Introduction
Gastric cancer (GC) is the second leading cause of
cancer-related deaths worldwide and imposes a major health burden
due to its poor prognosis (1).
Although surgery is a relatively effective treatment option for GC,
the 5-year survival rate is only 29% and the median survival rate
is less than a year (2). Various
cytotoxic drugs, such as 5-fluorouracil, platinum and taxane, are
treatment options, but drug resistance is significant. Thus, the
effective management of advanced GC remains a significant
challenge, especially for aggressive subtypes (3), making the development of novel
therapies targeting refractory and resistant cases an urgent
need.
The expression of various oncogenic growth factors,
such as epidermal growth factor receptor (EGFR) and ErbB2, is
elevated in gastrointestinal carcinomas and is associated with poor
prognosis and reduced overall survival (4–6). The
overexpression of these proteins further activates downstream
signaling pathways, such as phosphatidylinositol 3-kinase
(PI3K)/Akt and mitogen-activated protein kinase (MAPK)/Erk
pathways, that mediate oncogenic cellular proliferation,
differentiation, angiogenesis, tumor metastasis and survival
(7). These proteins are client
proteins of heat shock protein 90 (HSP90) which help facilitate
them to escape normal proteolytic turnover and contribute to tumor
development and survival (4).
Moreover, the overexpression of HSP90 and its client proteins has
been associated with the development of GC and its
clinicopathological features, such as tumor size, lymph node
metastases and patient survival (8). Hence, targeting HSP90 and,
indirectly, its associated oncogenic client proteins is a promising
anti-GC therapeutic strategy.
Ganetespib (STA-9090) is a resorcinol-based second
generation HSP90 inhibitor with enhanced potency and a favorable
safety profile as compared to geldanamycin derivatives (9). In preclinical studies, ganetespib has
demonstrated significant inhibition of cell proliferation and tumor
growth in cell and xenograft models of multiple cancers (9). Currently a number of ongoing clinical
trials are investigating the effect of ganetespib in various
cancers, including rectal, ErbB2+ metastatic breast,
multiple myeloma and lung cancers (10). Recently, Liu et al reported
the efficacy of ganetespib in targeting EGFR-mediated GC in cell
lines and xenograft models (11).
Still, further preclinical studies are necessary to fully elucidate
other receptor tyrosine kinase (RTK) signaling pathways that may be
involved in ganetespib-mediated inhibition of GC. In the present
study, we demonstrate the efficacy of ganetespib in targeting
multiple oncogenic pathways associated with RTK signaling in GC
cells. Given the poor clinical outcomes associated with growth
factor-mediated GC and the lack of effective GC therapeutics,
ganetespib has the potential to be developed into a therapeutic
agent for GC.
Materials and methods
Materials
Ganetespib was purchased from Medkoo Biosciences,
Inc. (Chapel Hill, NC, USA). Primary antibodies specific to Cyclin
B1, cleaved caspase-3, cleaved caspase-8, cleaved caspase-9,
cleaved PARP, Akt, phospho Akt (pAkt), mTOR, pmTOR, ErbB2, pErbB2,
GSK3, pGSK3, Erk, pErk, Src and pSrc were purchased from Cell
Signaling Technology (Danvers, MA, USA); and cyclin D1, cyclin E,
Cdk1, E2F1, p27, survivin, caspase-8, caspase-9, EGFR and β-actin
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Secondary anti-mouse or anti-rabbit antibodies were purchased from
Thermo Scientific (Rockford, IL, USA).
Cell culture
Human AGS and N87 GC cell lines were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Cells were maintained in Dulbecco's modified Eagle's medium (DMEM)
(Life Technologies; Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum, 100 µg/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified incubator with a 5%
CO2 atmosphere.
MTT assay
MTT (3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide) assay (Sigma-Aldrich, St. Louis, MO,
USA) was used to determine the cytotoxicity of ganetespib in
different cell lines. AGS and N87 cells were seeded at a density of
1000 cells/well in a 96-well plate and incubated overnight. After 5
days of ganetespib treatment (0, 0.5, 1, 5, 10, 50 or 100 nM), MTT
was added to the cells at a final concentration of 0.5 mg/ml. After
3 h of incubation with MTT, media was aspirated and 50 µl of
DMSO was added to each well and kept on a shaker for 45 min. The
absorbance at 570 nm was then measured using a SynergyMx microplate
reader (BioTek; Winooski, VT, USA). All samples were analyzed in
five replicates. IC50 was calculated manually by linear
interpolation using the formula: IC50 = [(50−A) / (B−A)]
× (D−C) + C, where A = the first point on the curve, expressed as
percent inhibition, that is less than 50%; B = the first point on
the curve, expressed as percent inhibition, that is greater than or
equal to 50%; C = the concentration of inhibitor that gives A%
inhibition; and D = the concentration of inhibitor that gives B%
inhibition (12).
Clonogenic assay
Viable AGS and N87 cells were plated at a density of
300 cells/well and 1000 cells/well, respectively, and allowed to
adhere overnight. Cells were treated with ganetespib (0, 1.25, 2.5,
5 or 10 nM) for 10 days. After 10 days, colonies were washed twice
with phosphate buffered saline (PBS) and stained with 0.5% crystal
violet (1:1 methanol: H2O) for 30 min at room
temperature. The extra stain was aspirated, and the plates were
washed with tap water and air dried. Colonies were counted and
imaged with a digital camera mounted on a Nikon C-LEDS
microscope.
Cell cycle analysis
AGS and N87 cells were treated with ganetespib (0
and 500 nM) for 24 h in complete medium. Floating and adherent
cells were collected, washed twice with cold PBS and centrifuged.
Cells were then fixed in 70% (v/v) ethanol at −20°C. After
centrifugation, cells were washed with cold PBS and stained with
propidium iodide (PI) in RNase solution. After incubation for 45
min at 37°C, cells were strained with a 40 µm filter and the
cell cycle distribution was determined using a Guava EasyCyte 8
Flow Cytometer (Millipore; Billerica, MA, USA).
Apoptosis assay
Apoptosis was measured using an Annexin-V-Fluos
Staining kit (Sigma-Aldrich) according to the manufacturer's
protocol. Briefly, the AGS and N87 cells were treated with
ganetespib (0, 25 or 50 nM) for 24 h. Then, the cells were
harvested and washed with cold PBS. After staining with Annexin
V-FITC/PI, the percentage of apoptotic cells was quantified using a
Guava EasyCyte 8 Flow Cytometer (Millipore).
