Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related death in the United States, with a 5-year overall
survival rate of 3–5% (1). Most
pancreatic cancer patients have distant metastasis at diagnosis,
and the prognosis of patients with peritoneal dissemination is
extremely poor (2,3). Furthermore, peritoneal dissemination
induces bowel obstruction and formation of malignant ascites, which
leads to poor performance status (3). Therefore, it is important to
determine a mechanism to control the peritoneal dissemination of
pancreatic cancer.
Pancreatic cancer is characterized by excessive
desmoplasia, which plays a crucial role in its aggressive behavior
through the tumor-stroma interaction (4). In the process of peritoneal
dissemination, cancer cells detached from the primary tumor are
transported by peritoneal fluid and disseminate in the peritoneum
(5). The peritoneum consists of a
monolayer of peritoneal mesothelial cells (PMCs) supported by a
basement membrane that rest on a layer of connective tissue with
fibroblasts, mast cells, macrophages and vessels (6). Therefore, the tumor-stroma
interaction is important in the process of peritoneal dissemination
(7). We recently investigated the
tumor-stroma interaction in peritoneal dissemination in pancreatic
cancer and found that peritoneal myofibroblasts contributed to the
promotion of peritoneal dissemination in pancreatic cancer
(8). Previous studies reported
that myofibroblasts were derived from resident peritoneal
fibroblasts, bone marrow progenitor cells, the primary tumor itself
or PMCs (9–12). However, the origin of peritoneal
myofibroblasts in pancreatic cancer is not yet clearly
understood.
PMCs generally act as a passive barrier and play
important roles in the response to wound healing and infection
(13). Other reports also showed
that PMCs are converted into myofibroblasts via
mesothelial-to-mesenchymal transition (MMT) by peritoneal dialysis
(9) and in ovarian and gastric
cancers (14,15). PMCs that are converted into
myofibroblasts via MMT affect cancer cells as cancer associated
fibroblasts (CAFs) in ovarian and gastric cancers (14,15).
CAFs support the malignant progression of tumors by promoting
growth, survival, angiogenesis, inflammation, drug resistance and
invasion and metastasis of tumors (16). A previous study showed that
heat-shock-factor-1 was one of the potent activator of CAFs
promoting malignancy (17). On the
other hand, the interaction between PMCs and cancer cells promoted
the proliferation and invasiveness of cancer cells in ovarian
(14,18,19)
and gastric (10,20) cancer. Moreover, adhesive
interaction between cancer cells and PMCs played an important role
in peritoneal dissemination (21).
A recent study revealed molecular mechanisms of peritoneal
dissemination in gastric cancer (22). However, the role of PMCs in the
peritoneal dissemination of pancreatic cancer remains unclear.
In the present study, we revealed the interaction
between the pancreatic cancer cells (PCCs) and PMCs in the process
of peritoneal dissemination in vitro and in vivo.
Materials and methods
Cell isolation and culture
conditions
PMCs were isolated from ascites recovered from
patients with no evidence of distant metastasis who underwent
curative resection for pancreatic cancer, as previously described
(9,23–25).
Briefly, after ascites were centrifuged at 1,500 rpm for 10 min,
the cell pellets were resuspended in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS),
penicillin and streptomycin and cultured in Collagen type I coated
dishes (Iwaki, Co., Ltd., Tokyo, Japan). The isolated cells were
identified as PMCs by their polygonal morphology and the expression
of calretinin and cells between passages 2 and 5 were used for
assays. We confirmed that there was no contamination with
fibroblasts, endothelial cells or malignant cells. PMCs were
activated by transforming growth factor-β1 (TGF-β1) administration
and these cells were regarded as activated PMCs (aPMCs), as
previously described (9,10). Briefly, PMCs were incubated with or
without 10 ng/ml TGF-β1 (R&D Systems, Oxon, UK) in DMEM with 1%
FBS for 48 h. We also used the following three PCC lines: SUIT-2
(Health Science Research Bank, Osaka, Japan), AsPC-1 (American Type
Culture Collection, Manassas, VA, USA) and PANC-1 (RIKEN
BioResource Center, Tsukuba, Japan). These cells were maintained as
previously described (26).
Establishment of immortalized PMCs
We cloned the DNA encoding hTERT and SV40 Large T in
the pLVSIN vector. We used these vectors to construct lentiviral
particles for infection of PMCs, followed by G418 selection.
Stable luciferase-expressing SUIT-2
cells
The firefly luciferase (GenTarget, #LVP326)
expression vector was transfected into SUIT-2 cells according to
the manufacturer's instructions. Blastidine S hydrochloride
(#15205; Sigma-Aldrich) was used for selection for more than 3
weeks. Luciferase expression was confirmed by a significant
increase in emission after adding 150 µg/ml D-Luciferin
potassium salt (#LK10000; OZ Biosciences, Marseille, France) as
compared with wild-type SUIT-2 cells.
Immunohistochemical analysis
Tissues of peritoneal dissemination of pancreatic
cancer in KPC (LSL-Kras G12D/+;
LSL-Trp53R172H/+;Pdx-1-Cre) transgenic mice (27) were evaluated by hematoxylin and
eosin (H&E), CK19, calretinin and α-smooth muscle actin (α-SMA)
immunohistochemical staining. Tissues were sliced to a thickness of
4 µm and incubated with rabbit anti-CK19 antibody (#133496,
1:100; Abcam), mouse anti-calretinin antibody (#M7245, 1:25; Dako)
or mouse anti-α-SMA antibody (#M0851, 1:100; Dako) overnight at
4°C. The staining was performed using serial sections.
Conditioned medium of PMCs
To obtain conditioned medium of PMCs (PMCs-CM), PMCs
were seeded and cultured until subconfluent. The medium was then
replaced with DMEM serum-free medium and the supernatants were
collected after 48 h of incubation.
Matrigel invasion and migration
assays
The invasiveness and migration capacities of PCCs
and PMCs were assessed by determining the number of cells that
invaded or migrated across Transwell chambers, as previously
described (26). For co-cultures,
PMCs (4.0×104/well) or aPMCs (4.0×104/well)
in 750 µl of DMEM supplemented with 10% FBS or medium alone
were seeded in the lower chambers for 24 h, and PCCs
(4.0×104/well) in 250 µl of DMEM supplemented
with 10% FBS were placed in the upper Transwell chamber (8
µm pore size; Becton-Dickinson, Franklin Lakes, NJ, USA)
containing 100 ml of reconstituted Matrigel-coated membrane (20
mg/well; BD Biosciences, Bedford, MA, USA). After incubation for 48
h, cell invasion was evaluated by counting the number of cells that
invaded through the Transwell chambers. Cell migration was assessed
after incubation for 24 h using uncoated Transwell chambers. To
assess the invasiveness of PMCs, each lower well was seeded with
PCCs (4.0×104/well) in 750 µl of DMEM
supplemented with 10% FBS or medium alone and incubated for 24 h.
PMCs (4.0×104/well) in 250 µl of DMEM
supplemented with 10% FBS were seeded in each upper well and
incubated for 24 h for the invasion assay and 12 h for the
migration assay. In both assays and at each time-point, invading or
migrated cells at the bottom of the chamber were fixed with 70%
ethanol and stained with H&E, and five random fields at ×200
magnification were counted under a light microscope. Each
experiment was performed in triplicate and repeated at least three
times.
Adhesion assay
The adhesion ability of PCCs was determined as
previously described (28).
