Introduction
Glioblastoma (GBM) is one of the most devastating
malignant neoplasms in the central nervous system with increasing
risk and incidence (1). Despite
recent therapeutic advances, the prognosis of patients afflicted by
GBM remains poor, even with multimodal therapy including maximal
surgical resection followed by concurrent radiation and
chemotherapy with alkylating drugs (2). Temozolomide (TMZ), an oral alkylating
agent used as the first-line therapy for GBM treatment, is
frequently limited in durability of treatment response because of
acquired drug resistance (3).
Thus, identifying novel mechanisms underlying acquired TMZ
resistance may allow for a more durable benefit from the
anti-glioma properties of TMZ (4).
Long non-coding RNAs (lncRNAs), longer than 200 bp
in length and lacking significant protein coding open reading
frames, transcribed from intergenic and intronic regions in human
genome and participating in various biological or pathological
processes including cancers (5,6). In
the past few years, deregulated lncRNA has been widely reported to
be involved in cancer occurrence, progression and metastasis
(7–9). Evidence linking lncRNAs to tumor drug
resistance have also emerged (10–12).
For instance, lncRNA UCA1 enhanced cisplatin resistance in bladder
cancer by CREB activation (13),
and lncRNA GAS5 downregulation causes trastuzumab resistance in
breast cancer (14). However, few
studies have focused on the occurrence and development of TMZ
resistance in GBM related to lncRNAs.
In this study, we profiled the expression of lncRNAs
and mRNAs in U87 TMZ-resistant (U87TR) cells compared to the
parental cells by microarray method. We showed various
differentially expressed lncRNAs and mRNAs and found multiple
dysregulated signal pathways that associated with TMZ resistance.
These findings may provide us novel insights and potential targets
for overcoming acquired TMZ resistance in GBM chemotherapy.
Materials and methods
Cell culture and TR cell
establishment
The human GBM cell line U87 was obtained from the
American Type Culture Collection (ATCC, USA) and U251 was purchased
from the CLS Cell Lines Service GmbH (Eppelheim, Germany). The TR
cells were generated by repetitive pulse exposure of U87 and U251
GBM cells to TMZ (48 h every 2 weeks) and with increasing TMZ
concentrations for 6 months. For TR phenotype maintenance, U87TR
and U251TR cells were alternately treated with TMZ (500 µM)
for 48 h. The corresponding methods were mainly based on the
previous study of Monoz et al (15–17)
and with minor adjustment in this study. The parental and TR cells
were maintained in DMEM (Hyclone, USA) with 10% (v/v) FBS (Hyclone)
and 1% (v/v) penicillin/streptomycin (Gibco, USA) at 37°C in 5%
CO2 humidified air incubator (Thermo Scientific,
USA).
Cell survival assay
The parental and TR cells were plated in 96-well
plate and treated with TMZ in different concentrations,
respectively. After 48-h incubation, cells were replaced with fresh
medium with CCK-8 solution (v/v 10%; Dojindo, Japan) and incubated
at 37°C for 2 h. Then the absorbance was measured at 450 nm
(reference, 620 nm) using Multiscan GO microplate reader (Thermo
Fisher Scientific, Finland). Cells without TMZ treatment were set
as the control and the result was shown as cell viability ratio
towards the control group.
RNA extraction and qPCR analysis
Total RNA was extracted by TRIzol reagent
(Invitrogen, USA) and the absorbance was measured with OD260/280
ratio higher than 1.8. For qPCR analysis, 1 µg total RNA was
subjected to the synthesis of cDNA by using RevertAid First Strand
cDNA Synthesis kit (Thermo Scientific, Germany). Reactions were
initiated by incubation at 65°C for 5 min, followed by 60 min at
42°C and terminated the reaction by heating at 70°C for 5 min. The
cDNA performed to PCR by using the Maxima SYBR Green/ROX qPCR
Master Mix (Thermo Scientific, Germany). Total reaction volume (25
µl) included 12.5 µl mix (2X), 1.5 µl (10 mM)
primers (Table I) synthesized by
Sangon Biological Engineering Technology and Services Co., Ltd.
(Shanghai, China), 8.5 µl nuclease-free water and 0.8
µg/1 µl cDNA. PCR reaction was run in Step-one Plus
Real-Time PCR system (Applied Biosystems, Germany) and analyzed
using Step-one software. The qPCR protocol contained initial
denaturation at 95°C 10 min, then 40 cycles including 95°C for
5-sec denaturation, 60°C for 30 sec annealing and 72°C for 30-sec
extension. qPCR assays were carried out in triplicate, and the
specificity of the PCR products was verified with melting curve
analysis. The amount of each respective amplification product was
determined relative to the gene β-actin. The fold change in gene
expression relative to control was calculated by 2−ΔΔCT
(18).
 | Table IPrimers used to perform qPCR
analysis. |
Table I
Primers used to perform qPCR
analysis.
| mRNA name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| ABCB1 |
CCCATCATTGCAATAGCAGG |
TGTTCAAACTTCTGCTCCTGA |
| ABCC |
ATGTCACGTGGAATACCAGC |
GAAGACTGAACTCCCTTCCT |
| BCRP |
ATGTCACGTGGAATACCAGC |
GAAGACTGAACTCCCTTCCT |
| MGMT |
ACCGTTTGCGACTTGGTACTT |
GGAGCTTTATTTCGTGCAGACC |
| DNMT1 |
ACCAGGGAGAAGGACAGG |
CTCACAGACGCCACATCG |
| TP53 |
GTGGTGGTGCCCTATGAG |
TGTTCCGTCCCAGTAGATTA |
| HIF-1A |
CATCTCCATCTCCTACCCACA |
CTTTTCCTGCTCTGTTTGGTG |
| CA9 |
GCTGCTTCTGGTGCCTGTC |
GGAGCCCTCTTCTTCTGATTTA |
| Bcl2L1 |
TGGAACTCTATGGGAACAATG |
TGAGCCCAGCAGAACCAC |
| VEGFA |
TTGCCTTGCTGCTCTACC |
ATGTCCACCAGGGTCTCG |
| GAPDH |
GACCTGACCTGCCGTCTA |
AGGAGTGGGTGTCGCTGT |
Microarray profiling and data
analysis
For microarray, Arraystar Human LncRNA Microarray
V3.0 covering ~30,586 lncRNAs and 26,109 coding transcripts was
designed to detect the profile of human lncRNAs and protein-coding
transcripts. Sample labeling and array hybridization were performed
according to the Agilent One-Color Microarray-Based Gene Expression
Analysis protocol (Agilent Technology). The array images were
further analyzed by Agilent Feature Extraction software (version
11.0.1.1). Quantile normalization and subsequent data processing
were applied with GeneSpring GX v11.5.1 software (Agilent
Technologies). Distinct lncRNAs and mRNAs between U87 and U87TR
were presented by hierarchical clustering and volcano plot
filtering. The gene ontology (GO) analysis and pathway analysis
were performed in the standard enrichment computation method
according to the latest KEGG database (Kyoto Encyclopedia of Genes
and Genomes, http:/www.genome.jp/kegg).
Western blot analysis
Total protein of cells was extracted by Cell Lysis
and Protein Extraction kit (Keygen Biotech Co., China) and
concentration was measured by a BCA Protein Detection kit (Keygen
Biotech). Total protein (40 µg) was subjected to 10%
SDS-polyacrylamide gel electrophoresis and transferred to PVDF
membrane (Millipore Corp., USA). The blots were blocked for 1 h at
RT with 5% non-fat milk (Bio-Rad, USA) in Tris-buffered saline
containing 0.1% Tween-20 (TBST) and probed with following primary
antibodies: MDR1/ABCB1(E1Y7B) (142 kDa), MRP1/ABCC1(D708N) (173
kDa), ABCG (66 kDa) (Cell Signaling Technology, USA), MGMT (25
kDa), collagen I (139 kDa), fibronectin (262 kDa), laminin (198
kDa) (Abcam, USA), CD44 (82 kDa) (Abnova, USA) in 5% non-fat milk
in TBST overnight at 4°C. Anti-GAPDH antibody (37 kDa) (Cell
Signaling Technology) was used as a loading control. Subsequently,
the blots were washed in TBST and incubated with goat anti-rabbit
or mouse IgG (H+L) horseradish peroxidase-conjugated secondary
antibody (Fdbio, China) for 1 h at RT. Then the blots were washed
with TBST and visualized using Immobilon Western HRP Substrate
(Millipore).