Western blotting
The protein concentrations of ganetespib-treated AGS
and N87 cell extracts were determined using the BCA assay (Thermo
Scientific). Protein from each sample (50 µg) was resolved
using SDS-PAGE and then transferred to a nitrocellulose membrane.
The membranes were blocked in 5% non-fat milk in Tris-buffered
saline with Tween (TBST) for 1 h, followed by overnight incubation
in appropriate primary antibodies at 4°C. After washing, membranes
were incubated in species-specific horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. After further washing, specific protein bands were
detected with enhanced chemiluminescence reagents and imaged with a
FluorChemE imager (Cell Biosciences; Santa Clara, CA, USA). β-actin
was used as a loading control.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted from the treated cells and
purified using an RNeasy mini kit (Qiagen; Germantown, MD, USA), as
per the manufacturer's instructions. Purified RNA (1 µg) was
used to synthesize cDNA using iScript cDNA Synthesis kit (Bio-Rad;
Hercules, CA, USA). The qPCR assay of triplicate samples was
carried out on a Bio-Rad CFX96 system using SYBR green (Bio-Rad).
The primer sequences used are as follows: CCNB1-forward primer
(F)-GACAACTTGAGGAAGAGCAAGC, reverse primer
(R)-ATGGTCTCCTGCAACAACCT; CCND1-F-GGCGGATTGGAAATGAACTT,
R-TCCTCTCCAAA ATGCCAGAG; CCNE1-F-GAAATGGCCAAAATCGA CAG,
R-TCTTTGTCAGGTGTGGGGA; CDK4-F-GTCGGCTTCAGAGTTTCCAC,
R-TGCAGTCCACATATGCA ACA; CDKN1B-F-TGGAGAAGCACTGCAGAGAC, R-GCG
TGTCCTCAGAGTTAGCC; and β-actin-F-GCACCACA CCTTCTACAATGAGC,
R-GACGTAGCACAGCTTCTCC TTAATG. Relative mRNA levels were quantified
based on the cycle threshold (Ct) values of the tested genes as
normalized to the control β-actin gene.
Statistical analysis
A Student's t-test for comparison of two groups was
used for statistical analysis. Calculations were performed using
GraphPad Prism software (GraphPad; La Jolla, CA, USA) and data were
expressed as means ± standard error of the mean (SEM) of at least
three independent experiments. A P-value of ≤0.05 was considered to
indicate a statistically significant difference.
Results
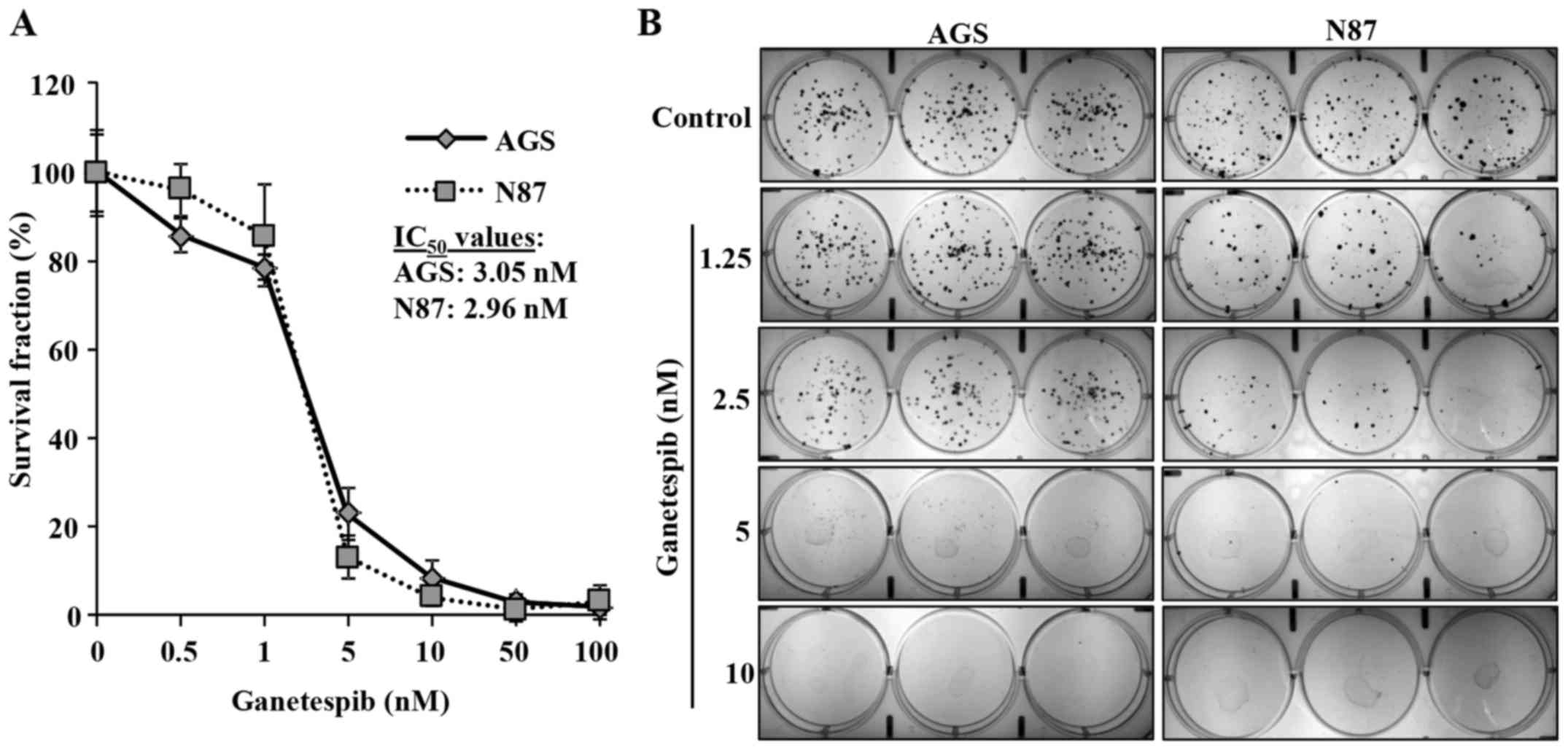
Ganetespib inhibits proliferation and
colony formation in AGS and N87 cell lines
Ganetespib inhibits the proliferation of various
cancer cells at low nanomolar concentrations (9). To evaluate the effects of ganetespib
on GC cell proliferation, we utilized AGS and N87 human GC cell
lines that express high levels of EGFR (11) and ErbB2, respectively. AGS and N87
cells were treated with low concentrations of ganetespib (0, 0.5,
1, 5, 10, 50 or 100 nM) for 5 days to determine its effect on cell
proliferation. MTT assay revealed that ganetespib dose-dependently
induced significant cell growth inhibition in both AGS and N87 cell
lines with IC50 of 3.05 and 2.96 nM, respectively
(Fig. 1A). To confirm our results,
a clonogenic assay was performed on AGS and N87 cells treated with
ganetespib (0, 1.25, 2.5, 5, or 10 nM) for 10 days. Consistent with
the MTT results, ganetespib also significantly inhibited colony
formation at low concentrations (5 and 2.5 nM) in AGS and N87
cells, respectively (Fig. 1B).