Briefly, PMCs and aPMCs (8.0×104/well) were cultured in
a monolayer in 96-well Collagen I coated plates overnight. Collagen
I was used as the principal extracellular matrix molecule. PCCs
were labeled with CellTracker™ Green CMFDA (Life Technologies,
Eugene OR, USA). PCCs (4.0×104/well) were added to the
96-well Collagen I coated plates containing confluent PMCs or aPMCs
or without cells, and cells were incubated for 3 h at 37°C. The
plates were then washed three times with 200 µl of
phosphate-buffered saline (PBS) to remove the non-adherent tumor
cells. The number of adhered PCCs was determined in five random
fields at ×200 magnification using a fluorescent microscope
(BZ-9000; Keyence Corp., Osaka, Japan). Each experiment was
performed in triplicate and repeated at least three times.
Cell viability assay (adhered and
non-adhered conditions)
PCCs (1.0×103/well) were seeded (Greiner
Bio-One GmbH, Frickenhausen, Germany) and cell viability examined
using the CellTiter-Glo® Luminescent Cell Viability
assay kit (G7570; Promega, Madison, WI, USA) after culture for 24,
48 and 72 h following the manufacturer's instructions. PCCs
(1.0×103/well) were incubated with or without PMC-CM in
10% FBS/DMEM in 96-well plates. In non-adhered conditions, PCCs
(1.0×103/well) were cultured in a 96-well ultra-low
adherence plate (Corning Costar CLS7007). Background was subtracted
using values from wells containing only culture medium. Each
experiment was performed in triplicate and repeated at least three
times.
Apoptosis assay
PCCs (1.0×106/well) were incubated with
or without PMCs-CM in DMEM serum-free medium in a 90 mm ultra-low
adherence plate (Nunclon Sphera Dishes; Thermo Fisher Scientific)
for 72 h and the cells were lysed and subjected to western blotting
to determine the expression of apoptosis regulators.
3D organotypic culture model
To assess the process of invasiveness of PCCs and
PMCs, the 3D organotypic culture model was set up as previously
described (20) with minor
modifications. Briefly, 1000 µl of the gel containing 2
mg/ml Collagen I (BD Biosciences) and 2.5 mg/ml Matrigel was laid
onto the upper Transwell chambers (6-well). PMCs
(6.0×105/well) were added on the gels in DMEM containing
1% FBS. After 6 h, PCCs (6.0×105/well) were added on the
monolayer of PMCs. The bottom well was filled with DMEM containing
10% FBS. SUIT-2 cells were labeled with CellTracker™ Green and PMCs
were labeled with CellTracker™ Red CMTPX (Life Technologies). A
laser-scanning confocal fluorescence microscope (A1R; Nikon) was
used for immunofluorescence microphotography. After incubation for
10 days, the gels were fixed in 4% paraformaldehyde and sections
were cut into 4 µm sections for H&E staining and
incubated with rabbit Ki-67 antibody (#16667, 1:100; Abcam) or
rabbit anti-CEA (#RB-368-A, 1:250; Thermo Fisher Scientific)
overnight at 4°C. The Ki-67 labeling index was calculated by
dividing the number of Ki-67 positive nuclei by the total number of
nuclei in five random fields at ×200 magnification.
Analysis of collagen fiber
orientation
The orientation of the 3D gel collagen fibers was
analyzed using OrientationJ (an ImageJ-plug-in) (v.1.48u; National
Institute of Health, Bethesda, MD, USA) (29). Counts of the total fibers as well
as each orientation angle were measured, and the angles were
determined by approximating the relative angle every 10°. To allow
for comparison of acquired images, we set the direction of the
invading cells to 0° in the invading area and the tangential
direction of the initial cell cluster surface to 0° in the
non-invading area.
Real-time quantitative reverse
transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using the iTaq Universal
SYBR-Green One-Step kit and CFX96 Touch Real-Time PCR Detection
systems (Bio-Rad Laboratories). The primers were purchased from
Takara Bio (Kusatsu, Japan). Human GAPDH gene was used as the
endogenous control gene. The following primers were used in the
present study: E-cadherin, forward, 5′-AAGTGCTGCAGCCAAAGACAGA-3′
and reverse, 5′-AAATTGCCAGGCTCAATGACAAG-3′; fibronectin 1 (FN1),
forward, 5′-ACAGAACTATGATGCCGACCAGAAG-3′ and reverse,
5′-ACTGATCTCCAATGCGGTACATGA-3′; GAPDH, forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
Whole cell lysates were prepared in PRO-PREP
solution (iNtRON Biotechnology, Seongnam, Korea) from PMCs, aPMCs
and PCCs. Proteins from cell lysates were fractioned by SDS-PAGE
and transferred to a polyvinylidene difluoride membrane (Bio-Rad
Laboratories). The membrane was incubated overnight at 4°C with the
following antibodies: anti-E-cadherin (#3195, 1:1,000; Cell
Signaling Technology, Danvers, MA, USA), anti-vimentin (#5741,
1:1,000; Cell Signaling Technology), anti-α-SMA (#M0851, 1:500;
Dako), anti-FN1 (#sc-9068, 1:200; Santa Cruz Biotechnology),
anti-calretinin (#92341, 1:1,000; Abcam), anti-CEA (#RB-368-A,
1:200; Thermo Fisher Scientific), anti-cleaved caspase-3 (#9661,
1:1,000; Cell Signaling Technology) and anti-β-actin (#8227,
1:5,000; Abcam). The membrane was then probed with
horseradish-peroxidase-conjugated secondary antibodies (Cell
Signaling Technology). Immunoblots were detected by enhanced
chemiluminescence.
Animal experiments
All experiments with mice were conducted and
approved by the Ethics Committee of Kyushu University. The model of
intraperitoneal injection of BALB/c nu/nu mice (5–6 weeks of age;
Kyudo Co.) was used to analyze the dissemination activity of PCCs
alone and PCCs with PMCs. All animals were bred in laminar-flow
cabinets under specific pathogen-free conditions. The mice were
anesthetized with ether and suspensions of luciferase-expressing
SUIT-2 cells (2×105) alone in 200 µl PBS or with
PMCs (1×106) in 200 µl PBS were injected into the
peritoneal cavity of groups of 6 mice. Luciferin luminescence was
measured at 21 days after intraperitoneal injection using IVIS
Spectrum (Caliper Life Sciences, Waltham, MA, USA) after injecting
150 mg D-Luciferin (#LK10000; OZ Biosciences) into the
intraperitoneal cavity of anesthetized mice. Luminescence was
quantified using Living Image software version 4.4 (Summit
Pharmaceuticals International Corp., Tokyo, Japan). All mice were
sacrificed at 21 days after the evaluation of luciferin
luminescence and the disseminated nodules >3 mm in size were
counted.
Statistical analysis
Results are presented as means ± SD. Comparisons
between the two groups were conducted using the Student's t-test.
Tumor volume and disseminated nodules in vivo were analyzed
using the Wilcoxon test. Statistical significance was defined as
P<0.05. All statistical analyses were performed using JMP 11
software (SAS Institute, Cary, NC, USA).
