Introduction
Gastric cancer is the fourth most common type of
cancer and the second cause of cancer-related mortalities worldwide
(1). Many Asian countries,
including China, Japan and Korea, have extremely high rates of
gastric cancer (2). Despite the
use of multimodal therapy, there are no effective methods for the
treatment of gastric cancer. Therefore, identifying the new
therapeutic targets is needed.
Cadherins are transmembrane glycoproteins mediating
Ca2+-dependent adhesion of adjacent cells and have
strong implications in tumorigenesis (3). Liver-intestine cadherin (CDH17), also
known as LI-cadherin, is a new member of the cadherin superfamily.
It is distinguished from classical cadherins by its functional
features and unique structure, as it contains seven rather than
five molecular domains (3,4). Previous studies revealed that CDH17
is highly expressed in many types of cancer, such as gastric,
liver, intestinal and pancreatic (5). However, the expression of CDH17 is
negligible in the epithelium of the healthy human stomach. Ito et
al demonstrated that CDH17 was one of the most upregulated genes in
gastric carcinomas and its expression of CDH17 was correlated with
poor prognosis (6). CDH17 is also
a useful immunohistochemical marker for the diagnosis of gastric
metaplasia and neoplasia (5).
Despite these significant clinical findings, the functions of CDH17
in human cancers are unclear and controversial.
In the present study, we used a lentiviral system as
a delivery mediator of RNA interference (RNAi) and established a
stable CDH17-silencing gastric cell line, and evaluated the effects
of CDH17 suppression on the migration, invasion, proliferation and
apoptosis of gastric cancer cells in vitro as well as tumor
growth in vivo.
Materials and methods
Patients and tissue specimens
Paraffin-embedded tumors and their adjacent
non-cancerous tissues specimens were collected from a series of 79
patients with gastric carcinoma who had undergone surgical
resection between 1 January, 2007 and 31 December, 2007 at the
First Affiliated Hospital of Medicine School, Shihezi University,
Xinjiang, China. There were 56 men (71%) and 23 women (29%). The
mean age was 60.1 years (range, 27–80 years). The specimens were
classified by their tumor-node-metastasis (TNM) stage (UICC, 2010).
Detailed patient information such as gender, age, depth of invasion
and lymphatic invasion is listed in Table I.
 | Table IAssociation between CDH17 expression
and the clinicopathological characteristics of gastric
carcinoma. |
Table I
Association between CDH17 expression
and the clinicopathological characteristics of gastric
carcinoma.
| Parameters | CDH17 expression
| P-value |
|---|
| Negative (n=30) | Positive (n=49) |
|---|
| Age (mean ± SD,
years) | 59.4±12.0 | 60.6±13.7 | |
| Gender | | | 0.248 |
| Male | 19 | 37 | |
| Female | 11 | 12 | |
| Histologic type | | | 0.329 |
|
Well-differentiated | 1 | 4 | |
| Moderately | 13 | 14 | |
| Poorly
differentiated | 16 | 31 | |
| Depth of
invasion | | | 0.020a |
| T1 | 5 | 6 | |
| T2 | 24 | 30 | |
| T3 | 1 | 6 | |
| T4 | 0 | 7 | |
| Lymph node
metastasis | | | 0.022a |
| Absent | 17 | 15 | |
| Present | 13 | 34 | |
| TNM stage | | | 0.021a |
| 0+I+II | 19 | 18 | |
| III+IV | 11 | 31 | |
| Distant
metastasis | | | 0.243 |
| M0 | 29 | 43 | |
| M1 | 1 | 6 | |
Immunohistochemistry (IHC)
Paraffin-embedded 4-µm tissue sections were
stained for CDH17 using anti-LI cadherin antibody (ab109220; Abcam,
Cambridge, UK). In brief, paraffin-embedded tissues were
deparaffinized through graded xylene, treated with 3% hydrogen
peroxide in methanol for 10 min, and immersed in 10 mmol/l citrate
buffer (pH 6.0). The specimens were incubated with 10% normal goat
serum (catalog no.: SA1022, Boster, Wuhan, China), followed by the
primary rabbit polyclonal antibody against CDH17 (diluted in 1:400;
catalog no.: ab3163-1, Abcam, Cambridge, MA, USA) at 4°C overnight
and the biotinylated goat anti-rabbit IgG secondary antibody
(catalog no.: SA1022, Boster, Wuhan, China) at 37°C for 30 min.
After washing, 3,3′-diaminobenzidine tetrahydrochloride solution
was used to visualise the staining. The stained slides were
evaluated by standard light microscopy (Eclipse 80i; Nikon, Tokyo,
Japan).
Cell culture, lentivirus production and
transduction
Human MKN28 gastric cancer cells were cultured in
RPMI-l640 with 10% fetal bovine serum, 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified incubator supplemented
with 5% CO2 at 37°C. The lentiviral RNAi expression
vector with specific miRNA against CDH17 (lenti-shCDH17) was
constructed as previously described (7–9). The
lentiviral vector was used to transduce MKN28 cells and the stably
transfected cell line termed lenti-shCDH17, in which CDH17 had been
knocked down by RNAi. The other two groups comprised MKN28 cells
received no treatment and the empty vector-transfected control
cells (lenti-shCDH17-neg).