Enzyme-linked immunosorbent assay
(ELISA)
During 3-day TMZ treatment, the culture supernatants
of U87 and U87TR cells were collected respectively and the level of
total collagen I was measured with Collagen I ELISA kit (R&D
Systems, USA). The assay was carried out as recommended by the kit
protocol.
Immunofluorescence staining
For immunofluorescence analysis, GBM cells were
seeded on glass coverslips (0.17 mm thickness, 14 mm diameter) in
6-well plate overnight and then treated with TMZ for 3 days,
respectively. After treatments, cells were performed by PBS
washing, 4% paraformaldehyde fixation (30 min), 0.1% Triton X-100
permeating (5 min) and 2% bovine serum albumin (BSA) blocking (30
min). Then the cells were incubated with anti-collagen I antibody
(1:2,000) and anti-CD44 antibody (1:1,000) diluted in 2% BSA at 4°C
overnight. After 3 times PBS rinsing, appropriate fluorescent
secondary antibodies were added to cell samples and incubated at
37°C in the dark for 1 h. Coverslips were mounted on slides using
mounting medium (Santa Cruz, USA) containing DAPI DNA counterstain.
Images were captured by a fluorescence microscopy (IX-70, Olympus,
Japan).
Statistical analysis
Results are presented as mean ± standard deviation
(SD) for three separate experiments and analyzed by SPSS 13.0
software with two-sample t-test assuming unequal variances.
p<0.05 was considered as statistically significant.
Results
TMZ-resistant phenotype in U87TR and
U251TR cells
U87 and U251 GBM cells were repetitively
pulse-exposed to increasing TMZ concentrations for 6 months until a
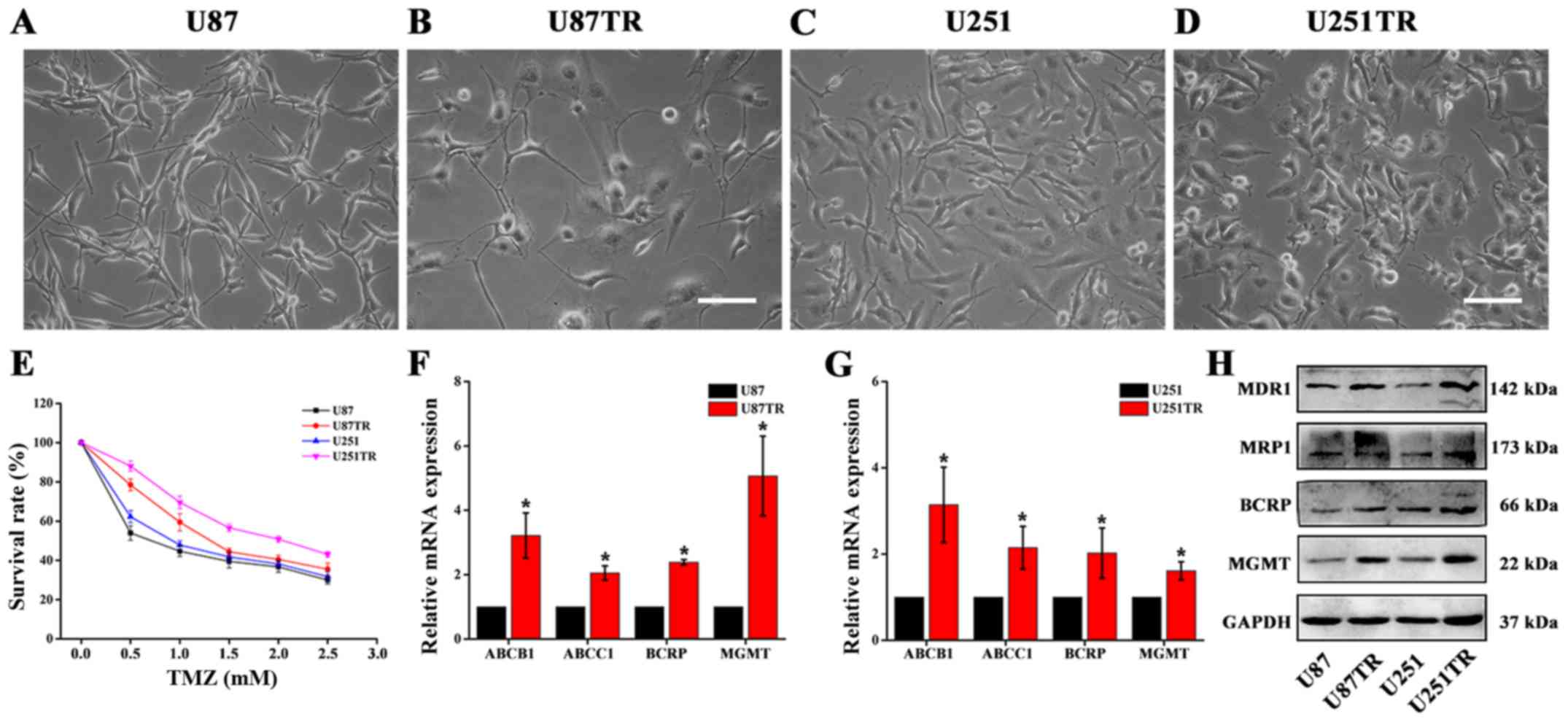
stable resistant phenotype was obtained. Through light microscopy,
we observed that the cellular morphology of U87TR and U251TR cells
differed from its parental cells with larger, irregular morphology
and long protrusions (Fig. 1A–D),
especially in U87TR cells. To examine the chemoresistant
properties, CCK-8 assay was used to characterize chemosensitivity
of these cells to TMZ. We noted that TMZ led to a
concentration-dependent decrease in both TR and parental cells and
the TR cells showed higer resistant level towards TMZ (U87TR
1.74±0.15 mM, U251TR 2.43±0.01 mM) when compared to its conterpart
parental cells (Fig. 1E).
Additionally, the expression of related multidrug-resistant (MDR)
phenotypes was also analyzed by qPCR and western blot analyses. As
shown in Fig. 1F and G, we found
the expression of ATP-binding cassette transporters (ABCB1, ABCC
and BCRP) and MGMT were significantly upregulated in TR cells and
further protein analysis comfirmed these results (Fig. 1H). Together, we demonstrated that
repetitive pulse-exposure of TMZ to GBM cells could establish
stable TR phenotype and these sublines were suitable for further
experiments.
