Introduction
Lung cancer has been reported as a major cause of
mortality with a high metastasis rate compared with other types of
cancers (1). Lung cancer cells
contain a small population called tumor-initiating cells or cancer
stem cells (CSCs) that have extremely tumorigenic, self-renewal and
differentiating properties, resulting in a prolonged tumor status,
resistance to chemotherapy and cancer relapse (2,3).
Clinical observation has reported that this CSC subpopulation is
found in cancer specimens and remains an obstacle for cancer
treatment (4). In vitro and
in vivo studies have revealed that lung cancer stem cells
are extensively resistant to the first-line therapy cisplatin
compared with neighboring cancer cells (5,6).
These CSCs can maintain the cell survival signaling that provides
their strength under severe environments (7). Therefore, the attenuation or removal
of CSCs would be likely to improve patient outcome.
Like other types of stem cells, CSCs express
specific surface markers, including octamer-binding transcription
factor 4 (Oct4), Nanog, ATP-binding cassette subfamily G member 2
(ABCG2), and CD133. Oct4 and Nanog are transcription factors that
are responsible for maintaining pluripotency, self-renewal
proliferation and tumorigenicity in both normal stem cells and
cancer stem cells (8,9). Several studies have reported that an
elevation of Oct4 and Nanog is tightly related to a low survival
rate and high incidence of cancer metastases (10,11).
In lung cancer, both Oct4 and Nanog are required to maintain the
CSC-like phenotype (12).
Overexpression of Oct4 and Nanog enhances spheroid formation in
vitro and significantly increases new tumor formation in
vivo. Furthermore, CD133-positive lung cancer cells isolated
from patients exhibited a high level of Oct4 and ABCG2, and
displayed a clear resistance to chemotherapy and radiotherapy
(13). Conversely, the attenuation
of Oct4 and Nanog expression levels using RNA interference in CSCs
led to the loss of the ability to form spheroids and enhanced the
sensitivity of these cells to chemotherapy (12,14).
Cumulative research has identified the mechanism
regulating cancer stemness properties. Recently, the Akt pathway
was demonstrated to be an upstream signaling pathway of Oct4, which
is linked to the CSC-like phenotype (15). The phosphorylation of Akt promotes
stemness through the upregulation of both the mRNA and protein
levels of Oct4 and Nanog (15,16).
Akt-positive cancers that express CSC markers can establish
colonies in vitro and in vivo (17,18).
In contrast, Akt-knockdown experiments using RNA silencing resulted
in an extensive suppression of Oct4 as well as an impeded stemness
behavior (19). The alteration of
both Akt activity and expression may disrupt Oct4 and Nanog
functions, and consequently, the CSC properties of cancer.
Several strategies to the suppression of the CSC
mechanism have been intensively emphasized. Natural substances from
plant sources, including vanillin, have gained increased interest
in an alternative therapy due to their various pharmacological
activities (20). Vanillin,
4-hydroxy-3-methoxybenzaldehyde (Fig.
1), is the main active ingredient found in the Vanilla
planifolia seed. It is widely used as a flavoring agent in many
products including food and cosmetics (21). Previous reports have demonstrated
several biological activities of vanillin such as antimicrobial,
hypotriglyceridemic, anti-inflammatory and antimutagenic activities
in rodents and humans (22–24).
Vanillin has been shown to inhibit cancer invasion and migration
through the reduction of matrix metalloprotease activity and
downregulation of nuclear factor-κB in hepatocellular carcinoma
cells (25). Vanillin could also
attenuate the formation of lamellipodia and angiogenesis in lung
cancer via the suppression of PI3K activity and inducing the
apoptosis of various cancer types, such as human cervical cancer
and breast cancer (26–28). However, the pharmacological effect
of vanillin and its mechanism on CSC-like properties in lung cancer
has not been elucidated. In this study, we investigated the effects
of vanillin on the CSC phenotypes in the non-small cell lung cancer
(NSCLC) NCI-H460 cell line. We herein demonstrated for the first
time that vanillin can suppress the stemness behavior of NCI-H460
cells, including spheroid formation, with a reduction in CSC
markers. Furthermore, our data showed that Akt is the primary
target of the vanillin-attenuating CSC behavior, suggesting that
vanillin exhibits potential for further anticancer development.
Materials and methods
Cells and culture conditions
The human NSCLC cell line NCI-H460 was purchased
from the American Type Culture Collection, ATCC (Manassas, VA, USA)
and was cultured as monolayers in Roswell Park Memorial
Institute-1640 medium (RPMI) supplemented with 10% fetal bovine
serum (FBS), 2 mM L-glutamine and 100 U/ml penicillin and
streptomycin. Cell cultures were incubated at 37°C in a humidified
incubator filled with 5% CO2. Cells were subcultured
routinely with 0.25% (w/v) trypsin in 0.53 mM ethylenediamine
tetraacetic acid (EDTA) and were seeded according to the
manufacturer's instructions at ~70% confluence.
Reagents and antibodies
Vanillin (Fig. 1),
as USP-secondary standard purity grade (>99.0% purity) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). The vanillin
stock solution was freshly diluted with RPMI medium, and the
treatment solution was prepared by dilution of the stock solution
with culture media to the desired concentrations. Cell culture
media were also used as the control solvent for the experiments.
Glutamate, penicillin-streptomycin antibiotics, and
phosphate-buffered saline (PBS) were purchased from Gibco (Grand
Island, NY, USA). Methanol, dimethyl sulfoxide (DMSO) and mouse
monoclonal antibodies to Oct4 (1:1,000) and ubiquitin (1:1,000)
were purchased from Sigma-Aldrich. Rabbit monoclonal antibodies to
ABCG2 (1:1,000), β-catenin (1:1,000), Nanog (1:1,000) and
phosphorylated Akt (P-Akt, (1:1,000) in Ser473 and Thr308 were from
Cell Signaling Technology, Inc. (MA, USA). A goat monoclonal
antibody against ALDH1A1 (1:1,000) was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA), and rabbit monoclonal antibody
against CD133 (1:1,000) was purchased from United States Biological
(MA, USA).
