Introduction
Colorectal cancer (CRC) is one of the most common
malignancies of gastrointestinal system (1). Although the treatment of CRC has been
developed substantially in the past decades, the prognosis of CRC
remains unsatisfactory (2,3). Therefore, there is still an urgent
clinical need to explore new and effective drugs for the treatment
of CRC.
The traditional medicine (TM) and their derivatives
is one of the most important source for anticancer drugs and
multiple drugs from TM have been used for the treatment of CRC for
decades, such as camptothecin, vincristine and paclitaxel (4–6).
Honokiol (HNK) is a biphenolic natural product, which was extracted
from the bark and leaves of Magnoliaplant spp (7). During the past decades, HNK has been
extensively studied for its multiple pharmacological activities
against several diseases such as anticancer, anti-oxidative,
anti-angiogenesis, anti-inflammatory and inhibition the
transformation of malignant papillae to carcinomas in vitro
and in vivo assays (8,9). HNK
may exerts such activities through various signaling pathways and
molecules, such as STAT3, epidermal growth factor receptor (EGFR),
nuclear factor-κB (NF-κB), cell survival signaling, cell cycle, and
inflammatory mediators (10–13).
It has been reported that HNK shows anticancer activities in CRC
(14–16), but the explicit mechanism under
this effect remains unclear.
As a tumor suppressor, p53 has been targeted by
various anticancer reagents. Evidence indicated that the anticancer
activity of HNK can also be mediated by upregulating p53 in CRC
(14), but how HNK regulates p53
is unknown. Bone morphogenetic proteins (BMPs) belong to the
transforming growth factor-β (TGF-β) super-family and plays an
important role in regulating the proliferation and differentiation
in epithelium of colon and rectum (17,18).
The aberrant signal of BMPs has been involved in the cause and
progress of CRC (19,20). BMPs can inhibit Wnt pathway
p53-dependently (21). BMP7, as a
member of BMPs, plays an important role in regulating the
osteogenic differentiation in mesenchymal stem cells (22). Apart from this, it has been
reported that BMP7 also possesses anticancer activity in CRC, which
may be mediated through the non-canonical BMPs/Smads pathway
(23,24). However, the concrete molecular
mechanism need to be deciphered further.
In this study, we determined the anticancer effect
of HNK in HCT116 cells and analyzed the role of p53 or BMP7 in the
anticancer activity of HNK. Moreover, we dissected the possible
relationship between p53 and BMP7 in HCT116 cells.
Materials and methods
Cell culture and drug preparations
The HCT116 cell line was obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) with 10%
fetal bovine serum(FBS), 100 U/ml of penicillin and 100
µg/ml of streptomycin at 37°C with 5% CO2. HNK
was obtained from Hao-Xuan Bio-Tech Co., Ltd. (Xi'an, China) and
dissolved with DMSO to 10 mmol/l as stock, stored at −20°C.
Cell proliferation assay
Cell proliferation and viability assay was measured
with crystal violet staining and CCK-8 assay. Briefly, cells were
plated at a density of 5000 cells per well in 96-well plates with
300 µl medium in the presence of different concentrations of
HNK for 24, 48 and 72 h. 10 µl CCK-8 per 100 µl cell
medium was added to each well and cells were incubated for 2 h at
37°C. The optical absorbance of each well was measured with a
microplate reader (BioTek, Winooski, VT, USA) at 450 nm. Each assay
was carried out in triplicate.
Flow cytometric analysis of cell
cycle
Cells were seeded into 6-well plates and treated
with different concentrations of HNK for 48 h. Cells were collected
and washed with phosphate buffered saline (PBS, 4°C), fixed with
cold (4°C) 70% ethanol, washed with 50% ethanol, 30% ethanol and
PBS sequentially. Finally, cells were stained with 1 ml propidium
iodide (PI, 20 mg/ml) containing RNase (1 mg/ml) in PBS for 30 min
and followed by flow cytometry analysis. Each assay was carried out
in triplicate.
Annexin V-EGFP staining
Cells were cultured in 24-well plates and treated
with different concentrations of HNK for 24 h. Cells were stained
with Annexin V-EGFP Apoptosis Detection kits (Keygen, Nanjing,
China) according to the manufacturer's instructions. Briefly, cells
were washed with cold PBS and then treated with 200 µl
binding buffer. Then, 2 µl Annexin V-EGFP working solution
was added to each well and incubated for 15 min. Finally, the plate
was washed extensively and images were taken with a fluorescent
microscope. Each assay was carried out in triplicate.
