Introduction
Cancer is an obstinate disease with high morbidity
and mortality, and the incidence rate is predicted to increase over
the coming years (1). Although
various therapies have been developed for the treatment of cancer,
the mortality rate remains high (2,3).
Hepatocellular carcinoma is one of the most common gastrointestinal
malignancies. It is the sixth-leading cause of cancer-related death
in the United States (4). The
prognosis of hepatocellular carcinoma is poor due to high
malignancy. Although biochemical and clinical studies have led to
significant advances, the 7-year (2004–2010) survival rate remains
<18% (4). Most of the poor
prognoses were associated with recurrence and metastasis following
treatment, including curative resection (5). Common treatments including surgery,
chemotherapy, radiotherapy, interventional treatment and liver
transplantation could only provide limited clinical results
(6). Traditional chemotherapy and
radiotherapy cause intrinsic and potential cytotoxicity in normal
cells, and extended use of these therapies can lead to drug
resistance and side effects, such as hair loss, vomiting, nausea,
and the occurrence of secondary cancers (1). Due to the limitations of conventional
therapies, it is important to find safer, more targeted anticancer
agents. New outstanding strategy for cancer chemoprevention and
chemotherapeutic are also required to including the cell cycle
arrest and apoptosis of cancer cells that grow abnormally by
deregulating the cell cycle control. Recently, there has been
growing interest in searching for novel and effective anticancer
agents from natural sources, especially from marine organisms
(7–19).
Halophytes are plants that tolerate high salt
concentrations, and can grow in salt marshes, mangrove swamps,
seashores, coastal sand dune regions, and estuarine environments
(20). Environmental stress and
ecological factors, such as drought, salt spray, floods, high
temperature, low capillary water holding activity of sandy soil,
low nutrients, and water availability affect the plant's metabolism
and survival (21–23). In these environments, halophytes
need to conform to develop stress adaptation responses for
survival. As a result, these salt marsh plants are predicted to be
an important source in the search for novel and unique bioactive
secondary metabolites (24–30).
Calystegia soldanella (L) Roem. et Schult
(Convolvulaceae), a representative halophyte and endemic plant, is
found on coastal sand dunes and foredunes where the environmental
stresses are significant. These plants are perennial vine herbs
with ubiquitous distribution in the coastal dune areas of South
Korea, East Asia, Europe, and the Pacific (31). This plant has long been used as an
edible and medicinal herb to cure rheumatic arthritis, sore throat,
dropsy, and scurvy (32). Some
studies have shown that this plant species exhibits various
biological activities. Another species, C. japonica, which
has been used as a traditional medicine to treat urination
problems, fever, or diarrhea in Chinese and oriental herb medicine
(33,34). Moreover, C. soldanella has
been shown to exhibit a number of biological activities, including
anti-inflammatory, antiviral, antifungal, anticancer, and analgesic
properties, and more specifically, inhibition of protein tyrosine
phosphate 1B (PTP1B) (35–42). Methanol extracts of C.
soldanella decreased NO production, iNOS protein, and mRNA
expression in LPS-activated Raw 264.7 cells (35). Water extracts of C.
soldanella induced anti-inflammatory and analgesic effects in
mice (36). Alkyl
p-coumarates of an n-hexane fraction from a C.
soldanella extract inhibited PTP1B activity in vitro
(37). Resin glycosides from C.
soldanella, calysolins V-IX, X-XIII, and XIV-XVII, induced
antiviral activity against the herpes simplex virus type 1 (HSV-1)
(39–41,43–46).
An active fraction of Ipomoea carnea subsp. fistulosa
(Convulvulaceae) induced antifungal activity in Colletotrichum
gloeosporioides and Cladosporium cucumerinum (42).
Active components from C. soldanella are
nortropane alkaloids, anthocyanin, coumaric acids, and flavonoids
(47–50). Moreover, chloroform extracts showed
both cytotoxic activities [ED50 2 μg/ml in UISO
(squamous cell cervix carcinoma); ED50 7 μg/ml in
KB (nasopharyngeal carcinoma)] and antibacterial (MIC 14.7
μg/ml in Bacillus subtilis) (43,44).
Methanol extract also exhibited potential cytotoxicity against A549
lung (IC50 8.0 μg/ml) and Col2 colon
(IC50 27.4 μg/ml) cancer cells (38). However, studies of the anticancer
effect of C. soldanella have not been extensive focused on
cytotoxicity. To find active components with anticancer activity,
this study investigated the cytotoxic activity of crude extract and
four solvent-partitioned fractions of C. soldanella in HepG2
human hepatocellular carcinoma cells. Furthermore, the 85% aqueous
methanol (aq. MeOH) fraction, which exhibited the greatest
cytotoxic effect, was evaluated for cell cycle distribution and the
expression of several cell cycle checkpoint proteins.
Materials and methods
Plant material
The C. soldanella whole plant was collected
from Gijang, Busan, Korea in July, 2013 by Professor Y. Seo. A
voucher specimen was deposited at the Herbarium of the Division of
Marine Environment and Bioscience, Korea Maritime and Ocean
University, Korea. The collected sample was briefly air-dried under
shade, chopped into small pieces, ground into a powder, and stored
at −25°C.
Extraction and fractions
Samples (800 g) were extracted for 2 days with
methylene chloride (CH2Cl2; 10 L × 2) and
methanol (MeOH; 10 L × 2). The combined crude extracts (106.51 g)
were evaporated under reduced pressure and partitioned between
CH2Cl2 and water. The organic layer was
further partitioned into n-hexane (19.19 g) and 85% aq. MeOH
(22.47 g). The aqueous layer was also fractionated with
n-butanol (BuOH; 10.48 g) and water (57.66 g),
successively.
Cell culture
The HepG2 human hepatocellular carcinoma cells (ATCC
HB-8065) were obtained from the American Type Culture Collection
(ATCC; MD, USA). Cells were cultured in modified essential medium
(MEM) supplemented with 10% fetal bovine serum containing 50
μg/ml penicillin, 25 μg/ml amphotericin B, and 50
μg/ml streptomycin in a humidified atmosphere with 5%
CO2 at 37°C. The medium was changed 2 or 3 times every
week.
Cell viability assay
Cell viability was evaluated using the CytoX cell
viability assay kit (LPS solution, Daejeon, Korea). The cells were
seeded at a density of 1×105 cells/well in a 96-well
plate. After 24 h, the cells were washed with serum-free medium
(SFM) for 4 h and the media were replaced with fresh SFM containing
different concentrations of samples. After 24 h of incubation, 20
μl of CytoX solution was added to each well and incubated
for 4 h. The amount of formazan crystals was determined by
measuring the absorbance at 450 nm using a FilterMax F5 microplate
reader (Molecular Devices LLC, CA, USA). Cell viability was
estimated by comparison with the relative absorbance value of the
untreated sample.