Together, these data indicate the anti-proliferative capacity of
ganetespib in GC cell lines.
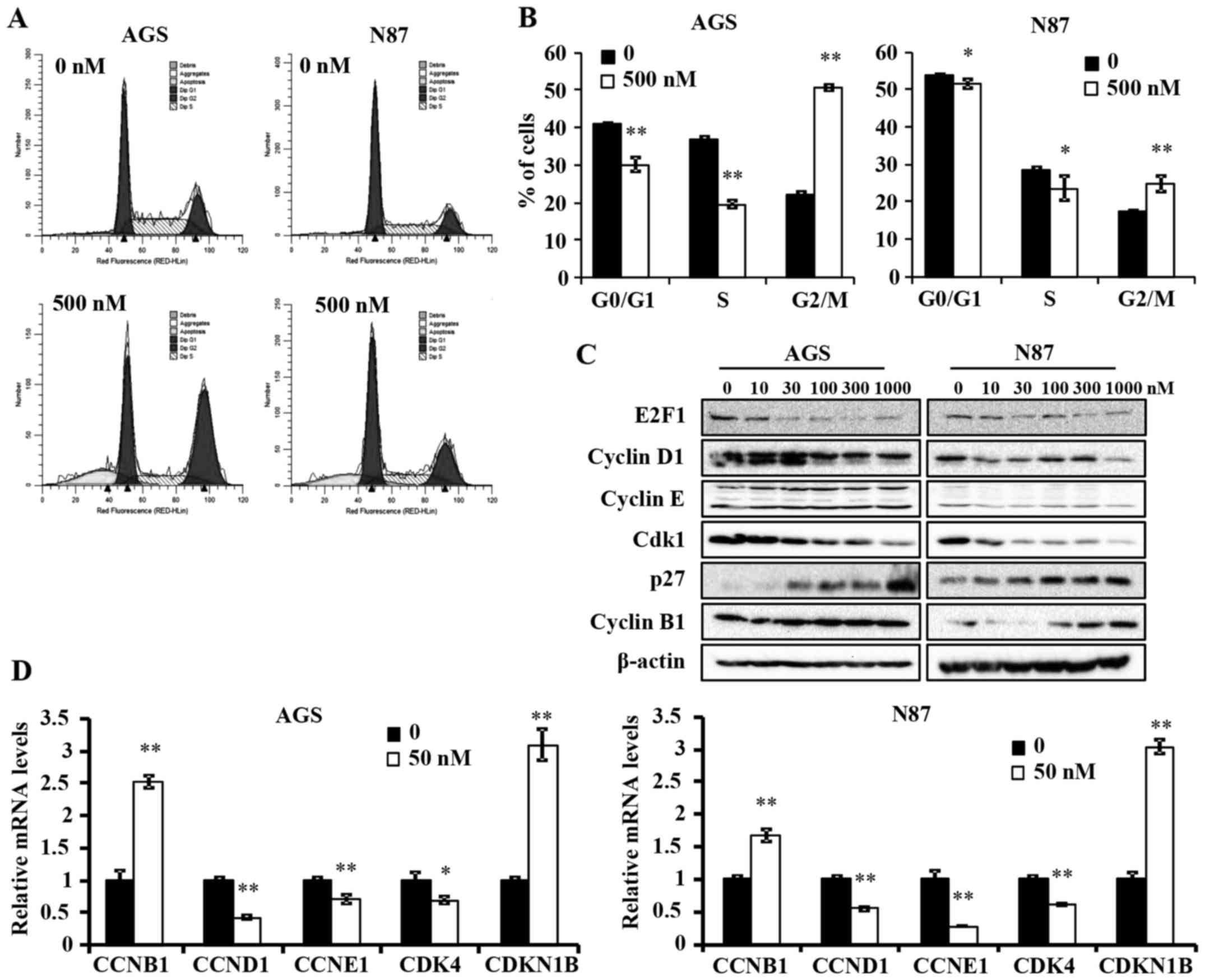
Ganetespib induces G2/M cell cycle arrest
in AGS and N87 GC cell lines
To further understand the anti-proliferative
mechanism of ganetespib, we examined its effects on cell cycle
progression in AGS and N87 cells. A high concentration of
ganetespib was used in both cell lines to ensure significant
inhibition of treated cells as compared to controls. Cell cycle
analysis using flow cytometry indicated that ganetespib (500 nM for
24 h) induced a significant reduction in G0/G1 and S phase, which
is indicative of a decreased proliferative cell population
(Fig. 2A and B). Importantly, a
significant accumulation of cells in G2/M phase was evident in both
cell lines, but at different intensities. This suggests that
ganetespib induces a specific pattern of cell cycle arrest in a
cell line-specific manner and prevents entry into G0/G1 phase by
potentially regulating the associated checkpoint regulators.
Various cell cycle regulators are involved in the
progression of cells from one phase to another. To elucidate the
mechanism of ganetespib-induced cell cycle arrest, we examined the
expression levels of several key cell cycle regulators in cells
treated with a range of ganetespib concentrations (0–1000 nM) for
24 h. In AGS and N87 cell lines, ganetespib downregulated the
protein levels of E2F1, cyclin D1 and cyclin-dependent kinase 1
(Cdk1) (Fig. 2C). Notably, cyclin
B1 and p27 were dose-dependently upregulated in both cell lines.