Results
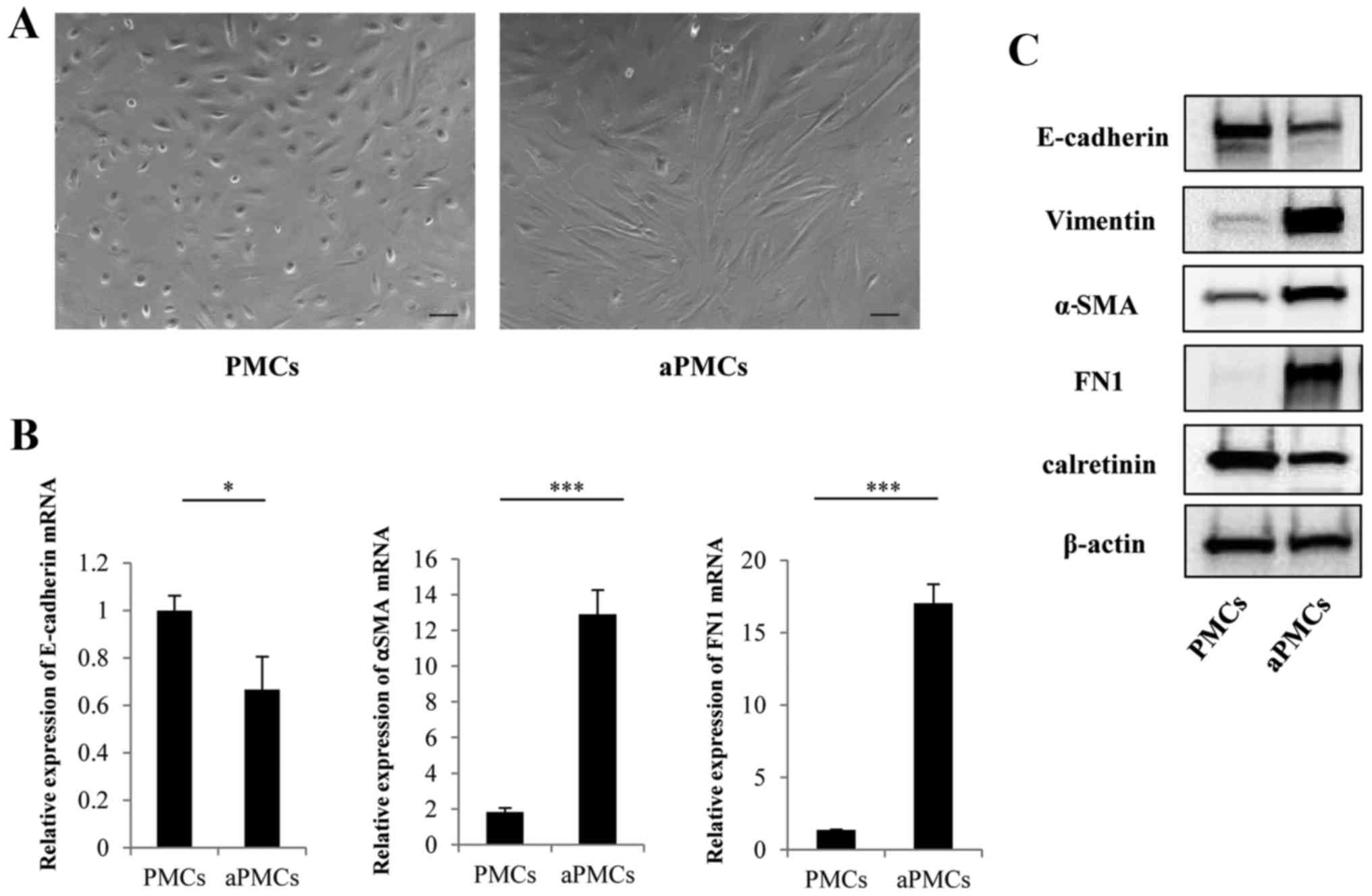
Verification of PMCs and aPMCs
The isolated cells were identified as PMCs by their
polygonal morphology and the expression of calretinin (Fig. 1A and C). Representative
microphotographs of aPMCs showed spindle-like morphology compared
with PMCs (Fig. 1A). To verify the
MMT of PMCs, the expressions of MMT markers were analyzed by
qRT-PCR and western blotting. The gene and protein expression of
E-cadherin was decreased in PMCs, whereas the expression levels of
α-SMA, FN1 and vimentin were increased (Fig. 1B and C) confirming MMT of PMCs.
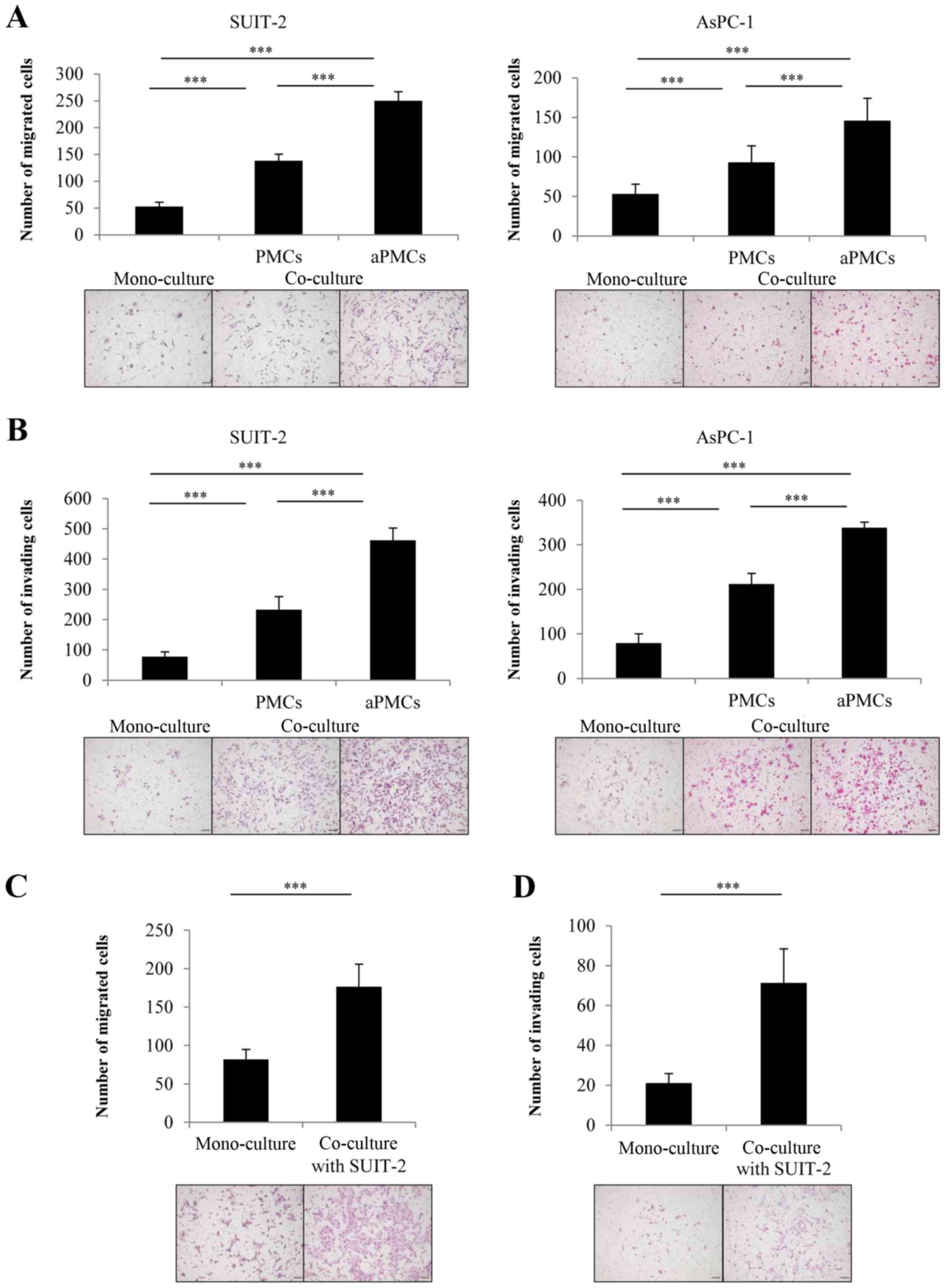
The tumor-stromal interaction of PCCs and
PMCs significantly enhances their migration and invasiveness in
indirect co-culture
To investigate the tumor-stromal interaction between
PMCs and PCCs, we evaluated the effect of PMCs on PCC migration and
invasiveness using indirect co-culture. We found that PMCs
significantly enhanced the migration (Fig. 2A) and the invasiveness (Fig. 2B) of PCCs in indirect co-culture
(both P<0.001). Furthermore, co-culture with aPMCs significantly
enhanced the migration (Fig. 2A)
and invasiveness (Fig. 2B) of PCCs
compared with the co-culture with PMCs (both P<0.001). We also
investigated the effect of co-cultured PCCs on the mobility and
invasiveness of PMCs and found that PCCs significantly enhanced the
migration (Fig. 2C) and the
invasiveness (Fig. 2D) of PMCs
(both P<0.001).
