Introduction
Epithelial ovarian cancer (EOC) is the third most
common gynecological malignancy worldwide, and it is also one of
the most fatal gynecological carcinomas in women (1), causing an estimated 125,000 deaths
each year globally (2) and 14,240
deaths in the United States in 2015 alone (3). Currently, platinum-based chemotherapy
acts as the primary treatment for this cancer (4). Although patient prognosis has
improved significantly for various types of solid cancers, women
suffering from ovarian cancer exhibited only a slight change in
survival rate since platinum-based treatment was introduced more
than 30 years ago (5,6). Furthermore, recent studies have
revealed that some genetic and epigenetic alterations contribute to
the survival of EOC (7). Thus,
further understanding of the molecular basis of ovarian cancer is
needed.
The anti-apoptotic effect resulting from the
augmented expression of certain proteins involved in the apoptosis
pathways is one of the most significant hallmarks for cancer cells.
X-linked inhibitor of apoptosis protein (XIAP), BCL2-like 2
(BCL2L2) and Baculoviral IAP repeat-containing protein 5 (BIRC5)
are three important anti-apoptotic proteins in EOC development.
Overexpression of XIAP, the most potent mammalian IAP, contributes
to the abnormal survival of EOC cells via inhibiting caspase
activity (8). Previous studies
indicated that by downregulating XIAP, apoptosis is induced both
in vitro and in vivo (9). Furthermore, XIAP has been found to be
the target of miR-24 leading to the decrease of apoptotic threshold
in cancer cells (10). BCL2L2, a
pro-survival member of the BCL2 family of proteins, is associated
with the ability of cancer cells to evade apoptotic signals
(11,12). Additionally, researchers have found
that miR-15a can induce cell apoptosis by targeting BCL2L2 in
non-small cell lung cancer (13),
and miR-214 is able to enhance cisplatin-induced cytotoxicity via
downregulation of BCL2L2 in cervical cancer cells (14).
Furthermore, a higher level of BCL2L2 also
contributes to cancer cell resistance to drugs, such as
camptothecin, cisplatin, etoposide (VP-16), adriamycin, or
1-D-arabinofuranosylcyto-sine (4).
BIRC5 (survivin) is one of the eight well-studied members of the
inhibitor of apoptosis protein (IAP) family, sharing a baculovirus
IAP repeat (BIR), and has important roles in apoptosis (15). IAPs act as endogenous inhibitors of
caspases, which are evolutionarily conserved (16,17).
BIRC5 has a cell cycle-dependent expression pattern during mitosis,
but it is also regulated by other non-cell cycle-dependent
mechanisms (18). Previous studies
suggested that BIRC5 counteracts apoptosis through interactions
with caspases or initiators (19,20).
BIRC5 is strongly expressed in embryonic and fetal organs but not
in most differentiated normal tissues (21,22).
Augmented expression of BIRC5 has been identified in tumors of
breast, stomach, lung, pancreas and liver (23–27).
Importantly, the aberrant expression of BIRC5 also correlates with
human EOC in different aspects. BIRC5 promoter polymorphisms are
found to be associated with age of onset of the disease (28). Furthermore, the overexpression of
BIRC5 desensitizes EOC cell response to S-allylmercaptocysteine, a
drug that inhibits the proliferation and metastasis of ovarian
cancer cells (29). These findings
make BIRC5 a key molecule in EOC research.
MicroRNA (miRNA), a type of small (20–25
nucleotides), noncoding RNA, acts as the main regulatory factor of
gene expression by binding to the 3′-untranslated regions (3′UTRs)
of their targeted mRNAs (30), and
this has been found to be relevant to various diseases in humans
(31). To date, more than 2588
human miRNAs have been described in the miRBase database
(http://www.mirbase.org/) with each miRNA possibly
regulating the expression pattern of hundreds of target genes
(31,32). Moreover, numerous studies have
shown that miRNA functions in oncogenic or cancer suppressor
activities, including apoptosis (33). The downregulation of some miRNAs
has been observed in a variety of cancers such as hepatocellular
carcinoma and ovarian cancer, and others (33,34).
MicroRNA-146a-5p (miR-146a-5p) is a highly important miRNA, the
dysregulation of which underlies the pathogenesis of peripartum
cardiomyopathy, osteoarthritis, and the development or metastasis
of various cancers, including papillary thyroid carcinoma and
breast cancer (31,35–38).
Additionally, miR-146a-5p is also critical for the suppressor
function of Foxp3+ regulatory T cells, indicating the
indispensability of a single miRNA in immune homeostasis (39). In ovarian cancer, Cui et al
discovered that augmented expression of miR-146a-5p prohibits cell
proliferation, enhances apoptosis, and increases sensitivity to
chemotherapy drugs by reducing SOD2 (40).