Cell viability and cell proliferation
assays
Cell viability and proliferation assays were
performed using the surrogate
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. Briefly, NCI-H460 cells were seeded onto
96-well plates at 10,000 cells/well and 2,500 cells/well for the
cell viability and proliferation assays, respectively. After cell
attachment, various concentrations of vanillin (0–400 µM)
were added for 24 h for the cell viability assay. Cells pretreated
with the same concentration of vanillin for 1 and 3 days were
subcultured and incubated for 24, 48 and 72 h for the cell
proliferation assays. At the end of each incubation time, the
medium was removed and replaced with MTT solution (Life
Technologies, Carlsbad, CA, USA) and incubated for 3 h at 37°C. The
MTT solution was then substituted with 100 µl of DMSO to
dissolve the formazan crystals, and then the absorbance was
measured at 570 nm using a microplate reader. The absorbance was
calculated and represented as the % viability and % relative
proliferation, respectively, compared with the untreated (control)
cells that were set at 100%.
Determination of apoptotic and necrotic
cell death
Hoechst 33342 and propidium iodide (PI)
(Sigma-Aldrich) were used to stain the nuclei to identify necrotic
and apoptotic cell death, respectively. Cells were treated with
vanillin for 24 h and then were washed with PBS, followed by
incubation with either 5 µM PI or 10 µM Hoechst 33342
at 37°C for 30 min. Cells were visualized and captured under a
fluorescence microscope at ×10 magnification (Olympus IX5; 10X with
DP70 digital camera system; Olympus, Tokyo, Japan). PI-positive
necrotic cells and nuclear condensation of apoptotic cells were
scored and reported as the % of all cells viewed.
Anchorage-independent growth assay
The NCI-H460 cells were pretreated with vanillin
(0–50 µM) for 1 and 3 days before being subjected to the
soft agar colony-formation assay to determine the
anchorage-independent growth property of cancer stemness. Soft agar
was prepared using a combination of complete media and 1% (w/v)
agarose at a 1:1 ratio. This mixture was allowed to solidify in
24-well plates as a bottom layer. Next, pretreated cells suspended
in RPMI complete media were mixed with 0.3% (w/v) agarose, added
onto the prepared bottom layer and then incubated at 37°C. Further
complete media were applied every 2 days to prevent the soft agar
drying. After 2 weeks, the colony was examined under a
phase-contrast microscope at ×4 magnification (Olympus IX5; 4X with
DP70 digital camera system; Olympus). The relative colony number
and size were analyzed compared with those of the control
group.
Spheroid formation assay
Vanillin-pretreated cells at a cell density of 2,500
cells/well were cultured in 24-well ultralow attachment plates in
RPMI serum-free medium for 7 days. Next, the spheroids were
disaggregated by trypsinization, resuspended in RPMI serum-free
medium as a single cell suspension and then cultured onto 24-well
ultralow attachment plates. After an additional 14 days, the
presence of secondary spheroids was investigated by phase-contrast
microscopy at ×4 magnification (Olympus 1X51 with DP70). The
relative spheroid number and size were analyzed compared with those
of the control group.
Western blot analysis
Cells were lysed using lysis buffer containing 20 mM
Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM
Na3VO4, 10% glycerol, 50 mM NaF, 100 mM
phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich), and a protease
inhibitor cocktail (Merck Millipore, MA, USA), sonicated and
incubated on an ice bath for 45 min. The protein content was
measured using the BCA protein assay kit (Pierce™ Thermo Fisher
Scientific Inc., IL, USA). An equal amount of denatured protein was
separated by electrophoresis [10% (w/v) acrylamide resolving gel]
and transferred to a 0.45-µm nitrocellulose membrane
(Bio-Rad, Hercules, CA, USA). Each membrane was blocked with 5%
(w/v) nonfat-milk in Tris-buffered saline solution containing 1%
(v/v) Tween-20 (TBST) for at least 30 min and then incubated with
the indicated primary antibody at 4°C overnight. Thereafter, the
membranes were washed three times with TBST and then incubated with
secondary antibody at room temperature for 2 h. The
antigen-antibody complexes were detected, after washing with TBST
as above, using chemiluminescent solution (Pierce Biotechnology)
and were exposed to film (Carestream Health, Inc., Rochester, NY,
USA). The densitometry of the target protein was measured using the
NIH ImageJ program and quantified as the relative expression level
to that of the control group.
Immunoprecipitation assay
Protein interaction was examined by
immunoprecipitation. Cells were incubated with 10 µM
lactacystin for 1 h prior to treatment with vanillin to inhibit
proteasome function while preserving the ubiquitin-protein complex.
Treated cells were collected and lysed in TMN buffer (50 mM
Tris-HCl pH 7.5, 140 mM NaCl and 0.5 mM MgCl2)
containing 10% glycerol, a protease inhibitor cocktail, 1%
nonylphenylpolyethylene glycol (NP-40) and 1% PMSF for 30 min,
followed by centrifugation at 12,000 rpm at 4°C for 15 min. The
supernatants were blocked with protein G agarose beads (GE
Healthcare, Little Chalfont, UK) to remove non-specific binding.
After centrifugation at 3,000 rpm at 4°C for 5 min, the protein
contents were measured. The supernatants were collected and stored
at 4°C as an input for immunoblotting where 300 µg of
protein was incubated with anti-Akt antibody at 4°C overnight and
then incubated with protein G agarose beads for 2 h at 4°C. The
immunoprecipitates were collected, washed with TMN buffer and
resuspended in 30 µl of 2X SDS sample buffer. The samples
were boiled at 95°C for 5 min and then were subjected to western
blot analysis using antibodies against ubiquitin.
Statistical analysis
The data were collected from at least four
independent experiments, normalized by the control groups and
presented as the means ± standard deviation (SD). The significant
difference among the groups was analyzed using one-way ANOVA
followed by the post hoc test. Statistical analysis was performed
using SPSS software (IBM Inc., NY, USA) and statistical
significance was accepted at the p<0.05 level.