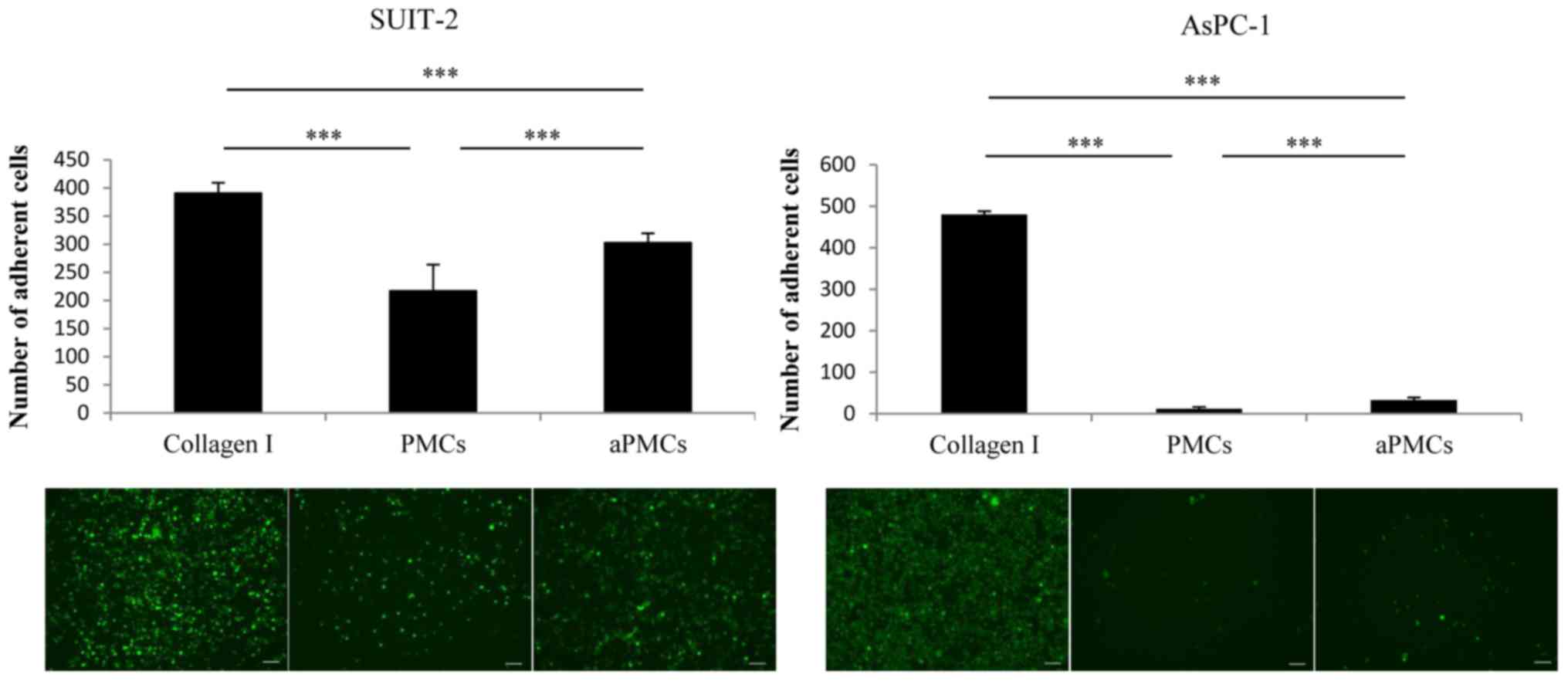
Adhesion ability of PCCs to PMCs, aPMCs
or Collagen I
Next, we assessed the adhesion ability of PCCs to
PMCs, aPMCs or Collagen I and found that the adhesion ability of
PCCs to PMCs was significantly decreased compared with the adhesion
to Collagen I, whereas the adhesion ability of PCCs to aPMCs was
significantly increased compared with the adhesion to PMCs
(Fig. 3; P<0.001).
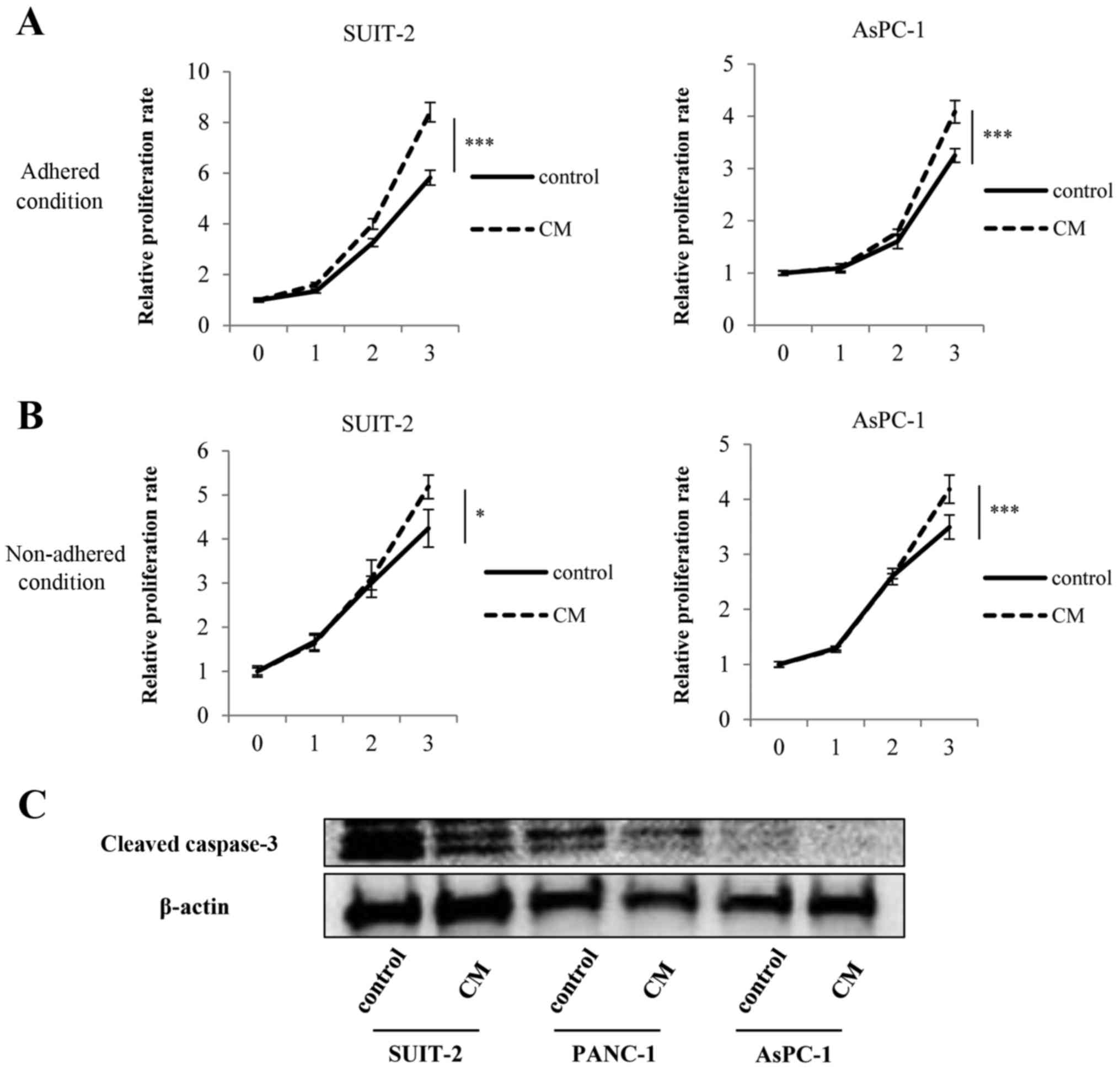
PMCs enhance proliferation and anoikis
resistance of PCCs
We found that PMCs significantly enhanced the
proliferation of PCCs compared with controls both in adhered
(Fig. 4A; P<0.001) and
non-adhered conditions (Fig. 4B;
SUIT-2 cells, P<0.05; AsPC-1 cells; P<0.001). Furthermore,
addition of PMCs-CM decreased the expression of cleaved caspase-3
of PCCs compared with controls (Fig.