Given the significant role of miR-146a-5p in EOC, we
hypothesized that miR-146a-5p accelerates cisplatin-induced
apoptosis via targeting certain anti-apoptotic genes. We found that
miR-146a-5p lowers the IC50 value of cisplatin in OVCAR3
and SKOV3 ovarian cancer cell lines and promotes apoptosis in
OVCAR3, SKOV3 and primary EOC cells. By computational predictions,
two genes in the IAP family (XIAP and BIRC5) along with one gene in
BCL2 family (BCL2L2) were predicted to be targets. Since miRNAs
usually work by binding to their target mRNAs, a dual-luciferase
assay was used to validate targets in our study. The results showed
miR-146a-5p downregulates XIAP, BCL2L2 and BIRC5 via their 3′UTRs.
After their 3′UTRs mutated, no differences were observed between
the control group and miR-146a-5p-treated group, demonstrating that
the 3′UTRs are the regulatory site of miR-146a-5p. For the most
strongly inhibited target XIAP, we further investigated the
mechanism using a lentivirus packaging system, western blotting and
DAPI staining. Overexpression of XIAP rescued the
apoptosis-promoting effect of a miR-146a-5p mimic, and suppression
of XIAP rescued the apoptosis-inhibiting effect of a miR-146a-5p
inhibitor in a dose-dependent fashion. These data together suggest
a pivotal role for miR-146a-5p in targeting several anti-apoptotic
genes in ovarian cancer cells, and this suggests a mechanism that
promotes apoptosis induced by cisplatin.
Materials and methods
Vector construction
To overexpress XIAP, human XIAP cDNA without its
native 3′UTR was cloned downstream of the CMV promoter in the
lentiviral expression vector pCDH-CMV-MCS-EF1-copGFP (pCDH; System
Biosciences, Mountain View, CA, USA), and the construct was named
LV-XIAP. To construct the luciferase reporter, the wild-type 3′UTRs
of XIAP, BIRC2, BIRC5 and BCL2L2, containing the putative
miR-146a-5p binding sites as well as the seed region mutated 3′UTR
of XIAP, BIRC5 and BCL2L2, were fused to the Renilla
luciferase reporter gene at the 3′UTR in the psiCHECK2 vector. The
primers used for developing the constructs above are listed in
Table I. DNA sequencing was
performed to confirm all constructs.
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Gene | Primer sequence (5′
to 3′) |
|---|
| LV-XIAP | S:
CACAATCTAGAGCCACCATGACTTTTAACAGTTTTGAA |
| AS:
AAGGATCCTTAAGACATAAAAATTTTTTGCTTG |
| XIAP-3′UTR2 | S:
CACAACTCGAGCAGAGGAAAGTTTGAGAGTAAAACTG |
| AS:
AAGGATCCTATATCATGTGAAACTAATGCTGGGG |
| XIAP-mutant | S:
TCCCAAGTCAAGAGAGTGTCTACATGTAGACTATTCCTTT |
| AS:
TAGACACTCTCTTGACTTGGGAGGGGGAAAAGATTTGGAT |
| BCL2L2-3′UTR | S:
CACAACTCGAGGTGTGGGCACATGAAACGAC |
| AS:
AAGGATCCATGCACAAGGAAGGGGGATG |
| BCL2L2-mutant | S:
GGGGGTCAAGAGTGTCCCTCCTCCCAACCC |
| AS:
GACACTCTTGACCCCCTAGTTCTTGCCATT |
| BIRC2-3′UTR | S:
CACAACTCGAGTGTTGAACACTTGAAGCCATCT |
| AS:
AAGGATCCGCACCAAAGACAATTCGGCA |
| BIRC5-3′UTR | S:
CACAACTCGAGTTGAAAGTGGCACCAGAGGT |
| AS:
AAGGATCCCTTTCCACATGGCGACAGC |
| BIRC5-mutant | S:
ACATGTGGTATTAAGAGCAAGAGTAAGTTGGAGTGGAGT |
| AS:
TTACTCTTGCTCTTAATACCACATGTGGACATGTATCTGA |
Cell lines and cell culture
OVCAR3 and SKOV3 human ovarian cancer cell lines
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and were maintained in a 37°C, 5% CO2
incubator in DMEM or RPMI (Invitrogen, Carlsbad, CA, USA) with 10%
fetal bovine serum (FBS) and penicillin/streptomycin (Invitrogen)
(100 U/ml). To isolate primary epithelial ovarian cancer cells from
freshly collected malignant ascites from one patient, a previously
described method was used (41).
The pathological type of ovarian cancer of the patient was ovarian
serous adenocystic carcinoma. The patient signed an informed
consent form, and the use of the sample and study protocol were
approved by the ethics committee of the Second Affiliated Hospital
of Guangzhou Medical University (no. 2013034).