Differential expression of lncRNAs and
mRNAs in U87TR compared to U87 cells
Arraystar probe dataset was applied to screen
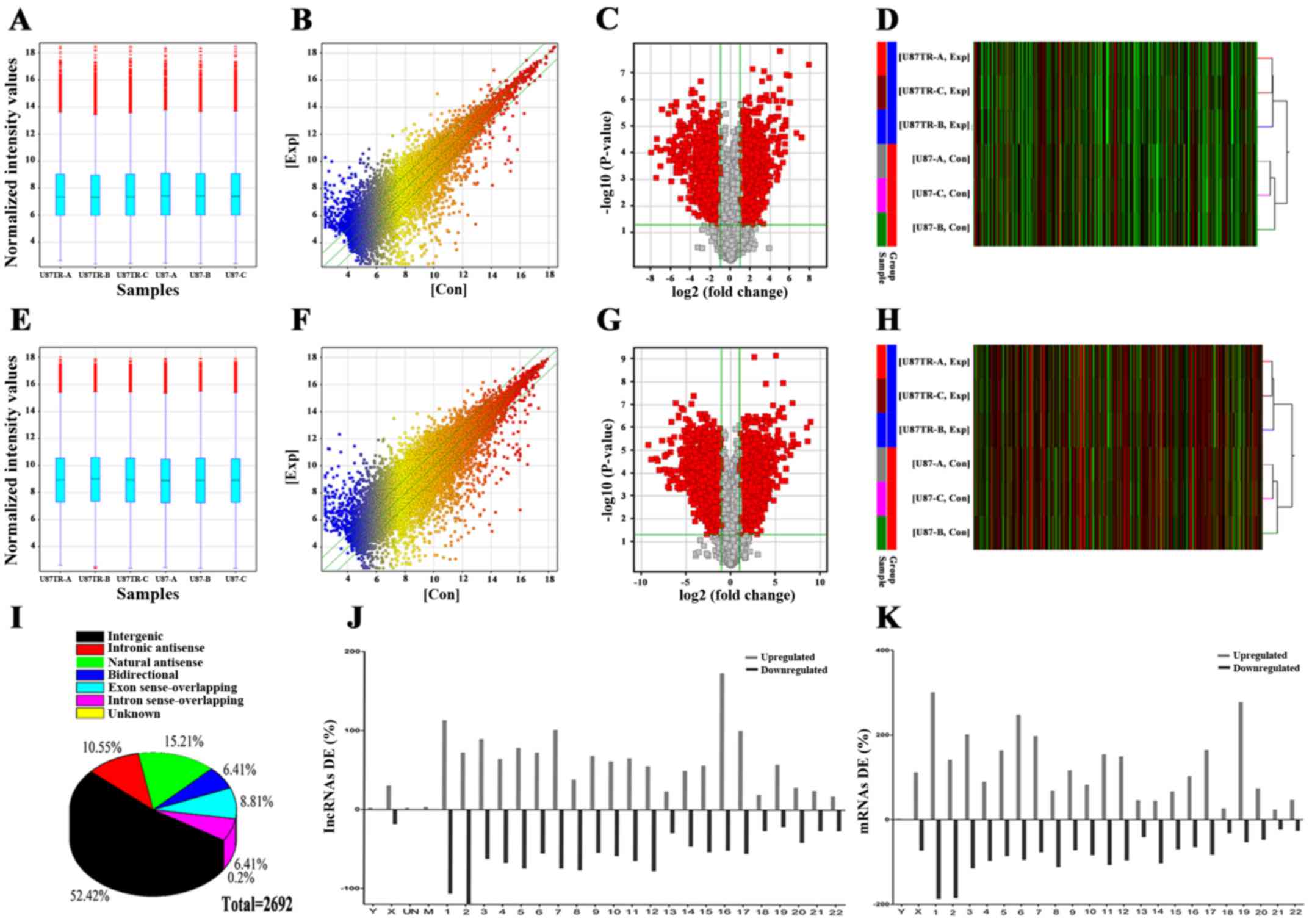
differentially expressed lncRNAs and mRNAs in U87 and U87TR cells.
After normalization and data filtering (Fig. 2A–C and E–G), we found that 2,692
distinct lncRNAs demonstrated >2-fold differential expression
with 1,383 lncRNAs upregulated and 1,309 lncRNAs downregulated
(Fig. 2D), whereas, 4,886
differential mRNAs displayed 2,933 mRNAs upregulated and 1953 mRNAs
downregulated which was shown by the hierarchical clustering (fold
change ≥2.0 and p-value ≤0.05) (Fig.
2H). The top 10 significantly and dominant dysregulated lncRNAs
and mRNAs are listed (Tables II
and III). Among the distinct
lncRNAs, there were 1,410 intergenic, 284 intronic antisense, 409
natural antisense, 173 bidirectional, 238 exon sense and 173 intron
sense-overlapping (Fig. 2I).
Additionally, the chromosomal imbalances associated with drug
resistance was analyzed and aberrantly expressed lncRNAs and mRNAs
located on chromosome 1 were uneven with 8.90% in lncRNA and 10.02%
in mRNA (Fig. 2J and K).
 | Table IITop 10 up- and downregulated lncRNAs
in U87TR cells. |
Table II
Top 10 up- and downregulated lncRNAs
in U87TR cells.
| Seqname | Gene symbol | Fold change | Chromosome
strand | Relationship | p-value | Up/down |
|---|
|
ENST00000443252 | AL132709.5 | 239.63 | chr14+ | Intergenic | 4.79E-08 | Up |
| uc010ahe.1 | BC041856 | 141.54 | chr14− | Intergenic | 2.58E-05 | Up |
| TCONS_00008977 | XLOC_003829 | 99.74 | chr4+ | Intergenic | 1.76E-05 | Up |
| TCONS_00022632 | XLOC_010933 | 64.73 | chr14+ | Intergenic | 1.27E-06 | Up |
|
ENST00000556720 | AL132709.5 | 59.33 | chr14+ | Intergenic | 1.19E-07 | Up |
|
ENST00000513211 | RP11-734I18.1 | 48.96 | chr4+ | Intergenic | 1.16E-07 | Up |
| TCONS_00027642 | XLOC_013181 | 47.55 | chr19+ | Intergenic | 9.62E-06 | Up |
|
ENST00000570409 | RP11-461A8.4 | 46.57 | chr16− | Intronic
antisense | 5.7E-06 | Up |
|
ENST00000547898 | RP11-328C8.5 | 45.69 | chr12− | Intron sense | 2.39E-05 | Up |
|
ENST00000442197 | AL132709.8 | 44.46 | chr14+ | Intergenic | 4.36E-05 | Up |
| NR_033869 | LOC401164 | 264.52 | chr4+ | Intergenic | 9.29E-05 | Down |
| NR_038848 | LOC643401 | 181.11 | chr5+ | Intergenic | 0.000837 | Down |
|
ENST00000565689 | RP11-941F15.1 | 176.55 | chr15− | Intergenic | 6.18E-05 | Down |
|
ENST00000444963 | AC018866.1 | 171.92 | chr2− | Intergenic | 2.82E-05 | Down |
| uc003jgv.2 | LOC643401 | 137.76 | chr5+ | Intergenic | 7.83E-05 | Down |
| uc003jgx.2 | LOC643401 | 136.05 | chr5+ | Intergenic | 0.000304 | Down |
|
ENST00000503458 | RP11-219G10.3 | 120.27 | chr4+ | Intergenic | 2.19E-06 | Down |
|
ENST00000421067 | RP11-83J21.3 | 90.59 | chr9+ | Intronic
antisense | 6.65E-05 | Down |
|
ENST00000421067 | RP11-83J21.3 | 90.59 | chr9+ | Intronic
antisense | 6.65E-05 | Down |
|
ENST00000578278 | RP11-146G7.3 | 77.20 | chr18− | Intergenic | 3.3E-06 | Down |
 | Table IIITop 10 up- and downregulated mRNAs in
U87TR cells. |
Table III
Top 10 up- and downregulated mRNAs in
U87TR cells.
| Gene symbol | Fold change | Up/down | p-value | Description |
|---|
| IL18 | 490.19 | Up | 5.32535E-07 | Interleukin 18
(interferon-γ-inducing factor) |
| ZNF93 | 394.98 | Up | 3.9652E-06 | Zinc finger protein
93 |
| MTAP | 371.14 | Up | 9.4726E-07 |
S-methyl-5′-thioadenosine
phosphorylase |
| ZNF254 | 182.70 | Up | 5.17967E-06 | Zinc finger protein
254 |
| SOX2 | 129.30 | Up | 3.85212E-06 | Transcription
factor SOX-2 |
| ZNF765 | 121.16 | Up | 2.0173E-06 | Zinc finger protein
765 |
| ZNF845 | 120.54 | Up | 6.692E-06 | Zinc finger protein
845 |
| ZNF611 | 118.25 | Up | 8.22434E-08 | Zinc finger protein
611 |
| GPR160 | 106.29 | Up | 5.8696E-06 | Probable G-protein
coupled receptor 160 |
| ZNF675 | 99.27 | Up | 1.49803E-05 | Zinc finger protein
675 |
| TFPI2 | 607.04 | Down | 5.48207E-06 | Tissue factor
pathway inhibitor 2 precursor |
| BAALC | 471.68 | Down | 2.4074E-05 | Brain and acute
leukemia cytoplasmic protein 2 |
| DPP4 | 330.97 | Down | 2.21878E-05 | Dipeptidyl
peptidase 4 |
| AHR | 292.93 | Down | 2.83673E-05 | Aryl hydrocarbon
receptor precursor |
| KYNU | 255.58 | Down | 6.99952E-05 | Kynureninase
isoform b |
| BDKRB1 | 206.12 | Down | 3.58205E-06 | B1 bradykinin
receptor |
| FOXD1 | 168.72 | Down | 6.46324E-05 | Forkhead box
protein D1 |
| EREG | 165.81 | Down | 1.73919E-05 | Proepiregulin
preproprotein |
| KYNU | 161.95 | Down | 1.72429E-05 | Kynureninase
isoform a |
| DCN | 142.10 | Down | 1.4439E-05 | Decorin isoform b
precursor |
LncRNAs classification and subgroup
analysis
For further investigation of potential-function of
lncRNAs, lncRNAs classification and subgroup analysis were
conducted. According to the Gencode annotation, lncRNAs were
devided into enhancer-like lncRNAs, large intergenic non-coding
RNAs (lincRNAs) and HOX lncRNAs. In enhancer lncRNA profiling, we
found 108 distinct enhancer lncRNAs with 266 nearby coding genes
transcription (distance <300 kb) and among these lncRNA-mRNA
relationships, up-up direction (87 pairs), up-down direction (67
pairs), down-up direction (56 pairs), down-down direction (56
pairs) (Table IV). However, in
lincRNA profiling, there were 809 distinct lincRNAs with 1,918
differentially expressed nearby coding genes (distance <300 kb)
including up-up direction (814 pairs), up-down direction (272
pairs), down-up direction (394 pairs) and down-down direction (438
pairs) of lncRNA-mRNA relationship (Table V). The data also showed 125 HOX
cluster transcribed regions in the four human HOX loci of both
lncRNAs and coding transcripts including 48 coding transcripts and
77 non-coding transcripts (Table
VI).