4C).
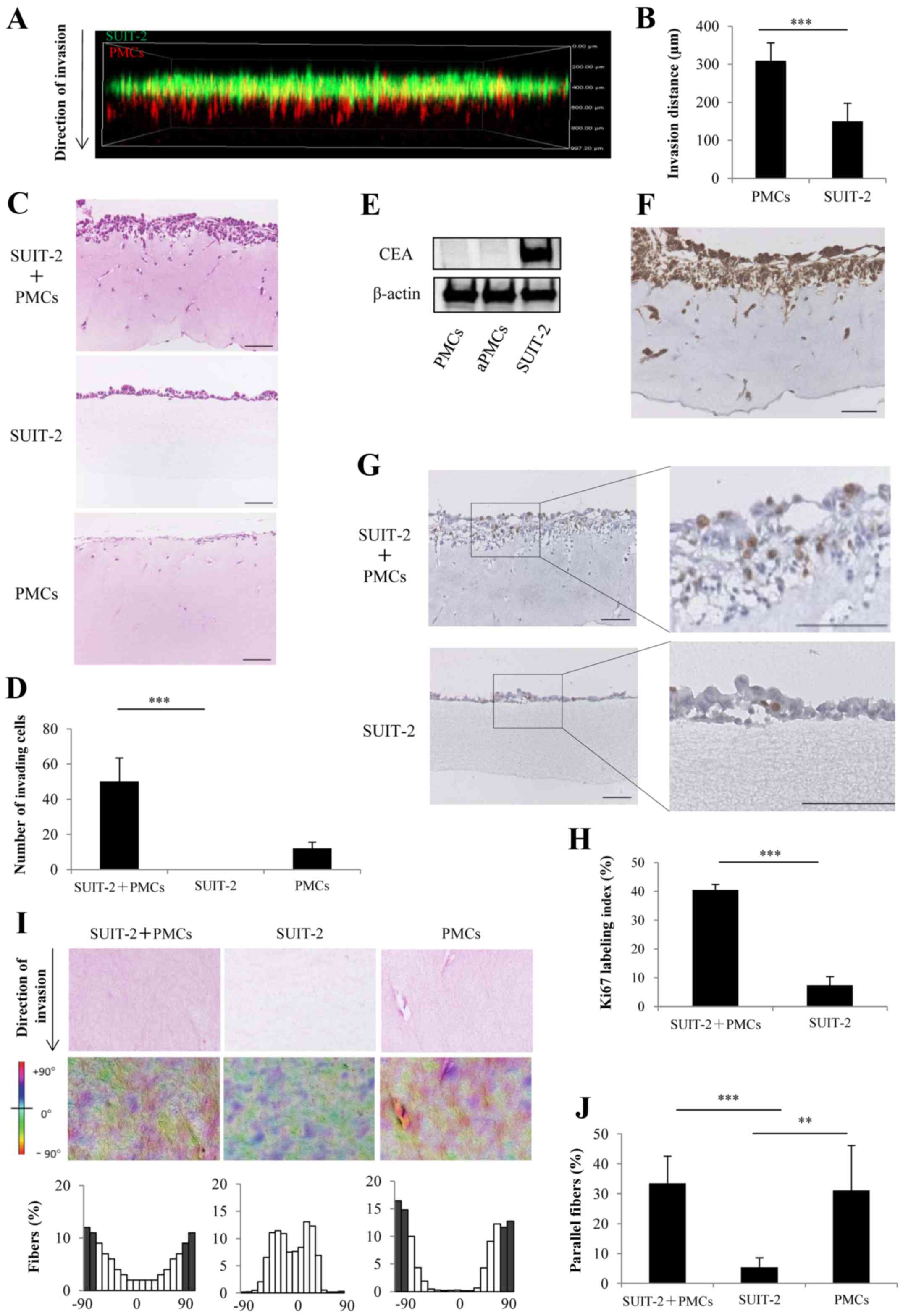
PMCs enhance invasiveness and
proliferation of PCCs and remodel collagen fibers in the 3D
organotypic culture model
We evaluated the invasion process of PCCs and PMCs
using a 3D organotypic culture model and confocal fluorescence
microscopy. We found that the invasion distance of PMCs was
significantly longer than that of SUIT-2 cells (Fig. 5A and B; P<0.001). H&E
sections showed that the co-culture of SUIT-2 cells and PMCs showed
a significant increase in the number of cells invading into the
collagen gel layer compared with mono-culture of SUIT-2 cells,
which did not invade into the collagen gel layer (Fig. 5C and D; P<0.001). To distinguish
between PCCs and PMCs, the expression of CEA was assessed by
western blotting and was positive only in SUIT-2 cells (Fig. 5E). Immunohistochemical staining
showed that SUIT-2 cells expressing CEA invaded into the collagen
gel layer upon co-culture with PMCs (Fig. 5F). Immunohistochemical staining of
Ki-67 showed that co-culture of SUIT-2 cells and PMCs significantly
enhanced proliferation compared with monoculture of SUIT-2 cells
(Fig. 5G and H; P<0.001). To
investigate the mechanism of PMC-induced invasiveness of PCCs, we
focused on the collagen fiber orientation. We found that the
collagen fibers in the co-cultures of SUIT-2 cells and PMCs or the
mon ocultures of PMCs displayed an organized parallel orientation
along the cells invading into collagen gel layer compared with the
random fiber arrangement detected in the monocultures of SUIT-2
cells (Fig. 5I and J).