Transfection of RNA oligonucleotides
A miR-146a-5p mimic and its control RNA, a
miR-146a-5p inhibitor and its control RNA, and an XIAP siRNA
(si-XIAP) and its control RNA were synthesized, purified and
annealed by GenePharma (Shanghai, China). The RNA sequences were as
follows. miR-146a-5p mimic: sense 5′-UGAGAACUGAAUUCCAUGG GUU-3′ and
antisense 5′-CCCAUGGAAUUCAGUUCUC AUU-3′; mimic control: sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′; miR-146a-5p inhibitor:
5′-AACCCAUGGAAUUCAGUUCUCA-3′; inhibitor control:
5′-CAGUACUUUUGUGUAGUACAA-3′; si-XIAP: sense
5′-CAUGCAGCUGUAGAUAGAUGGCAAU-3′ and antisense
5′-AUUGCCAUCUAUCUACAGCUGCAUG-3′; siRNA control: sense
5′-UUCUCCGAACGUGUCACGUTT-3′, antisense 5′-ACGUGACAC
GUUCGGAGAATT-3′. RNA oligonucleotides (100 or 50 nM) were used with
the X-tremeGENE siRNA Transfection Reagent (Roche, Basel,
Switzerland) to transfect the miRNA mimic or siRNA into OVCAR3 and
SKOV3 cells.
Western blotting
SKOV3 cells were seeded in 12-well plates at
2×105 cells/well and lysed using RIPA lysis buffer
(BioTeke Corp., Beijing, China) 48 h post-transfection, and a
bicinchoninic acid protein assay kit (Beyotime, Shanghai, China)
was used to measure protein concentration. The protein sample (20
µg per lane) was first heat-denatured and then separated by
12% SDS-polyacrylamide gel electrophoresis. Protein was transferred
to a PVDF membrane (Millipore, Bedford, MD, USA) and blocked with
3% BSA. After blocking, the PVDF membrane was incubated for 2 h at
room temperature with a mouse polyclonal antibody against human
XIAP (1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or a
mouse monoclonal antibody against human β-actin (1:3000; Abcam,
Cambridge, MA, USA) and incubated for 1 h with a goat anti-mouse
(1:5000; Abcam) secondary antibody. To detect the bound antibodies,
enhanced chemiluminescence detection reagents (Pierce, Rockford,
IL, USA) were used. Band intensities were quantified with a Kodak
Image Station 4000 MM Pro (Kodak, Tokyo, Japan).
Dual-luciferase assays
The dual-luciferase reporter assay was performed as
previously described (42). First,
293T cells were seeded in a 96-well plate at 1×104
cells/well, and cells were co-transfected with 50 ng of luciferase
reporter vector and 6 ng of synthetic miR-146a-5p mimic or mimic
control. The cells were harvested 48 h post-transfection, and the
luciferase activity was measured using a dual-luciferase reporter
assay system (Promega, Madison, WI, USA) following the
manufacturer's instructions. Renilla luciferase activities
were normalized to firefly luciferase activities.
Assay of IC50 value of
cisplatin
The 3-(4,5)-dimethylthiazol(-2-y1)
-3,5-di-phenyl-tetrazolium bromide (MTT) assay was used to measure
the IC50 value of cisplatin. OVCAR3 and SKOV3 cells were
transfected with miR-146a-5p mimic or mimic control one day after
seeding into a 96-well plate at 1×104 cells/well. One
day post-transfection, cisplatin was added to each well with a
concentration gradient of 0.625, 1.25, 2.50, 5.00, 10.0, 20.0, and
40.0 µM, 48 h later, 5 mg/ml of MTT reagent was added, and
the plate was incubated in a 5% CO2 incubator at 37°C
for 4 h. DMSO (100 µl) was added to each well, and the plate
was mixed for 10 min. The absorbance at 490 nm was detected with a
BioTek microplate reader (Winooski, VT, USA).
4′,6-Diamidino-2-phenylindole (DAPI)
staining
OVCAR3, SKOV3 or primary EOC cells were seeded in a
48-well plate with a cell density of 1.5×104 cells per
well and subsequently transfected with miR-146a-5p mimic or mimic
control. To achieve 10–30% apoptosis, 5 µM of cisplatin was
added to the medium 16–24 h after transfection. After another 24–48
h, the cells were stained with 1 µg/ml DAPI (Sigma, St.
Louis, MO, USA). A fluorescence microscope was used to observe
apoptotic cells, and the apoptotic ratio was calculated from
average 200 cellular nuclei in one image, and three images without
overlap were taken randomly in each experiment. The average values
± SD of three separate experiments were plotted.
Lentivirus packaging and infection
293T cells were seeded in a 6-cm dish at
8×105 cells/dish and co-transfected with 1.8 µg
of packaging plasmid pPAX2, 0.6 µg of envelope plasmid
pMD2.G and 2.5 µg of the XIAP expression vector LV-XIAP or
empty vector pCDH (LV-control) with the transfection reagent
Lipofectamine 2000 (Invitrogen). Viral supernatants were harvested
and stored at −80°C 48 h post-transfection. Before infection, 0.01
µg/ml of polybrane (Sigma) was added in order to improve the
infection efficiency.
Statistical analysis
The data were analyzed using Student's t-test and
are presented as the mean ± standard deviation (± SD) of three
separate experiments. P-values <0.05, 0.01, or 0.001 indicate
statistical significance. Prism software version 5.0 (GraphPad
Software, La Jolla, CA, USA) was used to analyze the data.