Immunofluorescent staining
The expression of CDH17 was determined by IF using
anti-LI cadherin antibody (ab69602; Abcam). Briefly, MKN28 cell
cultures, grown in 6-well plates, on coverslips, were fixed with 4%
paraformaldehyde for 10 min. Fixed cells were permeabilized in 0.1%
Triton X-100 for 5 min. Rabbit anti-LI cadherin sera at a dilution
of 1:500 were applied to these wells and incubated at 37°C for 2 h.
The cells were washed three times with PBS for 5 min each time.
Goat anti-rabbit IgG conjugated with cy3 (Sigma) at a dilution of
1:50 was added and incubated for 40 min at 37°C. After being washed
three times and stained with DAPI (diluted in 1:2,000) for 5 min,
the glass slides were mounted in glycerol with coverslips and the
cells were observed under an inverted fluorescence microscope
(Olympus, Tokyo, Japan).
Western blotting
The MKN28 cells of the different groups were
harvested, washed with phosphate-buffered saline [PBS, pH 7.4) and
treated with trypsin. All the cells were lysed in RIPA buffer [50
mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA, 30
µg/ml aprotinin, 50 µg/ml leupeptin and 1 mM PMSF].
Protein was loaded at a concentration of 30 µg per lane and
separated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE).
After electrophoresis, the protein was transferred onto a polyvinyl
difluoride membrane, incubated with 5% non-fat milk for 1 h and
then incubated overnight at 4°C with anti-CDH17 antibody (1:1,000;
Abcam). Horseradish peroxidase-conjugated IgG was used as the
secondary antibody. Protein was visualized by the enhanced
chemiluminescence kit.
Quantitative polymerase chain reaction
(qPCR)
We extracted total RNA using TRIzol reagent
(Invitrogen, Hong Kong, China), and single-stranded cDNA performed
with the cDNA synthesis kit (Takara Bio, Inc., Otsu, Japan)
according to standard protocols. We performed qPCR using
SYBR®Premix Ex Taq™ II (Takara Bio, Inc.).
The reactions were carried out in a 10-µl volume reaction
system. The PCR conditions consisted of 45 cycles, with 5 sec
denaturation at 95°C, 30 sec annealing at 60°C and 1 min at 72°C.
The primers used were: CDH17, forward: 5′-GCCAATCCTCCTGCTGTG-3′ and
reverse: 5′-GCAACCTGGAGATTGTGAGT-3′; GAPDH, forward:
5′-TGACTTCAACAGCGACACCCA-3′, and reverse:
5′-CACCCTGTTGCTGTAGCCAAA-3′. The reactions were performed in
triplicate. The relative expression level of CDH17 gene was
calculated based on the method of 2−ΔΔCq.
In vitro migration and invasion
assay
The inhibitory effect of RNAi on MKN28 migration and
invasion in vitro was demonstrated in transwell chambers
(8.0 µm pore size; Corning, NY, USA) according to the
manufacturer's instructions. First, transwell chambers were placed
onto 24-well plates. The cells were then resuspended in serum-free
medium (1×106 cells/ml), and 100 µl of this
suspension was added to the upper chambers. The lower chambers were
filled with 300 µl of RPMI-l640 medium containing 15% fetal
bovine serum. Transwell invasion chambers were coated on the upper
surface with 50 µl (1.25 mg/ml) BD Matrigel™
Matrix (BD Biosciences, San Diego, CA, USA). Following 24-h
incubation, the cells on the lower surface of the filters were
fixed in methanol, stained with 0.25% crystal violet for 15 min and
counted in five random fields at a magnification of ×200.
Flow cytometry for the determination of
MKN28 cell apoptosis
An Annexin V/propidium iodide (PI) apoptosis
detection kit I (BD Biosciences) was used to measure apoptosis. The
cells from each group were collected, washed twice with ice-cold
PBS and re-suspended in 200 µl binding buffer containing 10
µl Annexin V and 10 µl PI. Fluorescence intensity was
measured by flow cytometry (BD Biosciences). All the samples were
assayed in triplicate.
Measurement of cell proliferation by the
cell counting kit-8 (CCK-8) assay and colony formation assay
To observe the effect of the CDH17 gene
expression on cell proliferation in vitro, CCK-8 was
employed to draw cell growth curves. The cells from each group were
plated onto 96-well cell culture plates at a density of
2×104 cells/well in 100 µl of culture medium
containing 10% fetal bovine serum and grown overnight. Each group
had 5 replicates holes. At 24, 48, 72, 96 and 120 h, CCK-8 solution
was added to each well and the plate was placed in a CO2
incubator for 4 h. The plate was then placed in a microplate reader
(Bio Rad, Hercules, USA and the O.D. at 450 nm was read. A colony
formation assay was performed to analyze anchorage-dependent
growth. The cells were plated on a 6-well plate at 1×103
cells/well. Colonies were stained with 0.1% crystal violet and
counted on the 14th day after seeding. Colonies containing ≥50
cells were counted. The experiments were carried out in
triplicate.
