Introduction
Gastric cancer is the fifth most common malignancy
and the third leading cause of cancer-related death worldwide
(1). It was estimated that a total
of 952,000 new cases of gastric cancer were diagnosed in 2012 and
that 723,000 gastric cancer-related deaths occurred during the same
year (2). Despite advances in
surgical techniques and chemotherapies, metastasis remains the main
obstacle to effective gastric cancer treatment (3,4).
Therefore, the identification of novel biomarkers for gastric
cancer metastasis may facilitate the development of targeted
therapies useful for the treatment of gastric cancer.
Metadherin (MTDH), also known as astrocyte elevated
gene-1 (AEG-1) and lysine-rich CEACAM-1-associated protein (LYRIC),
was initially identified as an oncogene. Its expression was induced
in primary human fetal astrocytes by HIV-1 or treatment with HIV
envelope glycoproteins or tumor necrosis factor-α (5–7). In
recent years, studies have shown that MTDH expression levels were
elevated in many human malignancies, including breast cancer,
hepatocellular carcinoma, neuroblastoma, prostate cancer, and
esophageal squamous cell carcinoma (8–11).
Moreover, MTDH has been reported to play a vital role in
tumorigenesis, tumor progression and chemotherapy resistance
(12,13). A recent study (14) suggested that modulating the
AEG-1/EMT pathway may inhibit hepatocarcinoma growth and
metastasis. However, few studies have addressed whether MTDH
expression levels are upregulated in gastric cancer and whether the
protein participates in gastric cancer metastasis.
Britt et al (6) found that MTDH was mainly localized in
the cytoplasm and that the protein could be recruited to tight
junction complexes to serve as a marker for mature tight junctions.
It is well known that tight junctions are specific junctional
complexes that participate in cell-cell adhesion, which plays a key
role in preserving cell morphology. The cytoskeleton preserves
cellular structure. Several studies (15,16)
have demonstrated that protein-protein interactions in tight
junction complexes are probably related to actin cytoskeletal
remodeling, and tight junction disruption may cause the
cytoskeletons of epithelial cells to transform into cytoskeletons
characteristic of mesenchymal cells, leading to cell
metastasis.
It has been hypothesized (17) that tumor cell metastasis occurs via
the following steps: invasion in situ, intravasation,
survival in circulation, extravasation, micrometastasis and final
colonization. EMT is the process in which epithelial cells with
polarity transform into freely moving mesenchymal cells under
specific physiological and pathological conditions and is the most
important step in the induction of tumor cell invasion and
metastasis in situ (18).
Moreover, EMT was associated with altered gene
expression patterns resulting in the loss of E-cadherin and the
breakdown of cell-cell junctions as well as the acquisition of a
fibroblastic morphology including polarized actin cytoskeleton
assembly into protrusive and invasive pseudopodial structures
(19). Pseudopodial protrusion and
formation of related invadopodia have long been associated with
tumor cell migration and invasion (20,21).
Therefore, we surmised that MTDH can regulate
cytoskeletal remodeling, induce EMT and promote cancer cell
metastasis. We investigated the significance of MTDH in gastric
cancer, as well as explored the mechanism underlying actin
cytoskeletal remodeling and EMT-mediated gastric cancer
metastasis.
Materials and methods
Clinical samples
All experiments involving patients were approved by
the ethics committee of Shanghai Ninth People's Hospital. This
study was conducted on 83 paraffin-embedded primary gastric cancer
samples and 25 normal gastric mucosa samples whose diagnoses were
determined histopathologically and clinically at Shanghai Ninth
People's Hospital, School of Medicine between January 2009 and
December 2011. None of the patients from whom the samples were
originally obtained had undergone any preoperative chemotherapy or
radiotherapy. The patients in question were followed up until May
2016. A total of eleven patients (13.2% of patients) were lost to
followup. In addition, mRNA expression levels were assessed in
fresh tumor specimens from an additional 31 patients with
histological diagnoses of primary gastric carcinoma who underwent
surgical resection. All the patients had previously consented to
the use of the above clinical samples for research purposes.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Shanghai Ninth People's Hospital, School of Medicine, Shanghai Jiao
Tong University. Preoperative informed consent was obtained from
each patient enrolled in this study, in accordance with
institutional guidelines. The pathological samples were taken from
surgically resected specimens; thus, the corresponding experiments
did not pose any risks to the health or prognoses of the patients
enrolled herein. We are committed to maintaining the
confidentiality of the patient information. Furthermore, the
laboratory animals used in the study were cared for and handled in
accordance with the principles and standards set forth in the
Principles for the Use of Animals (National Guide for Grants and
Contracts).
Cell lines and cell culture
The gastric cancer cell lines AGS, KATO-III,
SGC7901, and MKN45 and the normal gastric mucosa cell line GES-1
were purchased from American Type Culture Collection (ATCC,
Manassas, VA, USA). The SGC7901, AGS, and MKN45 cell lines were
cultured in RPMI-1640 (Gibco) medium supplemented with 10% fetal
bovine serum (Gibco), the KATO-III cell line was cultured in IMDM
(ATCC) supplemented with 20% fetal bovine serum, and the GES-1 cell
line was cultured in DMEM (ATCC) supplemented with 10% fetal bovine
serum, according to the manufacturer's instructions. All the cell
lines were incubated in a humidified atmosphere at 37°C with 5%
CO2.
Histology and immunohistochemistry
Serial 4-µm-thick tissue sections were cut,
deparaffinized, rehydrated and then heated for 30 min in citrate
buffer (pH 6.0) for antigen retrieval. Endogenous peroxidase was
inactivated by 3% hydrogen peroxide for 10 min. The sections were
then blocked with 5% normal blocking serum before being incubated
with an MTDH mAb (1:500, Abcam, Cambridge, UK) overnight. The
following day, the sections were incubated with biotin-conjugated
anti-IgG serum (Boster, Wuhan, China) for 40 min before being
incubated with an SABC solution, according to the manufacturer's
instructions. The primary antibodies were visualized over a 10-min
period using a diaminobenzidine tetrachloride kit (Boster). The
sections were subsequently observed under a light microscope. In
addition, all the sections were independently assessed and scored
by two pathologists blinded to each patient's status, and the
staining results were assessed as described in our previous study
(22). The cell staining
percentage scores (0 point = 0–5%; 1 point = 6–25%; 2 points =
26–50%; and 3 points = >50%) and staining intensity scores (0
point = negative; 1 point = weak intensity; 2 points = moderate
intensity; and 3 points = strong intensity) were subsequently
summed to determine the total staining scores for each section.
Staining scores ≥3 points were indicative of positive staining,
while staining scores <3 points were indicative of negative
staining (23).