Construction of the BMP7 and p53
recombinant adenovirus
The recombinant adenovirses were constructed with
the AdEasy system (25). Briefly,
the coding sequence (CDS) of human BMP7, p53 and green fluorescent
protein (GFP) were amplified from the EST clone. Then, the
fragments were cloned into shuttle vector pAdTrace, respectively.
The shuttle vectors were linearized and transfected to HEK293 cells
for the package of the recombinant adenoviruses, which were
designated as AdBMP7 and Adp53. The recombinant adenoviruses were
tagged with green GFP for tracking the viruses, and the recombinant
adenovirus expressing GFP (AdGFP) only was used as vehicle
control.
Immunofluorescent staining
Cells were plated into 48-well plates and treated
with different concentrations of HNK as design. Following treatment
for 48 h, cells were fixed with cold (4°C) methanol for 15 min,
washed with cold PBS and permeablized with 0.5% Triton X-100. Cells
were blocked with 5% BSA at room temperature for 1 h, followed by
incubating with primary antibody for p53 or BMP7, the homologous
IgG were used as negative control, followed by incubating with
FITC-labeled anti-goat IgG for 1 h. Finally, cells were stained
with DAPI (1 µg/ml). The images were taken under an inverted
microscope. Each assay was carried out in triplicate.
Reverse transcription (RT) and real-time
polymerase chain reaction (PCR) analysis
The cells were plated in T25 flask and treated with
different concentration of HNK. At the scheduled time-point, total
RNA were extracted with TRIzol reagent (Invitrogen, Carlsbad, CA,
USA), and followed by RT reaction to generate cDNA template. The
cDNA products were used as templates for real-time PCR to detect
the expression level of target genes. All data of each sample were
normalized with expression of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). The primer sequences for this investigation
are presented in Table I.
 | Table IThe primers used for PCR assay. |
Table I
The primers used for PCR assay.
| Gene | Primer sequence
(5′-3′) |
|---|
| GAPDH | F:
CAACGAATTTGGCTACAGCA |
| R:
AGGGGAGATTCAGTGTGGTG |
| PCNA | F:
GGCTCTAGCCTGACAAATGC |
| R:
GCCTCCAACACCTTCTTGAG |
| Bad | F:
CGGAGGATGAGTGACGAGTT |
| R:
CGGAGGATGAGTGACGAGTT |
| P53 | F:
GTCGGTGGGTTGGTAGTTTCTA |
| R:
AAAAAGAAATTGACCCTGAGCA |
| BMP7 | F:
GGCAGGACTGGATCATCG |
| R:
AAGTGGACCAGCGTCTGC |
Western blot assay
Cells were seeded in 6-well plates and treated with
different concentrations of HNK and/or combined with corresponding
recombinant adenovirus or inhibitor. At the scheduled time point,
all cells were lysed, collected and the lysates were denatured by
boiling for 10 min. All samples were subjected to electrophoresis
with SDS-PAGE and transfered to polyvinylidene fluoride membranes,
blotted with corresponding primary antibodies and the secondary
antibodies conjugated with horseradish peroxidase successively.
Then, the target proteins were developed with SuperSignal West
Femto Substrate (#34095, Thermo Scientific, Rockford, IL, USA).
Each assay was done in triplicate.
Statistical analysis
The statistical analysis was performed with the
Student's t-test between the control and the treatment group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of HNK on the proliferation of
colon cancer cells
In this study, we first tested the
anti-proliferation effect of HNK in the three colon cancer cells
HCT116, LoVo and SW620. The results showed HNK can inhibit the
proliferation of all the three cell lines, and HCT116 cells are
more susceptible to HNK (Fig. 1A).
Therefore, we selected HCT116 cells for the following
investigation. The cell cycle analysis results showed that HNK
arrest cell cycle at G2 phase (Fig.
1B). The PCR and western blot analysis results showed that HNK
increases the expression of the proliferation cell nuclear antigen
(PCNA) in HCT116 cells (Fig. 1C and
D). These data indicated HNK can inhibit the proliferation of
colon cancer cells.
Effects of HNK on apoptosis in HCT116
cells
Next, we determined the effect of HNK on apoptosis
in HCT116 cells. The PCR and western blot assay showed that HNK can
upregulate the expression of Bad concentration-dependently
(Fig. 2A and B). Flow cytometry
analysis results showed that HNK can increase the ratio of
apoptotic cells (Fig. 2C). The
Annexin V-EGFP staining results showed that HNK can increase the
apoptosis in HCT116 cells (Fig.
2D). These data suggested that HNK can induce apoptosis in
colon cancer cells.