In vivo mouse models of gastric cancer
and tumorigenicity assay
Animal experiments were approved by the Animal
Experimental Ethics Committee of the Huazhong University. Gastric
cancer xenografts were established in 5-week-old male BALB/c nude
mice (Beijing HFK Bioscience Co. Ltd., Beijing, China). Briefly,
the cells from the three groups were harvested by trypsinization,
and washed with PBS. Single-cell suspensions (6×106
cells in 200 µl PBS) were injected into the nude mice (six
mice per group). Subcutaneous tumors were monitored every 3 days
with a caliper and tumor growth curves were calculated. Tumor size
was calculated using the formula: Length × width2 × 0.5.
After 4 weeks, the mice were sacrificed using cervical dislocation
and the xenograft tumor tissues were harvested, weighed, fixed, and
embedded. The slides were incubated with the primary antibody
against Ki-67 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
caspase-3 (Santa Cruz Biotechnology, Inc.) overnight at 4°C.
Biotinylated goat anti-rabbit IgGs or goat anti-mouse IgGs were
used as secondary antibodies. According to the manufacturer's
protocol of the TUNEL Apoptosis Assay kit (Roche Diagnostics,
Indianapolis, IN, USA), TUNEL was performed to detect apoptosis in
the tumor sections. Five fields were randomly selected from each
sample and 200 cells were randomly selected from every field under
microscopy. The apoptotic rate was calculated as the number of
total apoptotic cells/1,000) × 100%.
Statistical analysis
The experiments were repeated three times.
Statistical analyses were performed using SPSS 12.0 software (SPSS
Inc., Chicago, IL, USA). The results of the present study were
presented as mean ± SD. Statistically significant differences
between groups for each assay were analyzed by one-way analysis of
variance (ANOVA). The association between CDH17 protein expression
and the clinicopathological characteristics was assessed by the
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
CDH17 overexpression in gastric
carcinoma
CDH17 immunoreactivity was detected in 49/79 (62.1%)
gastric cancer tissues. The expression of CDH17 was localized in
the cell membrane and cytoplasm at various levels in gastric
carcinoma tissues. The high expression of CDH17 protein was
observed in the cell membrane of epithelial cells with intestinal