Cell cycle analysis
Cells were seeded at a density of 1×104
cells/well and treated with different concentrations of sample for
24 h. Control and treated cells were harvested, washed in cold
phosphate-buffered saline (PBS), fixed in 70% ethanol, and stored
at 4°C. The resulting cells were stained with 200 μl of Muse
cell cycle reagent at room temperature for 30 min in the dark prior
to analysis. DNA content was assessed with the Muse cell analyzer
(EMD Millipore Co., CA, USA).
Western blot analysis
Following treatment with different concentrations of
samples, cells were washed twice with PBS and lysed in RIPA buffer
[1% Nonidet™ P-40, 1 mM EDTA, 50 mM Tris (pH 7.4), 0.25%
Na-deoxycholate, 150 mM NaCl, 1 mM NaF, 1 mM sodium orthovanadate,
1 mM PMSF]. The cell lysates were centrifuged at 12,000 rpm for 15
min at 4°C and the supernatants were collected. The protein
concentrations were determined using a BCA protein assay kit
(Pierce Biotechnology, Inc., IL, USA). The proteins were treated
with SDS sample buffer and heated at 95°C for 10 min. The protein
samples were separated by 12% SDS-PAGE, and transferred to a
polyvinylidene difluoride membrane (Millipore Corp., MA, USA). The
membranes were blocked by incubation with 1% bovine serum albumin
(BSA) in Tris-buffered saline-Tween-20 [TBS-T; 10 mM Tris-HCl, 150
mM NaCl (pH 7.5) containing 0.1% Tween-20] at room temperature for
1 h and incubated for 3 h with primary antibodies against GAPDH,
cyclin D, cyclin E, cyclin A, cyclin-dependent kinase (CDK)2, CDK4,
CDK6, CDC25A, p21, p27, retinoblastoma (RB), and E2F (Santa Cruz
Biotechnology, Inc., TX, USA). The membranes were washed three
times with TBS-T and incubated for 2 h with the appropriate
HRP-conjugated goat anti-rabbit, goat anti-mouse, or rabbit
anti-goat secondary antibodies (Santa Cruz Biotechnology, Inc.)
diluted to 1:10,000 in TBS-T with 1% BSA. The respective proteins
were detected using a chemiluminescent substrate (Advansta, CA,
USA) and visualized on a GeneSys imaging system (SynGene Synoptics,
Ltd., London, UK).
Statistical analysis
The data are presented as mean ± standard deviation
(SD). Differences between the means of the individual groups were
analyzed using an analysis of variance (ANOVA) with Duncan's
multiple range tests performed in SPSS software (SPSS Inc., IL,
USA). A p-value <0.05 was considered to indicate statistical
significance.
Results
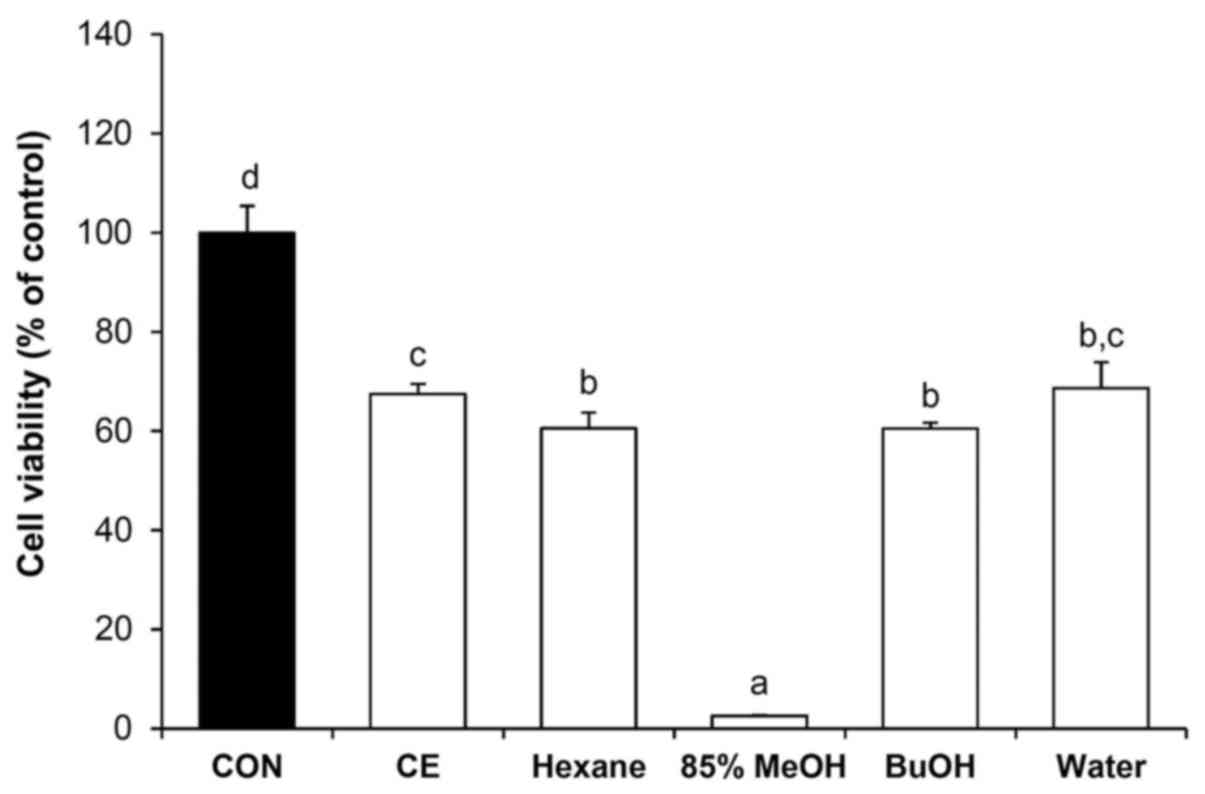
Crude extracts and solvent fractions of
C. soldanella decrease the viability of HepG2 cells
Effects of the crude extract and the four solvent
fractions of C. soldanella on the proliferation of HepG2
cells were examined using the CytoX cell viability assay kit. As
shown in Fig. 1, the growth of
HepG2 cells was inhibited at a concentration of 50 μg/ml.
The crude extract inhibited cell proliferation by 37%. The crude
extract was fractioned into n-hexane, 85% aq. MeOH,
n-BuOH, and water soluble fractions, treatment which
inhibited proliferation by 39, 97, 40 and 31%, respectively. Of the
four solvent fractions tested, the 85% aq. MeOH fraction caused the
greatest inhibition of HepG2 cell proliferation.