Results
Cytotoxicity and anti-proliferative
effect of vanillin on NCI-H460 cells
To diminish the interference of the cytotoxic effect
of vanillin on cancer stemness, a non-toxic concentration of
vanillin was used on the NCI-H460 cells, as determined by the
cytotoxicity assay. Cells were treated with various concentrations
of vanillin for 24 h and were then subjected to the MTT cell
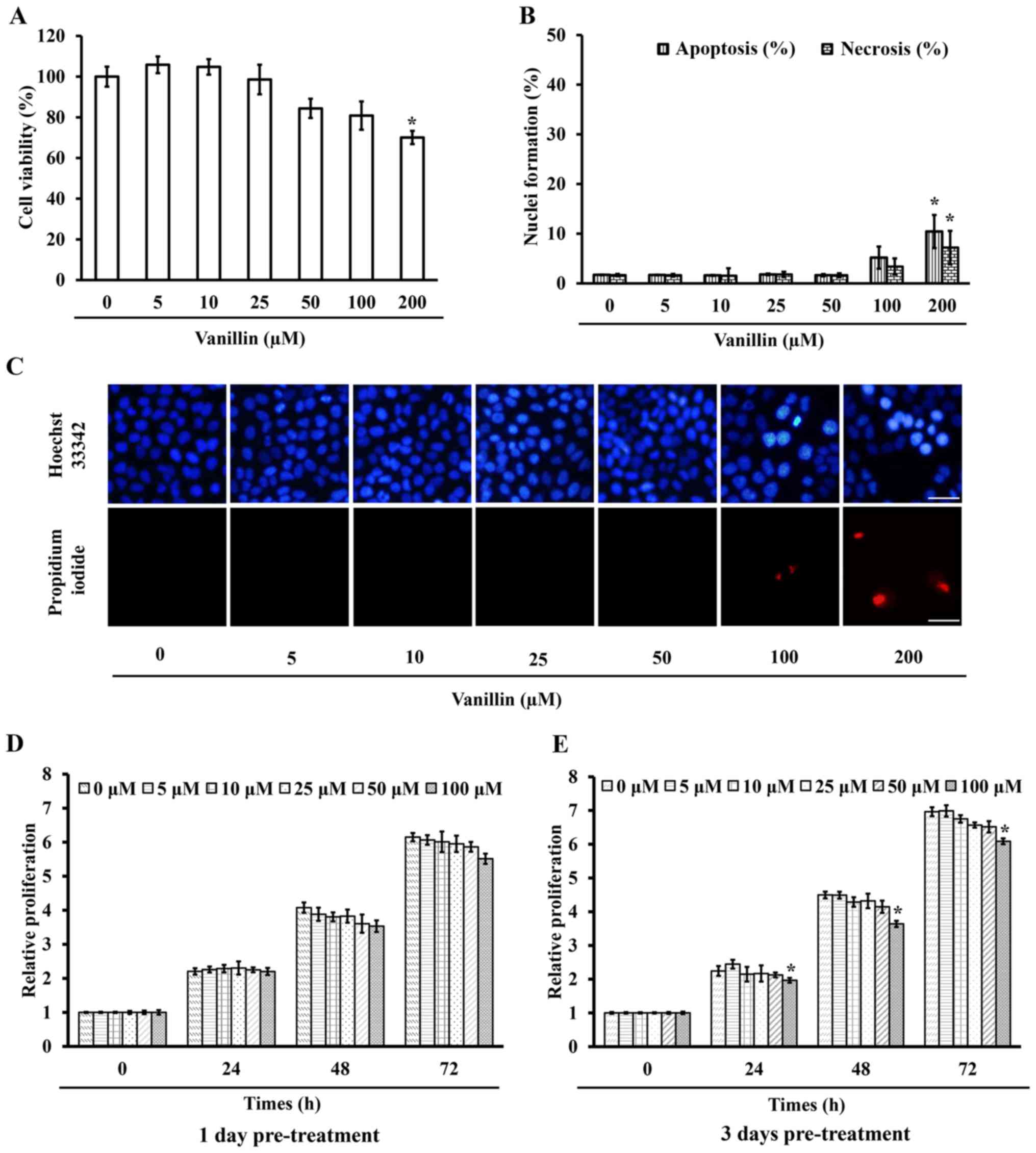
viability assay. Fig. 2A
demonstrates that 200 µM vanillin caused a significant
(p=0.003) mortality with ~30% cell death, whereas at a
concentration of <100 µM at least 80% viable cells
remained. To confirm the cell death mechanism, cells were similarly
treated with vanillin for 24 h and then were incubated with either
Hoechst 33342 or PI, where apoptotic nuclei and necrotic bodies
were clearly observed in the cells treated with a high (200
µM) dose of vanillin (Fig. 2B
and C). This result is consistent with the cell viability
testing, revealing that vanillin at concentrations of <100
µM are non-toxic to NCI-H460 cells, so these doses were used
for subsequent experiments.
The effect of vanillin on NCI-H460 cell
proliferation was also investigated. The cells were pre-treated
with vanillin for 1 and 3 days, prior to measuring their
proliferation after a further 24, 48 and 72 h. The proliferation of
the cells pretreated with 100 µM vanillin for 3 days was
significantly reduced as early as 24 h, whereas lower doses
(<100 µM) had no significant effect at any detection time
(Fig. 2E). Furthermore,
pretreatment with vanillin for 1 day showed non-detectable changes
compared with that in the control group (Fig. 2D). These results indicated that a
low dose of vanillin (0–50 µM) neither induced cell death
nor inhibited cell proliferation in the NCI-H460 cells. Cell growth
and survival in the absence of extracellular matrix is one of the
crucial behaviors of the cancer stem-like phenotype. Thus, to avoid
any anti-proliferative effect of vanillin on cancer stem cell
growth, vanillin doses that showed no such effect on NCI-H460 cells
under the attachment condition were then used for the cancer
stem-like phenotype characterization.
Inhibitory effect of vanillin on the
anchorage-independent growth and spheroid formation of NCI-H460
cells
Because self-renewal and tumorigenicity are
distinctive behaviors for the cancer stem-like phenotype,
anchorage-independent growth and spheroid formation have been used
to characterize these features. To investigate the suppressive
effect of vanillin on colony and spheroid formation, NCI-H460 cells
were pretreated with vanillin (0–50 µM) for 1 and 3 days,
followed by examination of their anchorage-independent growth and
spheroid formation. Fig. 3A shows
representative images in which both the colony size and number of
cells pretreated with vanillin for 1 day were not different from
those of untreated control cells. However, a significant and
vanillin dose-dependent reduction in the cell size and number was
observed when pre-incubated with vanillin for 3 days (Fig. 3B and C). Approximately 80 and 90%
reduction in the colony size and number, respectively, were
observed in the NCI-H460 cells pretreated with 50 µM
vanillin for 3 days (p=0.027).