metaplasia. CDH17 was detected in the tumors with
well-differentiated and moderately gastric cancer tissues. CDH17
staining intensity was relatively lower in the tumors with poor
differentiation. CDH17 was absent in normal gastric tissues.
Representative images of IHC staining are shown in Fig. 1.
The association between CDH17 expression and
clinicopathological parameters is summarized in Table I. Of the 79 cases, 49 (62.0%) were
classified as having positive CDH17 expression, whereas the
remaining 30 (38.0%) were classified as having a negative CDH17
expression. A positive expression of CDH17 was associated with the
depth of invasion (P=0.020). There was a significant relationship
between lymph node metastasis and the positive expression of CDH17
(P=0.022). The incidence of cases with CDH17-positive expression
was significantly higher in stage III–IV than in stages 0–II
(P=0.021). There was no significant correlation between CDH17 and
the other clinicopathological characteristics such as age, gender
and histologic type (P>0.05).
Knockdown of CDH17 inhibits gastric
cancer cell growth in vitro
In order to evaluate the effects of CDH17 knockdown
on the CDH17 gene, we selected the well-differentiated MKN28
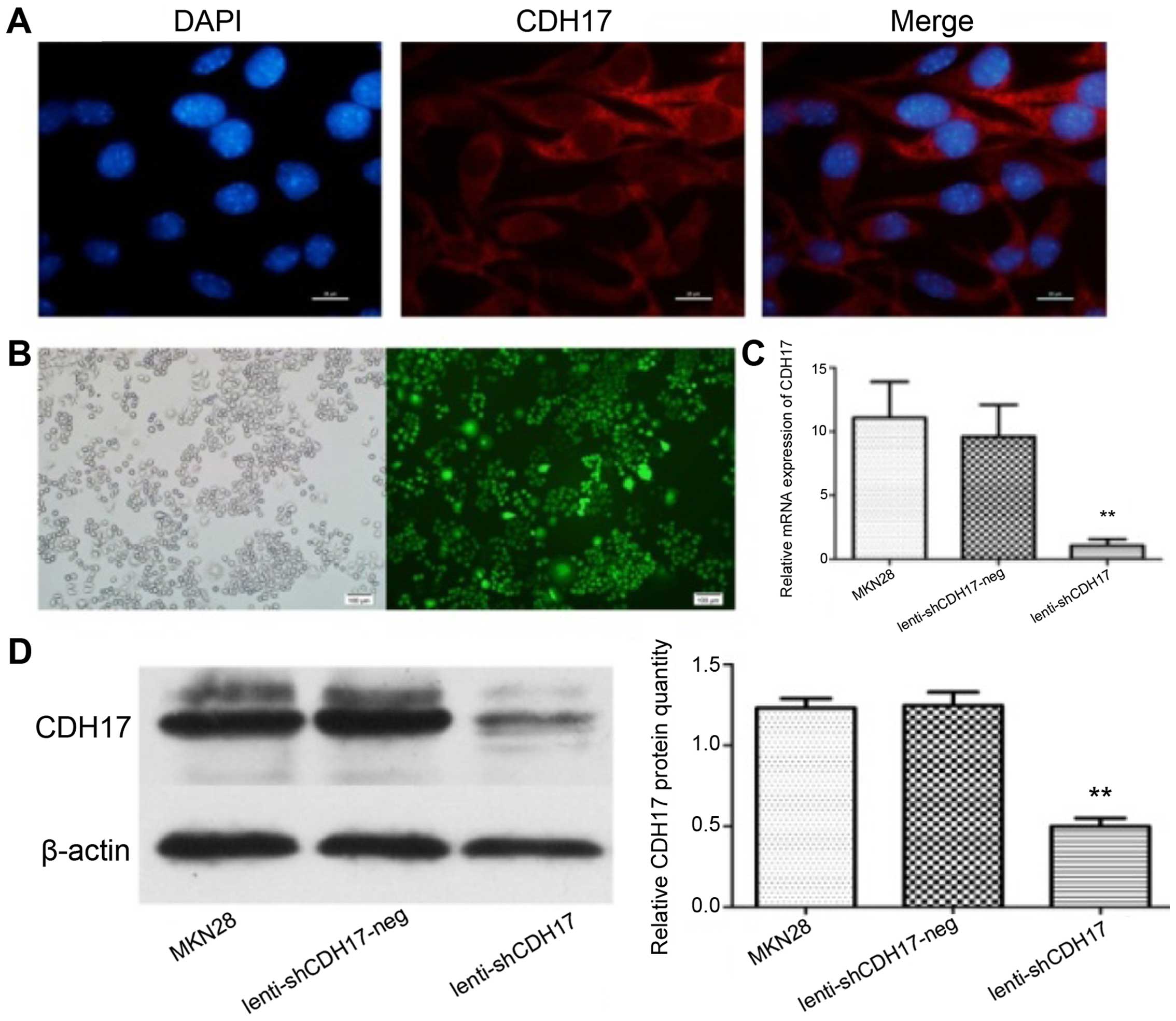
cell line, which has a strong expression of CDH17.
Immunofluorescent staining showed that CDH17 was mainly present on
the cell membrane and cytoplasm (Fig.
2A). As shown in Fig. 2B,
lenti-shCDH17 effectively infected MKN28 cell lines. The cells were
infected by lenti-CDH17 in terms of GFP expression as observed
under fluorescent microscope. Subsequent to the successful
infection of MKN28 cells, fluorogenic qPCR was performed to
evaluate the reduction in mRNA for CDH17 in lenti-shCDH17 cells.
Compared with the lenti-shCDH17-neg and untreated MKN28 cells, the
mRNA expression of CDH17 was suppressed efficiently in
lenti-shCDH17 cells (Fig. 2C). In
addition, western blot analysis revealed a reduced expression of
CDH17 protein in lenti-shCDH17 cells transfected with
lenti-shCDH17, whereas CDH17 protein expression in
lenti-shCDH17-neg cells and untreated MKN28 cells was not reduced
(Fig. 2D). These results
demonstrated that the expression of CDH17 may be downregulated
specifically and effectively by lenti-shCDH17. These infected cells
were used for subsequent experiments.