Quantitative real-time PCR (Q-PCR)
Total RNA was extracted from gastric cancer cells
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to
the manufacturer's protocol. Reverse transcription was performed
with a RevertAid™ First Strand cDNA Synthesis kit (Takara), and
then Q-PCR was performed using TaqMan assays and a QuantiTect SYBR
Green kit (Biotool, Houston, TX, USA). Relative mRNA expression
levels were determined using the comparative Ct-method after being
normalized to GADPH expression levels. The expression levels of
each gene were analyzed in triplicate. See Table I for the sequences of the primers
used for this experiment.
 | Table IPrimer sequences for qPCR. |
Table I
Primer sequences for qPCR.
| Human | Primer
sequence |
|---|
| GADPH | F:
ACGGATTTGGTCGTATTGGGCG |
| R:
CTCCTGGAAGATGGTGATGG |
| MTDH | F:
AAATGGGCGGACTGTTGAAGTG |
| R:
TGTCAATCTCTGGTGGCTGCTT |
| E-cadherin | F:
TGCCCAGAAAATGAAAAAGG |
| R:
GTGTATGTGGCAATGCGTTC |
| N-cadherin | F:
ACAGTGGCCACCTACAAAGG |
| R:
CCGAGATGGGGTTGATAATG |
| Snail | F:
CCTCCCTGTCAGATGAGGAC |
| R:
CCAGGCTGAGGTATTCCTTG |
| Slug | F:
GGGGAGAAGCCTTTTTCTTG |
| R:
TCCTCATGTTTGTGCAGGAG |
| ZO-1 | F:
GAGGACCAGCTGAAGGACAG |
| R:
ATATGGCTTGCCAATCGAAG |
Western blot assay
The cells were lysed with radioimmunoprecipitation
assay (RIPA) lysis buffer supplemented with RIPA 10 µl/ml
phenylmethanesulfonyl fluoride (PMSF), a protease inhibitor, and
protein concentrations were determined by a Bicinchoninic Acid
(BCA) Protein assay kit. All of these reagents were purchased from
the Beyotime Institute of Biotechnology, China. Equal amounts of
protein were separated by SDS-PAGE and then transferred to PVDF
membranes (Millipore, Billerica, MA, USA), which were blocked with
5% non-fat milk for 2 h before being incubated with the appropriate
primary antibodies overnight at 4°C. The membranes were
subsequently incubated with the appropriate HRP-conjugated
secondary antibodies for 2 h at room temperature. The protein bands
were visualized by an Enhanced Chemiluminescence Detection kit
(Thermo Scientific, Waltham, MA, USA) and photographed by a Gel
Logic 2200 PRO imaging system. The following primary antibodies
were used in this experiment: an MTDH rabbit mAb (1:2000, Abcam),
an E-cadherin rabbit mAb (1:1000, Cell Signaling Technology,
Beverly, MA, USA), a Slug rabbit mAb (1:1000, Cell Signaling
Technology), a Snail rabbit pAb (1:1000, Abcam), an N-cadherin
mouse mAb (1:1000, Cell Signaling Technology), a ZO-1 mouse mAb
(1:2000, Cell Signaling Technology), and a GAPDH mAb-HRP (1:5000,
Bioworld Technology, Minneapolis, MN, USA).
siRNA interference and stable knockdown
cell line establishment
Four siRNA sequences targeting human MTDH mRNA were
designed using RNAi designer (Oligo 6.0 software). A scrambled
sequence of targeting MTDH sequences was used as a negative
control. The control siRNA (MTDH-con si) targeting sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′, the MTDH-siRNA#1 (si#1) targeting
sequence was 5′-GCUGUUCGAACACCUCAAATT-3′, the MTDH-siRNA#2 (si#2)
targeting sequence was 5′-GCCGUAAUCAACCCUAUAUTT-3′, and the
MTDH-siRNA#3 (si#3) targeting sequence was
5′-GCCAUCUGUAAUCUUAUCATT-3′. The most effective siRNA sequence was
selected, after which a hairpin structure (shRNA) was added to it.
A recombinant lentivirus containing the corresponding MTDH-shRNA
construct was subsequently produced via co-transfection of 293T
cells with helper plasmids (psPAX2 and pMD2G) with Lipofier
(HanBio, Shanghai, China). After 48 h of transfection, the cultured
supernatant was harvested, filtered and concentrated by
ultracentrifugation. To establish stable MTDH-knockdown cell lines,
we transduced the MKN45 and AGS cell lines with lentiviral RNAi
vectors. After 72 h, the cells were treated with 2 µg/ml
puromycin. The puromycin-resistant clones were selected and
expanded for subsequent experiments.
Cell scratch-wound healing assay
The scratch-wound healing assay is one of the oldest
methods for directly assessing cell migration ability in
vitro (18). The cells were
seeded in 6-well plates and cultured in serum-free medium until
they reached 80–90% confluency. The resultant cell monolayers were
wounded by 10-µl plastic tips, which were used to produce
straight lines in the monolayers. To monitor the wound healing
process, we took photographs every 12 h using an Eclipse
TS100/100-F inverted research microscope (Nikon, Tokyo, Japan).
Transwell migration and invasion
assays
Migration assay was conducted using 24-well
transwell plates with pore sizes of 8 µm (Corning Inc.,
Corning, NY, USA). The upper chambers of these plates were not
coated with BD Matrigel™ Matrix Basement Membrane (BD Biosciences,
Franklin Lakes, NJ, USA). However, BD Matrigel™ Matrix Basement
Membrane was used for invasion assay. The upper chamber was filed
with 2×105 cells suspended in 200 µl of
serum-free medium, and the lower chamber was filed with 600
µl of medium containing 20% fetal bovine serum, which served
as a chemoattractant. After 24 and 48 h of incubation, the
migrating and invading cells, respectively, were fixed in 4%
paraformaldehyde and stained with 0.05% crystal violet (Beyotime).
The numbers of migrating cells and invading cells were subsequently
counted under a microscope (22).
Animals
Six-week-old male nude athymic BALB/c mice were used
to evaluate tumorigenicity. For the proliferation experiment, we
subcutaneously injected 1×106 cells in 200 µl of
PBS that had been stably transfected with MTDH-knockdown or empty
vectors into the left flanks of the nude mice. Mouse tumor volumes
and tumor weights were measured after 4 weeks. Tumor volumes were
measured using the following formula: volume = (short
diameter)2 × (long diameter)/2 (24). For the metastasis experiment, we
injected 1×106 cells in 200 µl of PBS that had
been stably transfected with MTDH-knockdown or empty vectors into
the lateral tail veins of nude mice. These mice were sacrificed 4
weeks later, after which their lungs were removed, and the number
of metastatic nodules in the lungs were counted. The lungs were
then fixed in 4% formaldehyde for additional examination. The
assays were performed using four nude mice per group, and all
animal experiments were conducted in strict accordance with
institutional guidelines.
Statistical analysis
Data are presented as the mean ± SD. One-way
analysis of variance and Student's t-test were performed to
determine significance, and Chi-square tests were used to compare
clinical characteristics of patients between different groups.
Overall survival curves were generated using the Kaplan-Meier
method and analyzed by the log-rank test. All the analyses were
performed using SPSS 19.0 software and Prism statistical program 5,
and all statistical tests were two-sided. P-values <0.05 were
considered statistically significant.
Results
MTDH expression is upregulated in gastric
cancer and is correlated with gastric cancer cell metastatic
ability
Among 31 pairs of gastric cancer tissues and
adjacent normal gastric mucosa tissues, 27 gastric cancer tissues
were found to have increased MTDH mRNA expression, and 4 gastric
cancer tissues were found to have decreased MTDH mRNA expression
after the mRNA expression levels of MTDH in these tissues were
normalized to the basal mRNA expression levels of MTDH in adjacent
normal gastric mucosa tissues (Fig.
1A). The mean relative mRNA expression level of MTDH was
1.960±0.770 in all pairs. The MTDH mRNA expression level in the 22
samples without lymph node metastasis (N0 stage) was lower than the
mean mRNA expression level in the 9 samples with lymph node
metastasis (Nn stage) (P=0.023; Fig.
1B).