The effect of vanillin on the self-renewal property
was evaluated by the spheroid formation assay. Primary spheroids,
consisting of several types of cells, including progenitors, mature
cells and stem cells (29), were
first initiated for 7 days and then dissociated to single cells.
The single cells were allowed to grow under detachment conditions
to form secondary spheroids over 14 days, reflecting the
pluripotency of CSCs. Interestingly, pretreatment with vanillin for
3 days markedly attenuated the number of secondary spheres formed
under detachment conditions, with only ~0.2-fold of the number of
sphere remaining after pretreatment with 50 µM vanillin for
3 days (p=0.022). In contrast, this effect was not found with a
1-day pretreatment with vanillin (Fig.
3D–F). These results suggest that vanillin can abolish the
cancer stem-like phenotype of CSCs, in terms of both spheroid
formation and anchorage-independent growth.
Vanillin downregulates stemness markers
in NCI-H460 cells
Cancer stem cell markers are differentially
expressed in different types of cancer, with CD133, ABCG2 and
ALDH1A1 being distinctly expressed in lung cancer stem cells
(3). Given the inhibitory effect
of vanillin on the CSC phenotype, its effect on stemness cell
markers was then evaluated in the NCI-H460 cells. NCI-H460 cells
were incubated with vanillin (0–50 µM) for 1 and 3 days, and
then the expression of CSCs markers was determined. Based on
western blot analysis (Fig. 4),
CSC protein marker expression was not significantly changed in
response to the treatment with vanillin for 1 day (50 µM,
p=0.124). However, the level of CSC markers, including those of
CD133, ABCG2 and ALDH1A1, were markedly attenuated in a
dose-dependent manner after treatment with vanillin for 3 days
(Fig. 4B). Quantitative analysis
demonstrated that a reduction in the expression level of these
proteins was observed in cells treated with vanillin for 3 days at
concentrations as low as 10 µM (p=0.021). Thus, vanillin
suppresses not only the CSC phenotype but also CSC markers in
NCI-H460 cells.
Vanillin suppression of Nanog and Oct4
expression is mediated by an Akt-dependent mechanism
The CSC phenotypes include not only an elevated
expression of stemness markers, but also high levels of the related
transcription factors, including Nanog and Oct4 (10–12)
that endow a self-renewal property to the cells (30). To assess whether the negative
regulation of CSCs properties by vanillin involved these
transcription factors, NCI-H460 cells were treated with various
concentrations of vanillin for 1 and 3 days, and then protein
expression levels of Oct4 and Nanog were evaluated. As shown in
Fig. 4B, the Oct4 and Nanog
protein expression levels were significantly decreased in the cells
treated with vanillin for 3 days, but not in the cells treated for
1 day (Fig. 4A). These results
illustrate a good correlation with the other stemness phenotypes
(Fig. 3 and 4), demonstrated by their suppression
after treatment with vanillin for 3 days.
Because the expression levels of Nanog and Oct4,
which contribute to the stemness behavior, are regulated directly
by Akt (15), whether vanillin
modulates Akt protein expression levels or its activity in terms of
P-Akt expression was evaluated next. NCI-H460 cells were treated
with vanillin for 3 days prior to determination of the Akt and
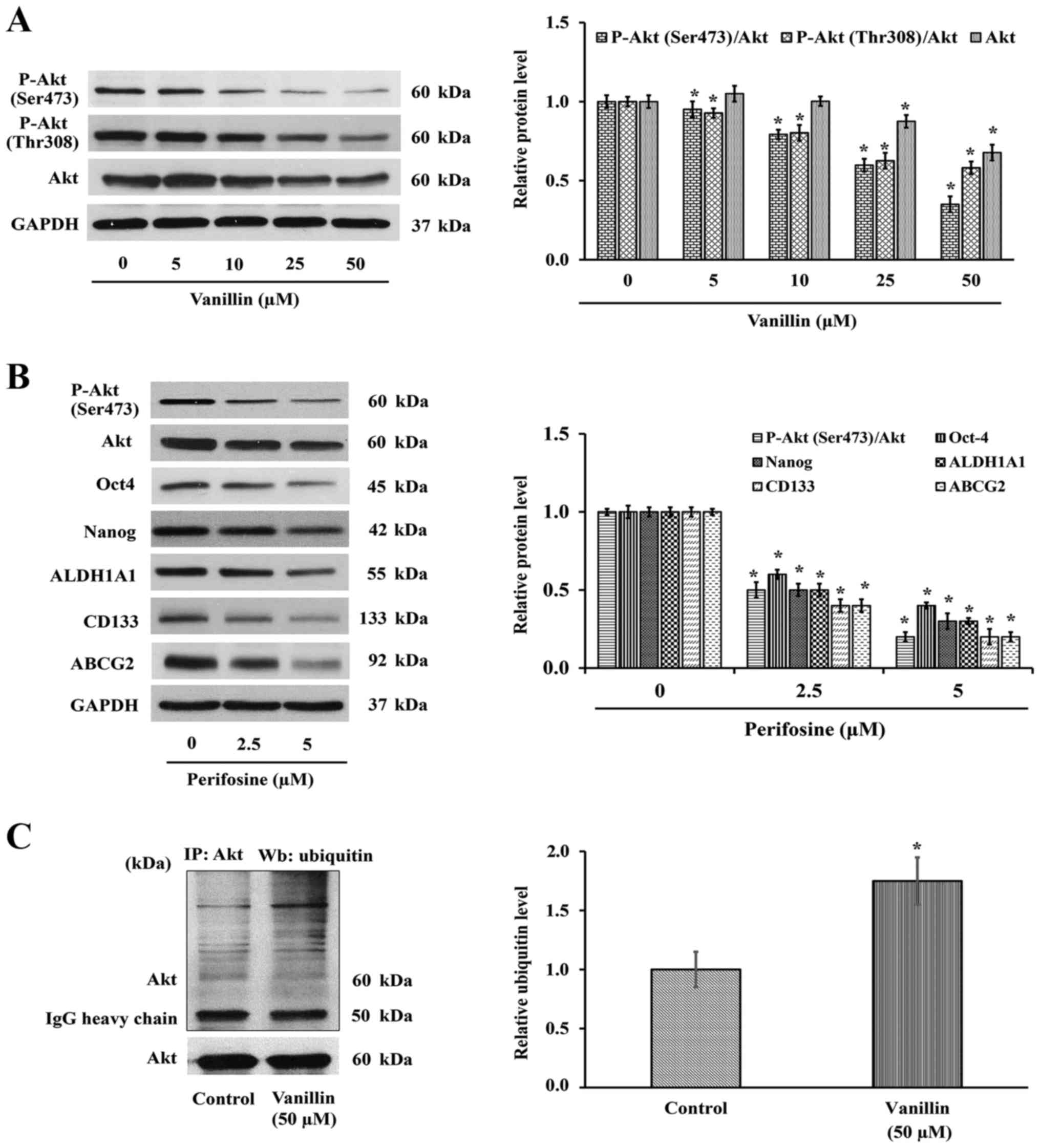
P-Akt expression levels. Western blot analysis showed that the
level of P-Akt (Ser473 and Thr308) or active Akt, was decreased
(Fig. 5A), suggesting that the
reduced CSCs phenotypes may result from an Akt-dependent mechanism.
