and inhibits proliferation in human colon cancer cells. Oncol Rep.
35:3341–3348. 2016.PubMed/NCBI
|
|
5
|
Gao S, Tan H, Zhu N, Gao H, Lv C, Gang J
and Ji Y: Oridonin induces apoptosis through the mitochondrial
pathway in human gastric cancer SGC-7901 cells. Int J Oncol.
48:2453–2460. 2016.PubMed/NCBI
|
|
6
|
Zhang XH, Liu YX, Jia M, Han JS, Zhao M,
Ji SP and Li AM: Oridonin inhibits tumor growth in glioma by
inducing cell cycle arrest and apoptosis. Cell Mol Biol. 60:29–36.
2014.
|
|
7
|
Dong Y, Zhang T, Li J, Deng H, Song Y,
Zhai D, Peng Y, Lu X, Liu M, Zhao Y, et al: Oridonin inhibits tumor
growth and metastasis through anti-angiogenesis by blocking the
Notch signaling. PLoS One. 9:e1138302014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kozomara A and Griffiths-Jones S: miRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39(Database): D152–D157. 2011. View Article : Google Scholar :
|
|
11
|
Zidar N, Boštjančič E, Jerala M, Kojc N,
Drobne D, Štabuc B and Glavač D: Down-regulation of microRNAs of
the miR-200 family and up-regulation of Snail and Slug in
inflammatory bowel diseases - hallmark of epithelial-mesenchymal
transition. J Cell Mol Med. 20:1813–1820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung VY, Tan TZ, Tan M, Wong MK, Kuay KT,
Yang Z, Ye J, Muller J, Koh CM, Guccione E, et al:
GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian
cancer through transcriptional regulation and histone modification.
Sci Rep. 6:199432016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cervantes-Arias A, Pang LY and Argyle DJ:
Epithelial-mesenchymal transition as a fundamental mechanism
underlying the cancer phenotype. Vet Comp Oncol. 11:169–184. 2013.
View Article : Google Scholar
|
|
15
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng F, Xue M, Xiao T, Li Y, Xiao S, Jiang
B and Ren C: MiR-200b promotes the cell proliferation and
metastasis of cervical cancer by inhibiting FOXG1. Biomed
Pharmacother. 79:294–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu H, Wang G, Wang Z, An S, Ye P and Luo
S: A negative feedback loop between miR-200b and the NF-kappaB
pathway via IKBKB/IKK-beta in breast cancer cells. FEBS J.
283:2259–2271. 2015. View Article : Google Scholar
|
|
18
|
Zhang HF, Alshareef A, Wu C, Li S, Jiao
JW, Cao HH, Lai R, Xu LY and Li EM: Loss of miR-200b promotes
invasion via activating the Kindlin-2/integrin β1/AKT pathway in
esophageal squamous cell carcinoma: An E-cadherin-independent
mechanism. Oncotarget. 6:28949–28960. 2015.PubMed/NCBI
|
|
19
|
Gui Z, Li S, Liu X, Xu B and Xu J:
Oridonin alters the expression profiles of microRNAs in BxPC-3
human pancreatic cancer cells. BMC Complement Altern Med.
15:1172015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Li M, Wang H, Fisher WE, Lin PH,
Yao Q and Chen C: Profiling of 95 microRNAs in pancreatic cancer
cell lines and surgical specimens by real-time PCR analysis. World
J Surg. 33:698–709. 2009. View Article : Google Scholar
|
|
22
|
Sun M, Estrov Z, Ji Y, Coombes KR, Harris
DH and Kurzrock R: Curcumin (diferuloylmethane) alters the
expression profiles of microRNAs in human pancreatic cancer cells.