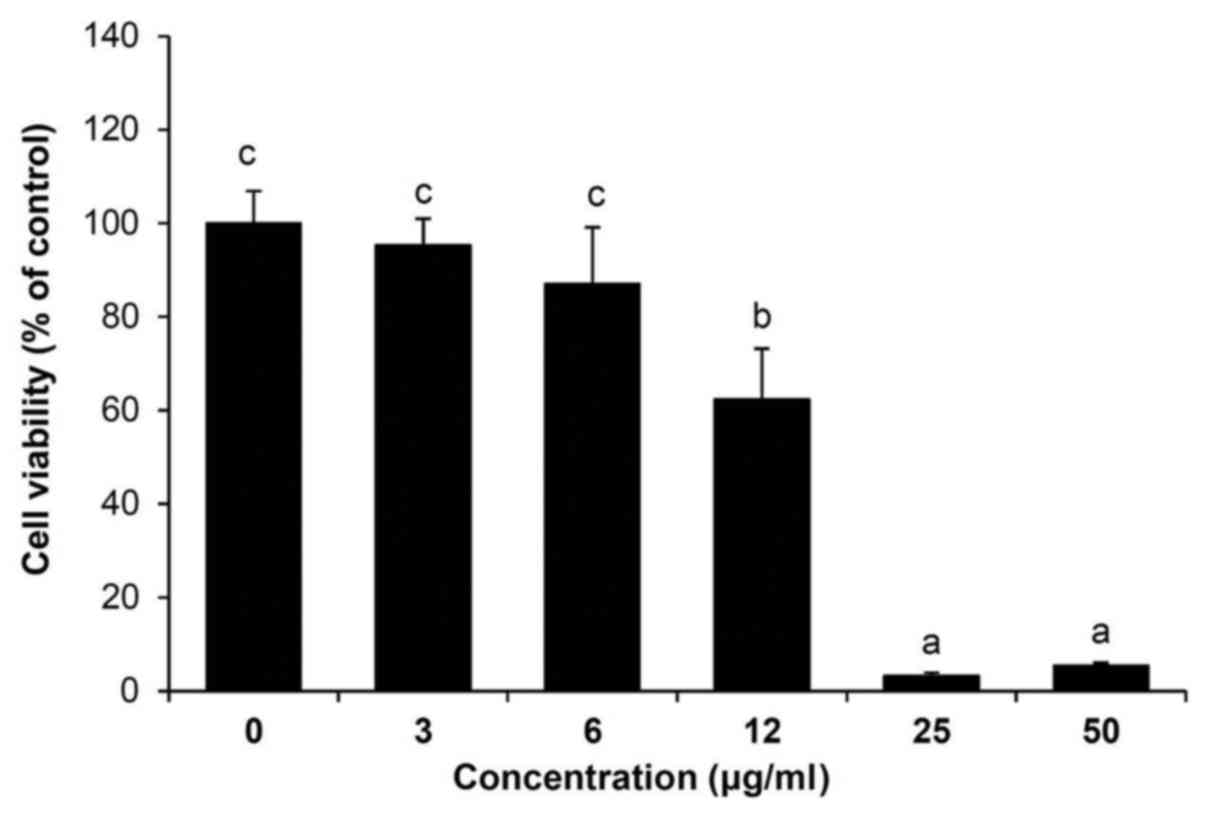
The 85% aq. MeOH fraction from C.
soldanella decreases the viability of HepG2 cells
To determine the effect of the 85% aq. MeOH fraction
from C. soldanella on the viability of HepG2 cells, the
cells were treated with 3, 6, 12, 25, or 50 μg/ml of the 85%
aq. MeOH fraction for 24 h. As shown in Fig. 2, treatment with 85% aq. MeOH
reduced the viability of HepG2 cells in a concentration-dependent
manner compared with the control group (3 μg/ml, 95.9%; 6
μg/ml, 90.6%; 12 μg/ml, 71.1%; 25 μg/ml,
20.8%; and 50 μg/ml, 22.7%). In subsequent experiments,
cells were treated with 3, 6, or 12 μg/ml of the 85% aq.
MeOH fraction from C. soldanella for 24 h.
The 85% aq. MeOH fraction from C.
soldanella induces a G0/G1 and S arrest in HepG2 cells
Flow cytometric analysis of the cell cycle of HepG2
cells showed that the number of cells in G0/G1 phase significantly
increased from 60.47±0.85% in the control group to 63.80±1.42,
64.97±0.90 and 69.60±3.44% in the groups treated with the various
concentrations of the C. soldanella 85% aq. MeOH fraction
(Table I). In addition, the number
of cells in S phase significantly increased from 12.87±0.21% in the
control group to 14.57±0.70, 16.10±2.16 and 16.77±1.59% in the
groups treated with the C. soldanella 85% aq. MeOH fraction.
The population of HepG2 cells in G2/M was significantly reduced
following treatment with the 85% aq. MeOH fraction from C.
soldanella. These results suggest that treatment with the C.
soldanella 85% aq. MeOH fraction arrests HepG2 cells in the
G0/G1 and S phases of the cell cycle, and that the reduced
viability of HepG2 cells following treatment with the 85% aq. MeOH
fraction is likely the result of these cell cycle blocks.
 | Table IInduction of G0/G1 and S arrest in
HepG2 cells following treatment with the 85% aq. MeOH fraction of
C. soldanella. |
Table I
Induction of G0/G1 and S arrest in
HepG2 cells following treatment with the 85% aq. MeOH fraction of
C. soldanella.
| Concentration
(μg/ml) | % of cells
|
|---|
| G0/G1 | S | G2/M |
|---|
| 0 |
60.47±0.85a |
12.87±0.21a |
25.20±0.87c |
| 3 |
63.80±1.42b |
14.57±0.71b |
19.60±1.37b |
| 6 |
64.97±0.90b |
16.10±2.16c |
15.43±0.31a |
| 12 |
69.60±3.44c |
16.77±1.59c |
16.40±0.70a |
The 85% aq. MeOH fraction from C.