To verify whether the reduction of P-Akt by vanillin is a result of
the suppression of P-Akt or total Akt expression levels, the total
Akt expression level was also investigated. As shown in Fig. 5A, the total Akt level was clearly
downregulated in a similar pattern. Densitometry analysis of P-Akt
relative to total Akt was then analyzed to confirm the hypothesis
that vanillin interferes with the Akt expression level. The
quantitative results demonstrated that the ratio of P-Akt over its
parental form are also changed, indicating the reduction of Akt
expression may be, at least in part, a mechanism by which vanillin
suppresses Akt activity.
To confirm the above finding that the downregulation
of Nanog and Oct4 by vanillin is by an Akt-dependent pathway,
perifosine (1,1-dimethylpiperidinium-4-yl octadecyl phosphate), an
Akt inhibitor, was used as a positive control. H460 cells were
treated with perifosine (0–5 µM) for 3 days, and the
mentioned proteins were observed by western blotting as before.
Interestingly, inactivation of Akt by perifosine caused a reduction
of its downstream signaling, including Oct4 and Nanog (Fig. 5B), similar to that with vanillin
(Fig. 4B). The CSC markers CD133,
ABCG2 and ALDH1A1 were also downregulated corresponding to the
suppression of Akt activity. Although the vanillin-blocked Akt
activation occurs partly through downregulating the parental form,
the total effect is similar to that by perifosine. These data
indicated the Akt function that promotes cancer stemness property
via Oct4 and Nanog, which was conversely inhibited by vanillin.
Vanillin mediates Akt degradation through
the ubiquitin-proteasomal pathway
Emerging evidence has indicated that the existence
of Akt is mainly governed by ubiquitin-proteasomal degradation via
E3 ligase (31,32). The reduction of Akt by the
enhancement of this mechanism results in the interference of its
downstream signaling mediators, including Oct4 and Nanog (11,15,16,33,34).
Accordingly, we next examined whether downregulation of Akt by
vanillin occurred through the ubiquitin proteasomal pathway.
Protein degradation by this pathway requires the protein to bind
with ubiquitin prior to recognition by the proteasome (35). NCI-H460 cells were pretreated with
10 µM lactacystin (Lac), a proteasomal inhibitor, prior to
vanillin treatment (50 µM) for 3 h, and the presence of
Akt-ubiquitin complex was determined by immunoprecipitation.
Fig. 5C shows that the level of
the Akt-ubiquitin interaction was markedly increased ~2-fold in the
vanillin-treated group, which indicated that suppression of CSCs by
vanillin may be a consequence of vanillin promoting Akt degradation
through the ubiquitin-proteasomal pathway.
Discussion
Taken together, cancers are one of the most common
non-accident related cause of deaths among human populations, but
the successful rate of treatment is low due to the resistance to
chemotherapy treatment, metastasis and the self-renewal properties
of cancer cells. It has been reported that these aggressive
behaviors are driven by CSCs (2),
which are a small population found within tumors, especially in
metastatic cancers (3). These CSCs
exhibit an overexpression of specific markers such as CD133,
ALDH1A1 and ABCG2 (2,36), and related proteins such as Akt and
mTOR (37). Recently, research
into drug discovery and development for cancer therapy has focused
on exploring new natural compounds that target CSCs and related
pathways. Several reports have suggested that many natural
substances, such as curcumin, resveratrol and pomegranate extract,
have the ability to inhibit CSCs (38–40).
Herein, we report for the first time that vanillin,
an active component in Vanilla planifolia, suppresses CSCs
in term of both their phenotype and related molecular mechanisms. A
non-cytotoxic concentration of vanillin (50 µM) attenuated
spheroid formation and anchorage-independent growth, which are the
two prominent characteristics of CSCs (Fig. 3). The inhibitory effects of
vanillin were clearly observed after treatment for 3 days,
suggesting that in the early phase of treatment, a compensation
mechanism by other kinase proteins, such as mitogen activated
protein kinase (MAPK) or ERK may exist (41,42).
Both PI3K/Akt and MAPK/ERK are known to play an important role in
the maintenance of cell survival, differentiation and proliferation
of cancer (43,44). A recent study reported that
epidermal growth factor (EGF) could induce Akt activation through
the MEK inhibitor (45). This
phenomenon may occur during the 1-day treatment course of vanillin.
An alternative possibility is that cellular signaling requires a
longer activation time to induce the phenotypic changes. For
example, the inhibitory effect of gigantol on CSCs was found only
after treatment for at least 2 days (46). The ability of cancer cells to form
neurospheres also indicates the pluripotency of CSCs (45), and our finding here demonstrated
that vanillin greatly suppresses the in vitro formation of
secondary spheroids consisting of NCI-H460 cells (Fig. 3D).
CD133, ALDH1A1 and ABCG2 have been reported to be
specific markers in lung CSCs both in vivo and in
vitro (47–49). Lung CSCs with CD133+
implanted in mice exhibited a self-renewal property,
chemoresistance and a high tumorigenic capability. Like the in
vitro study, NCI-H460 and NCI-A549, which express high levels
of ABCG2 and CD133, can form spheroids, but this ability was
attenuated after reduction of ABCG2 and CD133 expression (50). We further evaluated the expression
of such markers in vanillin-treated cells. Corresponding to the
phenotypic changes, vanillin downregulated these lung CSC markers
(Fig. 4). As transcription
factors, Oct4 and Nanog are frequently found in both CSCs and
normal stem cells (49), and are
responsible for maintaining the self-renewal and pluripotent
properties of CSCs under regulation by the kinase activity of Akt
(51). These transcription factors
are upregulated in many cancers, especially lung cancer, and the
reduction of Oct4 and Nanog expression can directly inhibit CSC
phenotypes (11,30). Knockdown of these transcription
factors was shown to decrease the proliferation and spheroid
formation of cancer cells (15).