 | Table IVTop 10 distinct enhancer lncRNAs near
the coding gene data. |
Table IV
Top 10 distinct enhancer lncRNAs near
the coding gene data.
| Gene symbol | Fold change | Regulation -
lncRNAs | Genome
relationship | Nearby gene | Fold change | Regulation -
mRNAs |
|---|
| RP11-346D6.6 | 39.582054 | Up | Downstream | PRKG1 | 38.59571 | Down |
| RP11-346D6.6 | 39.582054 | Up | Downstream | PRKG1 | 10.437759 | Down |
| RP11-346D6.6 | 39.582054 | Up | Downstream | DKK1 | 2.0498126 | Up |
| RP4-737E23.2 | 39.0317 | Down | Downstream | NXT1 | 2.1266758 | Up |
| RP4-737E23.2 | 39.0317 | Down | Downstream | GZF1 | 2.2363193 | Up |
| AX746690 | 28.182703 | Up | Upstream | ADIG | 5.036083 | Down |
| RP13-16H11.2 | 27.392548 | Down | Upstream | ABI1 | 2.0226862 | Down |
| RP13-16H11.2 | 27.392548 | Down | Upstream | ABI1 | 2.0447593 | Down |
| RP13-16H11.2 | 27.392548 | Down | Upstream | ABI1 | 2.2119737 | Down |
| RP11-117P22.1 | 25.868437 | Up | Upstream | AKR1C1 | 5.1898365 | Down |
| RP11-445H22.4 | 20.70799 | Down | Downstream | ADA | 2.115311 | Down |
| RP11-445H22.4 | 20.70799 | Down | Downstream | PKIG | 2.4809623 | Down |
| RP11-445H22.4 | 20.70799 | Down | Upstream | WISP2 | 2.2126765 | Down |
|
XXyac-YM21GA2.4 | 20.248714 | Down | Upstream | CTSL1 | 4.7252097 | Down |
| RP11-160A10.2 | 15.469184 | Down | Upstream | CLVS2 | 8.49382 | Down |
| LOC285758 | 14.293567 | Up | Downstream | MARCKS | 2.3576546 | Up |
| LOC285758 | 14.293567 | Up | Upstream | HDAC2 | 2.666347 | Up |
| RP11-14N7.2 | 13.172412 | Down | Upstream | NBPF16 | 2.5870576 | Up |
 | Table VTop 10 distinct lincRNAs near the
coding gene data. |
Table V
Top 10 distinct lincRNAs near the
coding gene data.
| Gene symbol | Fold change | Regulation
lncRNAs | Genome
relationship | Nearby gene | Fold change
mRNAs |
|---|
| RP11-941F15.1 | 176.5588 | Down | Downstream | CD276 | 3.1752949 |
| RP11-146G7.3 | 77.20038 | Down | Downstream | ARHGAP28 | 5.5441136 |
| RP11-554A11.4 | 64.153145 | Down | Downstream | CPT1A | 3.7187796 |
| RP11-554A11.4 | 64.153145 | Down | Downstream | CPT1A | 2.3309202 |
| RP11-554A11.4 | 64.153145 | Down | Upstream | MRGPRF | 2.9983652 |
| RP11-113C12.3 | 52.048756 | Down | Upstream | C3AR1 | 4.6431336 |
| XLOC_013181 | 47.557842 | Up | Downstream | ZNF8 | 3.150816 |
| XLOC_013181 | 47.557842 | Up | Downstream | TRIM28 | 2.829188 |
| XLOC_013181 | 47.557842 | Up | Downstream | ZNF324 | 2.4305477 |
| XLOC_013181 | 47.557842 | Up | Upstream | ZNF544 | 30.045504 |
| XLOC_013181 | 47.557842 | Up | Upstream | ZNF274 | 2.003165 |
| XLOC_013181 | 47.557842 | Up | Upstream | ZNF274 | 4.3925347 |
| PRORSD1P | 46.21162 | Down | Upstream | RTN4 | 2.7800567 |
| PRORSD1P | 46.21162 | Down | Upstream | RTN4 | 2.2093842 |
| PRORSD1P | 46.21162 | Down | Upstream | CLHC1 | 2.0670087 |
| PRORSD1P | 46.21162 | Down | Upstream | RTN4 | 3.4394581 |
| AC003092.1 | 43.996338 | Down | Upstream | BET1 | 2.096403 |
| AC003092.1 | 43.996338 | Down | Upstream | TFPI2 | 607.0495 |
| AK123141 | 43.92919 | Up | Downstream | ZNF680 | 5.060253 |
| AK123141 | 43.92919 | Up | Downstream | ZNF680 | 29.15355 |
| AK123141 | 43.92919 | Up | Upstream | ZNF736 | 11.060797 |
| AK123141 | 43.92919 | Up | Upstream | ZNF679 | 3.0256562 |
| AK123141 | 43.92919 | Up | Upstream | ZNF727 | 39.63797 |
| AK123141 | 43.92919 | Up | Upstream | ZNF735 | 4.4493775 |
| RP11-346D6.6 | 39.582054 | Up | Downstream | PRKG1 | 38.59571 |
| RP11-346D6.6 | 39.582054 | Up | Downstream | PRKG1 | 10.437759 |
| RP11-346D6.6 | 39.582054 | Up | Downstream | DKK1 | 2.0498126 |
| RP4-737E23.2 | 39.0317 | Down | Downstream | NXT1 | 2.1266758 |
| RP4-737E23.2 | 39.0317 | Down | Downstream | GZF1 | 2.2363193 |
 | Table VIHOX cluster profiling (part). |
Table VI
HOX cluster profiling (part).
| Probe name | Seqname | Gene symbol | Product |
|---|
| ASHGA5P021981 | NM_014212 | HOXC11 | Homeobox protein
Hox-C11 |
| ASHGA5P053003 | NM_006735 | HOXA2 | Homeobox protein
Hox-A2 |
| ASHGA5P005899 | NM_024017 | HOXB9 | Homeobox protein
Hox-B9 |
| ASHGA5P032505 | NM_024015 | HOXB4 | Homeobox protein
Hox-B4 |
| ASHGA5P036264 | NM_002148 | HOXD10 | Homeobox protein
Hox-D10 |
| ASHGA5P053006 | NM_006896 | HOXA7 | Homeobox protein
Hox-A7 |
| ASHGA5P036265 | NM_014213 | HOXD9 | Homeobox protein
Hox-D9 |
| ASHGA5P032508 | NM_032391 | PRAC | small nuclear
protein PRAC |
| ASHGA5P032507 | NM_004502 | HOXB7 | Homeobox protein
Hox-B7 |
| ASHGA5P036262 | NM_021192 | HOXD11 | Homeobox protein
Hox-D11 |
| ASHGA5P042956 | NM_024014 | HOXA6 | Homeobox protein
Hox-A6 |
| ASHGA5P028030 | NM_017410 | HOXC13 | Homeobox protein
Hox-C13 |
| ASHGA5P001267 | NM_018952 | HOXB6 | Homeobox protein
Hox-B6 |
| ASHGA5P055442 | NM_153693 | HOXC6 | Homeobox protein
Hox-C6 isoform 2 |
| ASHGA5P042958 | NM_005523 | HOXA11 | Homeobox protein
Hox-A11 |
| ASHGA5P006323 | NM_030661 | HOXA3 | Homeobox protein
Hox-A3 isoform a |
GO and pathway analysis of differentially
expressed mRNAs
Previous studies have revealed that the coding and
non-coding RNA can interact with each other in gene expression and
dictate final protein output (19,20).