Effects of HNK on p53 in HCT116
cells
It is well known that p53 is a tumor suppressor,
which has been the target of many anticancer agents. Therefore, we
scheduled to determine whether p53 is involved in the anticancer
effect of HNK in HCT116 cells. Western blot analysis results showed
that HNK increases the level of p53-concentration-dependently, as
well as the phosphorylation of p53 (Fig. 3A). The real-time PCR assay results
showed that HNK can also upregulate the mRNA expression of p53 in
HCT116 cells (Fig. 3B). To confirm
the effect of HNK on p53, we introduced immunofluorescent staining.
The results recaptured the effect of HNK on increasing the level of
p53 in HCT116 cells (Fig. 3C).
With further western blot assay, we found the same effect of HNK on
p53 in LoVo and SW620 colon cancer cells (Fig. 3D and E). All the data indicated
that p53 may play an essential role in the anti-proliferation
effect of HNK in HCT116 cells.
Effects of p53 on the anti-proliferation
effect of HNK in colon cancer cells
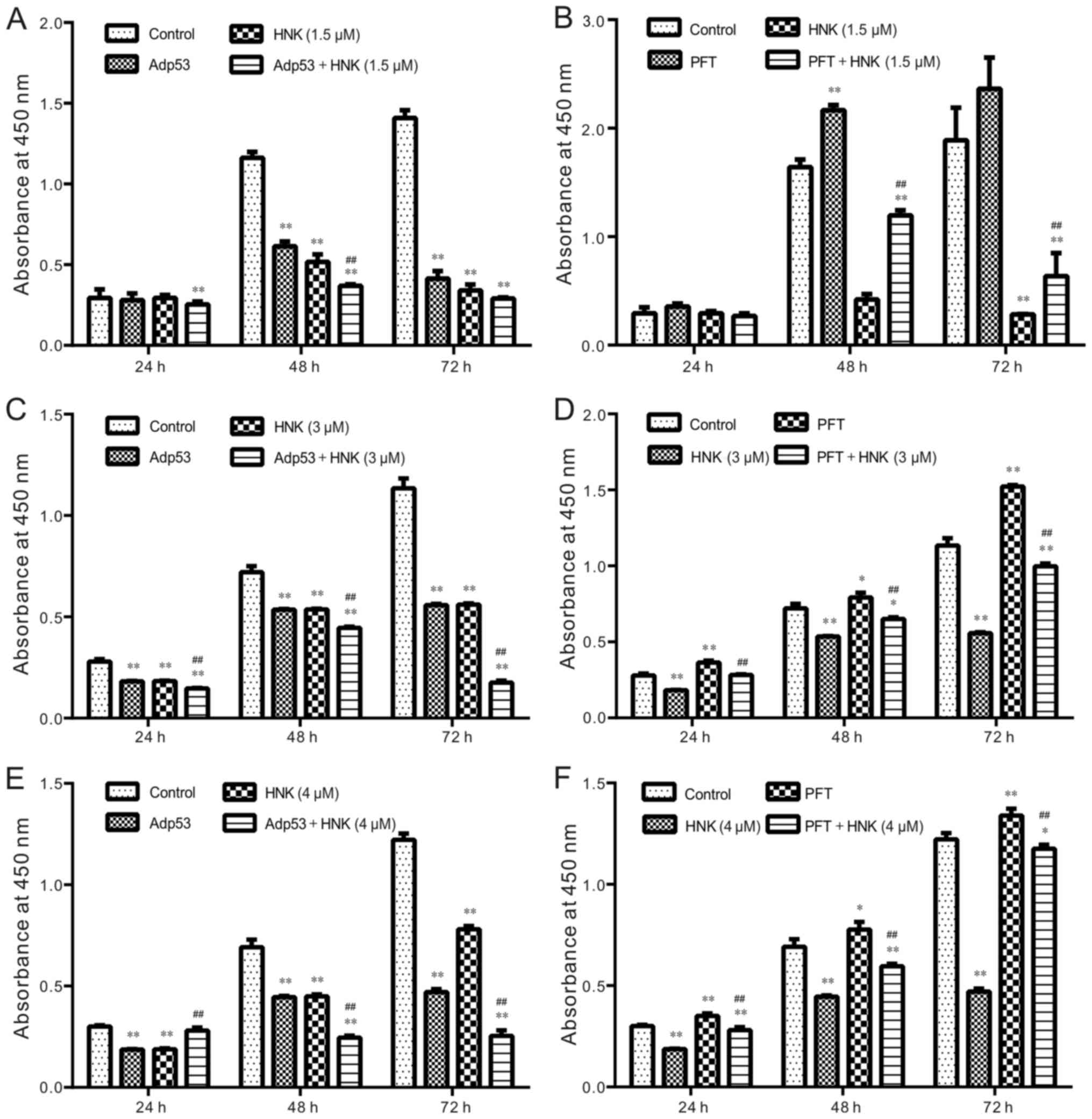
We next evaluated the effect of p53 on the
anticancer effect of HNK in the available colon cancer cells. The
CCK-8 assay results showed that exogenous p53 or HNK can both
inhibit the proliferation of HCT116 cells, while the combination of
exogenous p53 can apparently enhance the anti-proliferation effect
of HNK; specific inhibitor of p53 can not only partly increase the
proliferation of HCT116 cells, but also greatly attenuate the
anti-proliferation effect of HNK (Fig.