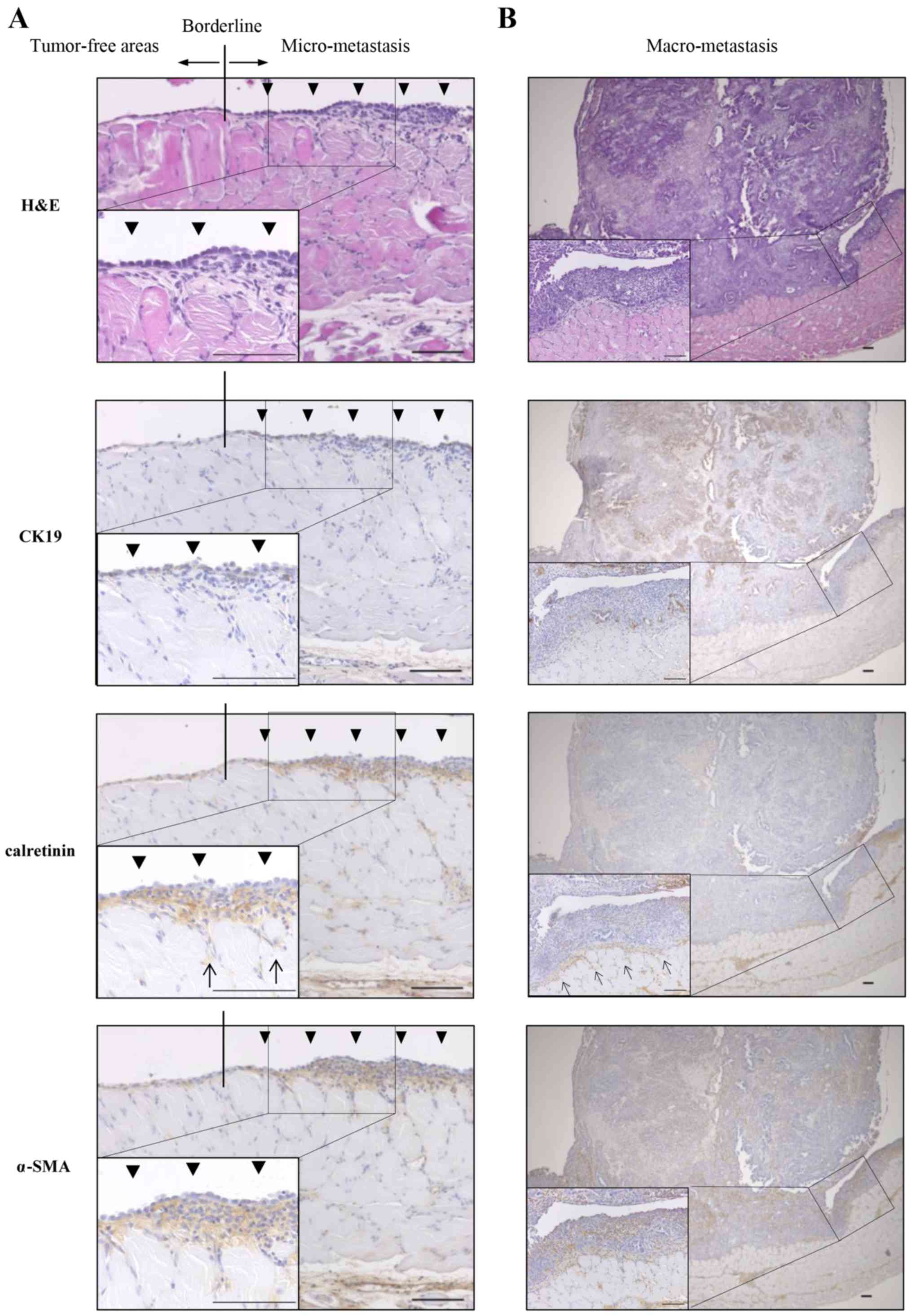
PMCs exist in the invasive front of
peritoneal dissemination of pancreatic cancer derived from KPC
mice
To assess the role of PMC in peritoneal
dissemination of pancreatic cancer in the KPC mouse, we focused on
the invasive front of peritoneal dissemination. We performed
H&E staining and immunohistochemical staining of CK19 as a PCC
marker, calretinin as a PMC marker and α-SMA as a myofibroblast
marker. The monolayer of PMCs was preserved in tumor-free areas,
whereas in micro-peritoneal dissemination, multiple layers of PMCs
were observed and a subset of PMCs invaded into the muscle layer
(Fig. 6A). In macro-peritoneal
dissemination, PMCs existed in the invasive front and pre-invaded
into the muscle layer, whereas α-SMA-positive and
calretinin-negative stromal cells existed in the tumor (Fig. 6B).
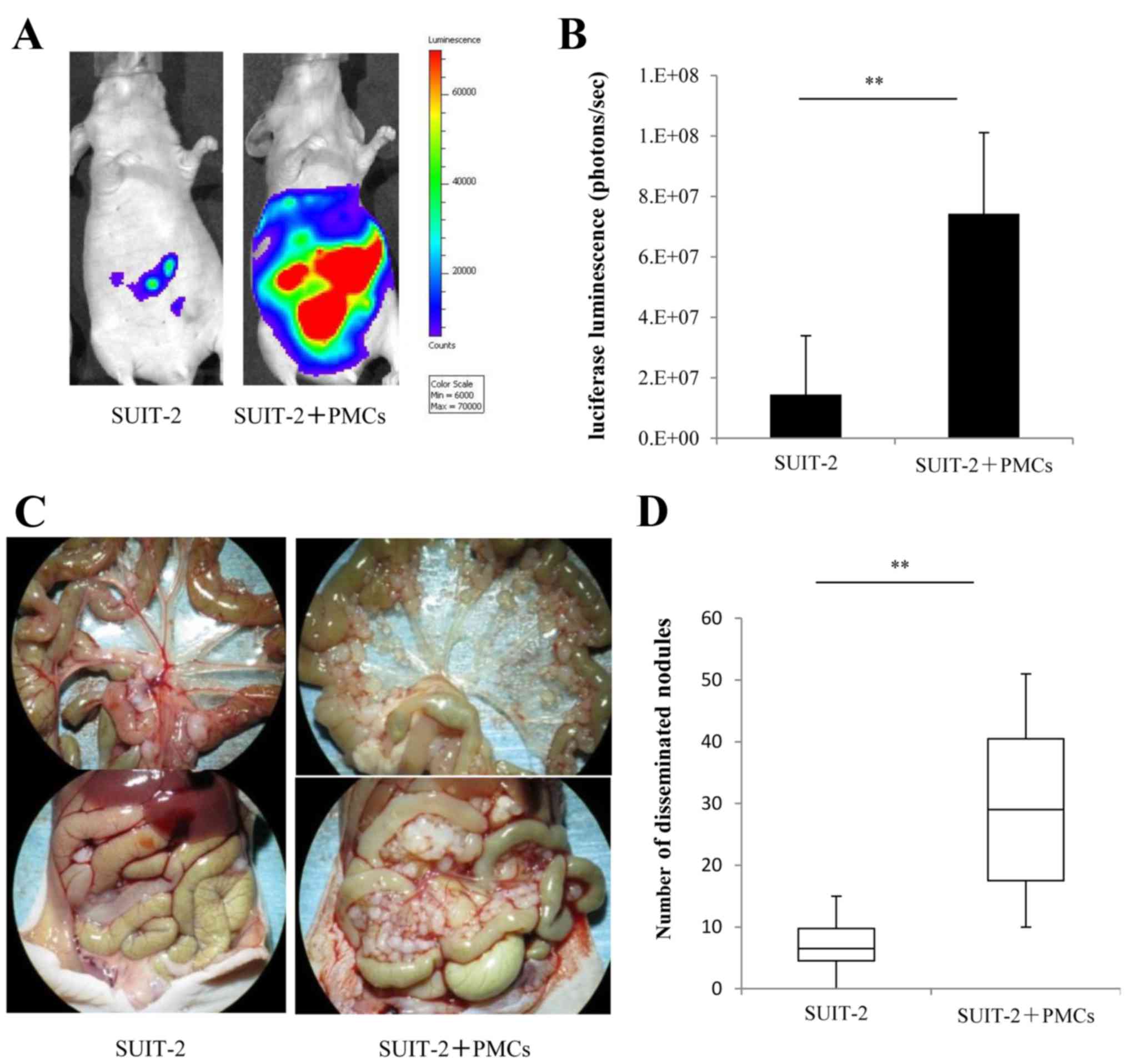
PMCs promote peritoneal dissemination of
PCCs in vivo
To investigate the functional role of PMCs on the
dissemination of PCCs in vivo, we intraperitoneally injected
luciferase-expressing SUIT-2 cells alone or with PMCs in nude mice
and measured the luciferase luminescence to evaluate the growth of
disseminated nodules of SUIT-2 cells. We found that intraperitoneal
injection of SUIT-2 cells with PMCs significantly promoted
peritoneal dissemination compared with SUIT-2 cells alone (Fig. 7A and B; P<0.01). Intraperitoneal
injection of SUIT-2 cells with PMCs (Fig. 7C, right panel) yielded a mean
29.3±14.1 peritoneal disseminated nodules >3 mm compared with
7.0±4.8 nodules in mice injected with SUIT-2 cells alone (Fig. 7C, left panel), a difference that
was statistically significant (Fig.