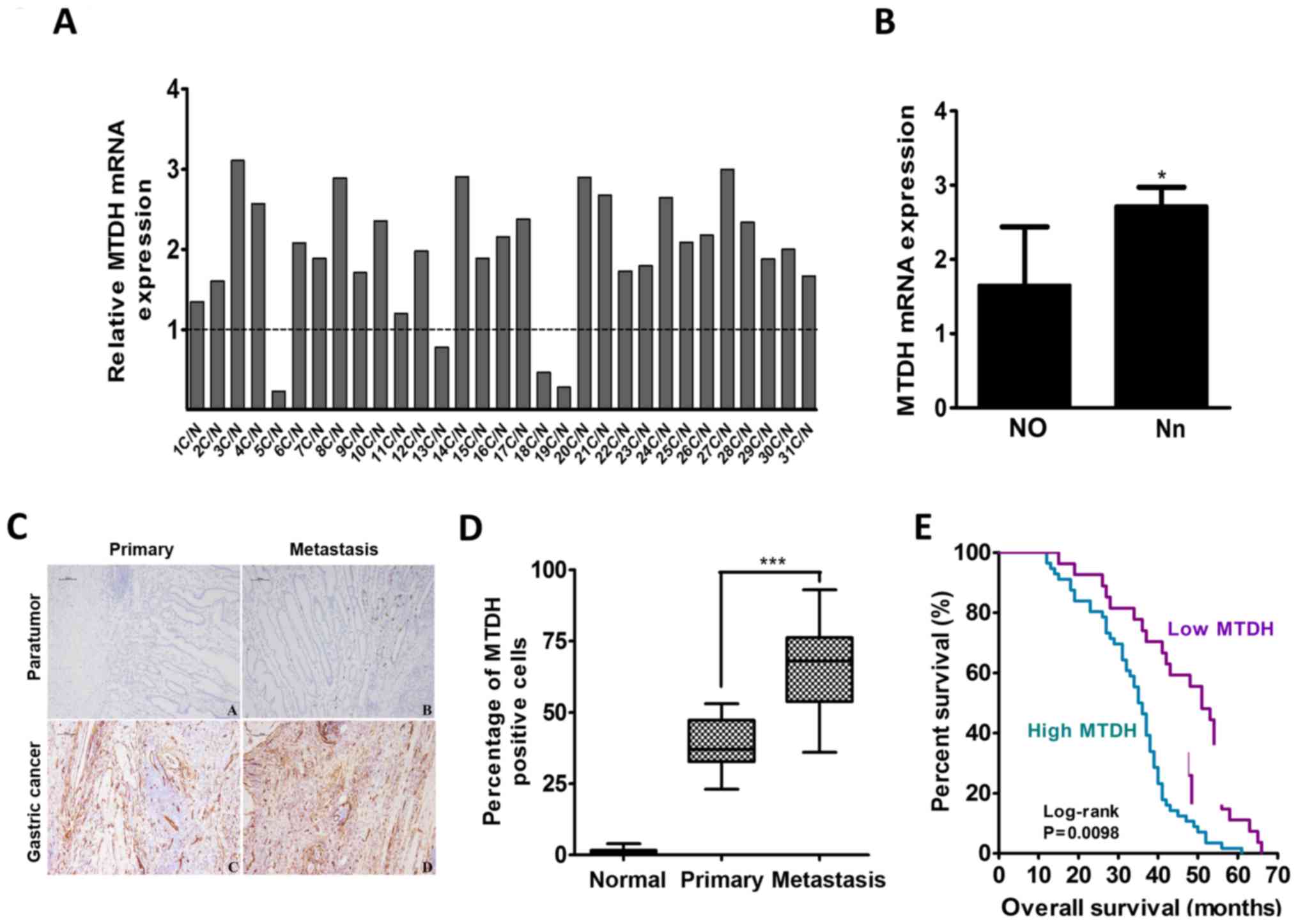 | Figure 1MTDH expression is upregulated in
gastric cancer and is positively correlated with gastric cancer
metastasis ability. (A) qPCR analysis of the expression levels of
MTDH mRNA in 31 fresh pairs of primary gastric cancer tissues after
the expression levels were normalized against the basal expression
levels of MTDH mRNA in adjacent normal gastric mucosal tissues. (B)
MTDH mRNA expression levels in primary gastric cancer patients with
or without lymph node metastasis (n=9 and n=22, respectively). Data
are the mean ± SD. (C) Immunohistochemistry staining for MTDH
expression in adjacent normal gastric mucosal tissues, primary
gastric cancer tissues without metastasis, and gastric cancer
tissues with metastasis. Scale bar, 100 µm. (D) The
percentages of MTDH-positive cells were different among the normal,
primary tumor, and metastasis groups. Data are the mean ± SD. (E).
Kaplan-Meier survival curves based on MTDH expression in gastric
cancer samples. Blue line: patients with high MTDH expression,
median overall survival time=36 months and n=56; Purple line:
patients with low MTDH expression, median overall survival time 51
months and n=27. *P<0.05, **P<0.01,
***P<0.001. |
To determine the correlation between MTDH expression
and gastric cancer progression, we performed immunohistochemical
(IHC) analysis to detect MTDH expression in 83 primary gastric
cancer samples with or without lymph node or vascular metastasis
and 25 normal gastric mucosa samples. MTDH expression was almost
non-existent in the normal gastric mucosa tissue samples. The mean
MTDH expression was 38.89±3.123% in the 19 gastric cancer tissues
without metastasis and 66.10±3.552% in the 64 gastric cancer
tissues with lymph node or vascular metastasis, a difference that
was statistically significant (P<0.001; Fig. 1D). The abovementioned 83 gastric
cancer tissue samples were subsequently divided into the following
two groups: a low MTDH expression group, which included 27 tissues,
and a high MTDH expression group, which included 56 tissues. As
shown in Table II, MTDH
expression levels were significantly correlated with lymph node
metastasis and TNM stages (P=0.002 and P<0.001, respectively).
The median overall survival (OS) time in the above-mentioned 83
cases was 44 months, and the median OS times in the low and high
MTDH expression groups were 51 and 36 months, respectively. High
MTDH protein expression was significantly associated with decreased
OS (P=0.0098; Fig. 1E).
 | Table IIDistribution of clinical
characteristics stratified by MTDH protein expression in 83
patients with gastric cancer. |
Table II
Distribution of clinical
characteristics stratified by MTDH protein expression in 83
patients with gastric cancer.
| MTDH
expression | High | Low | P-value |
|---|
| No. of
patients | 56 | 27 | – |
| Age (year) | | | 0.545 |
| ≥60 | 37 | 16 | |
| <60 | 19 | 11 | |
| Sex | | | 0.737 |
| Female | 25 | 11 | |
| Male | 31 | 16 | |
| Tumor size
(cm) | | | 0.725 |
| ≥5 | 23 | 10 | |
| <5 | 33 | 17 | |
| Lymph node
metastasis | | | 0.002 |
| Without | 15 | 17 | |
| With | 39 | 9 | |
| TNM stage | | | <0.001 |
| I | 5 | 2 | |
| II | 19 | 8 | |
| III | 24 | 11 | |
| IV | 8 | 6 | |
| Overall
survival | | | 0.0098 |
| Months
(median) | 36 | 51 | |
MTDH expression in gastric cancer cell
lines and stable shMTDH gastric cancer cells
MTDH mRNA and protein expression levels in four
gastric cancer cell lines (MKN45, AGS, SGC7901, and KATO-III) and a
normal gastric mucosa cell line (GES-1) were examined by qPCR and
western blotting, respectively (Fig.