soldanella regulates cell cycle checkpoint proteins in HepG2
cells
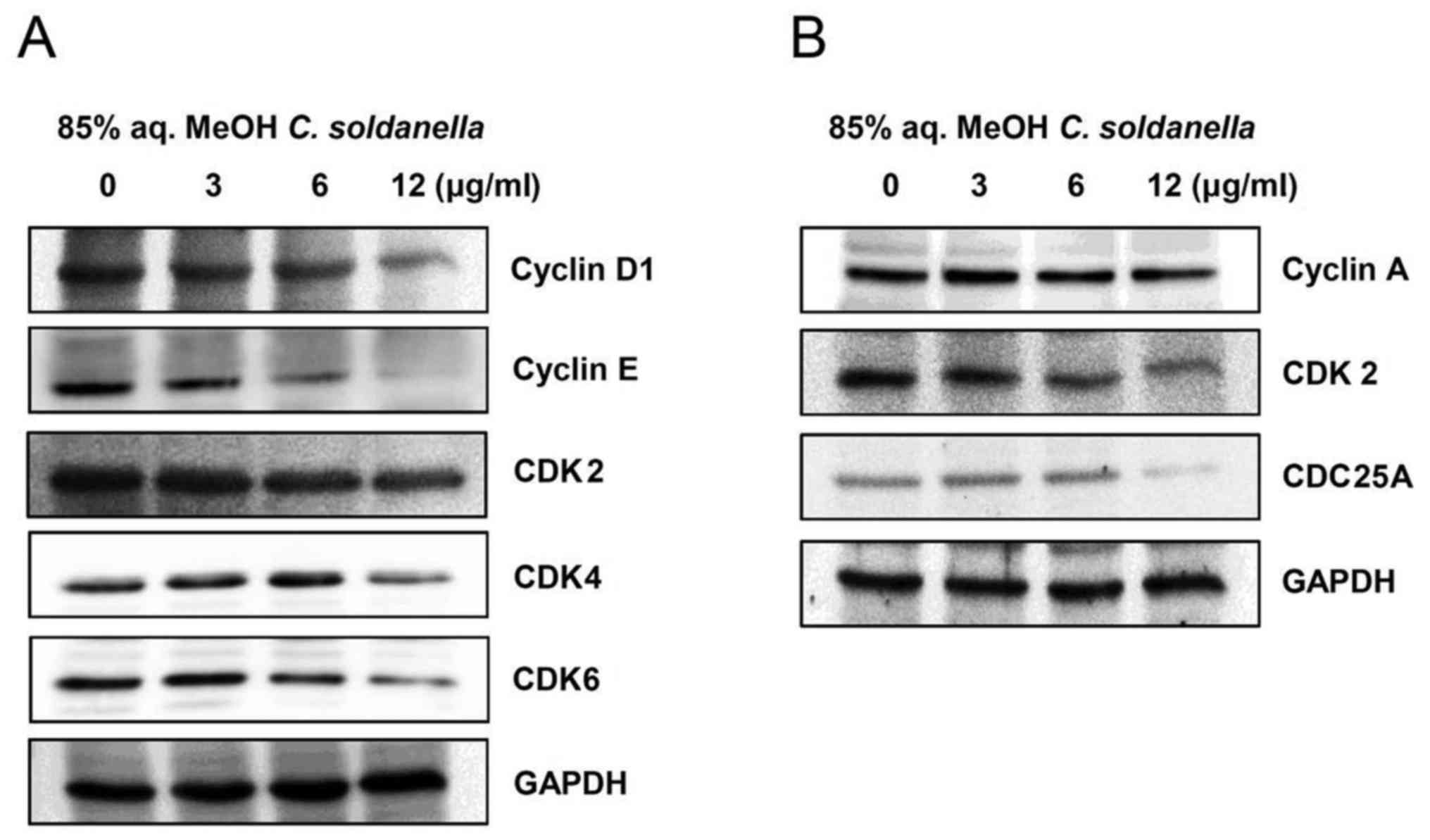
To investigate the cell cycle arrest induced by the
85% aq. MeOH fraction from C. soldanella in HepG2 cells, the
expression of G0/G1 phase cell cycle checkpoint proteins, including
cyclin D1, cyclin E, CDK2, CDK4, and CDK6, was examined. As shown
in Fig. 3A, the 85% aq. MeOH
fraction of C. soldanella significantly decreased the
protein levels of cyclin D1, cyclin E, CDK2, CDK4 and CDK6.
Treatment with 3, 6, or 12 μg/ml of the C.
soldanella 85% aq. MeOH fraction significantly reduced
cyclin D1 (81.9, 64.2 and 23.5%) and cyclin E (62.5, 50.4 and
24.0%) expression in a concentration-dependent manner. Also,
treatment with 3, 6, or 12 μg/ml of the 85% aq. MeOH
fraction reduced CDK4 expression in HepG2 cells compared with the
control group by 114.1, 109.7 and 78.5%, respectively. Moreover,
treatment with 3, 6, or 12 μg/ml of the 85% aq. MeOH
fraction reduced CDK6 expression in HepG2 cells compared with the
control group by 96.3, 68.7 and 46.5%, respectively.
To investigate the cell cycle arrest of HepG2 cells
induced by treatment with the 85% aq. MeOH fraction from C.
soldanella, the expression of S phase cell cycle checkpoint
proteins, including cyclin A, CDK2, and CDC25A, was examined. As
shown in Fig. 3B, the 85% aq. MeOH
fraction from C. soldanella significantly decreased the
protein levels of cyclin A, CDK2, and CDC25A. In particular,
treatment with 3, 6, or 12 μg/ml of the C. soldanella
85% aq. MeOH fraction resulted in significantly reduced CDK2
expression in a concentration-dependent manner with values of 78.5,
56.8 and 47.5%, respectively.
Treatment with the C. soldanella 85% aq.