7D; P<0.01).
Discussion
In the present study, we found that the
tumor-stromal interaction of PCCs and PMCs significantly enhanced
their migration and invasiveness and enhanced proliferation and
anoikis resistance of PCCs. In the 3D organotypic culture model, we
found that co-culture with PCCs and PMCs significantly increased
the numbers of cells invading into the collagen gel layer compared
with monoculture of PCCs. We also found that PMCs pre-invaded into
the collagen gel, remodeled collagen fibers, and increased parallel
fiber orientation along the direction of cell invasion. We recently
reported that 3D matrices with the parallel fiber architecture
derived from pancreatic stellate cells (PSCs) under hypoxia
promoted cancer cell motility by inducing directional migration of
PCCs (30). These findings suggest
that PMCs might enhance the directional invasion of PCCs by
increasing parallel fiber orientation besides growth factors
associated with the tumor-stromal interaction.
Kasagi et al (31) showed that peritoneal lavage fluid
that contained peritoneal collagen type IV and plasma fibronectin
facilitated spheroid formation of colon cancer cells. Moreover,
Condello et al (32)
revealed that spheroid formation of cancer cells under non-adherent
conditions served to protect cells from environmental-induced
anoikis. We demonstrated that PMCs enhanced proliferation in
non-adhered conditions and anoikis resistance of PCCs. These
findings suggest that PMCs promote spheroid formation of cancer
cells and contribute to survival of cancer cells in the peritoneal
fluid.
Next, we demonstrated that the adhesion ability of
PCCs to PMCs was significantly decreased compared with Collagen I,
whereas the adhesion ability of PCCs to aPMCs was significantly
increased compared with PMCs. These findings were similar to
previous reports in gastric cancer (33). A previous study also showed that
the expression of FN1 in PMCs that was stimulated by ovarian cancer
cells promoted the adhesion of cancer cells in ovarian cancer
(33,34). In the present study, the expression
of FN1 in PMCs with MMT was significantly increased compared with
PMCs (Fig. 1B and C). These
findings indicate that the expression of FN1 in PMCs with MMT might
be involved in promoting the adhesion of PCCs.
We demonstrated that growth factors associated with
tumorstromal interaction of PCCs and PMCs mutually enhanced their
migration and invasiveness in indirect co-culture. Moreover, aPMCs
significantly enhanced the migration and invasiveness of PCCs. In
the 3D organotypic culture model of peritoneal dissemination,
co-culture of SUIT-2 and PMCs significantly increased the number of
cells invading into the collagen gel layer compared with
monoculture of SUIT-2 cells, which did not invade into the collagen
gel layer. The interaction of cancer cells and PMCs enhanced the
invasiveness of cancer cells in ovarian (14) and gastric (20) cancer in the 3D organotypic culture
model. Moreover Satoyoshi et al (20) revealed that Tks5 activation in PMCs
created the invasion front of peritoneal metastasis, which guided
invasiveness of cancer cells. In the present study, we revealed
that PMCs pre-invaded into the collagen gel, remodeled collagen
fibers, and increased parallel fiber orientation along the
direction of cell invasion. Conklin et al (35) revealed that the presence of
straightened collagen fibers is a predictor of breast cancer
survival. Furthermore, we previously reported that 3D matrices with
the parallel fiber architecture derived from PSCs under hypoxia
promoted cancer cell motility by inducing directional migration of
PCCs (30). These findings suggest
that a subset of PMCs enhanced the directional invasion of PCCs by
increasing parallel fiber orientation besides growth factors
associated with tumor-stromal interaction.
In previous studies, the interaction of cancer cells
and PMCs in the murine tissues of peritoneal dissemination was
assessed in vivo using intraperitoneal injection of cancer
cells into nude mice (14,20). However, these models might not
reflect the spontaneous formation of peritoneal dissemination
because peritoneal dissemination of these models was artificially
created. Therefore, in the present study, we assessed the tissues
of peritoneal dissemination that were spontaneously developed in
the KPC mouse with pancreatic cancer, which histologically
recapitulates the human tumors. Similar to our findings in the 3D
organotypic culture model, the monolayer of PMCs was preserved in
tumor-free areas, whereas PMCs were present in the invasive front
of micro-peritoneal dissemination and proliferated there, and a
subset of PMCs invaded into the muscle layer. These findings
indicate that PMCs pre-invade, possibly to lead invasiveness of
PCCs through the tumorstroma interaction and the matrices
remodeling.
We also showed that intraperitoneal injection of
PCCs and PMCs significantly promoted peritoneal dissemination
compared with PCCs alone in vivo. Similar results were
reported in gastric (20) and
ovarian cancer (19). In the
present study, exogenous PMCs promoted peritoneal dissemination
in vivo possibly by enhancing proliferation, anoikis
resistance and invasiveness of PCCs.
In conclusion, our results suggest that a subset of
PMCs promote the formation of peritoneal dissemination through the
tumor-stromal interaction and the matrices remodeling although PMCs
were generally thought to play a protective role for peritoneal
dissemination. Therapy targeting this specific subset of PMCs may
improve the prognosis of patients with pancreatic cancer.
Acknowledgments
The authors thank E. Manabe, S. Sadatomi and M.
Ohmori (Department of Surgery and Oncology, Kyushu University
Hospital). The present study was supported in part by a Japan
Society for the Promotion of Science Grant-in-Aid for Fellows (no.
16J03962) and Scientific Research (B) and (C) and Scientific
Research on Innovative Areas (grant nos. 26293305, 15K10185,
25713050, 16K15621, 16K10601, 16K10600, 16H05417, 15H04933,
15K15498 and 16H05418).
Abbreviations:
|
PMCs
|
peritoneal mesothelial cells
|
|
PCCs
|
pancreatic cancer cells
|
|
KPC
|
(LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre)
mouse model
|
|
MMT
|
mesothelial-to-mesenchymal
transition
|
|
CAFs
|
cancer associated fibroblasts
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
TGF-β1
|
transforming growth factor-β1
|
|
aPMCs
|
activated PMCs
|
|
H&E
|
hematoxylin and eosin
|
|
α-SMA
|
α-smooth muscle actin
|
|
CM
|
conditioned medium
|
|
PBS
|
phosphate-buffered saline
|
|
FN1
|
fibronectin 1
|
|
qRT-PCR
|
real-time quantitative reverse
transcription polymerase chain reaction
|
|
PSCs
|
pancreatic stellate cells
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomassen I, Lemmens VE, Nienhuijs SW,
Luyer MD, Klaver YL and de Hingh IH: Incidence, prognosis, and
possible treatment strategies of peritoneal carcinomatosis of
pancreatic origin: A population-based study. Pancreas. 42:72–75.
2013. View Article : Google Scholar
|
|
3
|
Sadeghi B, Arvieux C, Glehen O, Beaujard
AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL,
Faure JL, et al: Peritoneal carcinomatosis from non-gynecologic
malignancies: Results of the EVOCAPE 1 multicentric prospective
study. Cancer. 88:358–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neesse A, Michl P, Frese KK, Feig C, Cook
N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, et al:
Stromal biology and therapy in pancreatic cancer. Gut. 60:861–868.
2011. View Article : Google Scholar
|
|
5
|
Tan DS, Agarwal R and Kaye SB: Mechanisms
of transcoelomic metastasis in ovarian cancer. Lancet Oncol.
7:925–934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugarbaker PH: Peritoneum as the
first-line of defense in carcinomatosis. J Surg Oncol. 95:93–96.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yashiro M and Hirakawa K: Cancer-stromal
interactions in scirrhous gastric carcinoma. Cancer Microenviron.