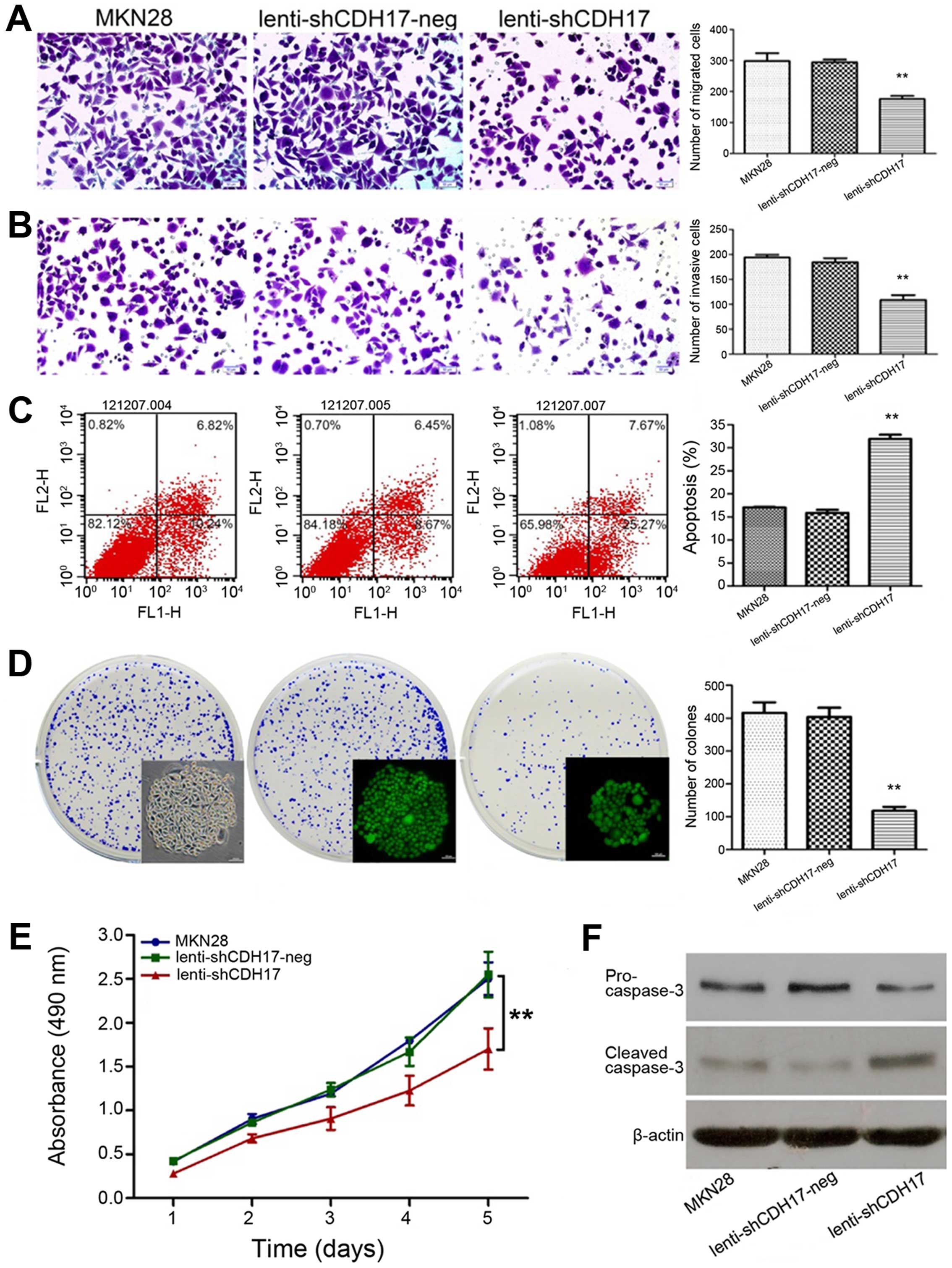
We investigated the tumorigenic and metastatic
properties (cell migration, invasion, colony formation, apoptosis
and proliferation) of the MKN28 cells transfected with
lenti-shCDH17 compared with the control cells-lenti-shCDH17-neg
cells and untreated MKN28 cells. Lenti-shCDH17 cells in culture
showed a decreased rate of migration and invasion ability
(P<0.01) (Figs. 3A and B). As
shown in Fig. 3C, CDH17 could
significantly increase the apoptotic rate of lenti-shCDH17 cells
(P<0.01). To investigate the mechanism of apoptosis in
vitro, we examined the expression of related proteins by
western blot analysis. The results revealed that knockdown of CDH17
protein markedly decreased the levels of pro-caspase-3 and
increased the levels of cleaved caspase-3 compared with the control
cells (Fig. 3F). In the colony
forming assay, the colony numbers of lenti-shCDH17 cells
(108.67±9.64/well) were much lower than those of the control group
treated with GFP-lentivirus (lenti-shCDH17-neg) cells
(184.33±8.14/well) and the MKN28 cells (193.80±5.37/well)
(P<0.01), which suggests that RNAi with specific sequence of
CDH17 may significantly suppress the colony-forming ability of
MKN28 cells. No significant difference was found between the
lenti-shCDH17-neg and MKN28 groups (P>0.05) (Fig. 3D). Empty lentiviral vector had no
effect on the proliferative ability of MKN28 cells, whereas RNAi
specific to MKN28 caused a marked reduction in cell proliferation
(P<0.01) (Fig. 3E).
Knockdown of CDH17 inhibits the growth of
the tumor derived from MKN28 cells in vivo
Based on the data obtained from the in vitro
experiments, we investigated whether the knockdown of CDH17
suppressed tumor growth and induced apoptosis in vivo.
Single-cell suspensions were injected subcutaneously into the
BALB/c nude mice. Four weeks after inoculation in nude mice, we
found that the mean tumor volume of the lenti-shCDH17 group was
180.23±115.16 mm3, much smaller than that of the MKN28
group (1708.2±1118.85 mm3) and the lenti-shCDH17-neg
group (1988.30±500.50 mm3) (P<0.01) (Fig. 4). Thus, these data indicate that
knockdown of CDH17 in gastric cancer cells can inhibit the tumor
formation of gastric cancer cells in vivo.