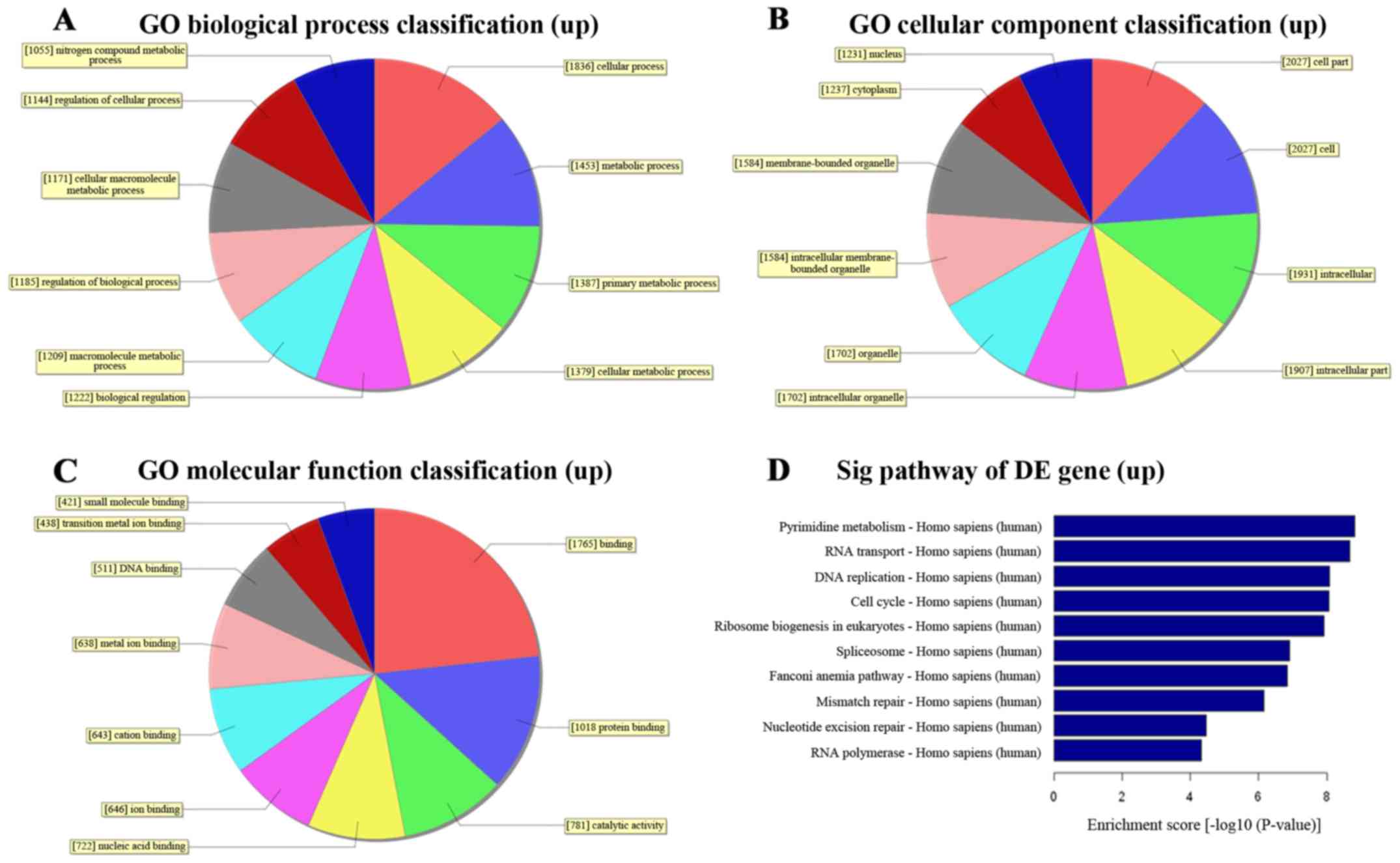
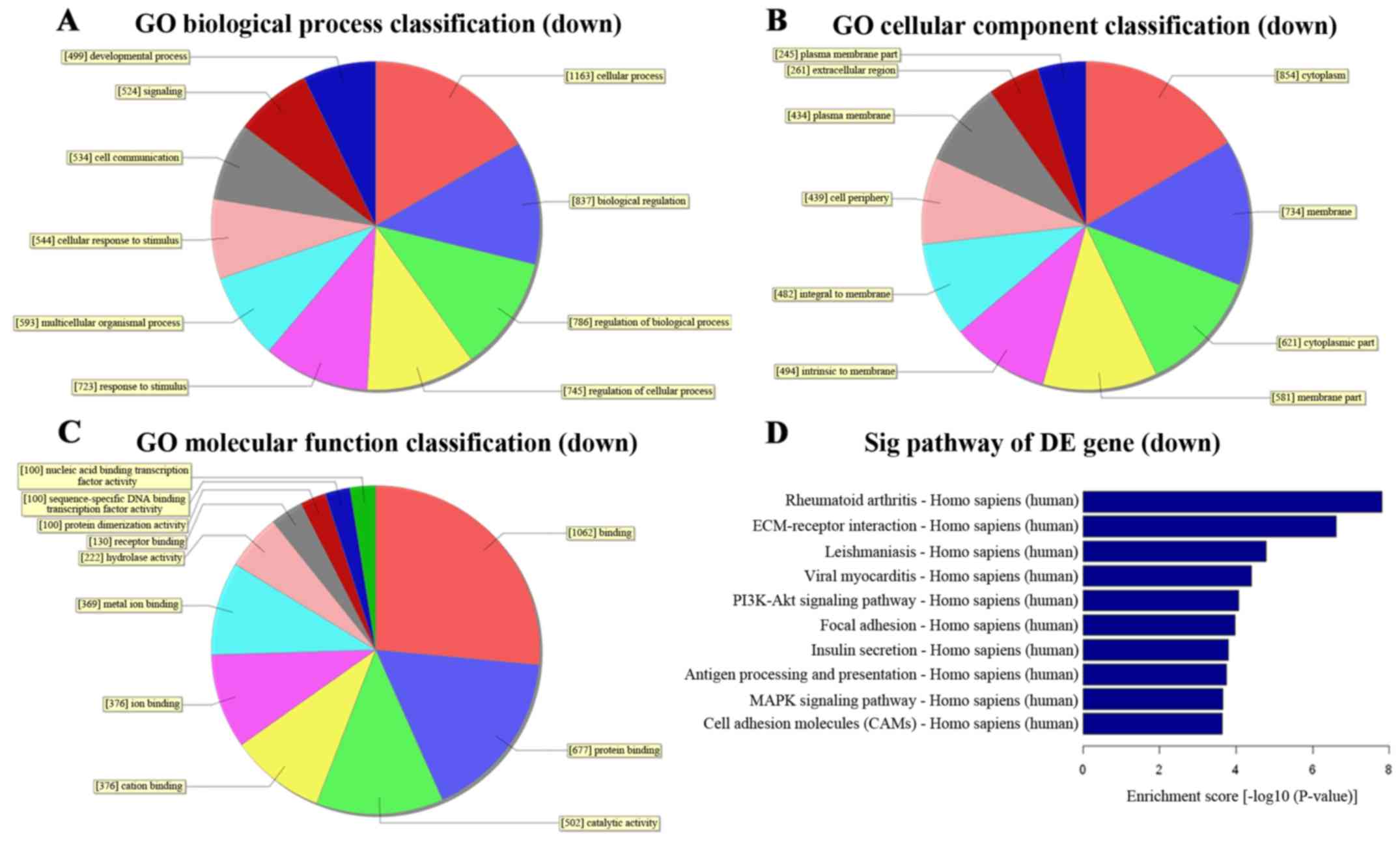
To better understand the function of distinct lncRNAs, we first
performed GO function analysis associating differentially expressed
mRNAs with GO categories. The GO categories were generally
comprised of 3 structured networks: biological processes, cellular
components and molecular function (21). In our study, the differentially
expressed mRNAs were mainly enriched for GO terms related to the
nucleic acid metabolic process and response to chemical stimulus
involved in biological processes, nucleus and extracellular region
part involved in cellular components as well as nucleic acid
binding and protein binding involved in molecular function
(Figs. 3A–C and 4A–C).