MeOH fraction decreases the expression of CDK inhibitors in HepG2
cells
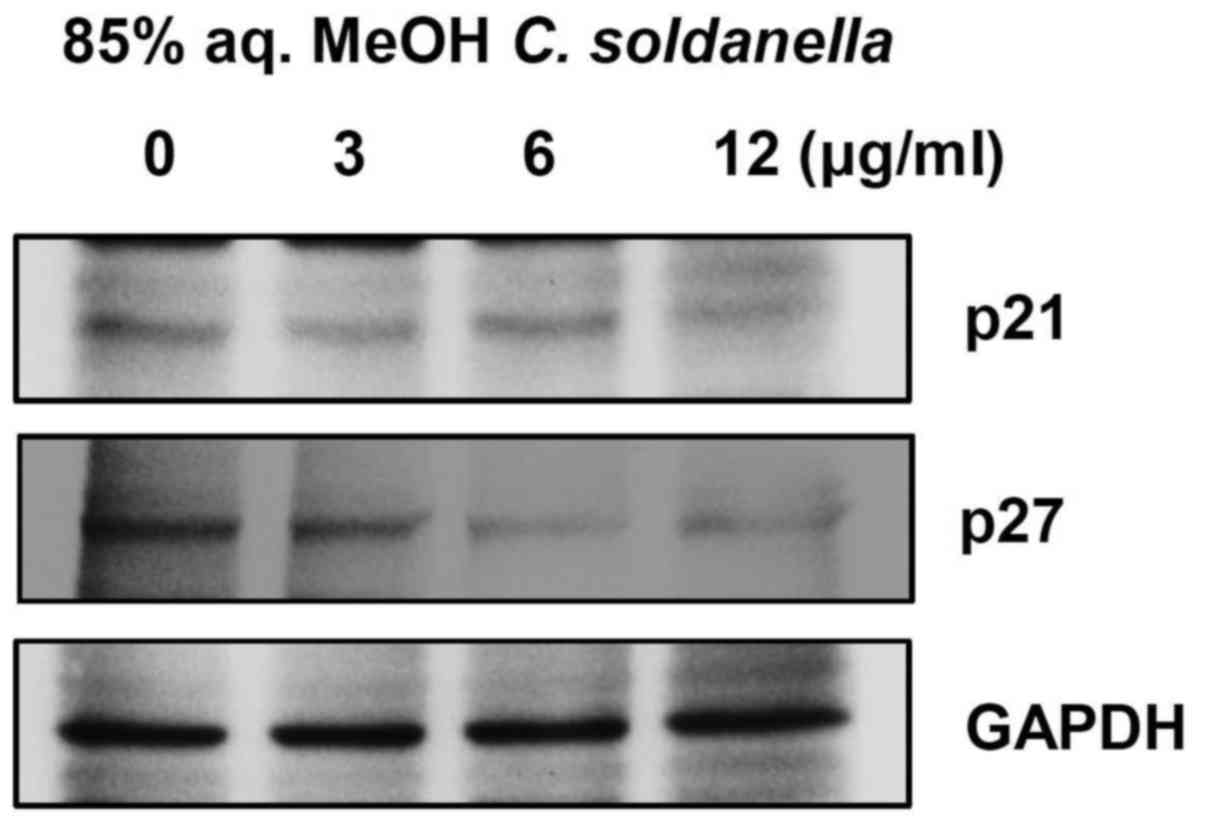
Cyclin D/CDK4/6 and cyclin E/CDK2 complexes are
important for the cell cycle transition from G1 into S phase, and
these complexes are negatively regulated by CDK inhibitors, such as
p21 and p27. As shown in Fig. 4,
treatment with the 85% aq. MeOH fraction of C. soldanella
significantly decreased the expression of p21 and p27.
Treatment with 3, 6, or 12 μg/ml of the 85%
aq. MeOH fraction from C. soldanella significantly reduced
p21 expression in a concentration-dependent manner, with values of
85.6, 86.1 and 70.4%, respectively. Also, treatment with 3, 6, or
12 μg/ml of the 85% aq. MeOH fraction from C.
soldanella reduced p27 expression in HepG2 cells compared with
the control group by 89.4, 74.3 and 50.9%, respectively.
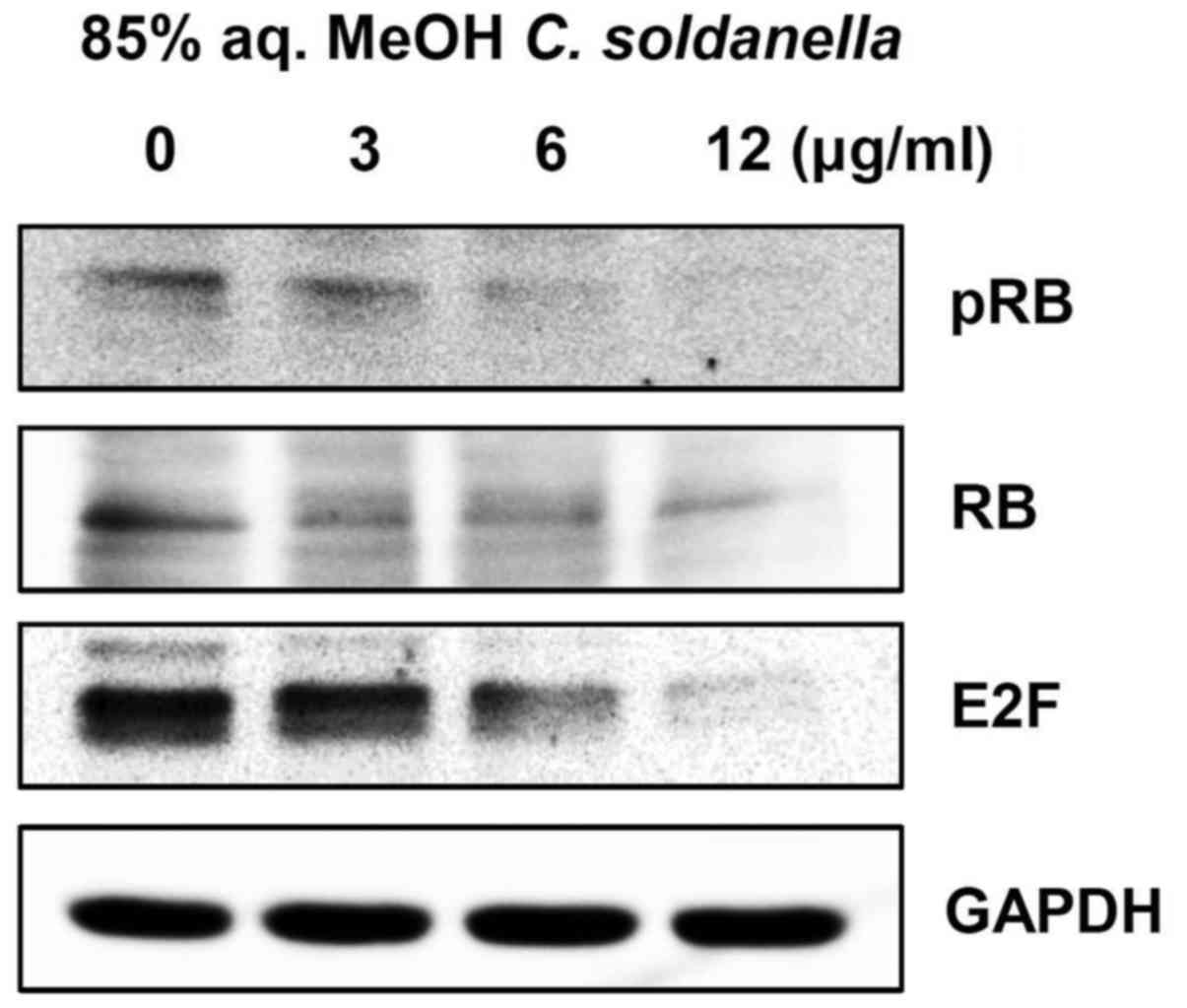
Treatment with the 85% aq. MeOH fraction
of C. soldanella downregulates RB phosphorylation and E2F
expression in HepG2 cells
As cyclin D and cyclin E-induced CDK activity
converges in hyperphosphorylation of the RB protein, the effect of
treatment with the 85% aq. MeOH fraction from C. soldanella
on the phosphorylation status of RB was examined using western
blotting. As shown in Fig. 5,
treatment with the 85% aq. MeOH fraction significantly decreased
the expression of phosphorylated RB (pRB) and RB.