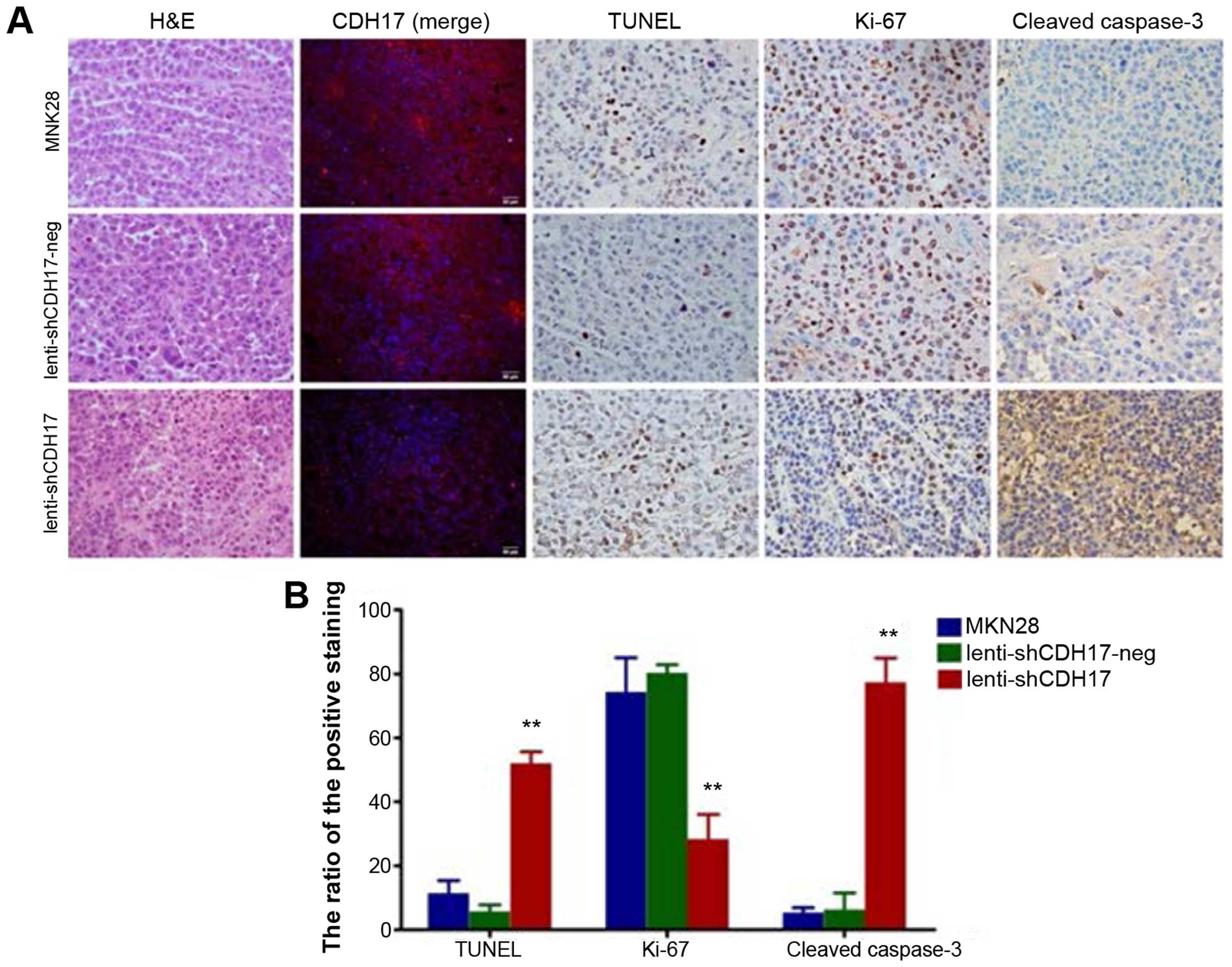
To confirm the effect of CDH17 knockdown on
apoptosis in vivo, immunohistochemical analyses on the
xenograft tissues were conducted. The expression of CDH17 was
reduced in the lenti-shCDH17 group as is shown in the IF staining.
In addition, the area of apoptosis in the lenti-shCDH17 group was
significantly larger than the other two groups. Apoptotic cells in
xenograft sections were analyzed by TUNEL staining. We observed
positive cells (52±3.60) in the lenti-shCDH17 group, which was more
significant than that in the MKN28 group (11.33±4.04) and
lenti-shCDH17-neg group (5.67±2.08). The number of Ki-67-positive
cells was lower in the tumor section derived from lenti-shCDH17
cells than that in lenti-shCDH17-neg or MKN28 cells (P<0.01). A
decreased expression of Ki-67 antigen indicated that reduced tumor
growth in mice was partly due to lower proliferation caused by the
knockdown of CDH17. By contrast, knockdown of CDH17 induced tumor
growth and widespread staining for cleaved caspase-3, compared to
others, in which the staining intensity in the lenti-shCDH17 group
was significantly higher than that in the lenti-shCDH17-neg and
MKN28 groups (Fig. 5).