4A and B). Similar results were found in LoVo cells (Fig. 4C and D) and SW620 cells (Fig. 4E and F). These data suggested that
p53 plays an important role in the anti-proliferation effect of HNK
in HCT116 cell, but how HNK upregulates p53 remains unknown.
Effects of HNK on BMP7 in colon cancer
cells
Although p53 may mediate the anti-proliferation
effect of HNK in colon cancer cells, the mechanism underlying this
process is unclear. Thus, we next tried to unveil how HNK
upregulates p53 in colon cancer cells. BMPs is one of the
sub-groups of TGF-β super-family, which play an important role in
regulating proliferation, and differentiation. Our previous studies
demonstrated that exogenous BMP7 can inhibit the proliferation of
colon cancer cells. Therefore, we determined whether HNK can affect
the expression of BMP7 in HCT116 cells. The real-time PCR analysis
results showed that HNK increases the mRNA expression of BMP7
notably (Fig. 5A). The western
blot and immunofluorescent staining results confirmed that HNK can
upregulate the expression of BMP7 in HCT116 cells (Fig. 5B and C). Western blot assay results
showed the endogenous level of BMP7 in HCT116 cells is higher than
that of FHC cells (Fig. 5D).
Further analysis of the results exhibited that HNK can also
increase the protein level of BMP7 in LoVo and SW620 cells
(Fig. 5E and F). These data
suggested that BMP7 may also be involved in the anti-proliferation
effect of HNK in colon cancer cells.
Effects of BMP7 on the anti-proliferation
effect of HNK in colon cancer cells
We next analyzed the effect of BMP7 on the
anti-proliferation effect of HNK in HCT116 cells. The CCK-8 assay
results showed that either HNK or exogenous BMP7 can inhibit the
proliferation of HCT116 cell, but the BMP7 specific antibody can
promote the proliferation of the cells; Combination of exogenous
BMP7 enhances the anti-proliferation effect of HNK, while the
anti-proliferation effect of HNK in HCT116 cells can be
substantially reversed by the combination of BMP7 specific antibody
(Fig. 6A and B). Simiar results
were recaptured in LoVo cells (Fig. 6C
and D) and SW620 cells (Fig. 6E
and F). Therefore, these data indicated that the
anti-proliferation effect of HNK may be partly mediated by
upregulating BMP7 in colon cancer cells, but how BMP7 exerts such
function remains unknown.
Effects of BMP7 on p53 affected by HNK in
colon cancer cells
As BMPs usually exert their biological functions
through BMPs/Smads pathway and HNK can upregulate the expression of
BMP7, we first detected whether HNK can activate BMPs/Smads signal
pathway in HCT116 cells. The western blot analysis results showed
that there are no substantial change either on the total level of
Smad1/5/8 or the level of phosphorylated Smad1/5/8 (Fig. 7A), which implied that the
anti-proliferation effect of BMP7 may not be mediated through the
canonical BMPs/Smads pathway. Since HNK upregulates p53 as well as
BMP7, we speculated that BMP7 may be associated with the
upregulation of p53 induced by HNK. Western blot assay results
showed that exogenous BMP7 increases the level of total and
phosphorylated p53 in HCT116 cells, which become more pronounced
when combined with HNK (Fig. 7B).
Specific antibody of BMP7 reduces the total level of p53 and
markedly decreases the level of phosphorylated p53 induced by HNK
in HCT116 cells (Fig. 7C). Similar
results showed that specific antibody of BMP7 can also decrease the
total and phosphorylated level of p53 in LoVo and SW620 cells,
respectively (Fig. 7D and E).
These results strongly suggested that the effect of HNK on
upregulating p53 may be partly mediated by the HNK-induced
BMP7.
Discussion
HNK, as a phenolic compounds, is extracted from the
bark and leaves of Chinese medicinal Magnolia plant spp. Increasing
evidence indicated that HNK exhibits anticancer activity in various
types of cancer cells, such as breast cancer, lung cancer,
leukemia, colon cancer and other types of cancers (16,26–29).
Therefore, HNK may be a promising candidate for chemotherapy.
However, the explicit mechanism for this activity remains unclear.