Mol Cancer Ther. 7:464–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakurai MA, Ozaki Y, Okuzaki D, Naito Y,
Sasakura T, Okamoto A, Tabara H, Inoue T, Hagiyama M, Ito A, et al:
Gefitinib and luteolin cause growth arrest of human prostate cancer
PC-3 cells via inhibition of cyclin G-associated kinase and
induction of miR-630. PLoS One. 9:e1001242014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu N, Wu GC, Hu R, Li M and Feng H:
Ginsenoside Rh2 inhibits glioma cell proliferation by targeting
microRNA-128. Acta Pharmacol Sin. 32:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Liu G, Wu S, Jiang F, Xie J and
Wang Y: Zinc finger E-box-binding homeobox 1: Its clinical
significance and functional role in human thyroid cancer. Onco
Targets Ther. 9:1303–1310. 2016.PubMed/NCBI
|
|
26
|
Li YJ, Ping C, Tang J and Zhang W:
MicroRNA-455 suppresses non-small cell lung cancer through
targeting ZEB1. Cell Biol Int. 40:621–628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou L, Li Q, Yu Y, Li M and Zhang D: SET8
induces epithelial-mesenchymal transition and enhances prostate
cancer cell metastasis by cooperating with ZEB1. Mol Med Rep.
13:1681–1688. 2016.PubMed/NCBI
|
|
28
|
Zhang G, An H and Fang X: MicroRNA-144
regulates proliferation, invasion, and apoptosis of cells in
malignant solitary pulmonary nodule via zinc finger E-box-binding
homeobox 1. Int J Clin Exp Pathol. 8:5960–5967. 2015.PubMed/NCBI
|
|
29
|
Inuzuka T, Tsuda M, Kawaguchi H and Ohba
Y: Transcription factor 8 activates R-Ras to regulate angiogenesis.
Biochem Biophys Res Commun. 379:510–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kundu ST, Byers LA, Peng DH, Roybal JD,
Diao L, Wang J, Tong P, Creighton CJ and Gibbons DL: The miR-200
family and the miR-183~96~182 cluster target Foxf2 to inhibit
invasion and metastasis in lung cancers. Oncogene. 35:173–186.
2016. View Article : Google Scholar
|
|
31
|
Xue L, Su D, Li D, Gao W, Yuan R and Pang
W: MiR-200 regulates epithelial-mesenchymal transition in
anaplastic thyroid cancer via EGF/EGFR signaling. Cell Biochem
Biophys. 72:185–190. 2015. View Article : Google Scholar
|
|
32
|
Shen A, Lin W, Chen Y, Liu L, Chen H,
Zhuang Q, Lin J, Sferra TJ and Peng J: Pien Tze Huang inhibits
metastasis of human colorectal carcinoma cells via modulation of
TGF-β1/ZEB/miR-200 signaling network. Int J Oncol. 46:685–690.
2015.
|
|
33
|
Rhodes LV, Martin EC, Segar HC, Miller DF,
Buechlein A, Rusch DB, Nephew KP, Burow ME and Collins-Burow BM:
Dual regulation by microRNA-200b-3p and microRNA-200b-5p in the
inhibition of epithelial-to-mesenchymal transition in
triple-negative breast cancer. Oncotarget. 6:16638–16652. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Qiu M, Tan G, Liang Z, Qin Y, Chen
L, Chen H and Liu J: miR-200c inhibits invasion, migration and
proliferation of bladder cancer cells through down-regulation of
BMI-1 and E2F3. J Transl Med. 12:3052014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Williams LV, Veliceasa D, Vinokour E and
Volpert OV: miR-200b inhibits prostate cancer EMT, growth and
metastasis. PLoS One. 8:e839912013. View Article : Google Scholar
|
|
36
|
Lu Y, Lu J, Li X, Zhu H, Fan X, Zhu S,
Wang Y, Guo Q, Wang L, Huang Y, et al: MiR-200a inhibits
epithelial-mesenchymal transition of pancreatic cancer stem cell.
BMC Cancer. 14:852014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu L, Li Q, Xu D, Wang Q, An Y, Du Q,
Zhang J, Zhu Y and Miao Y: hsa-miR-141 downregulates TM4SF1 to
inhibit pancreatic cancer cell invasion and migration. Int J Oncol.