E2F is an important transcription factor for cell
cycle progression from G1 to S phase and DNA synthesis. The effect
of the 85% aq. MeOH fraction from C. soldanella on the level
of E2F was examined. Treatment with the C. soldanella 85%
aq. MeOH fraction significantly reduced the expression of E2F. In
particular, treatment with 3, 6, or 12 μg/ml of the 85% aq.
MeOH fraction resulted in significantly reduced E2F expression in a
concentration-dependent manner, with values of 84.6, 65.1 and
42.1%, respectively.
Discussion
We screened cytotoxicity of crude extract from C.
soldanella against various human cancer cell including HepG2
hepatocellular, AGS gastric, HT-29 colon, and MCF-7 breast cancer
cell in 50 μg/ml concentration. As a result, the
cytotoxicity against HepG2 (37%) and HT-29 (36%) cancer cells was
the greater compared to AGS (27%) and MCF-7 (14%) cancer cells
(data not shown). This study reports the anticancer effect in HepG2
human cancer cells for the first time.
The purpose of this study was to investigate the
viability of crude extracts and four solvent-partitioned fractions
from C. soldanella in HepG2 human hepatocellular carcinoma
cells. C. soldanella was extracted with methylene chloride
and methanol, and the combined extract was partitioned into the
n-hexane, 85% aq. MeOH, n-BuOH, and water fractions.
The crude extract and four solvent fractions were examined using a
cell viability assay, in which the 85% aq. MeOH fraction showed the
greatest inhibition of proliferation in HepG2 cells at a
concentration of 50 μg/ml (97% compared with the control
group; Fig. 1). The effect of the
85% aq. MeOH fraction from C. soldanella on cell viability
was examined in HepG2 cells. Treatment with the C.
soldanella 85% aq. MeOH fraction reduced viability
concentration-dependently (Fig.
2).
Apoptosis (regulated cell death), occurs during
normal homeostasis, disease, and development, and is characterized
by morphological changes, including cell shrinkage, membrane
blebbing, nuclear fragmentation, chromatin condensation, and an
increase in the population of sub-G1 cells (51,52).
The 85% aq. MeOH fraction of C. soldanella induced apoptotic
nuclear morphological changes in HepG2 cells. Thus, the 85% aq.
MeOH fraction increased the rate of apoptosis compared with the
control (12 μg/ml, 44.27%; and control, 20.85%; data not
shown).
Because treatment with the 85% aq. MeOH fraction
resulted in the greatest inhibition of cell growth, we evaluated
the cell cycle distribution and expression of cell cycle checkpoint
proteins. As shown in Table I,
treatment with the C. soldanella 85% aq. MeOH fraction
induced G0/G1 arrest (12 μg/ml, 69.60%; and control, 60.47%)
and S phase arrest (12 μg/ml, 16.77%; and control, 12.87%)
in HepG2 cells. Therefore, our results suggest that treatment with
the 85% aq. MeOH fraction reduces cell growth of HepG2 cells
through cell cycle arrest in G0/G1 and S phase and induces
apoptosis.
Cancer cells exhibit deregulation of the cell cycle,
increased apoptosis, and activation of signaling pathways that
result in abnormal growth. Cyclins and CDKs are critical for
appropriate regulation of the cell cycle, and altered formation of
cyclin/CDK complexes has been shown to increase or decrease cell
growth and affect proliferation and/or differentiation by apoptosis
(53,54). Cyclin D/CDK4/6 complexes and cyclin
E/CDK2 complexes are critical factors for progression through the
G0/G1 phase of the cell cycle. These factors are negatively
regulated by CDK inhibitors, such as p21 and p27 (55,56).
To investigate the cell cycle arrest induced by treatment with the
85% aq. MeOH fraction from C. soldanella in HepG2 cells,
expression of the G0/G1 phase cell cycle proteins, including cyclin
D1, cyclin E, CDK2, CDK4, CDK6, p21, and p27, was examined. As
shown in Figs. 3A and 4, the 85% aq. MeOH fraction of C.
soldanella significantly decreased the protein levels of cyclin
D1, cyclin E, CDK2, CDK4, CDK6, p21, and p27.
Cyclin A, CDK2, and CDC25A are important factors for
the S phase of the cell cycle. CDC25A is activated by cyclin A/CDK2
complexes. These complexes allow for progression of the cell cycle,
and increased expression of CDC25A promotes cell growth (57,58).
We have demonstrated that treatment with the C. soldanella
85% aq. MeOH fraction significantly decreased the protein levels of
cyclin A, CDK2, and CDC25A (Fig.
3B).
The cell cycle proteins E2F and pRB are known to
play important roles in cell cycle progression from G1 to S phase.
Dephosphorylation of RB inhibits cell cycle progression by
interacting with transcription factors of the E2F family, but
phosphorylation of RB induces cell cycle progression by reducing
pRB/E2F complexes (55,56). We showed that treatment with the
85% aq. MeOH fraction decreased expression of E2F and pRB, thus
inhibiting the G1-S phase transition in HepG2 cells (Fig. 5). Overall, the C. soldanella
85% aq. MeOH fraction exhibited anticancer activity in HepG2 cells
by blocking the G0/G1 and S phases of the cell cycle and by
decreasing the expression of important cell cycle check point
proteins.
Previous studies have investigated the potential
cytotoxic effects of MeOH and chloroform extracts from C.
soldanella against human cancer cells, including A549 lung
cancer cells and Col2 colon cancer cells (38). This report reveals for the first
time the anticancer effect in HepG2 human hepatocellular carcinoma
cells. The 85% aq. MeOH fraction from C. soldanella should
be considered for its therapeutic potential in hepatocellular
cancer treatment. It will be necessary to identify the components
of the 85% aq. MeOH fraction with high performance liquid
chromatography (HPLC), nuclear magnetic resonance spectroscopy
(NMR), and mass spectroscopy (MS). Determining the composition of
the C. soldanella 85% aq. MeOH fraction is important.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
2012R1A6A1028677).
References
|
1
|
Li YL, Zhang J, Min D, Hongyan Z, Lin N
and Li QS: Anticancer effects of
1,3-Dihydroxy-2-Methylanthraquinone and the ethyl acetate fraction
of Hedyotis Diffusa Willd against HepG2 carcinoma cells mediated
via Apoptosis. PLoS One. 11:e01515022016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin S, Park HJ, Oh YN, Kwon HJ, Kim JH,
Choi YH and Kim BW: Anti-cancer activity of osmanthus matsumuranus
extract by inducing G2/M arrest and apoptosis in human
hepatocellular carcinoma Hep G2 cells. J Cancer Prev. 20:241–249.