This observation will be addressed in the Discussion. Consistent
with the western blot data, qPCR analysis revealed that ganetespib
also induces a significant increase in the mRNA levels of cyclin B1
(CCNB1) and p27 (CDKN1B) and a concomitant reduction in cyclin D1
(CCND1), cyclin E1 (CCNE1) and CDK4 mRNA expression in both cell
lines (Fig. 2D). Taken together,
these data suggest that ganetespib inhibits GC cell cycle
progression by transcriptionally, as well as translationally,
modifying key cell cycle regulators in vitro.
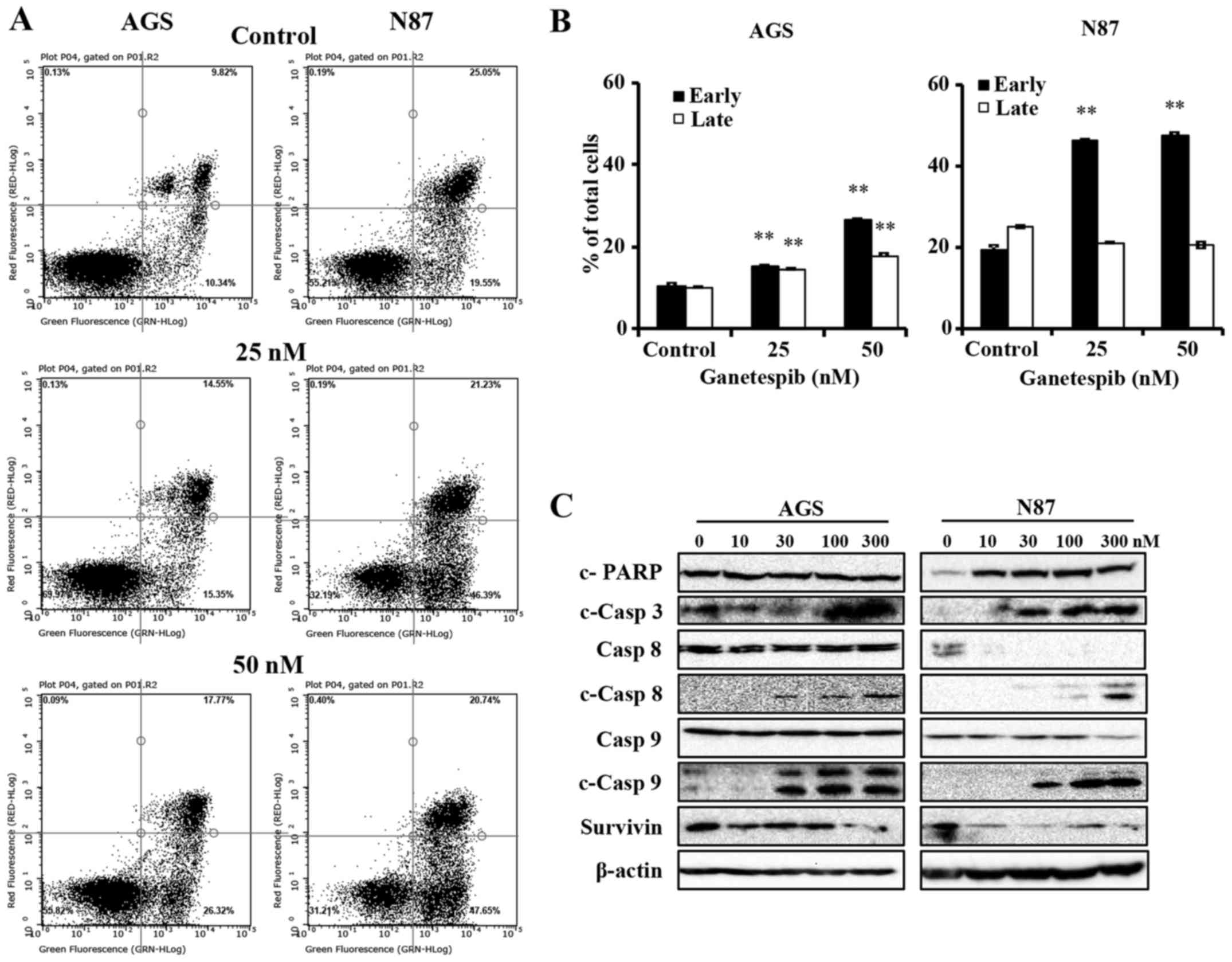
Ganetespib-treated AGS and N87 cells
undergo apoptosis through the activation of mitochondrial and death
receptor pathways
Apoptosis is a common mechanism of protein
degradation associated with HSP90 inhibition (13). To better understand the mechanisms
of ganetespib-induced cell death in AGS and N87 cells, we used flow
cytometry to determine the degree of apoptosis. To this end, the
cells were treated with 0–50 nM of ganetespib for 24 h, followed by
analysis of apoptotic cells using Annexin V/PI staining. Cells in
the early apoptotic stage are detected by Annexin
V+/PI− staining, while cells in the late
stages of apoptosis are marked by Annexin
V+/PI+ staining. In Fig. 3A and B, ganetespib significantly
increased the early apoptotic cell population, as compared to the
untreated control cells, in both AGS and N87 cell lines. Of note,
the percentage of cells in late stage apoptosis was only
significantly increased in AGS cells (Fig. 3B). Although ganetespib did not
significantly modify the percentage of cells in late stage
apoptosis in N87 cells, ganetespib-induced early stage apoptosis
was more striking. Together, these results markedly demonstrate
that ganetespib induces cell death via apoptosis in GC cell
lines.
Caspases are the mediators of drug-induced cell
death and ultimate dissociation. In order to identify the key
mechanism of apoptosis-mediated cell death with ganetespib
treatment, we next examined the activation of caspases and PARP in
ganetespib-treated cells. As in Fig.