2A and B). We found that MTDH mRNA and protein expression
levels were low in GES-1 and KATO-III cells and high in AGS and
MKN45 cells. According to ATCC and the results of our previous
study (23), MKN45 and AGS cells
were highly invasive, but KATO-III cells were poorly invasive,
suggesting that MTDH expression levels are correlated with gastric
cancer cell metastasis. Moreover, both the MKN45 and the AGS cell
lines, especially the MKN45 cell line, displayed easily observable
morphological changes, such as migration-related protrusions.
Therefore, the MKN45 and AGS cell lines were selected to establish
stable shMTDH cells, which were used in subsequent experiments. As
shown in Fig. 2C and D,
MTDH-siRNA3 (si#3) displayed maximal knockdown efficiency, making
it the ideal siRNA to which a hairpin structure could be added, as
well as the ideal siRNA with which stable shMTDH-MKN45 (MKN45-sh#3)
and shMTDH-AGS cell lines (AGS-sh#3) could be established (Fig. 2E and F).
 | Figure 2MTDH expression in gastric cancer
cells and stable shMTDH gastric cancer cells. (A and B) qPCR and
western blot analysis of MTDH expression levels in the gastric
cancer cell lines MKN45, AGS, SGC7901, and KATO-III and the normal
gastric mucosa cell line GES-1. (C and D) MKN45 and AGS cells were
transfected with three MTDH-targeting siRNAs (si#1, si#2, si#3) and
a negative control siRNA (con si). After 24 h, MTDH protein and
mRNA expression levels were determined by western blotting and
qPCR, respectively. Data are the mean ± SD. (E and F) The stable
shMTDH gastric cancer cell lines, namely, the MKN45-sh#3 and
AGS-sh#3 cell lines, were established, and immunofluorescence
staining and western blot analysis demonstrated the interfering
efficiency of the MTDH-targeting shRNA. *P<0.05,
**P<0.01, ***P<0.001. |
MTDH promotes gastric cancer cell
invasion and migration and regulates actin cytoskeletal remodeling
in vitro
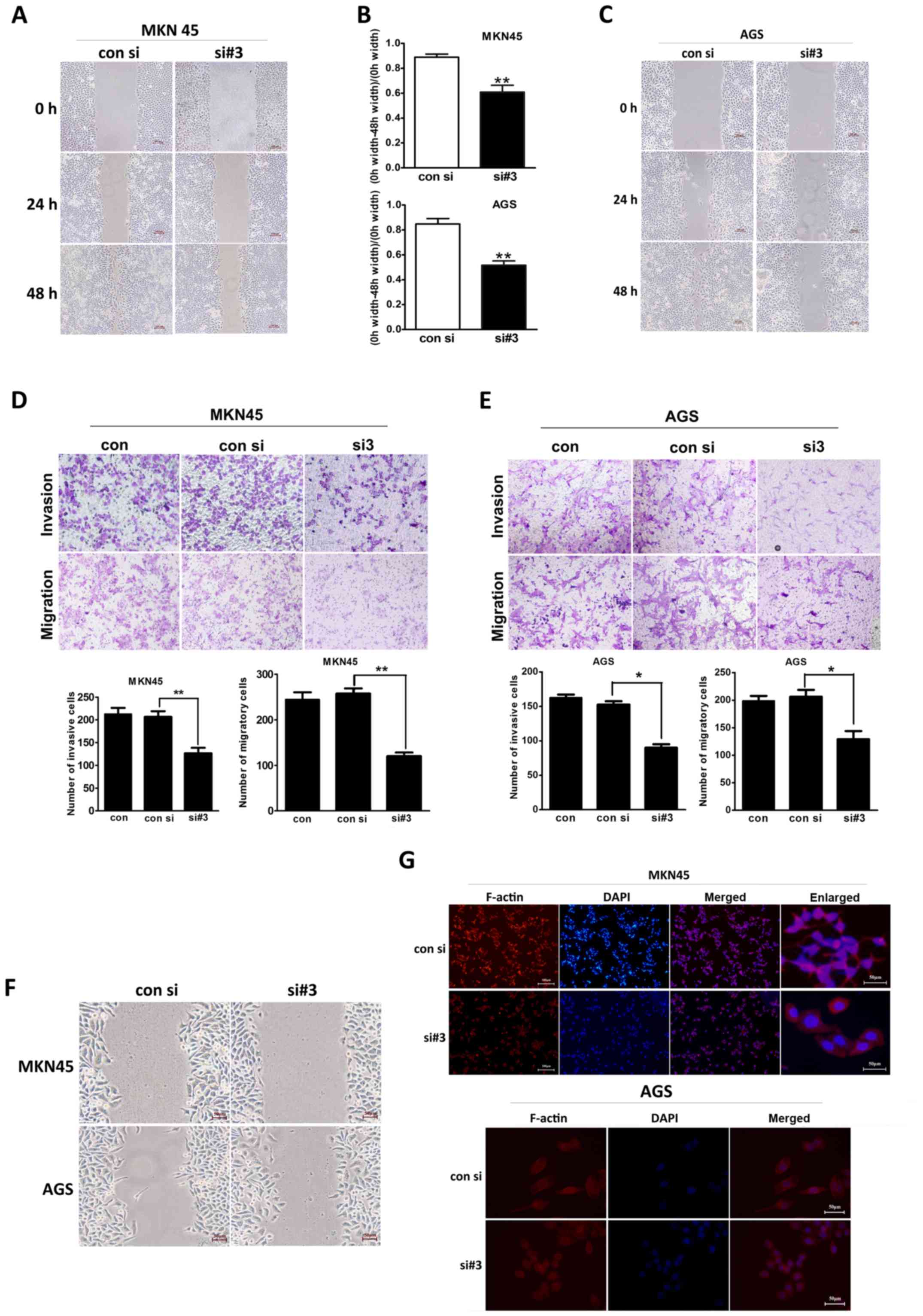
To investigate the invasion and the migration
ability of different gastric cancer cells, we conducted
scratch-wound healing and transwell assays. As shown in Fig. 3A, in the scratch-wound healing
assay, the migratory range of the MKN45 cells was decreased after
MTDH expression levels were downregulated. Consistent with this
finding, scratch-wound coverage was significantly decreased in this
cell line at 48 h post-wound placement (P=0.010; Fig. 3B). Similar results were observed in
the experiments involving the AGS cells (P=0.004; Fig. 3B and C).
In the Transwell assay (Fig. 3D), the number of siMTDH-MKN45 cells
that invaded the Matrigel was significantly reduced after 48 h
compared with the numbers of normal and negative control cells that
invaded the Matrigel after the same period of time (P=0.005).
Moreover, the number of siMTDH-MKN45 cells that migrated through
the chamber after 24 h was also decreased compared with the number
of normal and negative control cells that migrated through the
chamber after the same period of time (P=0.008). Similar results
were observed in the experiments involving the AGS cell line
(P=0.021 and P=0.034, respectively; Fig. 3E).
Early cell migration was observed via light
microscopy at 12 h after scratch-wound placement. As shown in
Fig. 3F, both MKN45 and AGS
control cells appeared to be distorted and elongated as they moved
toward the scratch-wound area and pseudopodial protrusions were
observed in these cells. However, the forward-most siMTDH cells
exhibited an epithelial-like polygonal appearance, with less
pseudopodial protrusions. As shown in Fig. 3G, both the MKN45 and the AGS cells
appeared smooth and displayed decreased number of migration-related
protrusions after MTDH downregulation.