2015. View Article : Google Scholar
|
|
3
|
Lee JI, Kwak MK, Park HY and Seo Y:
Cytotoxicity of meroterpenoids from Sargassum siliquastrum against
human cancer cells. Nat Prod Commun. 8:431–432. 2013.PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang Z, Zhou X, Lin Z, Yang B, Ma Z, Ye S,
Wu Z, Fan J, Liu Y, Liu K, et al: Surgical treatment of
hepatocellular carcinoma and related basic research with special
reference to recurrence and metastasis. Chin Med J (Engl).
112:887–891. 1999.
|
|
6
|
Yu Z, Luo X, Wang C, Ye J, Liu S, Xie L,
Wang F and Bao J: Baicalin promoted site-2 protease and not site-1
protease in endoplasmic reticulum stress-induced apoptosis of human
hepatocellular carcinoma cells. FEBS Open Bio. 6:1093–1101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen NH and Zhong JJ: Ganoderic acid Me
induces G1 arrest in wild-type p53 human tumor cells while G1/S
transition arrest in p53-null cells. Process Biochem. 44:928–933.
2009. View Article : Google Scholar
|
|
8
|
Kong CS, Um YR, Lee JI, Kim YA, Yea SS and
Seo Y: Constituents isolated from Glehnia littoralis suppress
proliferations of human cancer cells and MMP expression in HT1080
cells. Food Chem. 120:385–394. 2010. View Article : Google Scholar
|
|
9
|
Stan SD, Kar S, Stoner GD and Singh SV:
Bioactive food components and cancer risk reduction. J Cell
Biochem. 104:339–356. 2008. View Article : Google Scholar
|
|
10
|
Um YR, Kong CS, Lee JI, Kim YA, Nam TJ and
Seo Y: Evaluation of chemical constituents from Glehnia littoralis
for antiproliferative activity against HT-29 human colon cancer
cells. Process Biochem. 45:114–119. 2010. View Article : Google Scholar
|
|
11
|
Mary JS, Vinotha P and Pradeep AM:
Screening for in vitro cytotoxic activity of seaweed, Sargassum sp.
against Hep-2 and MCF-7 cancer cell lines. Asian Pac J Cancer Prev.
13:6073–6076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shamsabadi FT, Khoddami A, Fard SG,
Abdullah R, Othman HH and Mohamed S: Comparison of tamoxifen with
edible seaweed (Eucheuma cottonii L.) extract in suppressing breast
tumor. Nutr Cancer. 65:255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rubiolo JA, López-Alonso H, Roel M,
Vieytes MR, Thomas O, Ternon E, Vega FV and Botana LM: Mechanism of
cytotoxic action of crambescidin-816 on human liver-derived tumour
cells. Br J Pharmacol. 171:1655–1667. 2014. View Article : Google Scholar :
|
|
14
|
Russo GL, Russo M, Castellano I,
Napolitano A and Palumbo A: Ovothiol isolated from sea urchin
oocytes induces autophagy in the Hep-G2 cell line. Mar Drugs.
12:4069–4085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawee-Ai A and Kim SM: Application of
microalgal fucoxanthin for the reduction of colon cancer risk:
Inhibitory activity of fucoxanthin against beta-glucuronidase and
DLD-1 cancer cells. Nat Prod Commun. 9:921–924. 2014.PubMed/NCBI
|
|
16
|
Malve H: Exploring the ocean for new drug
developments: Marine pharmacology. J Pharm Bioallied Sci. 8:83–91.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gudiña EJ, Teixeira JA and Rodrigues LR:
Biosurfactants produced by marine microorganisms with therapeutic
applications. Mar Drugs. 14:382016. View Article : Google Scholar :
|
|
18
|
Talero E, García-Mauriño S, Ávila-Román J,
Rodríguez-Luna A, Alcaide A and Motilva V: Bioactive compounds
isolated from microalgae in chronic inflammation and cancer. Mar
Drugs. 13:6152–6209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li R: Marinopyrroles: Unique drug
discoveries based on marine natural products. Med Res Rev.
36:169–189. 2016. View Article : Google Scholar
|
|
20
|
Kong CS, Lee JI, Kim YA, Kim JA, Bak SS,
Hong JW, Park HY, Yea SS and Seo Y: Evaluation on anti-adipogenic
acitivity of flavonoids glucopyranosides from Salicornia herbacea.
Process Biochem. 47:1073–1078. 2012. View Article : Google Scholar
|
|
21
|
Hesp PA: Ecological processes and plant
adaptations on coastal dunes. J Arid Environ. 21:165–191. 1991.
|
|
22
|
Maun MA: Adaptations of plants to burial
in coastal sand dunes. Can J Bot. 76:713–738. 1998.
|
|
23
|
Lawlor DW and Cornic G: Photosynthetic
carbon assimilation and associated metabolism in relation to water
deficits in higher plants. Plant Cell Environ. 25:275–294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JI, Kong CS, Jung ME, Hong JW, Lim SY
and Seo Y: Antioxidant activity of the halophyte Limonium
tetragonum and its major active components. Biotechnol Bioprocess
Eng; BBE. 16:992–999. 2011. View Article : Google Scholar
|
|
25
|
Ksouri R, Megdiche W, Falleh H, Trabelsi
N, Boulaaba M, Smaoui A and Abdelly C: Influence of biological,
environmental and technical factors on phenolic content and
antioxidant activities of Tunisian halophytes. C R Biol.
331:865–873. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim YA, Kong CS, Lee JI, Kim H, Park HY,
Lee HS, Lee C and Seo Y: Evaluation of novel antioxidant
triterpenoid saponins from the halophyte Salicornia herbacea.
Bioorg Med Chem Lett. 22:4318–4322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim YA, Kong CS, Yea SS and Seo Y:
Constituents of Corydalis heterocarpa and their anti-proliferative
effects on human cancer cells. Food Chem Toxicol. 48:722–728. 2010.
View Article : Google Scholar
|
|
28
|
Oueslati S, Ksouri R, Pichette A, Lavoie
S, Girard-Lalancette K, Mshvildadze V, Abdelly C and Legault J: A
new flavonol glycoside from the medicinal halophyte Suaeda
fruticosa. Nat Prod Res. 28:960–966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong NN, Fang ST, Wang JH, Wang ZH and Xia
CH: Two new flavonoid glycosides from the halophyte Limonium
franchetii. J Asian Nat Prod Res. 16:370–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang ST, Liu X, Kong NN, Liu SJ and Xia
CH: Two new withanolides from the halophyte Datura stramonium L.
Nat Prod Res. 27:1965–1970. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bae CY, Hwang JS, Bae JJ, Choi SC, Lim SH,
Choi DG, Kim JG and Choo YS: Physiological responses of Calystegia
soldanella under drought stress. J Ecol Environ. 36:255–265. 2013.