Discussion
Previous findings have clearly demonstrated that
CDH17 is linked significantly to a high incidence of tumorigenesis
in the human stomach, liver, pancreas and intestine by displaying
an aberrant expression in their cancerous state. CDH17 was strongly
regulated by CDX2 in normal, metaplastic, and neoplastic tissues of
the gastrointestinal tract (10–12).
After the first report of CDH17 as a useful marker for the
diagnosis of gastric metaplasia and neoplasia (13), there is accumulating evidence that
CDH17 was expressed in 61–65% of gastric cancer tissues (9,12,14,15),
similar to our data here. In addition, we found that CDH17 was
overexpressed in gastric cancer and its overexpression was
associated with lymph node metastasis and TNM stage of the
patients. Recent studies showed that long-term intestinal
metaplasia of gastric mucosa often led to gastric adenocarcinoma
(16). Furthermore, the expression
of CDH17 was higher in the intestinal type than that in the diffuse
type of gastric cancer (17). A
high level of CDH17 tends to be correlated with advanced tumor
stages and is associated with lymph node metastasis and poor
prognosis (6,18,19).
Although it has been shown that CDH17 has a role in the invasion
and migration of gastric cancer cells (8,9,20),
the molecular pathogenesis of CDH17 has not been fully clarified
(21). In this report, we
investigated whether the downregulation of CDH17 inhibited gastric
cancer progression and attempted to elucidate its unclarified
mechanisms. The powerful lentiviral RNAi expression vector with
specific miRNA against CDH17 gene expression was used in the
present study.
In the present study, we carried out transwell
migration, invasion, cell proliferation and colony formation assays
after CDH17 knockdown in the highly tumorigenic MKN28 cell line.
Our present data show that inhibition of CDH17 by RNAi lead to less
tumorigenesis of MKN28 cells in vitro. In addition,
BALB/c-nu mice were used to investigate whether the downregulation
of CDH17 retarted the growth of gastric cancer derived from MKN28
cells. We found that the average tumor volume and weight were
significantly lower in the lenti-shCDH17 group compared with the
MKN28 group and the control group treated with GFP lentivirus. The
results of the present study, suggest that CDH17 knockdown did not
eliminate the tumor completely, however, it might be more
reasonable to obstruct multiple pathways simultaneously (22). In addition, CDH17 is not the only
protease involved in the invasion and growth of gastric cancer.
Unlike gastric cancer and hepatocellular carcinoma
(23), the expression of CDH17 is
reduced in colorectal carcinoma tissues, and this low level of
CDH17 is correlated to dedifferentiation, advanced tumor stage,
tumor invasion and poor survival (24,25).
Taken together, CDH17 manifests unique and distinct roles in
tumorigenesis originated from different organs and its molecular
pathogenesis has not been fully clarified. In the present study, we
investigated whether the downregulation of CDH17 induced apoptosis
and inhibited the proliferation of gastric cancer cells. In
vitro, CDH17 suppressed cell viability through activation of
cleaved caspase-3 and eventually induced apoptosis of MKN28 cells.
In gastric cancer xenografts, we found that CDH17 prominently
suppressed xenograft growth by inducing apoptosis, which was
consistent with our study in vitro. Induction of apoptosis
is considered a requirement for the inhibition of tumor growth.
CDH17 can block caspase-dependent apoptosis by binding to
caspase-3. Caspase-3 is activated in the typical apoptosis pathways
(26). In addition, we observed a
significant decrease in Ki-67 in lenti-shCDH17 xenograft tissue,
which indicated that reduced tumor growth was partly due to cell
growth inhibition, although the underlying mechanisms need further
elucidation.
In conclusion, the present study demonstrates that
CDH17 functions as a tumor suppressor protein in gastric cancer
cells in vitro and in vivo, at least in part, by
inhibiting proliferation, migration and invasion via the
suppression of apoptosis.
Acknowledgments
This project was partly supported by the National
Natural Science Foundation of China (no. 81172294). We would like
to thank Ming Yuan, La-ti Mu, Shao-xiong Niu, Yu Xi, Jia-geng He
and Jun-feng Kang from the Department of General Surgery, the First
Affiliated Hospital of Medicine School, Shihezi University,
Xinjiang, China.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sugano K: Screening of gastric cancer in
Asia. Best Pract Res Clin Gastroenterol. 29:895–905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gessner R and Tauber R: Intestinal cell
adhesion molecules. Liver-intestine cadherin. Ann N Y Acad Sci.