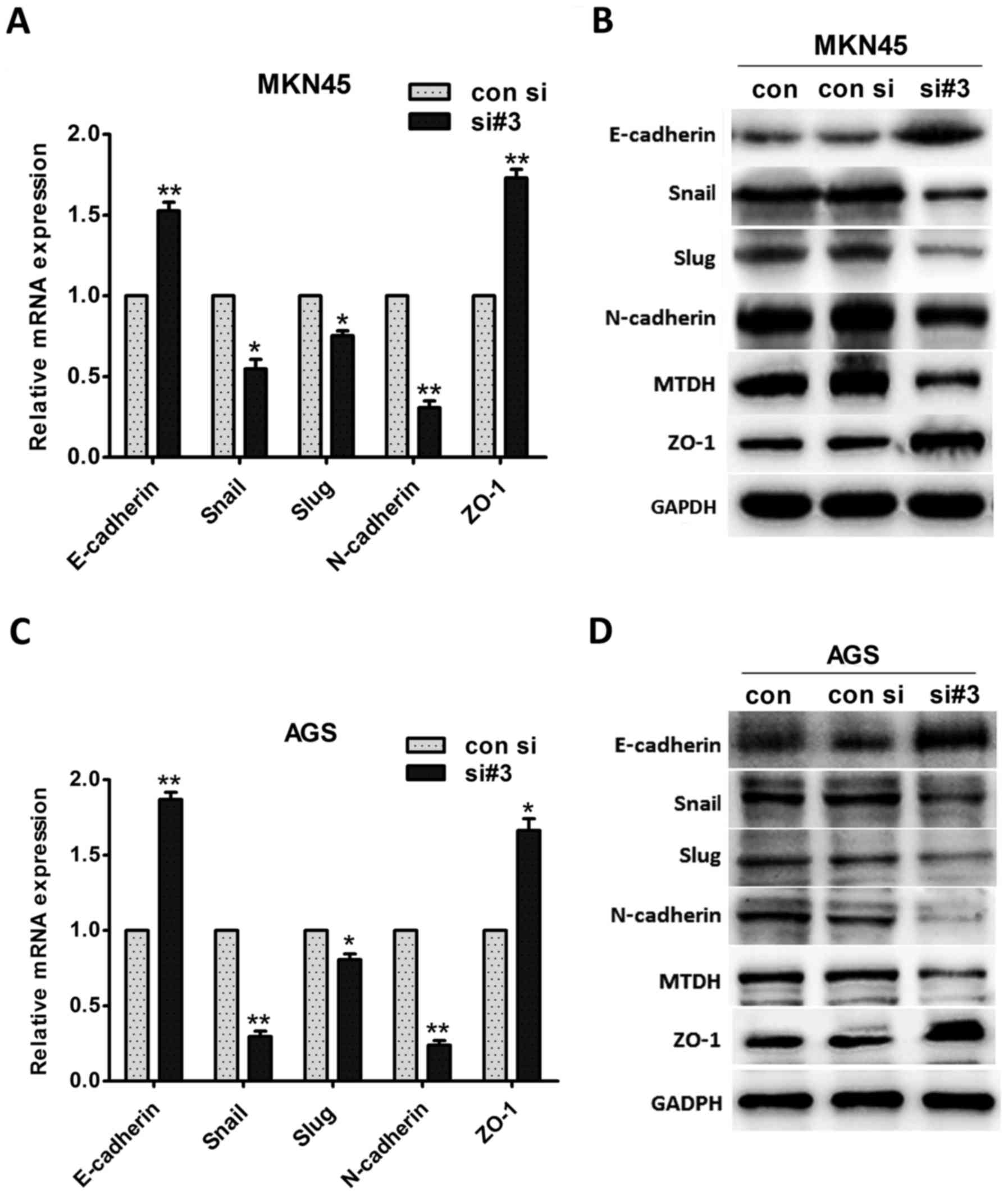
Downregulating MTDH expression inhibits
EMT in vitro
To confirm the existence of a relationship between
MTDH expression levels and gastric cancer cell metastasis, we
evaluated the changes in EMT marker expression levels. We found
that the mRNA expression levels of the epithelial markers
E-cadherin and ZO-1 were significantly increased in MKN45 cells
after MTDH downregulation (P=0.010 and P=0.005, respectively;
Fig. 4A). In contrast, the mRNA
expression levels of the mesenchymal marker N-cadherin and the
EMT-induced factors Slug and Snail were significantly inhibited
after MTDH down regulation (P=0.004, P=0.014, and P=0.017,
respectively; Fig. 4A). Moreover,
western blotting revealed that E-cadherin and ZO-1 protein
expression levels increased, whereas N-cadherin, Slug, and Snail
protein expression levels decreased after MTDH expression levels
were downregulated (Fig. 4B).
Similar results were observed in the AGS cell line,
as E-cadherin expression levels were increased, and N-cadherin
expression levels were decreased at both the mRNA and the protein
level when MTDH expression levels were downregulated (Fig. 4C and D). Taken together, the above
cell morphology and cell functional experimental results
demonstrated that MTDH regulates actin cytoskeletal remodeling and
promotes cell metastasis via EMT.
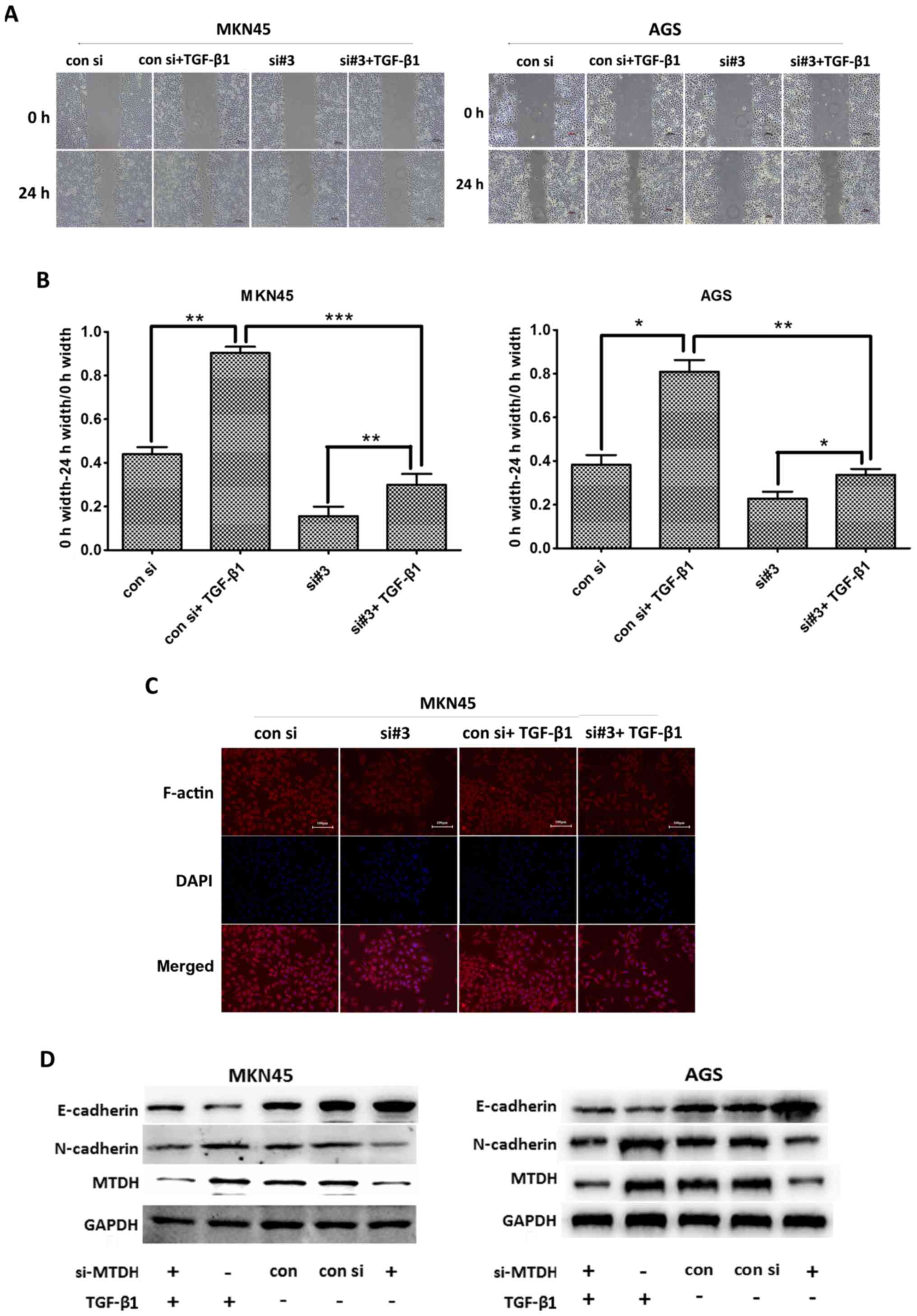
MTDH promotes EMT via actin cytoskeletal
remodeling in vitro
To confirm that MTDH promotes EMT via actin
cytoskeletal remodeling in gastric cancer cells, we stimulated
MKN45 and AGS cells with TGF-β1 (5 ng/ml), a well-known EMT
inducer, to actively induce EMT. As shown in Fig. 5A and B, the migratory range of the
siMTDH cell line decreased when MTDH expression was downregulated.