Supporting our finding, Oct4 and Nanog were extensively decreased
in response to vanillin treatment, and so likely contributed to the
impedance of neurosphere formation and colony-formation capability
of the NCI-H460 cells (Fig.
3).
Evidence has suggested that Akt plays a vital role
in cell survival and proliferation (52), and drive cancer cells to CSC-like
phenotypes including proliferation, migration and self-renewal
properties, through the action of the transcription factors Oct4
and Nanog (16). Blockade of Akt
was shown to inhibit the in vitro proliferation of spheroid
formation and enhanced Oct4 degradation (33). In breast cancer, inhibition of PI3K
or Akt activity led to pluripotency loss (37). Furthermore, in prostate cancer, the
alteration of the PTEN/PI3K/Akt pathway interfered with the CSC
phenotype (53). Likewise, in this
study, vanillin was found to diminish Akt function and its
expression, together with a decreased CSC behavior and marker
expression (Fig. 4). As a key
protein catabolism pathway, the ubiquitin-proteasomal pathway is
known to regulate several cellular signaling molecules, including
Akt (54). Targeted proteins for
degradation are poly-ubiquitinated by the ubiquitin ligase (E3) of
the proteasome prior to recognition by ubiquitin-activating E1 and
E2. The results of this study are consistent with the notion that
vanillin drives Akt degradation through elevation of the
ubiquitin-proteasomal pathway (Fig.
5). However, the mechanism that leads to ubiquitinized Akt is
still unknown. Recent studies reported that multiple growth factor
receptors such as EGFR and insulin-like growth factor 1 receptor,
might be possible targets of natural compounds (55,56).
Since the ubiquitination-proteasomal degradation of intracellular
proteins is modulated by the downstream signaling of these
receptors (57–60), it is possible that the mechanism of
vanillin action on ubiquitinized Akt might involve
vanillin-modulating of these receptors and their signaling
pathways. However, the ubiquitin-proteasomal pathway is also
regulated by the reactive oxygen species (61,62)
and the exogenous compounds, that alter the oxidative status of the
cells, affect this degradation pathway (63,64).
Since vanillin has been demonstrated to have a dual effect as an
oxidative stress scavenging and pro-oxidant activity, depending on
the cell type and vanillin concentration (65–67),
thus, it is also possible that vanillin may promote Akt degradation
through this mechanism.
In conclusion, this study demonstrates that vanillin
can suppress the CSC-like phenotype in NCI-H460 cells via the
enhancement of the Akt-ubiquitin proteasome degradation pathway,
resulting in the downregulation of the transcription factors Oct4
and Nanog (Fig. 6). This finding
may provide a new strategy to overcome CSCs in lung cancer.
Abbreviations:
|
ABCG2
|
ATP-binding cassette subfamily G
member 2
|
|
ALDH1A1
|
aldehyde dehydrogenase 1 family member
A1
|
|
Akt
|
protein kinase B
|
|
CSCs
|
cancer stem cells
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyl tetrazolium bromide
|
|
EDTA
|
ethylenediamine tetraacetic acid
|
|
FBS
|
fetal bovine serum
|
|
NSCLC
|
non-small cell lung cancer
|
|
Oct4
|
octamer-binding transcription factor
4
|
|
P-Akt
|
active Akt (phosphorylated at Ser473
and Thr308 residues)
|
|
PBS
|
phosphate-buffered saline
|
|
PI
|
propidium iodide
|
|
PMSF
|
phenylmethylsulfonyl fluoride
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
TBST
|
Tris-buffered saline solution with 1%
Tween-20
|
Acknowledgments
This study was supported by grant for International
Research Integration: Chula Research Scholar, Ratchadaphiseksomphot
Endowment Fund, and the 90th Anniversary Chulalongkorn University.
The English editing was proofed by The American Journal Expert
service.
References
|
1
|
Peters S, Adjei AA, Gridelli C, Reck M and
Kerr K: Metastatic non-small-cell lung cancer (NSCLC): ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23(Suppl 7): vii56–vii64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
3
|
Medema JP: Cancer stem cells: The
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pallini R, Ricci-Vitiani L, Banna GL,
Signore M, Lombardi D, Todaro M, Stassi G, Martini M, Maira G,
Larocca LM, et al: Cancer stem cell analysis and clinical outcome
in patients with glioblastoma multiforme. Clin Cancer Res.
14:8205–8212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopez-Ayllon BD, Moncho-Amor V, Abarrategi
A, Ibañez de Cáceres I, Castro-Carpeño J, Belda-Iniesta C, Perona R
and Sastre L: Cancer stem cells and cisplatin-resistant cells
isolated from non-small-lung cancer cell lines constitute related
cell populations. Cancer Med. 3:1099–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Guo W, Wang L, Yu L, Mei H, Fang
S, Ji P, Liu Y, Liu G and Song Q: Cisplatin-resistant osteosarcoma
cells possess cancer stem cell properties in a mouse model. Oncol
Lett. 12:2599–2605. 2016.PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin X, Zhang BH, Zheng SS, Gao DM, Qiu SJ,
Wu WZ and Ren ZG: Coexpression of gene Oct4 and Nanog initiates
stem cell characteristics in hepatocellular carcinoma and promotes
epithelial-mesenchymal transition through activation of Stat3/Snail
signaling. J Hematol Oncol. 8:232015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W,
Chen X, Bourque G, George J, Leong B, Liu J, et al: The Oct4 and
Nanog transcription network regulates pluripotency in mouse
embryonic stem cells. Nat Genet. 38:431–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He W, Li K, Wang F, Qin YR and Fan QX:
Expression of OCT4 in human esophageal squamous cell carcinoma is
significantly associated with poorer prognosis. World J
Gastroenterol. 18:712–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu X, Mazur SJ, Lin T, Appella E and Xu Y:
The pluripotency factor nanog promotes breast cancer tumorigenesis
and metastasis. Oncogene. 33:2655–2664. 2014. View Article : Google Scholar :
|
|
12
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YC, Hsu HS, Chen YW, Tsai TH, How CK,
Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X,
Lu J, Fan X, Zhu S, Wang Y, et al: Knockdown of Oct4 and Nanog
expression inhibits the stemness of pancreatic cancer cells. Cancer
Lett. 340:113–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao QW, Zhou YW, Li WX, Kang B, Zhang XQ,
Yang Y, Cheng J, Yin SY, Tong Y, He JQ, et al: Akt-mediated
phosphorylation of Oct4 is associated with the proliferation of
stem-like cancer cells. Oncol Rep. 33:1621–1629. 2015.PubMed/NCBI
|
|
16
|
Lin Y, Yang Y, Li W, Chen Q, Li J, Pan X,
Zhou L, Liu C, Chen C, He J, et al: Reciprocal regulation of Akt
and Oct4 promotes the self-renewal and survival of embryonal
carcinoma cells. Mol Cell. 48:627–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YK, Zhu YL, Qiu FM, Zhang T, Chen ZG,
Zheng S and Huang J: Activation of Akt and MAPK pathways enhances
the tumorigenicity of CD133+ primary colon cancer cells.