915:136–143. 2000. View Article : Google Scholar
|
|
4
|
Wendeler MW, Drenckhahn D, Gessner R and
Baumgartner W: Intestinal LI-cadherin acts as a
Ca2+-dependent adhesion switch. J Mol Biol. 370:220–230.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito R, Oue N, Yoshida K, Kunimitsu K,
Nakayama H, Nakachi K and Yasui W: Clinicopathological significant
and prognostic influence of cadherin-17 expression in gastric
cancer. Virchows Archiv. 447:717–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su MC, Yuan RH, Lin CY and Jeng YM:
Cadherin-17 is a useful diagnostic marker for adenocarcinomas of
the digestive system. Mod Pathol. 21:1379–1386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu QF, Dong WG and Ren JL: Knockdown of
Li-cadherin increases metastatic behaviors of LoVo cells. J Cancer
Res Clin Oncol. 136:1641–1649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu QS, Zhang J, Liu M and Dong WG:
Lentiviral-mediated miRNA against liver-intestine cadherin
suppresses tumor growth and invasiveness of human gastric cancer.
Cancer Sci. 101:1807–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Liu QS and Dong WG: Blockade of
proliferation and migration of gastric cancer via targeting CDH17
with an artificial microRNA. Med Oncol. 28:494–501. 2011.
View Article : Google Scholar
|
|
10
|
Hinoi T, Lucas PC, Kuick R, Hanash S, Cho
KR and Fearon ER: CDX2 regulates liver intestine-cadherin
expression in normal and malignant colon epithelium and intestinal
metaplasia. Gastroenterology. 123:1565–1577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barros R, da Costa LT, Pinto-de-Sousa J,
Duluc I, Freund JN, David L and Almeida R: CDX2 autoregulation in
human intestinal metaplasia of the stomach: Impact on the stability
of the phenotype. Gut. 60:290–298. 2011. View Article : Google Scholar :
|
|
12
|
Ge J and Chen Z, Wu S, Yuan W, Hu B and
Chen Z: A clinicopathological study on the expression of
cadherin-17 and caudal-related homeobox transcription factor (CDX2)
in human gastric carcinoma. Clin Oncol (R Coll Radiol). 20:275–283.
2008. View Article : Google Scholar
|
|
13
|
Grötzinger C, Kneifel J, Patschan D,
Schnoy N, Anagnostopoulos I, Faiss S, Tauber R, Wiedenmann B and
Gessner R: LI-cadherin: A marker of gastric metaplasia and
neoplasia. Gut. 49:73–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko S, Chu KM, Luk JM, Wong BW, Yuen ST,
Leung SY and Wong J: CDX2 co-localizes with liver-intestine
cadherin in intestinal metaplasia and adenocarcinoma of the
stomach. J Pathol. 205:615–622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui J, Chen Y, Chou WC, Sun L, Chen L, Suo
J, Ni Z, Zhang M, Kong X, Hoffman LL, et al: An integrated
transcriptomic and computational analysis for biomarker
identification in gastric cancer. Nucleic Acids Res. 39:1197–1207.
2011. View Article : Google Scholar :
|
|
16
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
17
|
Yasui W, Oue N, Sentani K, Sakamoto N and
Motoshita J: Transcriptome dissection of gastric cancer:
Identification of novel diagnostic and therapeutic targets from
pathology specimens. Pathol Int. 59:121–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ko S, Chu KM, Luk JM, Wong BW, Yuen ST,
Leung SY and Wong J: Overexpression of LI-cadherin in gastric
cancer is associated with lymph node metastasis. Biochem Biophys
Res Commun. 319:562–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Yu JC, Kang WM, Wang WZ, Liu YQ
and Gu P: The predictive effect of cadherin-17 on lymph node
micrometastasis in pN0 gastric cancer. Ann Surg Oncol.
19:1529–1534. 2012. View Article : Google Scholar
|
|
20
|
Xu Y, Zhang J, Liu QS and Dong WG:
Knockdown of liver-intestine cadherin decreases BGC823 cell
invasiveness and metastasis in vivo. World J Gastroenterol.
18:3129–3137. 2012. View Article : Google Scholar PubMed/NCBI
|
|
21
|
Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J
and Sung JJ: Dysregulation of cellular signaling in gastric cancer.
Cancer Lett. 295:144–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song Y, Dong MM and Yang HF: Effects of
RNA interference targeting four different genes on the growth and
proliferation of nasopharyngeal carcinoma CNE-2Z cells. Cancer Gene
Ther. 18:297–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu LX, Lee NP, Chan VW, Xue W, Zender L,
Zhang C, Mao M, Dai H, Wang XL, Xu MZ, et al: Targeting cadherin-17
inactivates Wnt signaling and inhibits tumor growth in liver
carcinoma. Hepatology. 50:1453–1463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwak JM, Min BW, Lee JH, Choi JS, Lee SI,
Park SS, Kim J, Um JW, Kim SH and Moon HY: The prognostic
significance of E-cadherin and liver intestine-cadherin expression
in colorectal cancer. Dis Colon Rectum. 50:1873–1880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takamura M, Ichida T, Matsuda Y, Kobayashi
M, Yamagiwa S, Genda T, Shioji K, Hashimoto S, Nomoto M, Hatakeyama
K, et al: Reduced expression of liver-intestine cadherin is
associated with progression and lymph node metastasis of human
colorectal carcinoma. Cancer Lett. 212:253–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|