Results
miR-146a-5p decreases the IC50
values of cisplatin in OVCAR3 and SKOV3 ovarian cancer cells
To investigate the function of miR-146a-5p in
ovarian cancer cells, we determined the IC50 values of
cisplatin in OVCAR3 and SKOV3 ovarian cancer cells transfected with
miR-146a-5p mimic or mimic control. After transfection, cisplatin
was added to each well with a concentration gradient of 40, 20, 10,
5, 2.5, 1.25, 0.625 and 0 µM to evaluate the IC50
values. We found that for the two cell lines, the IC50
values decreases with transfection of miR-146a-5p, and this is most
significant in the SKOV3 cell line (Fig. 1A and B). These results reveal that
miR-146a-5p can effectively increase the sensitivity of OVCAR3 and
SKOV3 ovarian cancer cells to cisplatin-induced apoptosis.
miR-146a-5p promotes apoptosis in OVCAR3,
SKOV3 and primary ovarian cancer cells
We hypothesized that miR-146a-5p lowers
IC50 values of cisplatin in ovarian cancer cells by
promoting apoptosis. To test this effect, two ovarian cancer cell
lines (OVCAR3 and SKOV3) and primary EOC cells were used. After
seeding onto a 48-well plate, OVCAR3 cells were transfected with
miR-146a-5p mimic, mimic control, miR-146a-5p inhibitor or
inhibitor control. Cisplatin treatment and DAPI staining were
performed as previously described (43). Our results show that more apoptotic
nuclei were observed in OVCAR3 cells transfected with miR-146a-5p
mimic compared to mimic control (Fig.
2A). Apoptosis was markedly suppressed in OVCAR3 cells
transfected with miR-146a-5p inhibitor compared to the inhibitor
control (Fig. 2B). These results
indicate that miR-146a-5p is the driving force of the observed
higher apoptosis rate.
In order to define the function of miR-146a-5p,
SKOV3 cells were transfected with mimic control and miR-146a-5p
mimic and treated with cisplatin. DAPI staining revealed a higher
apoptosis ratio in SKOV3 cells transfected with miR-146a-5p mimic
compared to mimic control (Fig.
2C). Primary EOC cells isolated from ascites were also
transfected with miR-146a-5p mimic or mimic control and treated
with 5 µM cisplatin for 24 or 48 h. We found that the
apoptosis rate in transfected primary cultured EOC cells was
increased approximately 50% after 24 h, and this rate almost
doubled after another 24 h (Fig.
3). Together, these robust results strongly suggest that
miR-146a-5p accelerates apoptosis by sensitizing EOC cells to
cisplatin.
Screening of anti-apoptotic genes
targeted by miR-146a-5p
Next, we wanted to identify genes targeted by
miR-146a-5p. Given its role in accelerating apoptosis, we predicted
that the target genes would be anti-apoptotic. Scores of studies
have confirmed the tight connection between proteins of the IAP
family and BCL2 family with carcinogenesis, including studies of
ovarian cancer (9,15,44–46).
We hypothesized that miR-146a-5p could possibly regulate these two
families, by decreasing expression of these proteins which are
often overexpressed in chemoresistant EOC cells. To elucidate which
proteins could be targeted by miR-146a-5p, a computational method
was utilized to predict potential miR-146a-5p-binding
anti-apoptotic genes (Table II).
Four genes (XIAP, BIRC2 and BIRC5 from the IAP family and BCL2L2
from the BCL2 family) out of 13 candidate genes were predicted to
have miR-146a-5p binding sites in their 3′UTRs (Fig. 4A). XIAP and BIRC2 had one predicted
site, while BCL2L2 and BIRC5 had two potential target sites.
 | Table IIPredicted miR-146a-5p binding sites
in the 3′UTR of anti-apoptotic genes. |
Table II
Predicted miR-146a-5p binding sites
in the 3′UTR of anti-apoptotic genes.
| Genes | With miR-146a-5p
binding sites in 3′UTR | Without miR-146a-5p
binding sites in 3′UTR |
|---|
| IAP family | XIAP, BIRC2,
BIRC5 | BIRC3, NAIP, BIRC6,
BIRC7, BIRC8 |
| BCL2 family | BCL2L2 | BCL2L1, BCL2,
CCND1, MCL1 |
A dual-luciferase reporter assay was used to test if
miR-146a-5p regulates XIAP, BCLCL2, BIRC2 and BIRC5 by binding to
their 3′UTRs. 293T cells were seeded in a 96-well plate 24 h before
transfection, and cells were co-transfected with miR-146a-5p mimic
or mimic control and the psiCHECK2 vector containing a luciferase
reporter gene fused with the 3′UTR of each the four genes. After 48
h cells were harvested, and the luciferase activity was measured.