In the present study, we demonstrated that HNK can exert obvious
anti-proliferation activity in colon cancer cells. Mechanically, we
found that this effect of HNK in colon cancer cells may be partly
mediated by the activation of p53, which may be resulted from the
HNK-induced up regulation of BMP7. Our findings demonstrated that
HNK may be a potential candidate drug for colon cancer, and BMP7
may be a plausible target for colon cancer treatment, although the
mechanism of how HNK upregulates BMP7 remains unknown.
Colon cancer is one of the leading malignancies in
alimentary system. Although the treatment for colon cancer have
been developed greatly in the past decades, the prognosis remains
unsatisfactory. There is still an urgent need to develop new and
efficacious drugs for the treatment of colon cancer. Natural
products and/or their derivates have been validated for colon
cancer treatment for several decades, such as camptothecin,
vincristine and paclitaxel (4–6).
Therefore, the active compounds from natural product may also be an
important source for chemotherapy agents. HNK, a biphenolic natural
product isolated from the bark and leaves of Magnolia plant spp,
has been demonstrated possessing excellent anticancer activity for
various cancer cells (26). Our
data also confirmed that HNK exerts efficacious anticancer effect
in colon cells which suggested that HNK may be a potential
anticancer agent for colon cancer. However, the mechanism
underlying this biological function of HNK remains unknown.
Multiple molecules and signals have been shown involved in the
pathogenic cause of colon cancer, such as mutation in Wnt signal
pathway, TP53 gene, and disfunction of TGF-β pathway and PTEN
(30–32). As a well-known tumor suppressor,
p53 has shown function losses or mutation in colon cancer (33,34).
Therefore, p53 is thought as a classical target for the treatment
of colon cancer. For this reason, we investigated whether HNK can
affect the status of p53 in colon cells. The results showed that
HNK obviously increases the level of p53; exogenous p53 enhances
the anticancer effect of HNK obviously, while inhibition of p53
attenuates this effect of HNK in HCT116, LoVo and SW620 cells.
Therefore, the anti-proliferation activity of HNK in colon cancer
cells may be mediated by the up regulation of p53, but how HNK
activates p53 remains unclear.
Bone morphogenetic proteins (BMPs) belong to the
super-family of transform growth factor-β (TGF-β). BMPs play an
important role in regulating proliferation and differentiation
(35). BMPs usually exhibit their
biological function through BMPs/Smads signal pathway called the
canonical BMPs/Smads signal pathway. In this pathway, BMPs function
as ligands to bind with the BMP receptors, including BMPRI and
BMPRII. It will recruit and phosphorylate BMPR1 when BMPR2 binds
with corresponding ligands. Then, Smad1, Smad5 and Smad8 will
become phosphorylated and form complex with Smad4. Finally, the
complex translocates into nucleus and regulates the downstream
targets (36,37). It has been reported that the
aberrant BMP signaling pathway is associated with the cause of
colon cancer (38,39). Mutations or disfunction of BMP
pathway have been found in juvenile polyposis, an inherited
polyposis syndrome that predisposes to colorectal cancer (40). BMP2 can inhibit the proliferation
of colon cancer, inactivation of BMP3 is involved with the
development of colon cancer and BMP9 may mediate the anticancer
effect of resveratrol in colon cancer cells (41–43).
BMP7 is one of the BMPs members, also known as osteogenic protein-1
(OP-1). Increasing evidence indicated that BMP7 is also involved in
cancer. The expression of BMP7 in breast cancer cells was higher
than that of normal cells (44).
Manning et al analyzed the tumor or normal samples from
colon cancer patient with microarray and found that the mRNA level
of BMP7 was twice in colorectal tumors than that of normal tissues
(45). Our previous study
demonstrated that Oridonin, an extract of active compound from the
traditional Chinese medicine, exhibits efficacious anticancer
activity against colon cancer through upregulating the expression
of BMP7 (24). Thus, we speculated
that the anticancer activity of HNK may also be related with BMP7.
The PCR and western blot analysis results provided evidence that
BMP7 is a target of HNK. The cell viability assay results indicated
that upregulation of BMP7 may partly mediate the anti-proliferation
activity of HNK in colon cancer. However, the explicit mechanism
underlying this process is unknown.