To identify significant pathways associated with TMZ
resistance, pathway analysis was applied for the differentially
expressed mRNAs. We found a total of 97 pathways that showed
significant differences with 28 upregulated and 69 downregulated
pathways. The top 3 upregulated pathways were pyrimidine
metabolism, RNA transport and DNA replication signaling while the
top 3 downregulated pathways were rheumatoid arthritis,
ECM-receptor interaction and leishmaniasis signaling. The
predominant pathways are shown in (Figs. 3D and 4D) and it is noteworthy that the
validated MMR and NER pathways, which associated with TMZ
resistance, were upregulated with the false discovery rate of
Pathway ID at 2.298×10−5 and 9.769×10−4,
respectively (Table VII).
 | Table VIISignificant pathways associated with
TMZ resistance. |
Table VII
Significant pathways associated with
TMZ resistance.
| Pathway ID | Definition | Fisher p-value | FDR | Enrichment
score | Genes |
|---|
| hsa03430 | Mismatch repair
(MMR) | 7E-07 | 2E-05 | 6.16 |
EXO1/LIG1/MLH1/MSH3/PCNA/POLD2/POLD3/RFC2/RFC3/RFC4/RFC5/RPA1/RPA2 |
| hsa03420 | Nucleotide excision
repair (NER) | 3E-05 | 0.001 | 4.48 |
ERCC1/ERCC2/ERCC3/ERCC8/POLD3/POLE/POLE2/RFC2/RFC3/RFC4/RFC5/RPA1/RPA2 |
| hsa04512 | ECM-receptor
interaction | 2E-07 | 3E-05 | 6.60 |
CD44/CD47/COL1A1/COL1A2/COL3A1/COL4A5/COL5A1/COL5A2/COL6A1/COL6A2/COL6A3/FN1/HSPG2/ITGA1/ITGA11/ITGA5/ITGAV/ITGB1/LAMA2/LAMA4/LAMC1/LAMC2/SPP1 |
Downregulation of ECM-receptor
interaction pathway associated with TR phenotype
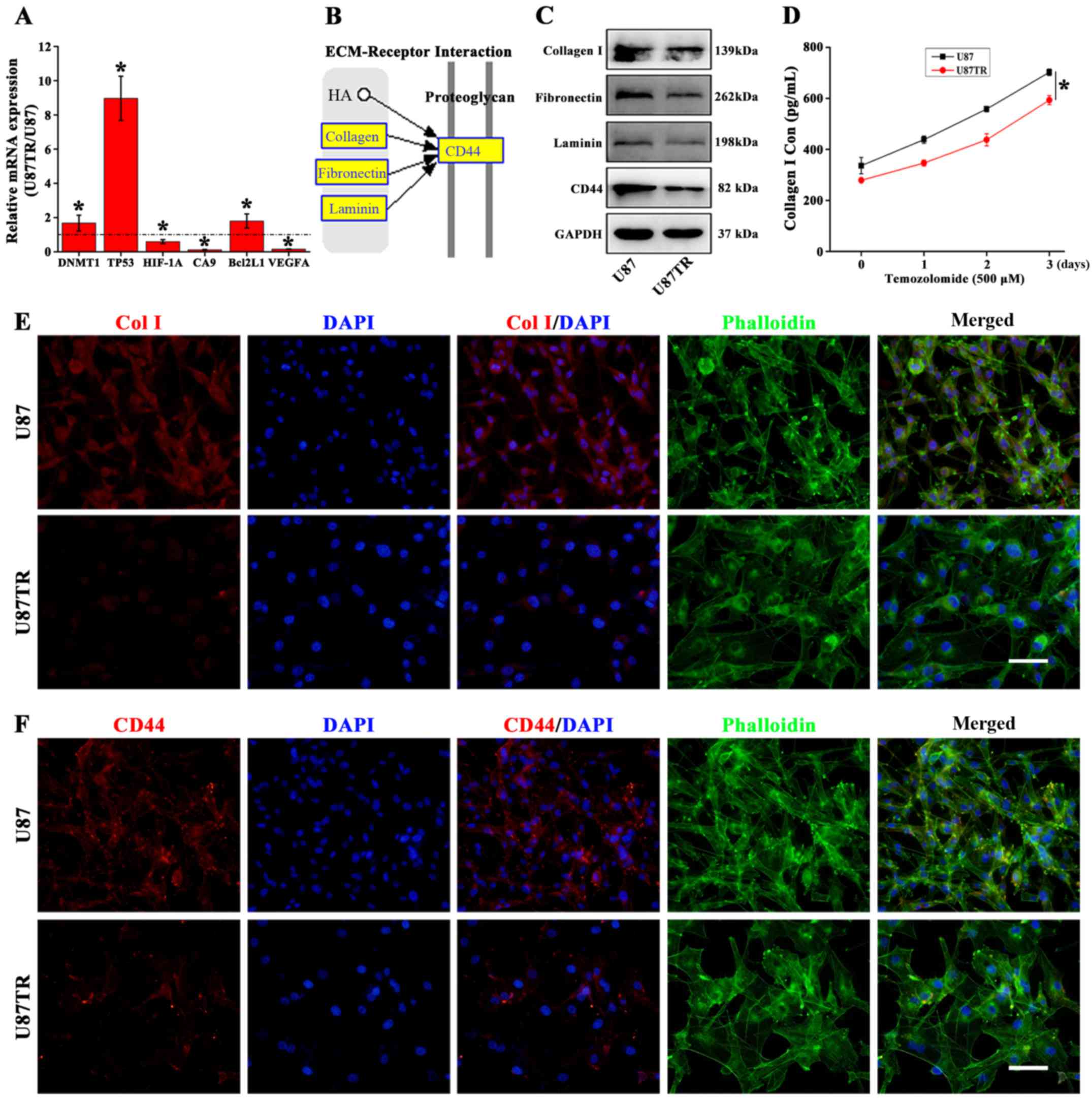
For microarray profile validation, six mRNAs (DNMT1,
TP53, HIF-1A, CA9, Bcl2L1 and VEGFA) were randomly selected and
performed for qPCR analysis in U87 and U87TR cells. Results showed
that the DNMT1, TP53 and Bcl2L1 were significantly upregulated
while the HIF-1A, CA9 and VEGFA were downregulated compared to the
parental U87 cells (Fig. 5A). With
the distinct cellular morphology (Fig.
1) and downregulation of ECM-related pathway (Fig. 4D) in TR cells, we speculated that
the ECM-related cellular morphology alterations may associate with
the TR properties. In the downregulated ECM-receptor interaction
pathway, we found collagen, integrin and laminin expression
downregulated with cell-surface glycoprotein CD44 and CD47
(Table VII). Moreover, the ECM
related receptor interaction from the KEGG Pathway Database
revealed that collagen, fibronectin and laminin could enhance its
downstream CD44 expression (Fig.
5B). To verify this, western blot analysis was applied and the
results showed the protein expression of collagen I, fibronectin,
laminin and CD44 were significantly decreased in U87TR cells as
compared to parental U87 cells (Fig.
5C). To comfirm this, ELISA assay was used to analyze the
collagen I expression in TMZ treated U87 and U87TR cell
supernatants during 3 days. The results showed U87TR cells with
significant downregulated collagen I expression when compared to
the parental U87 cells during TMZ treatment (Fig. 5D). In addition, the expression of
collagen I and CD44 were further confirmed by immunofluorescence
staining. Data showed that the U87TR cells presented with relative
larger and irregular cytoskeleton (phalloidin in green), decreased
secretion of collagen I (Fig. 5E)
and weaker CD44 expression (Fig.