We subsequently stimulated both control cells and siMTDH cells with
TGF-β1 for 24 h and found that the migratory range of the siMTDH
cell line increased. Similar results were noted in the experiments
involving the control cell line. We also found that migratory range
of the siMTDH cell line increased to a lesser extent than that of
the control cell line after TGF-β1 stimulation. These results
indicated that MTDH plays an important role in EMT.
As shown in Fig.
5C, our studies of MKN45 cell morphology revealed that siMTDH
cells displayed decreased numbers of migration-related protrusions
compared with control cells. Moreover, these cells exhibited a
polygonal, smooth-edge epithelial cell-like structure. After being
stimulated with TGF-β1 for 24 h, the above cells displayed
increased numbers of migration-related protrusions, changes similar
to those displayed by control cells stimulated with TGF-β1;
however, the control cell line displayed more migration-related
protrusions than the siMTDH cell line. These results indicated that
MTDH regulates actin cytoskeletal remodeling.
To observe the above effects at the protein level,
we performed western blot analysis to assess changes in EMT marker
protein expression levels (Fig.
5D). We found that the changes of EMT marker expression levels
were similar to the results of Fig. 5A
and C. Thus, we concluded that MTDH promoted EMT via actin
cytoskeletal remodeling in gastric cancer cells.
MTDH promotes tumor proliferation and
metastasis in vivo
To determine whether MTDH affects tumor formation
and growth in vivo, we subcutaneously injected the
established stable gastric cancer MKN45-sh#3 cells (Fig. 2E), and the MKN45-con sh negative
control cells into the left flanks of BALB/c mice and assessed
tumor growth after 4 weeks (Fig.
6A). We measured the maximum and minimum diameters of the
resultant tumors and determined that the mean tumor volume was 223
mm3 in the shMTDH group and 1385 mm3 in the
control group (P<0.001; Fig.
6B). Additionally, the average tumor weight was 238 g in the
shMTDH group and 528.6 g in the control group (P<0.001; Fig. 6B).
 | Figure 6Influence of MTDH knockdown on cancer
cell proliferation and metastasis in vivo. (A) Tumor
proliferation in nude mice. Negative control (con sh group) and
shMTDH (sh#3 group) cells derived from MKN45 cells were injected
subcutaneously into the left flanks of BALB/c mice. Tumor growth
was recorded, and all the mice were sacrificed after 4 weeks. (B)
Measurements of all the tumor volumes and weights in the con sh and
sh#3 group. Data are the mean ± SD. (C) Con sh and sh#3 cells
derived from MKN45 cells were injected into the tail veins of nude
mice. After 4 weeks, all the nude mice were sacrificed, and the
average numbers of metastatic nodules in the lungs (shown by the
black arrows) were counted. Data are the mean ± SD. (D) The
metastatic tumor nodules in the lungs of nude mice were identified
histologically via H&E staining. Upper panel: panoramic scans
of lung tissue slices; lower panel: enlarged images, ×100. Scale
bar, 200 µm. (E) Western blot analysis of MTDH, E-cadherin
and N-cadherin expression in both the con sh group and the sh#3
group. (F) Immunohistochemistry was performed to determine MTDH,
E-cadherin and N-cadherin expression levels in both the con sh
group and the sh#3 group. *P<0.05,
**P<0.01, ***P<0.001. Scale bar, 200
µm. |
To determine whether downregulating MTDH expression
inhibits cancer cell metastasis in vivo, we injected MKN45
cells from the control and shMTDH groups into the tail veins of
nude mice. After 4 weeks, the mice were euthanized, their lungs
were dissected, and the numbers of metastatic nodules in each lung
were counted (Fig. 6C). The mean
number of palpable nodules on the surface of the lung in the shMTDH
group was 12.667±2.517, and the mean number of palpable nodules on
the surface of the lung in the control group was 7.667±1.528
(P=0.043; Fig. 6C). As shown in
Fig. 6D, the metastatic tumor
nodules were identified histologically via H&E staining. The
images in the top panel represent panoramic scans of lung tissue
slices, and the images in the bottom panel are enlarged versions of
the scans. We found that lungs in the shMTDH group displayed fewer
cell clumps adjacent to their pulmonary vessels than the lungs in
the control group. Additionally, E-cadherin expression levels were
increased, and N-cadherin expression levels were decreased in the
metastatic nodules of the shMTDH group compared with those in the
control group (Fig. 6E and F), the
results are consistent with those of our in vitro
experiments.
Discussion
The results of studies aiming to identify novel
targets for the treatment and prevention of gastric cancer
metastasis are promising. This study demonstrated that MTDH played
a significant role in gastric cancer cell invasion and metastasis.
MTDH expression levels were elevated in gastric cancer tissues
compared with adjacent normal gastric mucosal tissues, and MTDH
expression levels were significantly increased in metastatic
gastric cancer tissues compared with non-metastatic gastric cancer
tissues. The OS time for patients with gastric cancer with high
MTDH expression levels was shorter than the OS time for patients
with gastric cancer with low MTDH expression levels. These results
suggested that MTDH expression levels were correlated with clinical
metastasis and patient survival. We also found that suppressing
MTDH expression could inhibit gastric cancer cell migration and
invasion ability in vitro and in vivo. Furthermore,
we demonstrated that MTDH expression can regulate cytoskeletal
remodeling and is strongly correlated with changes in EMT marker
(E-cadherin, ZO-1, Slug, Snail, and N-cadherin) expression. MTDH
downregulation decreased cell migratory protrusions and inhibit EMT
process in both MKN45 cells and AGS cells. Thus, our study not only
elucidated the role of MTDH in gastric cancer but also at least
partially elucidated the mechanism through which MTDH facilitates
gastric cancer metastasis.