Carcinogenesis. 31:1376–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noh KH, Kim BW, Song KH, Cho H, Lee YH,
Kim JH, Chung JY, Kim JH, Hewitt SM, Seong SY, et al: Nanog
signaling in cancer promotes stem-like phenotype and immune
evasion. J Clin Invest. 122:4077–4093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rajesh E, Sankari LS, Malathi L and Krupaa
JR: Naturally occurring products in cancer therapy. J Pharm
Bioallied Sci. 7(Suppl 1): S181–S183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walton NJ, Mayer MJ and Narbad A:
Vanillin. Phytochemistry. 63:505–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
King AA, Shaughnessy DT, Mure K,
Leszczynska J, Ward WO, Umbach DM, Xu Z, Ducharme D, Taylor JA,
Demarini DM, et al: Antimutagenicity of cinnamaldehyde and vanillin
in human cells: Global gene expression and possible role of DNA
damage and repair. Mutat Res. 616:60–69. 2007. View Article : Google Scholar :
|
|
23
|
Srinivasan K, Platel K and Rao MVL:
Hypotriglyceridemic effect of dietary vanillin in experimental
rats. Eur Food Res Technol. 228:103–108. 2008. View Article : Google Scholar
|
|
24
|
Imanishi H, Sasaki YF, Matsumoto K,
Watanabe M, Ohta T, Shirasu Y and Tutikawa K: Suppression of
6-TG-resistant mutations in V79 cells and recessive spot formations
in mice by vanillin. Mutat Res. 243:151–158. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang JA, Wu SL, Lo HY, Hsiang CY and Ho
TY: Vanillin inhibits matrix metalloproteinase-9 expression through
downregulation of nuclear factor-kappaB signaling pathway in human
hepatocellular carcinoma cells. Mol Pharmacol. 75:151–157. 2009.
View Article : Google Scholar
|
|
26
|
Lirdprapamongkol K, Sakurai H, Suzuki S,
Koizumi K, Prangsaengtong O, Viriyaroj A, Ruchirawat S, Svasti J
and Saiki I: Vanillin enhances TRAIL-induced apoptosis in cancer
cells through inhibition of NF-kappaB activation. In Vivo.
24:501–506. 2010.PubMed/NCBI
|
|
27
|
Lirdprapamongkol K, Sakurai H, Kawasaki N,
Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S,
Svasti J and Saiki I: Vanillin suppresses in vitro invasion and in
vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci.
25:57–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lirdprapamongkol K, Kramb JP,
Suthiphongchai T, Surarit R, Srisomsap C, Dannhardt G and Svasti J:
Vanillin suppresses metastatic potential of human cancer cells
through PI3K inhibition and decreases angiogenesis in vivo. J Agric
Food Chem. 57:3055–3063. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bachelard-Cascales E, Chapellier M, Delay
E and Maguer-Satta V: A protocol to quantify mammary early common
progenitors from long-term mammosphere culture. Curr Protoc Stem
Cell Biol. 1:p Unit 1E. 72012.PubMed/NCBI
|
|
30
|
Amini S, Fathi F, Mobalegi J,
Sofimajidpour H and Ghadimi T: The expressions of stem cell
markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3,
Dppa4, and Esrrb in bladder, colon, and prostate cancer, and
certain cancer cell lines. Anat Cell Biol. 47:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bae S, Kim SY, Jung JH, Yoon Y, Cha HJ,
Lee H, Kim K, Kim J, An IS, Kim J, et al: Akt is negatively
regulated by the MULAN E3 ligase. Cell Res. 22:873–885. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suizu F, Hiramuki Y, Okumura F, Matsuda M,
Okumura AJ, Hirata N, Narita M, Kohno T, Yokota J, Bohgaki M, et
al: The E3 ligase TTC3 facilitates ubiquitination and degradation
of phosphorylated Akt. Dev Cell. 17:800–810. 2009. View Article : Google Scholar
|
|
33
|
Campbell PA and Rudnicki MA: Oct4
interaction with Hmgb2 regulates Akt signaling and pluripotency.
Stem Cells. 31:1107–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
David O, Jett J, LeBeau H, Dy G, Hughes J,
Friedman M and Brody AR: Phospho-Akt overexpression in non-small
cell lung cancer confers significant stage-independent survival
disadvantage. Clin Cancer Res. 10:6865–6871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lecker SH, Goldberg AL and Mitch WE:
Protein degradation by the ubiquitin-proteasome pathway in normal
and disease states. J Am Soc Nephrol. 17:1807–1819. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao B, Ahmad A, Azmi AS, Ali S and Sarkar
FH: Cancer stem cells (CSCs) and mechanisms of their regulation:
Implications for cancer therapy. Curr Protoc Pharmacol.
14:p.Unit-14. 252013.
|
|
37
|
Gargini R, Cerliani JP, Escoll M, Antón IM
and Wandosell F: Cancer stem cell-like phenotype and survival are
coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells.
33:646–660. 2015. View Article : Google Scholar
|
|
38
|
Yu S, Shen G, Khor TO, Kim JH and Kong AN:
Curcumin inhibits Akt/mammalian target of rapamycin signaling
through protein phosphatase-dependent mechanism. Mol Cancer Ther.