It has been reported that BMP7 may exert the
anticancer effect with a Smad4-independent pathway (46). It remains unknown whether BMP7
mediates the anti-proliferation of HNK in colon cancer in the same
way. With western blot assay, we found that HNK exhibits no
significant effect on the total and phosphorylated level of
Smad1/5/8. This finding suggested that BMP7 may mediate the
anti-proliferation effect of HNK through non-canonical BMPs/Smads
pathway. However, the detail mechanism remains unclear. It has been
reported that p53 and ΔNp63α may be a subset of TGFβ and BMPs
regulated SMAD target genes in the mammary epithelium (47). In colorectal cancer, the effect of
BMPs' signal on Wnt pathway may dependent on the status of Smad4
and p53 (21). BMP4 induced
apoptosis in myeloma may also be mediated by p53 (48). The evidence suggested that the
effect of BMP7 on the anti-proliferation activity of HNK in colon
cancer may be mediated by p53. Our data demonstrated that HNK can
also upregulate p53 in HCT116 and other colon cancer cells. Herein,
we speculated that the HNK-induced upregulation of p53 may result
from BMP7. With western blot assay, we found that exogenous
expression of BMP7 can elevate the level of p53 induced by HNK,
while it can be dramatically diminished by the specific antibody of
BMP7 in HCT116, LoVo and SW620 cells. Therefore, the HNK-induced
upregulation of p53 may result from BMP7, which is upregulated by
HNK.
Taken together, our investigation demonstrated that
HNK may be an efficacious anticancer agent for colon cancer.
HNK-induced upregulation of p53 may partly mediate the anticancer
activity of HNK, and the effect of HNK on p53 may depend on the
upregulation of BMP7. The details about how HNK regulates the
expression of BMP7 need to be intensively deciphered.
Acknowledgments
The authors would like to thank Professor Tong-Chuan
He (Medical Center of the University of Chicago) for his generous
provision of the recombinant adenoviruses. The present study was
supported by research grants from the Natural Science Foundation of
China (grants nos. NSFC 81372120 and 81572226 to B.-C. He).
References
|
1
|
Aghagolzadeh P and Radpour R: New trends
in molecular and cellular biomarker discovery for colorectal
cancer. World J Gastroenterol. 22:5678–5693. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vinnakota K, Zhang Y, Selvanesan BC, Topi
G, Salim T, Sand-Dejmek J, Jönsson G and Sjölander A: M2-like
macrophages induce colon cancer cell invasion via matrix
metalloproteinases. J Cell Physiol. Jan 18–2017.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Na H, Liu X, Li X, Zhang X, Wang Y, Wang
Z, Yuan M, Zhang Y, Ren S and Zuo Y: Novel roles of DC-SIGNR in
colon cancer cell adhesion, migration, invasion, and liver
metastasis. J Hematol Oncol. 10:282017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chazin EL, Reis RR, Junior WT, Moor LF and
Vasconcelos TR: An overview on the development of new potentially
active camptothecin analogs against cancer. Mini Rev Med Chem.
14:953–962. 2014. View Article : Google Scholar
|
|
5
|
Xie X, Tang B, Zhou J, Gao Q and Zhang P:
Inhibition of the PI3K/Akt pathway increases the chemosensitivity
of gastric cancer to vincristine. Oncol Rep. 30:773–782.
2013.PubMed/NCBI
|
|
6
|
Baird RD, Tan DS and Kaye SB: Weekly
paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev
Clin Oncol. 7:575–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan J, Lee Y, Wang Y and You M: Honokiol
targets mitochondria to halt cancer progression and metastasis. Mol
Nutr Food Res. 60:1383–1395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu GJ, Lin CJ, Lin YW and Chen RM: Data
analyses of honokiol-induced autophagy of human glioma cells in
vitro and in vivo. Data Brief. 9:667–672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu CH, Chen SH, Chang YS, Liu YW, Wu JY,
Lim YP, Yu HI and Lee YR: Honokiol, a potential therapeutic agent,
induces cell cycle arrest and program cell death in vitro and in
vivo in human thyroid cancer cells. Pharmacol Res. 115:288–298.
2017. View Article : Google Scholar
|
|
10
|
Wen J, Wang X, Pei H, Xie C, Qiu N, Li S,
Wang W, Cheng X and Chen L: Anti-psoriatic effects of Honokiol
through the inhibition of NF-κB and VEGFR-2 in animal model of
K14-VEGF transgenic mouse. J Pharmacol Sci. 128:116–124. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prasad R and Katiyar SK: Honokiol, an
active compound of magnolia plant, inhibits growth, and progression
of cancers of different organs. Adv Exp Med Biol. 928:245–265.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan J, Lee Y, Zhang Q, Xiong D, Wan TC,
Wang Y and You M: Honokiol decreases lung cancer metastasis through
inhibition of the STAT3 signaling pathway. Cancer Prev Res (Phila).