MTDH was first identified as a novel protein known
as LYRIC, a known cell adhesion molecule and tumor suppressor
(25,26). Britt et al (6) found that LYRIC was colocalized with
the tight junction proteins ZO-1 and occludin in polarized
epithelial cells and showed that LYRIC can be recruited during
tight junction complex maturation. Other studies (27–29)
demonstrated that tight junction proteins, such as claudin-1,
occludin, and ZO-1, can act as tumor suppressors. In the event of
tight junction complex disruption (which is associated with
decrease in ZO-1 and claudin-1 expression), MTDH dissociates from
the cell adhesion protein complex, resulting in actin cytoskeletal
remodeling and EMT induction. In this study, we found MTDH promotes
gastric cancer cell metastasis. When we downregulated MTDH
expression in MKN45 and AGS cells, the cells displayed decreased
number of migration-related protrusions, and the EMT course was
inhibited. Our study first demonstrated that MTDH promotes gastric
cancer metastasis through actin cytoskeletal remodeling.
During EMT, cells undergo morphological changes
through which they lose their polarity, assume a mesenchymal
cell-like spindle shape and develop migratory protrusions.
Moreover, cells undergo changes in the expression levels of their
differentiation markers, resulting in a shift from an epithelial to
a mesenchymal phenotype, and functional changes leading to
extracellular matrix (ECM) invasion (30–32).
Disrupting cell-cell adhesion induced cell polarity loss and
cytoskeletal remodeling, during which cells lost their polygonal
epithelial cell-like features and developed mesenchymal cell-like
features (33,34). We also demonstrated that both MKN45
cells and AGS cells transformed from elongated spindle-shaped cells
with migratory protrusions into nearly polygonal, smooth-edged, and
epithelial-like cells upon MTDH downregulation. A well-known EMT
inducer (35), TGF-β1 promoted EMT
in MKN45 and AGS cells and induced comparable changes in cell
morphology and EMT marker protein expression in the MKN45 and AGS
siMTDH cell lines. Taken together, these data show that MTDH
upregulation regulates actin cytoskeletal remodeling and induces
EMT in gastric cancer cell metastasis. Likewise, many studies
(19,36,37)
reported that pseudopodial protrusion and formation of related
invadopodia have long been associated with tumor cell migration and
invasion, and dynamic actin cytoskeleton remodeling was closely
related to EMT. However, although our study indicated that
downregulating MTDH expression could induce actin cytoskeleton
remodeling and inhibit EMT in MKN45 cells and AGS cells, we thought
it was unable to completely block EMT. EMT could be regulated by
many pathway, such as Wnt signaling, cytokine signaling, Notch
signaling (38). Emdad et
al (39) reported that MTDH
promoted tumor invasion and metastasis by activating the nuclear
factor kappaB pathway. Hu et al (40) showed that MTDH may promote breast
cancer metastasis by facilitating interactions between a
lung-homing domain and an unknown receptor located on the surface
of endothelial cells, as well as NF-κB signaling pathway
activation. Hence, MTDH can promote cancer cell metastasis through
many different processes warranting further investigation.
However, MTDH is correlated not only with tumor
metastasis but also with tumorigenesis, cancer cell proliferation,
autophagy, and chemical drug resistance (8,41–44).
Liu et al (45)
demonstrated that AEG-1/MTDH promoted neuroblastoma cell
proliferation and that AEG-1 knockdown could enhance the
chemosensitivity of the above tumors to cisplatin and doxorubicin.
In our animal experiment, we also found that both tumor volume and
tumor weight in shMTDH mice were significantly decreased compared
with those in control mice. Moreover, Li et al and Shen
et al (46,47) showed that MTDH can promote tumor
proliferation in gastric cancer.
Taken together, our findings show that MTDH
regulated actin cytoskeletal remodeling and enhanced EMT in gastric
cancer metastasis. Moreover, our findings indicate that MTDH is a
crucial factor for predicting tumor metastasis and prognosis.
Therefore, MTDH may be a target for the efficient treatment of
gastric cancer metastasis.
Acknowledgments
This work was supported by Grants of the Shanghai
Municipal Health Bureau Foundation of China (201540202).
Glossary
Abbreviations
Abbreviations:
|
MTDH
|
metadherin
|
|
EMT
|
epithelial-mesenchymal transition
|
|
AEG-1
|
astrocyte elevated gene-1
|
|
Lyric
|
Lysine-rich CEACAM-1-associated
protein
|
|
TGF-β
|
transforming growth factor β
|
References
|
1
|
Jin P, Wong CC, Mei S, He X, Qian Y and
Sun L: MK-2206 co-treatment with 5-fluorouracil or doxorubicin
enhances chemosensitivity and apoptosis in gastric cancer by
attenuation of Akt phosphorylation. Onco Targets Ther. 9:4387–4396.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia H, Chen Q, Chen Y, Ge X, Leng W, Tang
Q, Ren M, Chen L, Yuan D, Zhang Y, et al: The lncRNA MALAT1 is a
novel biomarker for gastric cancer metastasis. Oncotarget.
7:56209–56218. 2016.PubMed/NCBI
|
|
4
|
Xu W, Yang Z and Lu N: Molecular targeted
therapy for the treatment of gastric cancer. J Exp Clin Cancer Res.
35:12016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown DM and Ruoslahti E: Metadherin, a
cell surface protein in breast tumors that mediates lung
metastasis. Cancer Cell. 5:365–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Britt DE, Yang DF, Yang DQ, Flanagan D,
Callanan H, Lim YP, Lin SH and Hixson DC: Identification of a novel
protein, LYRIC, localized to tight junctions of polarized
epithelial cells. Exp Cell Res. 300:134–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization. RaSH Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar
|
|
8
|
Zhou Z, Deng H, Yan W, Luo M, Tu W, Xia Y,
He J, Han P, Fu Y and Tian D: AEG-1 promotes anoikis resistance and
orientation chemotaxis in hepatocellular carcinoma cells. PLoS One.
9:e1003722014. View Article : Google Scholar :
|
|
9
|
Yu L, Liu X, Cui K, Di Y, Xin L, Sun X,
Zhang W, Yang X,, Wei M, Yao Z, et al SND1 acts downstream of TGFβ1
and upstream of Smurf1 to promote breast cancer metastasis. Cancer
Res. 75:1275–1286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu
G, Tian Y, Qiu Y and Zhang X: Metadherin regulates metastasis of
squamous cell carcinoma of the head and neck via AKT signalling
pathway-mediated epithelial-mesenchymal transition. Cancer Lett.
343:258–267. 2014. View Article : Google Scholar
|
|
11
|
Lupo J, Conti A, Sueur C, Coly PA, Couté
Y, Hunziker W, Burmeister WP, Germi R, Manet E, Gruffat H, et al:
Identification of new interacting partners of the shuttling protein
ubinuclein (Ubn-1). Exp Cell Res. 318:509–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC,
Bruce JN, Volsky DJ and Fisher PB: Astrocyte elevated gene-1:
Recent insights into a novel gene involved in tumor progression,
metastasis and neurodegeneration. Pharmacol Ther. 114:155–170.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Huaier polysaccharides
suppresses hepatocarcinoma MHCC97-H cell metastasis via
inactivation of EMT and AEG-1 pathway. Int J Biol Macromol.
64:106–110. 2014. View Article : Google Scholar
|
|
15
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar
|
|
16
|
Friedl P: Prespecification and plasticity:
Shifting mechanisms of cell migration. Curr Opin Cell Biol.
16:14–23. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995;
discussion 5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou M, Zhu W, Wang L, Shi L, Gao R, Ou Y,
Chen X, Wang Z, Jiang A, Liu K, et al: AEG-1/MTDH-activated
autophagy enhances human malignant glioma susceptibility to
TGF-β1-triggered epithelial-mesenchymal transition. Oncotarget.