5F). Together, these results indicated that the TR phenotype
was associated with the downregulation of ECM signaling and
ECM-related collagen or CD44 may act as TR phenotype molecular
markers.
Discussion
The emergence of acquired drug resistance in tumor
constantly leads to chemotherapy faliure or even tumor relapse.
Thus, fully understanding its mechanisms is urgent for improving
effective chemotherapy and overcoming tumor drug resistance. Here,
we sought to explore the mechanisms of acquired resistance to TMZ
in GBM through in vitro TMZ-resistant GBM cell lines
generated by repetitive exposure to increasing TMZ concentrations.
Although this approach may not closely reflect the situation in
vivo, it allows us for an assessment of mechanisms triggered by
repeated pulse-exposure to TMZ chemotherapy in vitro.
Previous studies have indicated a growing number of
molecular mechanisms contributing to TMZ resistance in GBM
including genetic and epigenetic, such as MGMT methylation
(22), IDH mutations (23), aberrant ABC transporter expression
(24,25), p53 mutations and deletions
(26), DNA repair deregulation
(27,28) and miRNAs (29,30).
Furthermore, evidence is now beginning to demonstrate lncRNAs as
having important roles in cancer therapy (31,32).
However, the relationships between lncRNAs and GBM acquired TMZ
resistance are rarely reported. In our study, the TMZ-resistant GBM
cell lines were first generated using stepwise selection and then
subjected to the Human lncRNA microarray. We found numerous
distinctly expressed lncRNAs and mRNAs with up- or downregulation
(Tables II and III). To our best knowledge, these
results may be the first reporting on expression profile of lncRNAs
and mRNAs associated with TMZ resistance in GBM cells in
vitro.
For preliminary understanding upon these
differential expressed lncRNAs and mRNAs towards TMZ resistance,
further functional analysis was processed. In lncRNA classification
and subgroup analysis, the lncRNAs were devided into three types,
the enhancer-like lncRNAs, lincRNAs and HOX lncRNAs, and each of
the function pattern was distinct. For example, lincRNAs regulate
the neighboring HOX gene expression via impacting chromatin
signature (33) while
enhancer-like lncRNAs function by interacting with their nearby
coding genes (34). Thus the exact
function of lncRNA clusters is not yet clear before every single
lncRNA is identified and still need further studies. According to
previous studies, we also found some distinct lncRNAs in our study,
which are consistent with other researchers, for example, the
lncRNA CRNDE (7), UCA-1 (6,35),
MEG3 (31,36) and HOTAIR (37). These suggest that the data obtained
in this study are reliable. Additionally, lncRNAs were also
reported to function in tumor drug resistance through coding
transcription modulation (38). In
our study, some function of molecules in the drug resistance
related MMR and NER signaling pathway were upregulated in U87TR
cells, e.g., MSH3 in MMR and ERCC1/2 in NER signaling, which were
revealed by the pathway analysis on mRNAs and were consistent with
previous reports (39,40).
With the great morphologic changes and
downregulation of ECM-receptor interaction pathway in TR cells as
compared with their parental cells, we speculate that the cell
morphology change may associate with drug resistance phenotype.
Previous reports demonstrated that drug resistance acquisition
showed statistically significant morphological changes in cell
membrane as well as biological parameters (41). Chemotherapy-induced phenotypic
reversion of cancer cells is accompanied by regression of several
morphological malignant features (42). Here, we have confirmed some
important molecules in ECM-receptor interaction pathway, e.g., the
collagen, fibronectin, laminin and CD44 were associated with TMZ
resistance phenotype. In this study, we showed that the collagen,
fibronectin and laminin could enhance its downstream CD44
expression in U87TR cells according to the KEGG pathway database.
Chetty et al reported that the CD44-mediated cell matrix
interaction in glioma perivascular niches enhances cancer stem cell
phenotype and promote tumor aggressiveness (43). Moreover, recent studies have shown
that the high level of CD44 was associated with a better survial
and better response to radiotherapy and TMZ and the CD44 could
establish a prognosis marker by predicting survival and response to
therapy for GBM patients (44,45).
In our study, the CD44 expression was downregulated in U87TR cells
and targeting CD44 expression might enhance the TMZ
chemosensitivity to TR cells. However, its role and underlying
mechanisms are not fully understood and more effort is still needed
for further studies. Therefore, these data suggest that acquired
TMZ resistance might be mirrored by the parallel changes in
cellular morphology associated with CD44 expression.
In conclusion, we showed differential lncRNAs and
mRNAs expression profiles associated with TMZ resistance in GBM
cells in vitro, and these dysregulated lncRNAs and mRNAs
identified in this work may represent good candidates for future
diagnostic or prognostic biomarkers and provide novel targets for
overcoming acquired TMZ resistance in GBM chemotherapy.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (81672477, 81372691, 81041068
and 30971183) and Guangdong Provincial Clinical Medical Centre for
Neurosurgery (no. 2013B020400005). We thank the Department of
Anatomy, Key Laboratory of Construction and Detection of Guangdong
Province, Southern Medical University, Guangzhou, for generous
help.
References
|
1
|
Thomas AA, Brennan CW, DeAngelis LM and
Omuro AM: Emerging therapies for glioblastoma. JAMA Neurol.
71:1437–1444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Preusser M, de Ribaupierre S, Wöhrer A,
Erridge SC, Hegi M, Weller M and Stupp R: Current concepts and
management of glioblastoma. Ann Neurol. 70:9–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stavrovskaya AA, Shushanov SS and
Rybalkina EY: Problems of glioblastoma multiforme drug resistance.
Biochemistry (Mosc). 81:91–100. 2016. View Article : Google Scholar
|
|
4
|
Sarkaria JN, Kitange GJ, James CD, Plummer
R, Calvert H, Weller M and Wick W: Mechanisms of chemoresistance to
alkylating agents in malignant glioma. Clin Cancer Res.
14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xue M, Chen W and Li X: Urothelial cancer
associated 1: A long noncoding RNA with a crucial role in cancer. J
Cancer Res Clin Oncol. 142:1407–1419. 2016. View Article : Google Scholar
|
|
7
|
Wang Y, Wang Y, Li J, Zhang Y, Yin H and
Han B: CRNDE, a long-noncoding RNA, promotes glioma cell growth and
invasion through mTOR signaling. Cancer Lett. 367:122–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni B, Yu X, Guo X, Fan X, Yang Z, Wu P,
Yuan Z, Deng Y, Wang J, Chen D, et al: Increased urothelial cancer
associated 1 is associated with tumor proliferation and metastasis
and predicts poor prognosis in colorectal cancer. Int J Oncol.
47:1329–1338. 2015.PubMed/NCBI
|
|
9
|
Zhang JY, Weng MZ, Song FB, Xu YG, Liu Q,
Wu JY, Qin J, Jin T and Xu JM: Long noncoding RNA AFAP1-AS1
indicates a poor prognosis of hepatocellular carcinoma and promotes
cell proliferation and invasion via upregulation of the RhoA/Rac2
signaling. Int J Oncol. 48:1590–1598. 2016.PubMed/NCBI
|
|
10
|
Sun QL, Zhao CP, Wang TY, Hao XB, Wang XY,
Zhang X and Li YC: Expression profile analysis of long non-coding
RNA associated with vincristine resistance in colon cancer cells by
next-generation sequencing. Gene. 572:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parasramka MA, Maji S, Matsuda A, Yan IK
and Patel T: Long non-coding RNAs as novel targets for therapy in
hepatocellular carcinoma. Pharmacol Ther. 161:67–78. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He DX, Zhang GY, Gu XT, Mao AQ, Lu CX, Jin
J, Liu DQ and Ma X: Genome-wide profiling of long non-coding RNA
expression patterns in anthracycline-resistant breast cancer cells.
Int J Oncol. 49:1695–1703. 2016.PubMed/NCBI
|
|
13
|
Pan J, Li X, Wu W, Xue M, Hou H, Zhai W
and Chen W: Long non-coding RNA UCA1 promotes cisplatin/gemcitabine
resistance through CREB modulating miR-196a-5p in bladder cancer
cells. Cancer Lett. 382:64–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Zhai L, Wang H, Liu C, Zhang J, Chen
W and Wei Q: Downregulation of LncRNA GAS5 causes trastuzumab
resistance in breast cancer. Oncotarget. 7:27778–27786.