10:133–141. 2017. View Article : Google Scholar
|
|
13
|
Leeman-Neill RJ, Cai Q, Joyce SC, Thomas
SM, Bhola NE, Neill DB, Arbiser JL and Grandis JR: Honokiol
inhibits epidermal growth factor receptor signaling and enhances
the antitumor effects of epidermal growth factor receptor
inhibitors. Clin Cancer Res. 16:2571–2579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai YJ, Lin CI, Wang CL and Chao JI:
Expression of survivin and p53 modulates honokiol-induced apoptosis
in colorectal cancer cells. J Cell Biochem. 115:1888–1899.
2014.PubMed/NCBI
|
|
15
|
Ponnurangam S, Mammen JM, Ramalingam S, He
Z, Zhang Y, Umar S, Subramaniam D and Anant S: Honokiol in
combination with radiation targets notch signaling to inhibit colon
cancer stem cells. Mol Cancer Ther. 11:963–972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wynn ML, Consul N, Merajver SD and Schnell
S: Inferring the effects of honokiol on the notch signaling pathway
in SW480 colon cancer cells. Cancer Inform. 13(Suppl 5): 1–12.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hardwick JC, Van Den Brink GR, Bleuming
SA, Ballester I, Van Den Brande JM, Keller JJ, Offerhaus GJ, Van
Deventer SJ and Peppelenbosch MP: Bone morphogenetic protein 2 is
expressed by, and acts upon, mature epithelial cells in the colon.
Gastroenterology. 126:111–121. 2004. View Article : Google Scholar
|
|
18
|
Ji T, Takabayashi H, Mao M, Han X, Xue X,
Brazil JC, Eaton KA, Shah YM and Todisco A: Regulation and function
of bone morphogenetic protein signaling in colonic injury and
inflammation. Am J Physiol Gastrointest Liver Physiol. 312:G24–G33.
2017. View Article : Google Scholar
|
|
19
|
Slattery ML, Lundgreen A, Herrick JS,
Kadlubar S, Caan BJ, Potter JD and Wolff RK: Genetic variation in
bone morphogenetic protein and colon and rectal cancer. Int J
Cancer. 130:653–664. 2012. View Article : Google Scholar
|
|
20
|
Voorneveld PW, Kodach LL, Jacobs RJ, Liv
N, Zonnevylle AC, Hoogenboom JP, Biemond I, Verspaget HW, Hommes
DW, de Rooij K, et al: Loss of SMAD4 alters BMP signaling to
promote colorectal cancer cell metastasis via activation of Rho and
ROCK. Gastroenterology. 147:196–208.e13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Voorneveld PW, Kodach LL, Jacobs RJ, van
Noesel CJ, Peppelenbosch MP, Korkmaz KS, Molendijk I, Dekker E,
Morreau H, van Pelt GW, et al: The BMP pathway either enhances or
inhibits the Wnt pathway depending on the SMAD4 and p53 status in
CRC. Br J Cancer. 112:122–130. 2015. View Article : Google Scholar :
|
|
22
|
Xue Z, Niu LY, An G, Guo YS, Lv SC and Ren
XP: Expression of recombinant BMP-7 gene increased ossification
activity in the rabbit bone mesenchymal stem cells. Eur Rev Med
Pharmacol Sci. 19:3056–3062. 2015.PubMed/NCBI
|
|
23
|
Hao Z, Yang X, Lv Y, Li S, Purbey BK and
Su H: Intracolonically administered adeno-associated virus-bone
morphogenetic protein-7 ameliorates dextran sulphate sodium-induced
acute colitis in rats. J Gene Med. 14:482–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren CM, Li Y, Chen QZ, Zeng YH, Shao Y, Wu
QX, Yuan SX, Yang JQ, Yu Y, Wu K, et al: Oridonin inhibits the
proliferation of human colon cancer cells by upregulating BMP7 to
activate p38 MAPK. Oncol Rep. 35:2691–2698. 2016.PubMed/NCBI
|
|
25
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh T and Katiyar SK: Honokiol, a
phytochemical from Magnolia spp., inhibits breast cancer cell
migration by targeting nitric oxide and cyclooxygenase-2. Int J
Oncol. 38:769–776. 2011.PubMed/NCBI
|
|
27
|
Singh T and Katiyar SK: Honokiol inhibits
non-small cell lung cancer cell migration by targeting
PGE2-mediated activation of β-catenin signaling. PLoS
One. 8:e607492013. View Article : Google Scholar
|
|
28
|
Li HY, Ye HG, Chen CQ, Yin LH, Wu JB, He
LC and Gao SM: Honokiol induces cell cycle arrest and apoptosis via
inhibiting class I histone deacetylases in acute myeloid leukemia.