3C, ganetespib induced remarkable cleavage of PARP (in N87
cells only) and caspase-3, which are common proteolytic markers of
apoptosis. Importantly, ganetespib treatment resulted in
significant accumulation of cleaved caspase-8 and caspase-9
(Fig. 3C), which are the apical
caspases for death receptor and mitochondrial pathways,
respectively. Moreover, ganetespib induced significant
downregulation of survivin, an apoptotic antagonist, in both cell
lines.
As we observed that ganetespib induced a significant
increase in cleaved caspase-8 and -9 without corresponding decrease
in zymogen levels, we examined the mRNA levels of caspase-8,
caspase-9 and other apoptotic regulators, including Bcl-2, BIRC5
and MCL1. When the cells were treated with 50 nM ganetespib for 16
h, mRNA levels of caspase-8 and -9 significantly increased and mRNA
levels of Bcl-2 and BIRC5 significantly decreased in both cell
lines (data not shown), suggesting that ganetespib regulates
apoptosis at both transcriptional and translational levels. In all,
these data indicate that ganetespib promotes the apoptotic cascade
at multiple regulatory levels.
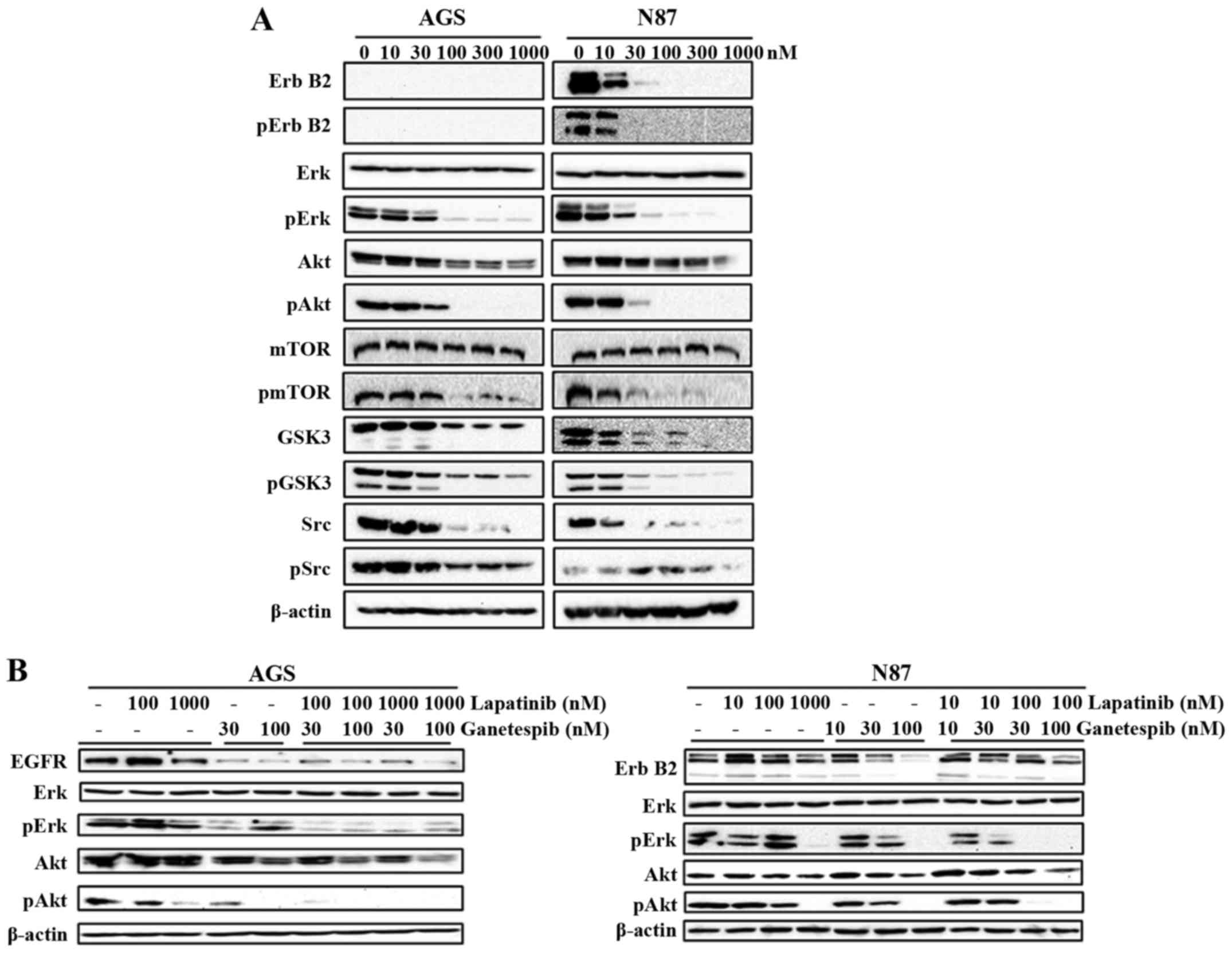
Ganetespib substantially suppresses RTK
signaling
Deregulation of RTKs, such as ErbB2 and EGFR, plays
a critical role in GC development, including cell proliferation,
angiogenesis and metastasis (8).
The role of ganetespib in inhibiting EGFR-mediated GC was
previously revealed by Liu et al (11). Therefore, we examined the effects
of ganetespib (0–1000 nM) on the expression and activation of a
series of markers representing different levels of the ErbB2
signaling cascade. We demonstrated that ganetespib remarkably
down-regulates the activation/phosphorylation of ErbB2 in N87 cells
and its downstream effector molecules Erk, Akt, mTOR, GSK3 and Src,
indicating the inhibitory effect of ganetespib on the kinase
activities of the RTK pathway (Fig.
4A). Importantly, total protein levels of ErbB2, Akt, GSK3 and
Src were also significantly downregulated in ganetespib-treated
cells. Although AGS cells do not express ErbB2, ganetespib
treatment significantly reduced the activation/phosphorylation of
Erk, Akt, mTOR, GSK3 and Src also in this cell line. To confirm
that RTK/ErbB2 signaling inhibition is a critical mechanism of
ganetespib-induced cellular responses, we used lapatinib, an
EGFR/ErbB2 dual inhibitor, to suppress EGFR and ErbB2 kinase
activity. Lapatinib and ganetespib induced similar effects on Erk
and Akt activation/phosphorylation (Fig. 4B), which indicates that the
inhibition of RTK signaling is necessary for the actions of both
drugs. Noteworthy, the combined treatment of ganetespib (30 or 100
nM) + lapatinib (100 nM) synergistically enhanced the inhibition of
Erk and Akt activation/phosphorylation. Thus, our data support that
ganetespib effectively inhibits HSP90 client growth factors leading
to RTK pathway inhibition and consequent cellular activities in GC
cells.