2016.PubMed/NCBI
|
|
15
|
Munoz JL, Rodriguez-Cruz V, Greco SJ,
Ramkissoon SH, Ligon KL and Rameshwar P: Temozolomide resistance in
glioblastoma cells occurs partly through epidermal growth factor
receptor-mediated induction of connexin 43. Cell Death Dis.
5:e11452014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Munoz JL, Rodriguez-Cruz V, Greco SJ,
Nagula V, Scotto KW and Rameshwar P: Temozolomide induces the
production of epidermal growth factor to regulate MDR1 expression
in glioblastoma cells. Mol Cancer Ther. 13:2399–2411. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Munoz JL, Walker ND, Scotto KW and
Rameshwar P: Temozolomide competes for P-glycoprotein and
contributes to chemoresistance in glioblastoma cells. Cancer Lett.
367:69–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Guil S and Esteller M: RNA-RNA
interactions in gene regulation: The coding and noncoding players.
Trends Biochem Sci. 40:248–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Y, Zhang L, Jiang Y, Xu T, Mei Q, Wang
H, Qin R, Zou Y, Hu G, Chen J, et al: LncRNA and mRNA interaction
study based on transcriptome profiles reveals potential core genes
in the pathogenesis of human glioblastoma multiforme. J Cancer Res
Clin Oncol. 141:827–838. 2015. View Article : Google Scholar
|
|
21
|
Cao G, Zhang J, Wang M, Song X, Liu W, Mao
C and Lv C: Differential expression of long non-coding RNAs in
bleomycin-induced lung fibrosis. Int J Mol Med. 32:355–364.
2013.PubMed/NCBI
|
|
22
|
Fan CH, Liu WL, Cao H, Wen C, Chen L and
Jiang G: O6-methylguanine DNA methyltransferase as a
promising target for the treatment of temozolomide-resistant
gliomas. Cell Death Dis. 4:e8762013. View Article : Google Scholar
|
|
23
|
Hartmann C, Hentschel B, Simon M, Westphal
M, Schackert G, Tonn JC, Loeffler M, Reifenberger G, Pietsch T, von
Deimling A, et al: German Glioma Network: Long-term survival in
primary glioblastoma with versus without isocitrate dehydrogenase
mutations. Clin Cancer Res. 19:5146–5157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schaich M, Kestel L, Pfirrmann M, Robel K,
Illmer T, Kramer M, Dill C, Ehninger G, Schackert G and Krex D: A
MDR1 (ABCB1) gene single nucleotide polymorphism predicts outcome
of temozolomide treatment in glioblastoma patients. Ann Oncol.
20:175–181. 2009. View Article : Google Scholar
|
|
25
|
Lin F, de Gooijer MC, Roig EM, Buil LC,
Christner SM, Beumer JH, Würdinger T, Beijnen JH and van Tellingen
O: ABCB1, ABCG2, and PTEN determine the response of glioblastoma to
temozolomide and ABT-888 therapy. Clin Cancer Res. 20:2703–2713.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YY, Zhang T, Li SW, Qian TY, Fan X,
Peng XX, Ma J, Wang L and Jiang T: Mapping p53 mutations in
low-grade glioma: A voxel-based neuroimaging analysis. AJNR Am J
Neuroradiol. 36:70–76. 2015. View Article : Google Scholar
|
|
27
|
Turner KM, Sun Y, Ji P, Granberg KJ,
Bernard B, Hu L, Cogdell DE, Zhou X, Yli-Harja O, Nykter M, et al:
Genomically amplified Akt3 activates DNA repair pathway and
promotes glioma progression. Proc Natl Acad Sci USA. 112:3421–3426.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trivedi RN, Almeida KH, Fornsaglio JL,
Schamus S and Sobol RW: The role of base excision repair in the
sensitivity and resistance to temozolomide-mediated cell death.
Cancer Res. 65:6394–6400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong ST, Zhang XQ, Zhuang JT, Chan HL, Li
CH and Leung GK: MicroRNA-21 inhibition enhances in vitro
chemosensitivity of temozolomide-resistant glioblastoma cells.
Anticancer Res. 32:2835–2841. 2012.PubMed/NCBI
|
|
30
|
Ujifuku K, Mitsutake N, Takakura S,
Matsuse M, Saenko V, Suzuki K, Hayashi K, Matsuo T, Kamada K,
Nagata I, et al: miR-195, miR-455-3p and miR-10a(*) are implicated
in acquired temozolomide resistance in glioblastoma multiforme
cells. Cancer Lett. 296:241–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P,
Lu B, Liu G and Wang Z: The long noncoding RNA MEG3 contributes to
cisplatin resistance of human lung adenocarcinoma. PLoS One.
10:e01145862015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsang WP, Wong TW, Cheung AH, Co CN and
Kwok TT: Induction of drug resistance and transformation in human
cancer cells by the noncoding RNA CUDR. RNA. 13:890–898. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng N, Li X, Zhao C, Ren S, Chen X, Cai
W, Zhao M, Zhang Y, Li J, Wang Q, et al: Microarray expression
profile of long non-coding RNAs in EGFR-TKIs resistance of human
non-small cell lung cancer. Oncol Rep. 33:833–839. 2015.
|
|
35
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: Long non-coding RNA UCA1 increases chemoresistance of
bladder cancer cells by regulating Wnt signaling. FEBS J.
281:1750–1758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Bian EB, He XJ, Ma CC, Zong G, Wang
HL and Zhao B: Epigenetic repression of long non-coding RNA MEG3
mediated by DNMT1 represses the p53 pathway in gliomas. Int J
Oncol. 48:723–733. 2016.
|
|
37
|
Zhang J, Zhang P, Wang L, Piao HL and Ma
L: Long non-coding RNA HOTAIR in carcinogenesis and metastasis.
Acta Biochim Biophys Sin (Shanghai). 46:1–5. 2014. View Article : Google Scholar
|
|
38
|
Xia H and Hui KM: Mechanism of cancer drug
resistance and the involvement of noncoding RNAs. Curr Med Chem.
21:3029–3041. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Idbaih A, Carvalho Silva R, Crinière E,
Marie Y, Carpentier C, Boisselier B, Taillibert S, Rousseau A,
Mokhtari K, Ducray F, et al: Genomic changes in progression of
low-grade gliomas. J Neurooncol. 90:133–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Geng P, Ou J, Li J, Liao Y, Wang N, Xie G,
Sa R, Liu C, Xiang L and Liang H: A comprehensive analysis of
influence ERCC polymorphisms confer on the development of brain
tumors. Mol Neurobiol. 53:2705–2714. 2016. View Article : Google Scholar
|
|
41
|
Pasqualato A, Palombo A, Cucina A,
Mariggiò MA, Galli L, Passaro D, Dinicola S, Proietti S, D'Anselmi
F, Coluccia P, et al: Quantitative shape analysis of chemoresistant
colon cancer cells: Correlation between morphotype and phenotype.
Exp Cell Res. 318:835–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uppal SO, Li Y, Wendt E, Cayer ML, Barnes
J, Conway D, Boudreau N and Heckman CA: Pattern analysis of
microtubule-polymerizing and -depolymerizing agent combinations as
cancer chemotherapies. Int J Oncol. 31:1281–1291. 2007.PubMed/NCBI
|
|
43
|
Chetty C, Vanamala SK, Gondi CS, Dinh DH,
Gujrati M and Rao JS: MMP-9 induces CD44 cleavage and CD44 mediated
cell migration in glioblastoma xenograft cells. Cell Signal.
24:549–559. 2012. View Article : Google Scholar :
|
|
44
|
Guadagno E, Borrelli G, Califano M, Calì
G, Solari D and Del Basso De Caro M: Immunohistochemical expression
of stem cell markers CD44 and nestin in glioblastomas: Evaluation
of their prognostic significance. Pathol Res Pract. 212:825–832.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pinel B, Duchesne M, Godet J, Milin S,
Berger A, Wager M and Karayan-Tapon L: Mesenchymal subtype of
glioblastomas with high DNA-PKcs expression is associated with
better response to radiotherapy and temozolomide. J Neurooncol.
132:287–294. 2017. View Article : Google Scholar : PubMed/NCBI
|