The luciferase activity data showed that the miR-146a-5p mimic
inhibited the three 3′UTRs of XIAP, BCL2L2 and BIRC5 to different
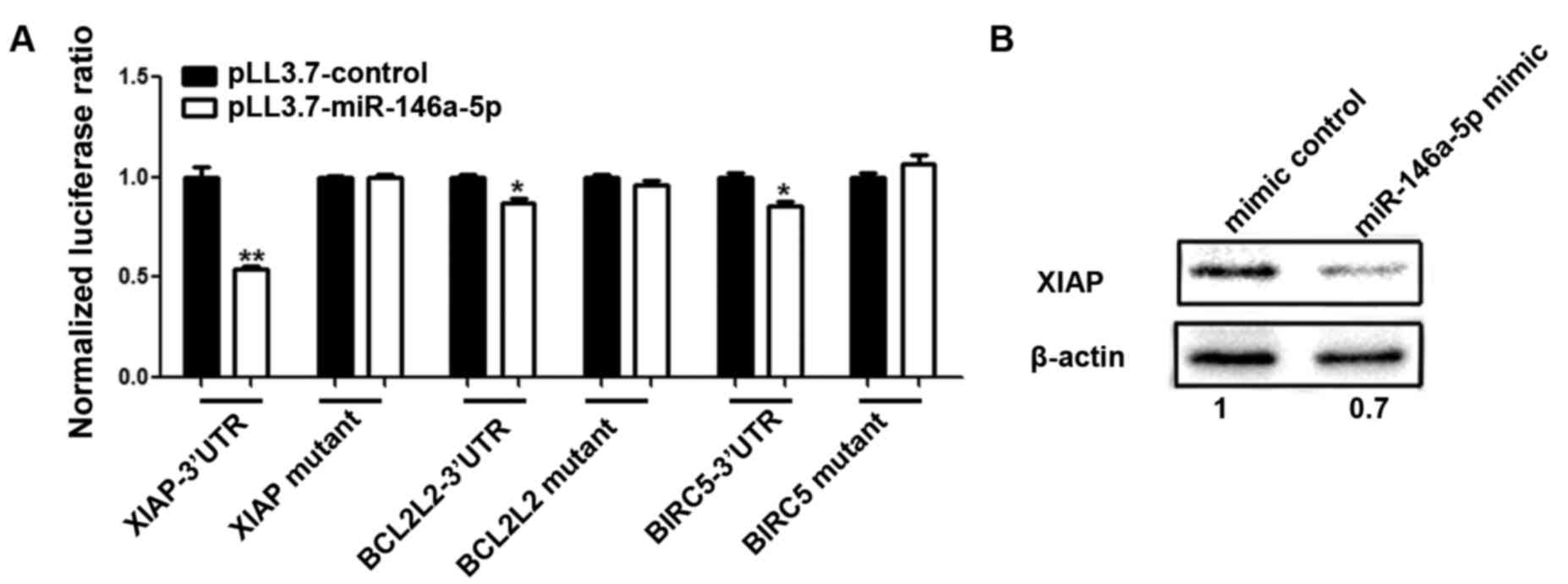
extents (Student's t-test, p<0.05) (Fig. 4B). This effect was most significant
with XIAP. The results for BCL2L2 showed a moderate influence,
which was followed by BIRC5. There was no interaction between
miR-146a-5p and the 3′UTR of BIRC2.
miR-146a-5p targets XIAP, BCL2L2 and
BIRC5
To determine whether miR-146a-5p recognizes the
predicted sites (XIAP 3′UTR 2781-2803; BCL2L2 3′UTR 394-420,
567-586; BIRC5 3′UTR 918-940, 1831-1852), we constructed the seed
region-mutated Renilla luciferase reporter for XIAP 3′UTR,
both seed region-mutated reporters for BCL2L2 and BIRC5 3′UTRs
(Fig. 4A). For each gene, both
wild-type and mutant reporters were co-transfected into 293T cells
with miR-146a-5p mimic or mimic control, respectively (Fig. 5A). By testing the luciferase
activity, we found that the ability of miR-146a-5p to inhibit XIAP,
BCL2L2 and BIRC5 were abrogated through a mutation for XIAP, and
mutations of both sites for BCL2L2 and BIRC5 (Fig. 5A). Since XIAP is the most
significantly inhibited gene, we chose XIAP for further
experiments.
To confirm whether miR-146a-5p could also influence
endogenous target expression, SKOV3 cells were transfected with
miR-146a-5p mimic or mimic control. Western blot analysis indicated
that the level of endogenous XIAP decreased, and this was ascribed
to the transfection of miR-146a-5p mimic compared to the control
group. The level of the internal reference GADPH was consistent
between the two groups (Fig. 5B).
Taken together, our data demonstrated that miR-146a-5p targets
XIAP, BCL2L2 and BIRC5 via their 3′UTRs in 293T and ovarian cancer
cells.