Discussion
HSP90 inhibitors have gained much attention over the
last few decades owing to their role in targeting HSP90 client
proteins, including Akt, Raf, Erk, ErbB2 and EGFR, that are
involved in various cancers (14).
Due to solubility and toxicity issues, the first generation of
geldanamycin-based HSP90 inhibitors were withdrawn from clinical
trials, but they provided a proof of concept for targeting HSP90 in
cancer. Promisingly, second generation HSP90 inhibitors exhibit
more efficacy and less toxicity than the former inhibitors
(15). In particular, ganetespib
(STA-9090) is a potent resorcinol-based second generation HSP90
inhibitor with a unique triazolone moiety and improved efficacy and
safety profile, without hepatotoxicity and ocular toxicity, than
earlier HSP90 inhibitors. Preclinical studies involving ganetespib,
alone or in combination with other drugs (16), reported improved efficacy and
cytotoxicity in various hematological and solid tumors, including
those with mutant kinases, such as B-RAF, EGFR and c-KIT (9,17,18).
The antitumor effects of ganetespib are attributed to its ability
to inhibit cell growth via cell cycle arrest, apoptosis and
PI3K/Akt, MAPK/Erk, mTOR and JAK2/STAT3 signaling inhibition
(9).
In ErbB2-amplified breast cancer cell and xenograft
models, ganetespib has demonstrated potential as a monotherapy or
in combination with other drugs, such as trastuzumab, to prevent
tumor resistance and regression (19). In addition, ganetespib has
exhibited greater efficacy than first generation inhibitors in
degrading HSP90 client proteins after short exposure (10). The ability of ganetespib to inhibit
multiple targets of growth and survival pathways, especially the
cell cycle and apoptosis, makes it an attractive strategy that can
be used to prevent advanced GC, including ErbB2-amplified and
mutant EGFR subtypes. Thus, in the present study, we investigated
the effects of ganetespib treatment on AGS and N87 human GC cell
lines. Our results determined that ganetespib inhibits cell
proliferation (Fig. 1), induces
G2/M cell cycle arrest (Fig. 2)
and activates both cell death receptor- and mitochondria-mediated
apoptotic pathways (Fig. 3),
alongside inhibition of RTK and PI3K/Akt/MAPK signaling (Fig. 4) in AGS and N87 GC cells.
HSP90 client proteins include several proteins
involved in cell cycle regulation and progression, such as Cdk1,
Cdk4, cyclin D, cyclin B and survivin. These client proteins play
important roles in driving mitogenic processes, as well as in G1/S,
G2/M and spindle checkpoint controls (20). HSP90 inhibition can thus induce
both G1/S and G2/M cell cycle arrest. The majority of the first
generation HSP90 inhibitors have been reported to induce G1 cell
cycle arrest in cancer cells. As such, geldanamycin and its
derivative, 17-AAG, cause Rb-dependent G1 arrest in breast cancer
cells, which is associated with downregulation of cyclin D
(21). In our study, our data
indicate that ganetespib inhibits cell proliferation by inducing
cell cycle arrest in G2/M phase with significant upregulation of
cyclin B1 and p27 at both transcriptional and translational levels
and concurrent downregulation of Cdk1, cyclin D1 and cyclin E
proteins, CDK4 (mRNA) and transcription factor E2F1. Cyclin B1 and
Cdk1 are major regulators of G2/M cell cycle checkpoint and their
interaction triggers the entry into the mitotic phase (22,23).
Although increased p27 levels are associated with G0/G1 arrest, we
do not see an accumulation of cells in G0/G1 phase.
Earlier studies have shown that in mitogen-starved
states, upregulated p27 can directly bind to cyclin B1 and block
the progression through G2/M phase (22,24,25).
Therefore, the overexpression of p27 that we observed in our study
might be accompanied by the blocking of cyclin B1 to induce G2/M
arrest. Nevertheless, further studies are required to understand
the role of elevated p27 and cyclin B1 levels in the current
scenario. Liu et al recently reported that G2/M cell cycle
arrest in ganetespib-treated MGC-803 GC cells was associated with
decreased cyclin B1, Cdk1 and Chk1 levels (11). Specifically, this
ganetespib-induced reduction in cyclin B1 expression in MGC-803
cells is inconsistent with our findings in AGS and N87 GC cells
(Fig. 2), suggesting cell
line-specific effects of ganetespib on cell cycle regulation. Thus,
we show that ganetespib induces G2/M cell cycle arrest in AGS and
N87 cell lines with concomitant increases in cyclin B1 and p27
levels. Additional studies are required to fully understand the
potential cell-specific mechanisms of ganetespib.
The cell cycle arrest induced by most HSP90
inhibitors advances the cells towards programmed cell death
(20). In this study, we found
that ganetespib-induced G2/M cell cycle arrest initiated a
signaling cascade leading to apoptosis in both AGS and N87 cell
lines. Our results demonstrated a dose-dependent increase in early
phase apoptosis in AGS and N87 cell lines and an increasing trend
for late phase apoptosis in AGS cells, but not in N87 cells
(Fig. 3). Since PI+
cells can also be undergoing necrosis (26), late phase apoptosis may not be the
most accurate indicator of continued apoptosis in cells. To further
investigate the mechanism of ganetespib-induced apoptosis, we
evaluated different proteins involved in caspase-mediated apoptotic
pathways. Our results showed an increase in cleaved caspase-3, -8
and -9 along with cleaved PARP (only in N87 cells), which are
indicative of the activation of both cell death receptor- and
mitochondria-mediated apoptotic pathways. Also, survivin, an
anti-apoptotic marker, is decreased in both cell lines, which
provides additional evidence of apoptotic induction. The variation
in the c-PARP levels between the AGS and N87 cell lines tested in
our study could be due to the short treatment time of ganetespib
(24 h) as compared to earlier reports that showed increased c-PARP
levels after 48 or 72 h of treatment (11). Thus, our data also suggest the
increased sensitivity of N87 cells, as compared to AGS cells, to
ganetespib after a short exposure time. Taken together, our results
support the activation of both cell death receptor- and
mitochondria-mediated apoptotic pathways in ganetespib-treated GC
cells.