J Cell Biochem. 116:287–298. 2015. View Article : Google Scholar
|
|
29
|
Hahm ER, Karlsson AI, Bonner MY, Arbiser
JL and Singh SV: Honokiol inhibits androgen receptor activity in
prostate cancer cells. Prostate. 74:408–420. 2014. View Article : Google Scholar :
|
|
30
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehlen P and Fearon ER: Role of the
dependence receptor DCC in colorectal cancer pathogenesis. J Clin
Oncol. 22:3420–3428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung B, Staudacher JJ and Beauchamp D:
Transforming growth factor β superfamily signaling in development
of colorectal cancer. Gastroenterology. 152:36–52. 2017. View Article : Google Scholar
|
|
33
|
Yamada H, Shinmura K, Yamamura Y, Kurachi
K, Nakamura T, Tsuneyoshi T, Yokota N, Maekawa M and Sugimura H:
Identification and characterization of a novel germline p53
mutation in a patient with glioblastoma and colon cancer. Int J
Cancer. 125:973–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Demir L, Ekinci N, Erten C, Somali I, Can
A, Dirican A, Cokmert S, Bayoglu V, Akyol M, Kucukzeybek Y, et al:
The impact of cell proliferation markers and p53 mutation status on
prognosis of non-metastatic colon cancer. J Surg Oncol.
109:665–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murray SS, Brochmann Murray EJ, Wang JC
and Duarte ME: The history and histology of bone morphogenetic
protein. Histol Histopathol. 31:721–732. 2016.PubMed/NCBI
|
|
36
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawabata M, Imamura T and Miyazono K:
Signal transduction by bone morphogenetic proteins. Cytokine growth
Factor Rev. 9:49–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grijelmo C, Rodrigue C, Svrcek M, Bruyneel
E, Hendrix A, de Wever O and Gespach C: Proinvasive activity of
BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent
signaling pathways in colon cancer cells. Cell Signal.
19:1722–1732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bertrand FE, Angus CW, Partis WJ and
Sigounas G: Developmental pathways in colon cancer: Crosstalk
between WNT, BMP, Hedgehog and Notch. Cell Cycle. 11:4344–4351.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brosens LA, Langeveld D, van Hattem WA,
Giardiello FM and Offerhaus GJ: Juvenile polyposis syndrome. World
J Gastroenterol. 17:4839–4844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Chen X, Qiao M, Zhang BQ, Wang N,
Zhang Z, Liao Z, Zeng L, Deng Y, Deng F, et al: Bone morphogenetic
protein 2 inhibits the proliferation and growth of human colorectal
cancer cells. Oncol Rep. 32:1013–1020. 2014.PubMed/NCBI
|
|
42
|
Loh K, Chia JA, Greco S, Cozzi SJ,
Buttenshaw RL, Bond CE, Simms LA, Pike T, Young JP, Jass JR, et al:
Bone morphogenic protein 3 inactivation is an early and frequent
event in colorectal cancer development. Genes Chromosomes Cancer.
47:449–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan SX, Wang DX, Wu QX, Ren CM, Li Y,
Chen QZ, Zeng YH, Shao Y, Yang JQ, Bai Y, et al: BMP9/p38 MAPK is
essential for the antiproliferative effect of resveratrol on human
colon cancer. Oncol Rep. 35:939–947. 2016.
|
|
44
|
Alarmo EL, Pärssinen J, Ketolainen JM,
Savinainen K, Karhu R and Kallioniemi A: BMP7 influences
proliferation, migration, and invasion of breast cancer cells.
Cancer Lett. 275:35–43. 2009. View Article : Google Scholar
|
|
45
|
Manning AM, Williams AC, Game SM and
Paraskeva C: Differential sensitivity of human colonic adenoma and
carcinoma cells to transforming growth factor beta (TGF-beta):
Conversion of an adenoma cell line to a tumorigenic phenotype is
accompanied by a reduced response to the inhibitory effects of
TGF-beta. Oncogene. 6:1471–1476. 1991.PubMed/NCBI
|
|
46
|
Beck SE, Jung BH, Fiorino A, Gomez J,
Rosario ED, Cabrera BL, Huang SC, Chow JY and Carethers JM: Bone
morphogenetic protein signaling and growth suppression in colon
cancer. Am J Physiol Gastrointest Liver Physiol. 291:G135–G145.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Balboni AL, Cherukuri P, Ung M, DeCastro
AJ, Cheng C and DiRenzo J: p53 and ΔNp63α coregulate the
transcriptional and cellular response to TGFβ and BMP signals. Mol
Cancer Res. 13:732–742. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukuda N, Saitoh M, Kobayashi N and
Miyazono K: Execution of BMP-4-induced apoptosis by p53-dependent
ER dysfunction in myeloma and B-cell hybridoma cells. Oncogene.
25:3509–3517. 2006. View Article : Google Scholar : PubMed/NCBI
|