3:127–135. 2010. View Article : Google Scholar
|
|
8
|
Akagawa S, Ohuchida K, Torata N, Hattori
M, Eguchi D, Fujiwara K, Kozono S, Cui L, Ikenaga N, Ohtsuka T, et
al: Peritoneal myofibroblasts at metastatic foci promote
dissemination of pancreatic cancer. Int J Oncol. 45:113–120.
2014.PubMed/NCBI
|
|
9
|
Yáñez-Mó M, Lara-Pezzi E, Selgas R,
Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA,
Aguilera A, Sánchez-Tomero JA, Bajo MA, Alvarez V, et al:
Peritoneal dialysis and epithelial-to-mesenchymal transition of
mesothelial cells. N Engl J Med. 348:403–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsukada T, Fushida S, Harada S, Yagi Y,
Kinoshita J, Oyama K, Tajima H, Fujita H, Ninomiya I, Fujimura T,
et al: The role of human peritoneal mesothelial cells in the
fibrosis and progression of gastric cancer. Int J Oncol.
41:476–482. 2012.PubMed/NCBI
|
|
11
|
Epperly MW, Guo H, Gretton JE and
Greenberger JS: Bone marrow origin of myofibroblasts in irradiation
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:213–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer
E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS,
et al: Role of pancreatic stellate cells in pancreatic cancer
metastasis. Am J Pathol. 177:2585–2596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mutsaers SE: The mesothelial cell. Int J
Biochem Cell Biol. 36:9–16. 2004. View Article : Google Scholar
|
|
14
|
Sandoval P, Jiménez-Heffernan JA,
Rynne-Vidal Á, Pérez-Lozano ML, Gilsanz Á, Ruiz-Carpio V, Reyes R,
García-Bordas J, Stamatakis K, Dotor J, et al: Carcinoma-associated
fibroblasts derive from mesothelial cells via
mesothelial-to-mesenchymal transition in peritoneal metastasis. J
Pathol. 231:517–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Na D, Lv ZD, Liu FN, Xu Y, Jiang CG, Sun
Z, Miao ZF, Li F and Xu HM: Transforming growth factor beta1
produced in autocrine/paracrine manner affects the morphology and
function of mesothelial cells and promotes peritoneal
carcinomatosis. Int J Mol Med. 26:325–332. 2010.PubMed/NCBI
|
|
16
|
Yamaguchi H and Sakai R: Direct
interaction between carcinoma cells and cancer associated
fibroblasts for the regulation of cancer invasion. Cancers (Basel).
7:2054–2062. 2015. View Article : Google Scholar
|
|
17
|
Scherz-Shouval R, Santagata S, Mendillo
ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M,
Stemmer SM, Whitesell L, et al: The reprogramming of tumor stroma
by HSF1 is a potent enabler of malignancy. Cell. 158:564–578. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ksiazek K, Mikula-Pietrasik J, Korybalska
K, Dworacki G, Jörres A and Witowski J: Senescent peritoneal
mesothelial cells promote ovarian cancer cell adhesion: The role of
oxidative stress-induced fibronectin. Am J Pathol. 174:1230–1240.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mikuła-Pietrasik J, Sosińska P, Kucińska
M, Murias M, Maksin K, Malińska A, Ziółkowska A, Piotrowska H,
Woźniak A and Książek K: Peritoneal mesothelium promotes the
progression of ovarian cancer cells in vitro and in a mice
xenograft model in vivo. Cancer Lett. 355:310–315. 2014. View Article : Google Scholar
|
|
20
|
Satoyoshi R, Aiba N, Yanagihara K, Yashiro
M and Tanaka M: Tks5 activation in mesothelial cells creates
invasion front of peritoneal carcinomatosis. Oncogene.
34:3176–3187. 2015. View Article : Google Scholar
|
|
21
|
Sluiter N, de Cuba E, Kwakman R, Kazemier
G, Meijer G and Te Velde EA: Adhesion molecules in peritoneal
dissemination: Function, prognostic relevance and therapeutic
options. Clin Exp Metastasis. 33:401–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanda M and Kodera Y: Molecular mechanisms
of peritoneal dissemination in gastric cancer. World J
Gastroenterol. 22:6829–6840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yung S, Li FK and Chan TM: Peritoneal
mesothelial cell culture and biology. Perit Dial Int. 26:162–173.
2006.PubMed/NCBI
|
|
24
|
Kitayama J, Emoto S, Yamaguchi H, Ishigami
H and Watanabe T: CD90+ mesothelial-like cells in
peritoneal fluid promote peritoneal metastasis by forming a tumor
permissive microenvironment. PLoS One. 9:e865162014. View Article : Google Scholar
|
|
25
|
Whitehead RH and Hughes LE: Tissue culture
studies. Br J Cancer. 32:512–518. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohuchida K, Mizumoto K, Murakami M, Qian
LW, Sato N, Nagai E, Matsumoto K, Nakamura T and Tanaka M:
Radiation to stromal fibroblasts increases invasiveness of
pancreatic cancer cells through tumor-stromal interactions. Cancer
Res. 64:3215–3222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hingorani SR, Wang L, Multani AS, Combs C,
Deramaudt TB, Hruban RH, Rustgi AK, Chang S and Tuveson DA:
Trp53R172H and KrasG12D cooperate to promote
chromosomal instability and widely metastatic pancreatic ductal
adenocarcinoma in mice. Cancer Cell. 7:469–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alkhamesi NA, Ziprin P, Pfistermuller K,
Peck DH and Darzi AW: ICAM-1 mediated peritoneal carcinomatosis, a
target for therapeutic intervention. Clin Exp Metastasis.
22:449–459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rezakhaniha R, Agianniotis A, Schrauwen
JTC, Griffa A, Sage D, Bouten CV, van de Vosse FN, Unser M and
Stergiopulos N: Experimental investigation of collagen waviness and
orientation in the arterial adventitia using confocal laser
scanning microscopy. Biomech Model Mechanobiol. 11:461–473. 2012.
View Article : Google Scholar
|
|
30
|
Sada M, Ohuchida K, Horioka K, Okumura T,
Moriyama T, Miyasaka Y, Ohtsuka T, Mizumoto K, Oda Y and Nakamura
M: Hypoxic stellate cells of pancreatic cancer stroma regulate
extracellular matrix fiber organization and cancer cell motility.
Cancer Lett. 372:210–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kasagi Y, Harada Y, Morodomi Y, Iwai T,
Saito S, Yoshida K, Oki E, Saeki H, Ohgaki K, Sugiyama M, et al:
Peritoneal dissemination requires an Sp1-dependent CXCR4/CXCL12
signaling axis and extracellular matrix-directed spheroid
formation. Cancer Res. 76:347–357. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Condello S, Morgan Ca, Nagdas S, Cao L,
Turek J, Hurley TD and Matei D: β-Catenin-regulated ALDH1A1 is a
target in ovarian cancer spheroids. Oncogene. 34:1–12. 2014.
|
|
33
|
Lv Z-D, Na D, Liu F-N, Du ZM, Sun Z, Li Z,
Ma XY, Wang ZN and Xu HM: Induction of gastric cancer cell adhesion
through transforming growth factor-beta1-mediated peritoneal
fibrosis. J Exp Clin Cancer Res. 29:1392010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kenny HA, Chiang CY, White EA, Schryver
EM, Habis M, Romero IL, Ladanyi A, Penicka CV, George J, Matlin K,
et al: Mesothelial cells promote early ovarian cancer metastasis
through fibronectin secretion. J Clin Invest. 124:4614–4628. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conklin MW, Eickhoff JC, Riching KM,
Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A and Keely PJ:
Aligned collagen is a prognostic signature for survival in human
breast carcinoma. Am J Pathol. 178:1221–1232. 2011. View Article : Google Scholar : PubMed/NCBI
|