RTK-mediated signaling pathways are activated
downstream of various HSP90 client proteins, including ErbB2, EGFR
and VEGF, and are thus sensitive to HSP90 inhibition. These
pathways, including PI3K/Akt and MAPK/Erk, are effectively
suppressed by various HSP90 inhibitors [such as 17-DMAG (27), NVP-AUY922 (19) and LD053 (28)] thereby inhibiting targets
associated with cell proliferation, survival and invasion (8). Our study revealed that ganetespib
treatment in GC cells (ErbB2 expression was only detected in N87
cells and not detected in AGS cells) downregulates both ErbB2
activation/phosphorylation and its downstream signaling effector
molecules, including Erk, Akt, mTOR, GSK3 and Src (Fig. 4). ErbB2 is one of the most
sensitive client proteins of HSP90, making various HSP90 inhibitors
remarkably effective in downregulating ErbB2-mediated
signaling.
NVP-AUY922 is a potent HSP90 inhibitor that has
shown efficacy in preclinical models of ErbB2-amplified,
trastuzumab-resistant GC by degrading ErbB2, Akt and Erk and
subsequently suppressing downstream Akt-mediated signaling
(19,29). Similarly, another HSP90 inhibitor,
LD053, exerts its anti-proliferative effects by promoting the
dissociation of the HSP90-Cdc37 complex, degrading c-Raf and
inhibiting Akt-mediated c-Raf/Mek/Erk and PI3K/Akt signaling
(28). Ganetespib is also shown to
inhibit HSP90-p23 complex formation, thereby inhibiting
HSP90-driven client proteins, including mutant EGFR, mutant ErbB2,
KRAS and associated downstream signaling pathways in mutant
ErbB2-driven non-small cell lung cancers (30). EGFR-mediated inhibition of
PI3K/Akt, Ras/Raf/Erk and JAK/STAT pathways was also reported in GC
cell and xenograft models (11).
Our results further support these data and demonstrate inhibition
of PI3K/Akt and MAPK/Erk signaling downstream of EGFR and ErbB2 in
GC cells, suggesting that the signaling regulation by ganetespib
may target both EGFR- and ErbB2-mediated pathways and subsequently
modulate the associated downstream oncogenic signaling.
Despite multiple clinical trials that have reported
a promising safety profile for ganetespib in the treatment of
non-small cell lung cancer and metastatic breast cancer, the
clinical application of ganetespib remains uncertain due to
marginal changes in patient outcomes (31–33).
Nevertheless, in support of our current study and previous reports
indicating that EGFR and/or ErbB2-overexpressing cancer subtypes
may be more responsive to ganetespib, an ongoing phase I clinical
trial is designed to test ganetespib in combination with other
therapeutic agents in ErbB2+ metastatic breast cancer
patients (ClinicalTrials.gov Identifier:
NCT02060253). To further extend the clinical potential of
ganetespib, our study helps to form the foundation for future
preclinical studies and ultimately clinical trials exploring the
application of ganetespib as a monotherapy or combinational therapy
for EGFR+/ErbB2+ gastric cancer patients.
Overall, our studies in AGS and N87 human cell line
models of GC indicate the effectiveness of ganetespib in inhibiting
proliferation and colony-forming ability of cells by inducing G2/M
cell cycle arrest and apoptosis. The anticancer capacity of
ganetespib was further corroborated by the inhibition of ErbB2/RTK
and downstream PI3K/Akt/MAPK signaling pathways. Thus, our data
provide additional mechanistic insights into the activity of
ganetespib and support its clinical development for effective
treatment of EGFR+/ErbB2+ GC.
Acknowledgments
We thank Dr Erin Howard for critical reading and
editing of the manuscript. This work was supported in part by the
American Cancer Society (grant no. RSG-08-138-01-CNE), the National
Institute of Environmental Health Sciences (grant no. R21ES025337),
the National Cancer Institute (grant no. 5U54CA156735), the
National Institute on Alcohol Abuse and Alcoholism (grant no. U54
AA019765), and a UNC GA Research Opportunities Initiative (ROI)
Grant to X.Y.
References
|
1
|
Okamoto W, Okamoto I, Yoshida T, Okamoto
K, Takezawa K, Hatashita E, Yamada Y, Kuwata K, Arao T, Yanagihara
K, et al: Identification of c-Src as a potential therapeutic target
for gastric cancer and of MET activation as a cause of resistance
to c-Src inhibition. Mol Cancer Ther. 9:1188–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society: Cancer Facts and
Figures 2016. ACS; Atlanta, GA: 2016, http://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html.
Accessed date January 24, 2017.
|
|
3
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasui W, Hata J, Yokozaki H, Nakatani H,
Ochiai A, Ito H and Tahara E: Interaction between epidermal growth
factor and its receptor in progression of human gastric carcinoma.
Int J Cancer. 41:211–217. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mi L, Ji X and Ji J: Prognostic biomarker
in advanced gastric cancer. Transl Gastrointest Cancer. 5:16–29.
2016.
|
|
6
|
Aydin K, Okutur SK, Bozkurt M, Turkmen I,
Namal E, Pilanci K, Ozturk A, Akcali Z, Dogusoy G and Demir OG:
Effect of epidermal growth factor receptor status on the outcomes
of patients with metastatic gastric cancer: A pilot study. Oncol
Lett. 7:255–259. 2014.
|
|
7
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moser C, Lang SA and Stoeltzing O:
Heat-shock protein 90 (Hsp90) as a molecular target for therapy of
gastrointestinal cancer. Anticancer Res. 29:2031–2042.
2009.PubMed/NCBI
|
|
9
|
Wang Y, Trepel JB, Neckers LM and Giaccone
G: STA-9090, a small-molecule Hsp90 inhibitor for the potential
treatment of cancer. Curr Opin Investig Drugs. 11:1466–1476.
2010.PubMed/NCBI
|
|
10
|
Jhaveri K and Modi S: Ganetespib: Research
and clinical development. Onco Targets Ther. 8:1849–1858.