XIAP rescues the effects of miR-146a-5p
on apoptosis
We showed that miR-146a-5p promoted
cisplatin-induced apoptosis in ovarian cancer cells (Fig. 2), and it was also able to inhibit
the expression of XIAP. To further validate that miR-146a-5p indeed
accelerates cisplatin-induced apoptosis via down-regulating XIAP, a
rescue experiment was performed. OVCAR3 cells were co-transfected
with 50 nM si-XIAP and different amounts of miR-146a-5p inhibitor
(100 and 200 nM). DAPI staining was used to allow quantification of
the state of apoptosis. When 50 nM si-XIAP and 100 nM miR-146a-5p
inhibitor was co-transfected, there was no significant difference
between the 100 nM miR-146a-5p inhibitor experimental group and
control group, but there was weaker apoptotic induction in the 200
nM miR-146a-5p inhibitor experimental group compared to the control
group (Fig. 6A). This suggests
that XIAP siRNA can counteract the effect of miR-146a-5p inhibitor
in suppressing apoptosis, and there is decreased apoptosis when a
higher amount of miR-146a-5p inhibitor is transfected. Similarly
(Fig. 6B), OVCAR3 cells were
infected with constant quantities of XIAP without its 3′UTR
(multiplicities of infection equal to 1) and were then transfected
with varying amounts of miR-146a-5p mimic (50 and 100 nM), and
there was no obvious difference between 50 nM miR-146a-5p mimic
group and control group and a higher apoptosis ratio in 100 nM
miR-146a-5p mimic group compared to control group (Fig. 6B). These results indicate that XIAP
can counteract the effect of miR-146a-5p in promoting apoptosis,
and there is enhanced apoptosis when miR-146a-5p is expressed
excessively. The western blot results from our previous study
indicated that XIAP was overexpressed with lentiviral infection,
and si-XIAP decreased XIAP expression in OVCAR3 cells (47). In summary, our results indicate
that these four anti-apoptotic proteins are targets of miR-146a-5p,
providing insights into how miR-146a-5p can promote apoptosis in
EOC cells.
Discussion
Many studies have illustrated that miR-146a-5p is
engaged in multiple diseases and the development of metastasis in
various cancer types (31,35–38).
Furthermore, augmented expression of miR-146a-5p can inhibit cell
proliferation, enhance apoptosis, and increase chemosensitivity
(40), which prompted us to
speculate that miR-146a-5p may play a suppressive role in ovarian
cancer. In support of this hypothesis, our results showed that
overexpression of miR-146a-5p reduces the IC50 values of
cisplatin in OVCAR3 and SKOV3 cells and enhances cisplatin-induced
apoptosis in OVCAR3, SKOV3 and primary ovarian cancer cells.
XIAP, BCL2L2 and BIRC5 are three important
anti-apoptotic proteins in a multitude of cancers (8,38).
Numerous studies have reported that XIAP, whose expression is at a
high level in ovarian cancer (48–50),
is essential for suppressing apoptosis and promoting cell
proliferation (8). Likewise, BIRC5
also have functions associated with apoptosis (19,51).
Overexpression of BIRC5 can decrease the sensitivity of EOC cells
to an anti-proliferative drug (29). BCL2L2 is also associated with
overriding apoptotic signals in cancer cells (11,12).
Bioinformatic predictions and luciferase assays demonstrated that
these three proteins can be targeted by miR-146a-5p via their
3′UTRs, and the effect on XIAP was the most significant (Figs. 4 and 5). Furthermore, the experiments showed
that XIAP functionally rescued the apoptotic effect of miR-146a-5p
(Fig. 6). Consequently, we deduced
that the potentially abnormal functions of these three proteins in
ovarian cancer might be partially ascribed to the dysregulation of
some miRNAs including miR-146a-5p. Several studies have illustrated
that XIAP is also regulated by other miRNAs such as miR-519d,
miR-509-3p, miR-155 and miR-7 (43,52–54),
which may allow us to construct a XIAP-regulating network.
Furthermore, this study provided novel targets for
EOC treatment. The five-year relative survival rate of ovarian
cancer patients is 46% from 2005 to 2011, without much increase
compared to that from 1987 to 1989 at 36% (3). Currently, standard platinum
chemotherapy leads to a recurring drug-resistant cancer in
approximately 25% of patients within six months (55). This relatively unsatisfactory
prognosis means that further understanding of the molecular
mechanisms of ovarian cancer is needed. Previous research revealed
that overexpression of miR-146a-5p in EOC cells inhibits cell
proliferation and enhances apoptosis and chemosensitivity through
reduction of SOD2 (40). Herein,
data from our work further showed that miR-146a-5p decreases the
IC50 value of cisplatin in ovarian cancer cells by
downregulating three anti-apoptotic genes including XIAP. Thus,
augmenting the level of miR-146a-5p by gene therapy combined with
cisplatin chemotherapy might be a more efficient treatment.
In conclusion, our study indicates that miR-146a-5p
promotes cisplatin-induced apoptosis in ovarian cancer cells by
repressing multiple anti-apoptotic genes, including XIAP, BCL2L2
and BIRC5, of which XIAP shows the strongest evidence. The
miR-146a-5p/three protein axis should be further tested by
evaluation of whether miR-146a-5p level correlates with prognosis
using clinical specimens. These studies may illuminate new targets
for treatment of ovarian cancer, which may facilitate
cisplatin-induced apoptosis and improve chemotherapy.
Acknowledgments
This study was supported by grants from the Natural
Science Foundation of China (grant no. 81572567).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Y, Chen Q, Qin R, Zhang K and Li H:
MicroRNA-449a reduces cell survival and enhances cisplatin-induced
cytotoxicity via downregulation of NOTCH1 in ovarian cancer cells.