7:13122–13138. 2016.PubMed/NCBI
|
|
19
|
Shankar J, Messenberg A, Chan J, Underhill
TM, Foster LJ and Nabi IR: Pseudopodial actin dynamics control
epithelial-mesenchymal transition in metastatic cancer cells.
Cancer Res. 70:3780–3790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guirguis R, Margulies I, Taraboletti G,
Schiffmann E and Liotta L: Cytokine-induced pseudopodial protrusion
is coupled to tumour cell migration. Nature. 329:261–263. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen WT: Proteolytic activity of
specialized surface protrusions formed at rosette contact sites of
transformed cells. J Exp Zool. 251:167–185. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu JW, Wu SH, Lu RQ, Wu JG, Ni XC, Zhou
GC, Jiang HG, Zheng LH, Li XQ, Du GY, et al: Expression and
significances of contactin-1 in human gastric cancer. Gastroenterol
Res Pract. 2013:2102052013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen DH, Yu JW, Wu JG, Wang SL and Jiang
BJ: Significances of contactin-1 expression in human gastric cancer
and knockdown of contactin-1 expression inhibits invasion and
metastasis of MKN45 gastric cancer cells. J Cancer Res Clin Oncol.
141:2109–2120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang M, Gu YY, Peng H, Zhao M, Wang J,
Huang SK, Yuan XH, Li J, Sang JL, Luo Q, et al: NAIF1 inhibits
gastric cancer cells migration and invasion via the MAPK pathways.
J Cancer Res Clin Oncol. 141:1037–1047. 2015. View Article : Google Scholar
|
|
25
|
Thompson NL, Lin SH, Panzica MA and Hixson
DC: Cell CAM 105 isoform RNA expression is differentially regulated
during rat liver regeneration and carcinogenesis. Pathobiology.
62:209–220. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kleinerman DI, Troncoso P, Lin SH, Pisters
LL, Sherwood ER, Brooks T, von Eschenbach AC and Hsieh JT:
Consistent expression of an epithelial cell adhesion molecule
(C-CAM) during human prostate development and loss of expression in
prostate cancer: Implication as a tumor suppressor. Cancer Res.
55:1215–1220. 1995.PubMed/NCBI
|
|
27
|
Kojima T, Takano K, Yamamoto T, Murata M,
Son S, Imamura M, Yamaguchi H, Osanai M, Chiba H, Himi T, et al:
Transforming growth factor-beta induces epithelial to mesenchymal
transition by down-regulation of claudin-1 expression and the fence
function in adult rat hepatocytes. Liver Int. 28:534–545. 2008.
View Article : Google Scholar
|
|
28
|
Martínez-Estrada OM, Cullerés A, Soriano
FX, Peinado H, Bolós V, Martínez FO, Reina M, Cano A, Fabre M and
Vilaró S: The transcription factors Slug and Snail act as
repressors of Claudin-1 expression in epithelial cells. Biochem J.
394:449–457. 2006. View Article : Google Scholar :
|
|
29
|
Singh AB and Harris RC: Epidermal growth
factor receptor activation differentially regulates claudin
expression and enhances transepithelial resistance in Madin-Darby
canine kidney cells. J Biol Chem. 279:3543–3552. 2004. View Article : Google Scholar
|
|
30
|
Yao Y, Gu X, Liu H, Wu G, Yuan D, Yang X
and Song Y: Metadherin regulates proliferation and metastasis via
actin cytoskeletal remodelling in non-small cell lung cancer. Br J
Cancer. 111:355–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janda E, Lehmann K, Killisch I, Jechlinger
M, Herzig M, Downward J, Beug H and Grünert S: Ras and TGF (beta)
cooperatively regulate epithelial cell plasticity and metastasis:
Dissection of Ras signaling pathways. J Cell Biol. 156:299–313.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boyer B and Thiery JP:
Epithelium-mesenchyme interconversion as example of epithelial
plasticity. APMIS. 101:257–268. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar
|
|
36
|
Lin YC, Tsai PH, Lin CY, Cheng CH, Lin TH,
Lee KP, Huang KY, Chen SH, Hwang JJ, Kandaswami CC, et al: Impact
of flavonoids on matrix metalloproteinase secretion and invadopodia
formation in highly invasive A431-III cancer cells. PLoS One.
8:e719032013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee MR and Jeon TJ: Cell migration:
Regulation of cytoskeleton by Rap1 in Dictyostelium discoideum. J
Microbiol. 50:555–561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moustakas A and Heldin C-H: Mechanisms of
TGFβ-induced epithelial-mesenchymal transition. J Clin Med. 5:1–34.
2016. View Article : Google Scholar
|
|
39
|
Emdad L, Sarkar D, Su ZZ, Randolph A,
Boukerche H, Valerie K and Fisher PB: Activation of the nuclear
factor kappaB pathway by astrocyte elevated gene-1: Implications
for tumor progression and metastasis. Cancer Res. 66:1509–1516.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu G, Chong RA, Yang Q, Wei Y, Blanco MA,
Li F, Reiss M, Au JL, Haffty BG and Kang Y: MTDH activation by 8q22
genomic gain promotes chemoresistance and metastasis of
poor-prognosis breast cancer. Cancer Cell. 15:9–20. 2009.
View Article : Google Scholar :
|
|
41
|
Liu X, Wang D, Liu H, Feng Y, Zhu T, Zhang
L, Zhu B and Zhang Y: Knockdown of astrocyte elevated gene-1
(AEG-1) in cervical cancer cells decreases their invasiveness,
epithelial to mesenchymal transition, and chemoresistance. Cell
Cycle. 13:1702–1707. 2014. View Article : Google Scholar
|
|
42
|
Zhang B, Liu XX, He JR, Zhou CX, Guo M, He
M, Li MF, Chen GQ and Zhao Q: Pathologically decreased miR-26a
antagonizes apoptosis and facilitates carcinogenesis by targeting
MTDH and EZH2 in breast cancer. Carcinogenesis. 32:2–9. 2011.
View Article : Google Scholar
|
|
43
|
Gu C, Feng L, Peng H, Yang H, Feng Z and
Yang Y: MTDH is an oncogene in multiple myeloma, which is
suppressed by Bortezomib treatment. Oncotarget. 7:4559–4569.
2016.
|
|
44
|
Li WF, Dai H, Ou Q, Zuo GQ and Liu CA:
Overexpression of microRNA-30a-5p inhibits liver cancer cell
proliferation and induces apoptosis by targeting MTDH/PTEN/AKT
pathway. Tumour Biol. 37:5885–5895. 2016. View Article : Google Scholar
|
|
45
|
Liu H, Song X, Liu C, Xie L, Wei L and Sun
R: Knockdown of astrocyte elevated gene-1 inhibits proliferation
and enhancing chemo-sensitivity to cisplatin or doxorubicin in
neuroblastoma cells. J Exp Clin Cancer Res. 28:192009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen X, Si Y, Yang Z, Wang Q, Yuan J and
Zhang X: MicroRNA-542-3p suppresses cell growth of gastric cancer
cells via targeting oncogene astrocyte-elevated gene-1. Med Oncol.
32:3612015. View Article : Google Scholar
|
|
47
|
Li W, Lin S, Li W, Wang W, Li X and Xu D:
IL-8 interacts with metadherin promoting proliferation and
migration in gastric cancer. Biochem Biophys Res Commun.
478:1330–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|