2015.PubMed/NCBI
|
|
11
|
Liu H, Lu J, Hua Y, Zhang P, Liang Z, Ruan
L, Lian C, Shi H, Chen K and Tu Z: Targeting heat-shock protein 90
with ganetespib for molecularly targeted therapy of gastric cancer.
Cell Death Dis. 6:e15952015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahata S, Maru S, Shukla S, Pandey A,
Mugesh G, Das BC and Bharti AC: Anticancer property of Bryophyllum
pinnata (Lam.) Oken. leaf on human cervical cancer cells. BMC
Complement Altern Med. 12:152012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takayama S, Reed JC and Homma S:
Heat-shock proteins as regulators of apoptosis. Oncogene.
22:9041–9047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jhaveri K, Ochiana SO, Dunphy MP,
Gerecitano JF, Corben AD, Peter RI, Janjigian YY, Gomes-DaGama EM,
Koren J III, Modi S, et al: Heat shock protein 90 inhibitors in the
treatment of cancer: Current status and future directions. Expert
Opin Investig Drugs. 23:611–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Den RB and Lu B: Heat shock protein 90
inhibition: Rationale and clinical potential. Ther Adv Med Oncol.
4:211–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai CH, Park KS, Lee DH, Alberobello AT,
Raffeld M, Pierobon M, Pin E, Petricoin Iii EF, Wang Y and Giaccone
G: HSP-90 inhibitor ganetespib is synergistic with doxorubicin in
small cell lung cancer. Oncogene. 33:4867–4876. 2014. View Article : Google Scholar :
|
|
17
|
Smith DL, Acquaviva J, Sequeira M, Jimenez
JP, Zhang C, Sang J, Bates RC and Proia DA: The HSP90 inhibitor
ganetespib potentiates the antitumor activity of EGFR tyrosine
kinase inhibition in mutant and wild-type non-small cell lung
cancer. Target Oncol. 10:235–245. 2015. View Article : Google Scholar :
|
|
18
|
Acquaviva J, Smith DL, Jimenez JP, Zhang
C, Sequeira M, He S, Sang J, Bates RC and Proia DA: Overcoming
acquired BRAF inhibitor resistance in melanoma via targeted
inhibition of Hsp90 with ganetespib. Mol Cancer Ther. 13:353–363.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wainberg ZA, Anghel A, Rogers AM, Desai
AJ, Kalous O, Conklin D, Ayala R, O'Brien NA, Quadt C, Akimov M, et
al: Inhibition of HSP90 with AUY922 induces synergy in
HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol
Cancer Ther. 12:509–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burrows F, Zhang H and Kamal A: Hsp90
activation and cell cycle regulation. Cell Cycle. 3:1530–1536.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Srethapakdi M, Liu F, Tavorath R and Rosen
N: Inhibition of Hsp90 function by ansamycins causes retinoblastoma
gene product-dependent G1 arrest. Cancer Res. 60:3940–3946.
2000.PubMed/NCBI
|
|
22
|
Foijer F and te Riele H: Check, double
check: The G2 barrier to cancer. Cell Cycle. 5:831–836. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
DiPaola RS: To arrest or not to G(2)-M
Cell-cycle arrest: commentary re: A.K. Tyagi et al., Silibinin
strongly synergizes human prostate carcinoma DU145 cells to
doxorubicin-induced growth inhibition, G(2)-M arrest, and
apoptosis. Clin Cancer Res. 8:3512–3519. 2002.
Clin Cancer Res. 8:3311–3314. 2002.
|
|
24
|
Toyoshima H and Hunter T: p27 a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Foijer F, Delzenne-Goette E, Dekker M and
Te Riele H: In vivo significance of the G2 restriction point.
Cancer Res. 67:9244–9247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sawai H and Domae N: Discrimination
between primary necrosis and apoptosis by necrostatin-1 in Annexin
V-positive/propidium iodide-negative cells. Biochem Biophys Res
Commun. 411:569–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lang SA, Klein D, Moser C, Gaumann A,
Glockzin G, Dahlke MH, Dietmaier W, Bolder U, Schlitt HJ, Geissler
EK, et al: Inhibition of heat shock protein 90 impairs epidermal
growth factor-mediated signaling in gastric cancer cells and
reduces tumor growth and vascularization in vivo. Mol Cancer Ther.
6:1123–1132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu C, Liu D, Jin J, Deokar H, Zhang Y,
Buolamwini JK, Yu X, Yan C and Chen X: Inhibition of gastric tumor
growth by a novel Hsp90 inhibitor. Biochem Pharmacol. 85:1246–1256.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee KH, Lee JH, Han SW, Im SA, Kim TY, Oh
DY and Bang YJ: Antitumor activity of NVP-AUY922, a novel heat
shock protein 90 inhibitor, in human gastric cancer cells is
mediated through proteasomal degradation of client proteins. Cancer
Sci. 102:1388–1395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimamura T, Perera SA, Foley KP, Sang J,
Rodig SJ, Inoue T, Chen L, Li D, Carretero J, Li YC, et al:
Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has
potent antitumor activity in in vitro and in vivo models of
non-small cell lung cancer. Clin Cancer Res. 18:4973–4985. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Socinski MA, Goldman J, El-Hariry I,
Koczywas M, Vukovic V, Horn L, Paschold E, Salgia R, West H,
Sequist LV, et al: A multi-center phase II study of ganetespib
monotherapy in patients with genotypically defined advanced
non-small cell lung cancer. Clin Cancer Res. 19:3068–3077. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramalingam S, Goss G, Rosell R,
Schmid-Bindert G, Zaric B, Andric Z, Bondarenko I, Komov D, Ceric
T, Khuri F, et al: A randomized phase II study of ganetespib, a
heat shock protein 90 inhibitor, in combination with docetaxel in
second-line therapy of advanced non-small cell lung cancer
(GALAXY-1). Ann Oncol. 26:1741–1748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jhaveri K, Chandarlapaty S, Lake D,
Gilewski T, Robson M, Goldfarb S, Drullinsky P, Sugarman S,
Wasserheit-Leiblich C, Fasano J, et al: A phase II open-label study
of ganetespib, a novel heat shock protein 90 inhibitor for patients
with metastatic breast cancer. Clin Breast Cancer. 14:154–160.
2014. View Article : Google Scholar : PubMed/NCBI
|