View Article : Google Scholar
|
|
32
|
Bae KH: The Medicinal Plants of Korea.
Korea: Kyo-Hak publishing, Seoul; 2000
|
|
33
|
Lee YS, Kwak CG and Kim NW: Nutritional
characteristics of Calystegia japonica. Korean J Food Preserv.
19:619–625. 2012. View Article : Google Scholar
|
|
34
|
Takagi S, Yamaki M, Masuda K and Kubota M:
Studies on the purgative drugs. IV. On the constituents of
Calystegia japonica Choisy (author's transl). Yakugaku Zasshi.
97:1369–1371. 1977.In Japanese. PubMed/NCBI
|
|
35
|
Kim Y, Min HY, Park HJ, Lee EJ, Park EJ,
Hwang HJ, Jin C, Lee YS and Lee SK: Suppressive effects of nitric
oxide production and inducible nitric oxide synthase (iNOS) gene
expression by Calystegia soldanella methanol extract on
lipopolysaccharide-activated RAW 264.7 cells. Eur J Cancer Prev.
13:419–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang Z and Feng C: Experimental study on
anti-inflammatory and analgesic effects of water extracts of
Calystegia soldanella. Chin Arch Tradit Chin Med. 6:72010.
|
|
37
|
Lee JI, Kim IH, Choi YH, Kim EY and Nam
TJ: PTP1B inhibitory effect of alkyl p-coumarates from Calystegia
soldanella. Nat Prod Commun. 9:1585–1588. 2014.PubMed/NCBI
|
|
38
|
Min HY, Kim Y, Lee EJ, Hwang HJ, Park EJ
and Lee SK: Cytotoxic activities of indigenous plant extracts in
cultured human cancer cells. Nat Prod Sci. 8:170–172. 2002.
|
|
39
|
Ono M, Takigawa A, Kanemaru Y, Kawakami G,
Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H and Nohara T:
Calysolins V–IX, resin glycosides from Calystegia soldanella and
their antiviral activity toward herpes. Chem Pharm Bull (Tokyo).
62:97–105. 2014. View Article : Google Scholar
|
|
40
|
Ono M, Kawakami G, Takigawa A, Kabata K,
Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H and Nohara T: Calysolins
X-XIII, resin glycosides from Calystegia soldanella, and their
antiviral activity toward herpes simplex virus. Chem Pharm Bull
(Tokyo). 62:839–844. 2014. View Article : Google Scholar
|
|
41
|
Ono M, Takigawa A, Muto H, Kabata K, Okawa
M, Kinjo J, Yokomizo K, Yoshimitsu H and Nohara T: Antiviral
activity of four new resin glycosides calysolins XIV-XVII from
Calystegia soldanella against Herpes Simplex Virus. Chem Pharm Bull
(Tokyo). 63:641–648. 2015. View Article : Google Scholar
|
|
42
|
Nidiry ES, Ganeshan G and Lokesha AN:
Antifungal activity and isomerization of octadecyl p-coumarates
from Ipomoea carnea subsp. fistulosa. Nat Prod Commun. 6:1889–1892.
2011.
|
|
43
|
Gaspar EMM: New pentasaccharide
macrolactone from the European Convolvulaceae Calystegia
soldanella. Tetrahedron Lett. 40:6861–6864. 1999. View Article : Google Scholar
|
|
44
|
Gaspar EMM: Soldanelline B-The first
acylated nonlinear tetrasaccharide macrolactone from the European
Convolvulaceae Calystegia soldanella. Eur J Org Chem. 2001:369–373.
2001. View Article : Google Scholar
|
|
45
|
Takigawa A, Setoguchi H, Okawa M, Kinjo J,
Miyashita H, Yokomizo K, Yoshimitsu H, Nohara T and Ono M:
Identification and characterization of component organic and
glycosidic acids of crude resin glycoside fraction from Calystegia
soldanella. Chem Pharm Bull (Tokyo). 59:1163–1168. 2011. View Article : Google Scholar
|
|
46
|
Takigawa A, Muto H, Kabata K, Okawa M,
Kinjo J, Yoshimitsu H, Nohara T and Ono M: Calysolins I–IV, resin
glycosides from Calystegia soldanella. J Nat Prod. 74:2414–2419.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asano N, Yokoyama K, Sakurai M, Ikeda K,
Kizu H, Kato A, Arisawa M, Höke D, Dräger B, Watson AA, et al:
Dihydroxynortropane alkaloids from calystegine-producing plants.
Phytochemistry. 57:721–726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tatsuzawa F, Mikanagi Y and Saito N:
Flower anthocyanins of Calystegia in Japan. Biochem Syst Ecol.
32:1235–1238. 2004. View Article : Google Scholar
|
|
49
|
Tori M, Ohara Y, Nakashima K and Sono M:
Caffeic and coumaric acid esters from Calystegia soldanella.
Fitoterapia. 71:353–359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ahn NR, Ko JM and Cha HC: Comparison of
flavonoid profiles between leaves and stems of Calystegia
soldanella and Calystegia japonica. Am J Plant Sci. 3:1073–1076.
2012. View Article : Google Scholar
|
|
51
|
Ashkenazi A: Targeting the extrinsic
apoptosis pathway in cancer. Cytokine Growth Factor Rev.
19:325–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang Y, Zhu X, Chen Y, Wang X and Chen R:
p38 and JNK MAPK, but not ERK1/2 MAPK, play important role in
colchicine-induced cortical neurons apoptosis. Eur J Pharmacol.
576:26–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Canavese M, Santo L and Raje N: Cyclin
dependent kinases in cancer: Potential for therapeutic
intervention. Cancer Biol Ther. 13:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sperka T, Wang J and Rudolph KL: DNA
damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol
Cell Biol. 13:579–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dobashi Y, Takehana T and Ooi A:
Perspectives on cancer therapy: Cell cycle blockers and
perturbators. Curr Med Chem. 10:2549–2558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Paternot S, Bockstaele L, Bisteau X,
Kooken H, Coulonval K and Roger PP: Rb inactivation in cell cycle
and cancer: The puzzle of highly regulated activating
phosphorylation of CDK4 versus constitutively active CDK-activating
kinase. Cell Cycle. 9:689–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
George Rosenker KM, Paquette WD, Johnston
PA, Sharlow ER, Vogt A, Bakan A, Lazo JS and Wipf P: Synthesis and
biological evaluation of 3-aminoisoquinolin-1(2H)-one based
inhibitors of the dual-specificity phosphatase Cdc25B. Bioorg Med
Chem. 23:2810–2818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tilaoui M, Mouse HA, Jaafari A and Zyad A:
Differential effect of artemisinin against cancer cell lines. Nat
Prod Bioprospect. 4:189–196. 2014. View Article : Google Scholar : PubMed/NCBI
|