7:2609–2620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang H, Shang X, Wu H, Gautam SC,
Al-Holou S, Li C, Kuo J, Zhang L and Chopp M: Resveratrol
downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma
cells. J Exp Ther Oncol. 8:25–33. 2009.PubMed/NCBI
|
|
40
|
Efferth T: Stem cells, cancer stem-like
cells, and natural products. Planta Med. 78:935–942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mendoza MC, Er EE and Blenis J: The
Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends
Biochem Sci. 36:320–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dent P: Crosstalk between ERK, AKT, and
cell survival. Cancer Biol Ther. 15:245–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
44
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu CF, Liu ZX and Cantley LG: ERK
negatively regulates the epidermal growth factor-mediated
interaction of Gab1 and the phosphatidylinositol 3-kinase. J Biol
Chem. 277:19382–19388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bhummaphan N and Chanvorachote P: Gigantol
suppresses cancer stem cell-like phenotypes in lung cancer cells.
Evid Based Complement Alternat Med. 2015:8365642015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
48
|
Sun FF, Hu YH, Xiong LP, Tu XY, Zhao JH,
Chen SS, Song J and Ye XQ: Enhanced expression of stem cell markers
and drug resistance in sphere-forming non-small cell lung cancer
cells. Int J Clin Exp Pathol. 8:6287–6300. 2015.PubMed/NCBI
|
|
49
|
Shi Y, Fu X, Hua Y, Han Y, Lu Y and Wang
J: The side population in human lung cancer cell line NCI-H460 is
enriched in stem-like cancer cells. PLoS One. 7:e333582012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bertolini G, Roz L, Perego P, Tortoreto M,
Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S,
et al: Highly tumorigenic lung cancer CD133+ cells
display stem-like features and are spared by cisplatin treatment.
Proc Natl Acad Sci USA. 106:16281–16286. 2009. View Article : Google Scholar
|
|
51
|
Mirza AM, Gysin S, Malek N, Nakayama K,
Roberts JM and McMahon M: Cooperative regulation of the cell
division cycle by the protein kinases RAF and AKT. Mol Cell Biol.
24:10868–10881. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Testa JR and Tsichlis PN: AKT signaling in
normal and malignant cells. Oncogene. 24:7391–7393. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dubrovska A, Kim S, Salamone RJ, Walker
JR, Maira SM, García-Echeverría C, Schultz PG and Reddy VA: The
role of PTEN/Akt/PI3K signaling in the maintenance and viability of
prostate cancer stem-like cell populations. Proc Natl Acad Sci USA.
106:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xiang T, Ohashi A, Huang Y, Pandita TK,
Ludwig T, Powell SN and Yang Q: Negative regulation of Akt
activation by BRCA1. Cancer Res. 68:10040–10044. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Millimouno FM, Dong J, Yang L, Li J and Li
X: Targeting apoptosis pathways in cancer and perspectives with
natural compounds from mother nature. Cancer Prev Res (Phila).
7:1081–1107. 2014. View Article : Google Scholar
|
|
56
|
Singh P and Bast F: Screening of
multi-targeted natural compounds for receptor tyrosine kinases
inhibitors and biological evaluation on cancer cell lines, in
silico and in vitro. Med Oncol. 32:2332015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
El Khattabi I and Sharma A: Preventing p38
MAPK-mediated MafA degradation ameliorates β-cell dysfunction under
oxidative stress. Mol Endocrinol. 27:1078–1090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Niu H, Ye BH and Dalla-Favera R: Antigen
receptor signaling induces MAP kinase-mediated phosphorylation and
degradation of the BCL-6 transcription factor. Genes Dev.
12:1953–1961. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Deschênes-Simard X, Gaumont-Leclerc MF,
Bourdeau V, Lessard F, Moiseeva O, Forest V, Igelmann S, Mallette
FA, Saba-El-Leil MK, Meloche S, et al: Tumor suppressor activity of
the ERK/MAPK pathway by promoting selective protein degradation.
Genes Dev. 27:900–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ley R, Balmanno K, Hadfield K, Weston C
and Cook SJ: Activation of the ERK1/2 signaling pathway promotes
phosphorylation and proteasome-dependent degradation of the
BH3-only protein, Bim. J Biol Chem. 278:18811–18816. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rungtabnapa P, Nimmannit U, Halim H,
Rojanasakul Y and Chanvorachote P: Hydrogen peroxide inhibits
non-small cell lung cancer cell anoikis through the inhibition of
caveolin-1 degradation. Am J Physiol Cell Physiol. 300:C235–C245.
2011. View Article : Google Scholar :
|
|
62
|
Chanvorachote P, Nimmannit U, Lu Y,
Talbott S, Jiang BH and Rojanasakul Y: Nitric oxide regulates lung
carcinoma cell anoikis through inhibition of ubiquitin-proteasomal
degradation of caveolin-1. J Biol Chem. 284:28476–28484. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pongrakhananon V, Stueckle TA, Wang HY,
O'Doherty GA, Dinu CZ, Chanvorachote P and Rojanasakul Y:
Monosaccharide digitoxin derivative sensitize human non-small cell
lung cancer cells to anoikis through Mcl-1 proteasomal degradation.
Biochem Pharmacol. 88:23–35. 2014. View Article : Google Scholar :
|
|
64
|
Pongrakhananon V, Nimmannit U, Luanpitpong
S, Rojanasakul Y and Chanvorachote P: Curcumin sensitizes non-small
cell lung cancer cell anoikis through reactive oxygen
species-mediated Bcl-2 downregulation. Apoptosis. 15:574–585. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Castor LR, Locatelli KA and Ximenes VF:
Pro-oxidant activity of apocynin radical. Free Radic Biol Med.
48:1636–1643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu J and Mori A: Antioxidant and
pro-oxidant activities of p-hydroxybenzyl alcohol and vanillin:
Effects on free radicals, brain peroxidation and degradation of
benzoate, deoxyribose, amino acids and DNA. Neuropharmacology.
32:659–669. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tai A, Sawano T, Yazama F and Ito H:
Evaluation of antioxidant activity of vanillin by using multiple
antioxidant assays. Biochim Biophys Acta. 1810:170–177. 2011.
View Article : Google Scholar
|