Tumour Biol. 35:12369–12378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGuire WP: Maintenance therapy for
ovarian cancer: Of Helsinki and Hippocrates. J Clin Oncol.
27:4633–4634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurman RJ, Visvanathan K, Roden R, Wu TC
and Shih IeM: Early detection and treatment of ovarian cancer:
Shifting from early stage to minimal volume of disease based on a
new model of carcinogenesis. Am J Obstet Gynecol. 198:351–356.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiraz Y, Adan A, Kartal Yandim M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaw TJ, Lacasse EC, Durkin JP and
Vanderhyden BC: Downregulation of XIAP expression in ovarian cancer
cells induces cell death in vitro and in vivo. Int J Cancer.
122:1430–1434. 2008. View Article : Google Scholar
|
|
10
|
Xie Y, Tobin LA, Camps J, Wangsa D, Yang
J, Rao M, Witasp E, Awad KS, Yoo N, Ried T, et al: MicroRNA-24
regulates XIAP to reduce the apoptosis threshold in cancer cells.
Oncogene. 32:2442–2451. 2013. View Article : Google Scholar
|
|
11
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Camisasca DR, Honorato J, Bernardo V, da
Silva LE, da Fonseca EC, de Faria PA, Dias FL and Lourenço SQ:
Expression of Bcl-2 family proteins and associated
clinicopathologic factors predict survival outcome in patients with
oral squamous cell carcinoma. Oral Oncol. 45:225–233. 2009.
View Article : Google Scholar
|
|
13
|
Yang T, Thakur A, Chen T, Yang L, Lei G,
Liang Y, Zhang S, Ren H and Chen M: MicroRNA-15a induces cell
apoptosis and inhibits metastasis by targeting BCL2L2 in non-small
cell lung cancer. Tumour Biol. 36:4357–4365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cory S, Huang DCS and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salvesen GS and Duckett CS: IAP proteins:
Blocking the road to death's door. Nat Rev Mol Cell Biol.
3:401–410. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saleem M, Qadir MI, Perveen N and Ahmad B,
Saleem U, Irshad T and Ahmad B: Inhibitors of apoptotic proteins:
New targets for anticancer therapy. Chem Biol Drug Des. 82:243–251.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
20
|
Kasof GM and Gomes BC: Livin, a novel
inhibitor of apoptosis protein family member. J Biol Chem.
276:3238–3246. 2001. View Article : Google Scholar
|
|
21
|
Adida C, Crotty PL, McGrath J, Berrebi D,
Diebold J and Altieri DC: Developmentally regulated expression of
the novel cancer anti-apoptosis gene survivin in human and mouse
differentiation. Am J Pathol. 152:43–49. 1998.PubMed/NCBI
|
|
22
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monzó M, Rosell R, Felip E, Astudillo J,
Sánchez JJ, Maestre J, Martín C, Font A, Barnadas A and Abad A: A
novel anti-apoptosis gene: Re-expression of survivin messenger RNA
as a prognosis marker in non-small-cell lung cancers. J Clin Oncol.
17:2100–2104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanaka K, Iwamoto S, Gon G, Nohara T,
Iwamoto M and Tanigawa N: Expression of survivin and its
relationship to loss of apoptosis in breast carcinomas. Clin Cancer
Res. 6:127–134. 2000.PubMed/NCBI
|
|
25
|
Lu CD, Altieri DC and Tanigawa N:
Expression of a novel anti-apoptosis gene, survivin, correlated
with tumor cell apoptosis and p53 accumulation in gastric
carcinomas. Cancer Res. 58:1808–1812. 1998.PubMed/NCBI
|
|
26
|
Satoh K, Kaneko K, Hirota M, Masamune A,
Satoh A and Shimosegawa T: Expression of survivin is correlated
with cancer cell apoptosis and is involved in the development of
human pancreatic duct cell tumors. Cancer. 92:271–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikeguchi M, Ueta T, Yamane Y, Hirooka Y
and Kaibara N: Inducible nitric oxide synthase and survivin
messenger RNA expression in hepatocellular carcinoma. Clin Cancer
Res. 8:3131–3136. 2002.PubMed/NCBI
|
|
28
|
Han CH, Wei Q, Lu KK, Liu Z, Mills GB and
Wang LE: Polymorphisms in the survivin promoter are associated with
age of onset of ovarian cancer. Int J Clin Exp Med. 2:289–299.
2009.
|
|
29
|
Wu J, Zhao S, Zhang J, Qu X, Jiang S,
Zhong Z, Zhang F, Wong Y and Chen H: Over-expression of survivin is
a factor responsible for differential responses of ovarian cancer
cells to S-allylmercaptocysteine (SAMC). Exp Mol Pathol.
100:294–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rusca N and Monticelli S: MiR-146a in
immunity and disease. Mol Biol Int. 2011:4373012011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Navarro F and Lieberman J: Small RNAs
guide hematopoietic cell differentiation and function. J Immunol.
184:5939–5947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, Kim JW and Kim S: MicroRNA expression profiles in serous
ovarian carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong
Q, Qin L, Wu X, Zheng Y, Yang Y, et al: Identification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinoma. Cancer Cell.