44:459–466. 2014.
|
|
38
|
Paik WH, Song BJ, Kim HW, Kim HR and Hwang
JH: MicroRNA-200c as a prognostic biomarker for pancreatic cancer.
Korean J Gastroenterol. 66:215–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song B, Zheng K, Ma H, Liu A, Jing W, Shao
C, Li G and Jin G: miR-429 determines poor outcome and inhibits
pancreatic ductal adenocarcinoma growth by targeting TBK1. Cell
Physiol Biochem. 35:1846–1856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen RY, Xu B, Chen SF, Chen SS, Zhang T,
Ren J and Xu J: Effect of oridonin-mediated hallmark changes on
inflammatory pathways in human pancreatic cancer (BxPC-3) cells.
World J Gastroenterol. 20:14895–14903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kamitani S, Yamauchi Y, Kawasaki S, Takami
K, Takizawa H, Nagase T and Kohyama T: Simultaneous stimulation
with TGF-β1 and TNF-α induces epithelial mesenchymal transition in
bronchial epithelial cells. Int Arch Allergy Immunol. 155:119–128.
2011. View Article : Google Scholar
|
|
42
|
Doerner AM and Zuraw BL: TGF-beta1 induced
epithelial to mesenchymal transition (EMT) in human bronchial
epithelial cells is enhanced by IL-1beta but not abrogated by
corticosteroids. Respir Res. 10:1002009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wilson CB, Leopard J, Cheresh DA and
Nakamura RM: Extracellular matrix and integrin composition of the
normal bladder wall. World J Urol. 14(Suppl 1): S30–S37. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Topalovski M and Brekken RA: Matrix
control of pancreatic cancer: New insights into fibronectin
signaling. Cancer Lett. 381:252–258. 2015. View Article : Google Scholar
|
|
45
|
Di D, Chen L, Wang L, Sun P, Liu Y, Xu Z
and Ju J: Downregulation of human intercellular adhesion molecule-1
attenuates the metastatic ability in human breast cancer cell
lines. Oncol Rep. 35:1541–1548. 2016.PubMed/NCBI
|
|
46
|
Liu JF, Tsao YT and Hou CH: Amphiregulin
enhances inter-cellular adhesion molecule-1 expression and promotes
tumor metastasis in human osteosarcoma. Oncotarget. 6:40880–40895.
2015.PubMed/NCBI
|
|
47
|
Tai HC, Chang AC, Yu HJ, Huang CY, Tsai
YC, Lai YW, Sun HL, Tang CH and Wang SW: Osteoblast-derived
WNT-induced secreted protein 1 increases VCAM-1 expression and
enhances prostate cancer metastasis by down-regulating miR-126.
Oncotarget. 5:7589–7598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Theurkauf WE, Smiley S, Wong ML and
Alberts BM: Reorganization of the cytoskeleton during Drosophila
oogenesis: Implications for axis specification and intercellular
transport. Development. 115:923–936. 1992.PubMed/NCBI
|
|
49
|
Wu L, Wang X, Liu Q, Wingnang Leung A,
Wang P and Xu C: Sinoporphyrin sodium mediated photodynamic therapy
inhibits the migration associated with collapse of F-actin
filaments cytoskeleton in MDA-MB-231 cells. Photodiagnosis Photodyn
Ther. 13:58–65. 2015. View Article : Google Scholar
|
|
50
|
da Silva PL, do Amaral VC, Gabrielli V,
Montt Guevara MM, Mannella P, Baracat EC, Soares-Jr JM and
Simoncini T: Prolactin promotes breast bancer bell migration
through actin cytoskeleton remodeling. Front Endocrinol (Lausanne).
6:1862015.
|
|
51
|
Li L and Yang XJ: Tubulin acetylation:
Responsible enzymes, biological functions and human diseases. Cell
Mol Life Sci. 72:4237–4255. 2015. View Article : Google Scholar : PubMed/NCBI
|