19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Halkein J, Tabruyn SP, Ricke-Hoch M,
Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert
V, Thiry M, et al: MicroRNA-146a is a therapeutic target and
biomarker for peripartum cardiomyopathy. J Clin Invest.
123:2143–2154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:19075–19080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y,
Shi J and Jia L: Upregulated miR-29b promotes neuronal cell death
by inhibiting Bcl2L2 after ischemic brain injury. Exp Brain Res.
216:225–230. 2012. View Article : Google Scholar
|
|
39
|
Lu LF, Boldin MP, Chaudhry A, Lin LL,
Taganov KD, Hanada T, Yoshimura A, Baltimore D and Rudensky AY:
Function of miR-146a in controlling Treg cell-mediated regulation
of Th1 responses. Cell. 142:914–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui Y, She K, Tian D, Zhang P and Xin X:
miR-146a inhibits proliferation and enhances chemosensitivity in
epithelial ovarian cancer via reduction of SOD2. Oncol Res.
23:275–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sapi E, Alvero AB, Chen W, O'Malley D, Hao
XY, Dwipoyono B, Garg M, Kamsteeg M, Rutherford T and Mor G:
Resistance of ovarian carcinoma cells to docetaxel is XIAP
dependent and reversible by phenoxodiol. Oncol Res. 14:567–578.
2004.
|
|
42
|
Zhou P, Xu W, Peng X, Luo Z, Xing Q, Chen
X, Hou C, Liang W, Zhou J, Wu X, et al: Large-scale screens of
miRNA-mRNA interactions unveiled that the-3′ UTR of a gene is
targeted by multiple miRNAs. PLoS One. 8:e682042013. View Article : Google Scholar
|
|
43
|
Chen W, Zeng W, Li X, Xiong W, Zhang M,
Huang Y, Zhou L and Jiang S: MicroRNA-509-3p increases the
sensitivity of epithelial ovarian cancer cells to cisplatin-induced
apoptosis. Pharmacogenomics. 17:187–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan WY, Cheung KK, Schorge JO, Huang LW,
Welch WR, Bell DA, Berkowitz RS and Mok SC: Bcl-2 and p53 protein
expression, apoptosis, and p53 mutation in human epithelial ovarian
cancers. Am J Pathol. 156:409–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qu Y, Xia P, Zhang S, Pan S and Zhao J:
Silencing XIAP suppresses osteosarcoma cell growth, and enhances
the sensitivity of osteosarcoma cells to doxorubicin and cisplatin.
Oncol Rep. 33:1177–1184. 2015.PubMed/NCBI
|
|
46
|
Chang JJ, Jeon SY, Song JY, Kim JH, Li L,
Park DH, Lee YL, Park JJ, Woo DW, Kim GJ, et al: Alteration of
X-linked inhibitors of apoptosis (XIAP) expression in rat model
with DEN-induced hepatocellular carcinogenesis. Mol Cell Toxicol.
4:278–284. 2008.
|
|
47
|
Li X, Chen W, Zeng W, Wan C, Duan S and
Jiang S: microRNA-137 promotes apoptosis in ovarian cancer cells
via the regulation of XIAP. Br J Cancer. 116:66–76. 2017.
View Article : Google Scholar
|
|
48
|
Tamm I, Kornblau SM, Segall H, Krajewski
S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
49
|
Sasaki H, Sheng Y, Kotsuji F and Tsang BK:
Down-regulation of X-linked inhibitor of apoptosis protein induces
apoptosis in chemoresistant human ovarian cancer cells. Cancer Res.
60:5659–5666. 2000.PubMed/NCBI
|
|
50
|
Li J, Sasaki H, Sheng YL, Schneiderman D,
Xiao CW, Kotsuji F and Tsang BK: Apoptosis and chemoresistance in
human ovarian cancer: Is Xiap a determinant? Biol Signals Recept.
9:122–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gyrd-Hansen M and Meier P: IAPs: From
caspase inhibitors to modulators of NF-kappaB, inflammation and
cancer. Nat Rev Cancer. 10:561–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pang Y, Mao H, Shen L, Zhao Z, Liu R and
Liu P: MiR-519d represses ovarian cancer cell proliferation and
enhances cisplatin-mediated cytotoxicity in vitro by targeting
XIAP. Onco Targets Ther. 7:587–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen W, Huang L, Hao C, Zeng W, Luo X, Li
X, Zhou L, Jiang S, Chen Z and He Y: MicroRNA-155 promotes
apoptosis in SKOV3, A2780, and primary cultured ovarian cancer
cells. Tumour Biol. 37:9289–9299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu S, Zhang P, Chen Z, Liu M, Li X and
Tang H: MicroRNA-7 downregulates XIAP expression to suppress cell
growth and promote apoptosis in cervical cancer cells. FEBS Lett.
587:2247–2253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bell D, Berchuck A, Birrer M, Chien J,
Cramer DW, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P, et al Cancer
Genome Atlas Research Network: Integrated genomic analyses of
ovarian carcinoma. Nature. 474:609–615. 2011. View Article : Google Scholar
|